Abstract
BACKGROUND
Cortical disease has emerged as a critical aspect of the pathogenesis of multiple sclerosis, being associated with disease progression and cognitive impairment. Most studies of cortical lesions have focused on autopsy findings in patients with long-standing, chronic, progressive multiple sclerosis, and the noninflammatory nature of these lesions has been emphasized. Magnetic resonance imaging studies indicate that cortical damage occurs early in the disease.
METHODS
We evaluated the prevalence and character of demyelinating cortical lesions in patients with multiple sclerosis. Cortical tissues were obtained in passing during biopsy sampling of white-matter lesions. In most cases, biopsy was done with the use of stereotactic procedures to diagnose suspected tumors. Patients with sufficient cortex (138 of 563 patients screened) were evaluated for cortical demyelination. Using immunohistochemistry, we characterized cortical lesions with respect to demyelinating activity, inflammatory infiltrates, the presence of meningeal inflammation, and a topographic association between cortical demyelination and meningeal inflammation. Diagnoses were ascertained in a subgroup of 77 patients (56%) at the last follow-up visit (at a median of 3.5 years).
RESULTS
Cortical demyelination was present in 53 patients (38%) (104 lesions and 222 tissue blocks) and was absent in 85 patients (121 tissue blocks). Twenty-five patients with cortical demyelination had definite multiple sclerosis (81% of 31 patients who underwent long-term follow-up), as did 33 patients without cortical demyelination (72% of 46 patients who underwent long-term follow-up). In representative tissues, 58 of 71 lesions (82%) showed CD3+ T-cell infiltrates, and 32 of 78 lesions (41%) showed macrophage-associated demyelination. Meningeal inflammation was topographically associated with cortical demyelination in patients who had sufficient meningeal tissue for study.
CONCLUSIONS
In this cohort of patients with early-stage multiple sclerosis, cortical demyelinating lesions were frequent, inflammatory, and strongly associated with meningeal inflammation. (Funded by the National Multiple Sclerosis Society and the National Institutes of Health.)
Diagnostic, therapeutic, and investigative efforts in multiple sclerosis have concentrated on disease of the white matter. Imaging and histopathological studies suggest that cortical damage is a correlate of cognitive dysfunction and disease progression, reflecting demyelination or secondary neurodegeneration.1–7 Three types of cortical plaques have been described: leukocortical lesions, which extend from the white matter into the cortex; intracortical lesions, which project radially from microvessels; and subpial lesions, which extend intracortically from the pia mater.5,8 Subpial, bandlike, demyelinated plaques often involve contiguous gyri,6 favoring those regions of the brain engaged in attention and memory processing.6,8,9
Studies of postmortem tissues from patients with long-standing multiple sclerosis have led to the suggestion that cortical demyelination contributes to disability in patients with progressive multiple sclerosis,6 occurs independently of white-matter lesions,6 is driven by organized meningeal inflammatory infiltrates10,11 and is devoid of parenchymal lymphocytes and macrophages,4,5,8 suggesting that neurodegeneration proceeds independently of parenchymal inflammation.
Little is known about cortical demyelination in early multiple sclerosis because conventional magnetic resonance imaging (MRI) does not reveal most lesions. Double inversion-recovery imaging (not routinely performed in practice) detects some but not all cortical lesions. Other neuroimaging studies in early multiple sclerosis have revealed varied, nonlesional cortical abnormalities,12–14 suggesting that the cortex may be damaged near the time of disease onset — a concept further supported by recent case reports of cortical-onset multiple sclerosis.15,16
Here we report the prevalence, histopathological features, and clinical correlates of cortical demyelination in a series of 563 patients with pathologically confirmed inflammatory demyelinating disease of the central nervous system, diagnosed by means of brain biopsy performed within days or weeks after presentation, to rule out diseases other than multiple sclerosis.
METHODS
STUDY DESIGN AND SERIES
This study was approved by the institutional review board of the Mayo Clinic. Brain-biopsy specimens were obtained within the context of routine clinical care, with written or oral consent to this surgical procedure obtained by the treating physicians. At the time of study entry, we obtained written informed consent from patients who underwent prospective blood sampling, underwent follow-up MRI, participated in a face-to-face encounter, were interviewed by telephone, or underwent a combination of these assessments. In the case of deceased patients, written informed consent was obtained from the next of kin. The institutional review board issued a waiver of the requirement of consent for the examination of archival pathological material when attempts to contact the patient had been exhausted; samples evaluated under this waiver were deidentified.
Inclusion criteria were a pathological diagnosis of inflammatory demyelinating disease consistent with multiple sclerosis and a sufficient amount of tissue for analyses (≥1 mm2). Exclusion criteria were the presence of acute disseminated encephalomyelitis, 17 neuromyelitis optica,18 or any other disease of the central nervous system. Clinical information was obtained from patients, family members, or physicians; a review of the patient’s medical record; face-to-face encounters; or a combination of these sources. Patients received the diagnosis of multiple sclerosis according to the McDonald19 or Poser20 criteria. A single episode indicated a clinically isolated syndrome.
NEUROPATHOLOGICAL EVALUATION
Formalin-fixed, paraffin-embedded sections were stained with hematoxylin and eosin, Luxol fast blue and periodic acid–Schiff, and Bielschowsky silver impregnation. Avidin–biotin immunohistochemical analysis was performed21 with the use of primary antibodies22 (see the Supplementary Appendix, available with the full text of this article at NEJM.org). Cortical plaque types were classified according to standard criteria,4,5,8 and demyelinating activity was staged according to published criteria.23 Grading of cortical inflammation and meningeal inflammation is described in the Supplementary Appendix.
STATISTICAL ANALYSIS
Statistical inference was made on the basis of logistic-regression analysis, and the strengths of associations were expressed as odds ratios along with 95% confidence intervals. Additional details are provided in the Supplementary Appendix.
RESULTS
PREVALENCE AND SPECTRUM OF CORTICAL DEMYELINATION
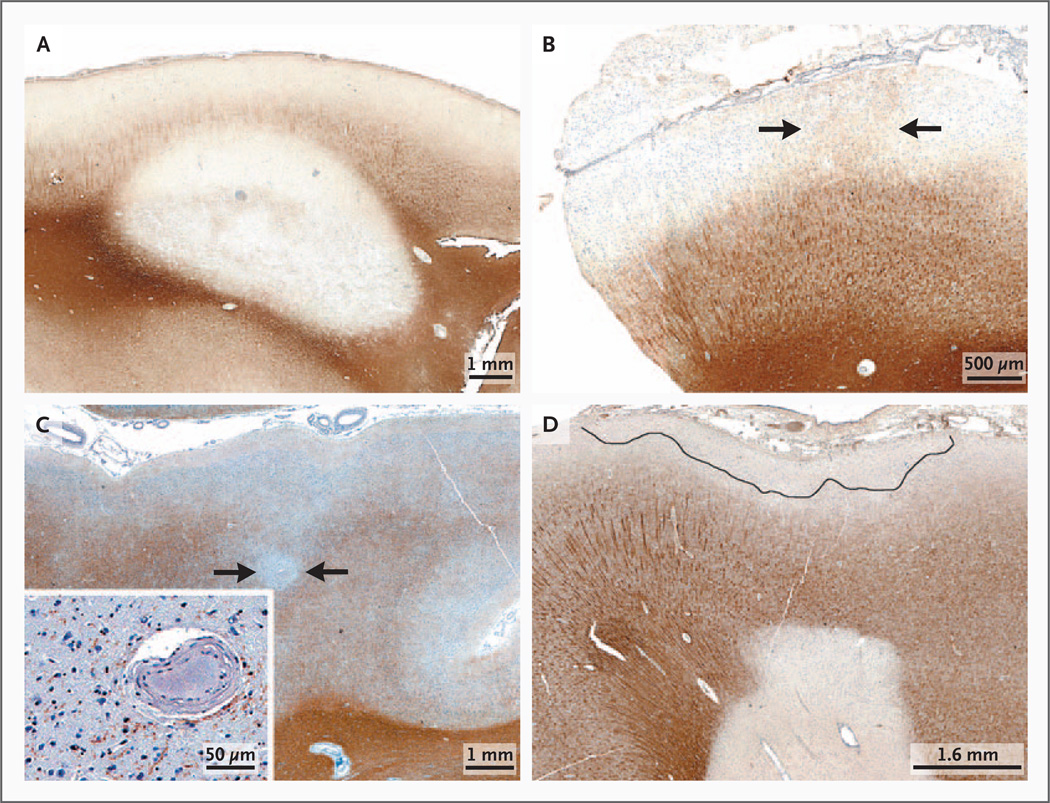
Figure 1 in the Supplementary Appendix shows the study design. Of the 138 patients for whom a sufficient amount of cortex was available for analysis, 53 (38%) had cortical demyelination. Of the 85 patients without cortical demyelination, 12 (14%) had cortical inflammation and 73 (86%) had cortex that appeared to be normal. All three types of plaque were observed in those with cortical demyelination (Fig. 1A, 1B, and 1C, and Fig. 2A in the Supplementary Appendix). Of 104 lesions studied, leukocortical lesions were the most common (52 [50%]), followed by subpial lesions (35 [34%]) and intracortical lesions (17 [16%]). Some single biopsy specimens contained more than one type of plaque (Fig. 1D).
Figure 1. Representative Types of Cortical Demyelinated Plaques in Early Multiple Sclerosis on Immunohistochemical Staining for Proteolipid Protein.
Panel A shows leukocortical demyelination; Panel B subpial demyelination (arrows delineate an area of cortex with preserved myelin); Panel C intracortical demyelination (arrows), with neurons in the demyelinated lesion (inset); and Panel D subpial and leukocortical demyelination in the same tissue section.
CLINICAL CHARACTERISTICS
Biopsies were typically performed at the time of presentation, with a median interval from symptom onset to biopsy of 27 days (interquartile range, 14 to 72). The median time to biopsy was 26 days (interquartile range, 13 to 73) for the group with cortical demyelination and 44 days (interquartile range, 15 to 64) for the group without cortical demyelination (area under the curve, 0.56; P = 0.59). Of the 138 patients, 77 (56%) underwent comprehensive clinical follow-up (median, 3.5 years; interquartile range, 1.3 to 7.5); multiple sclerosis was diagnosed in 58 of these patients (75%), and a clinically isolated syndrome was diagnosed in 19 (25%). Table 1 in the Supplementary Appendix shows the percentages of patients with and those without cortical demyelination according to diagnosis, which were not significantly different (P = 0.60 by Fisher’s exact test). Table 2 in the Supplementary Appendix shows the percentage of patients with cortical demyelination according to follow-up group (Fig. 1 in the Supplementary Appendix). Among those with definite multiple sclerosis or a clinically isolated syndrome, the prevalence of cortical demyelination was 40%, as compared with 38% in the total cohort.
DEMYELINATING ACTIVITY
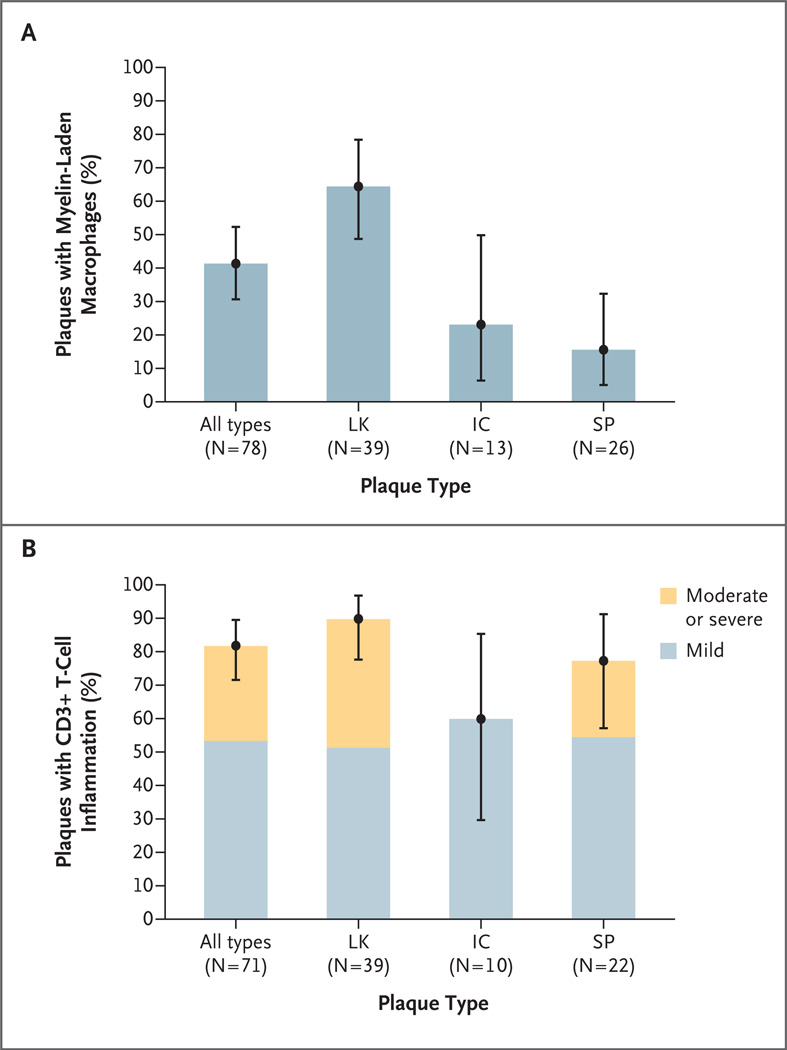
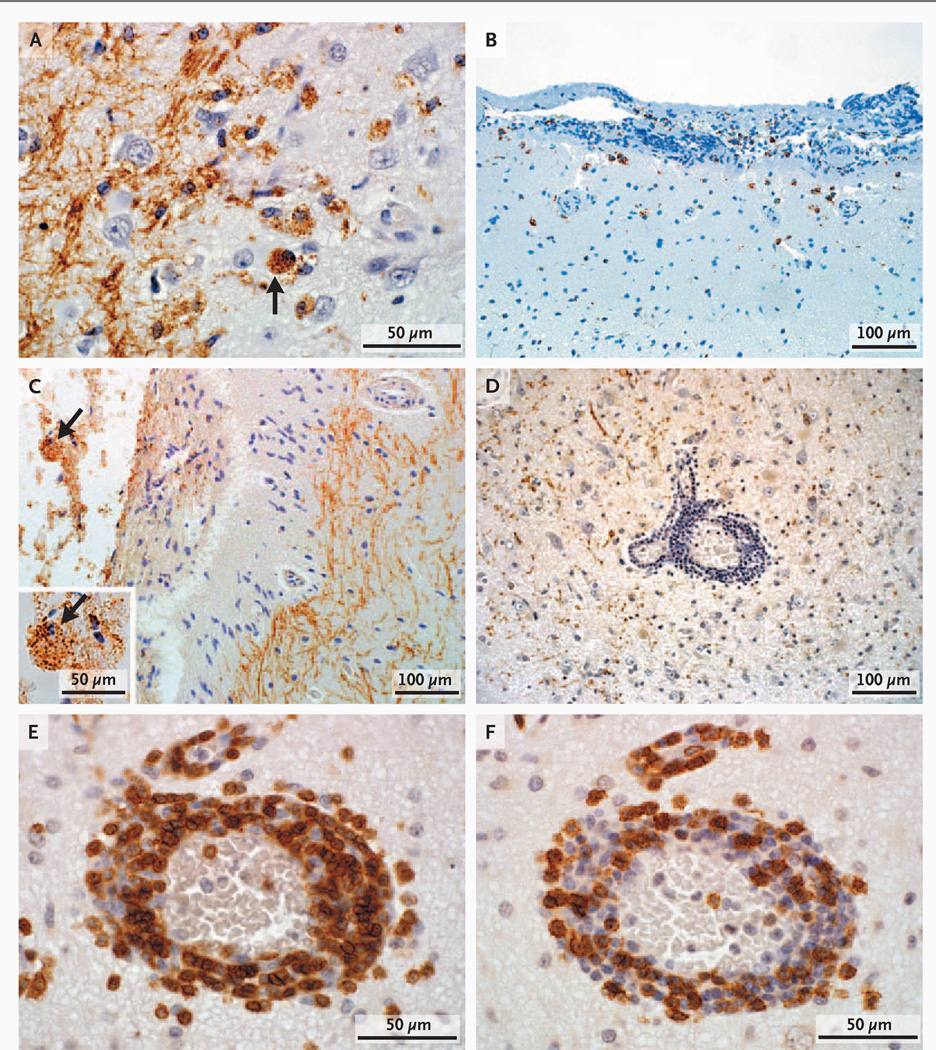
Cortical lesions in late-stage multiple sclerosis show few actively demyelinating plaques,4,5,8 precluding temporal associations between demyelination and phagocytic macrophages. Lesions were staged according to the presence or absence of myelin debris (indicating active or inactive demyelination, respectively) within macrophages in a subset of cortical lesions (78 lesions from 41 patients) with sufficient tissue for analysis and with a cortical-plaque distribution that was similar to the distribution in the overall cohort. Of these 41 patients, 27 (66%) had cortical lesions that contained foamy macrophages, indicating ongoing demyelination. (We observed 39 active lesions.) Myelin-laden macrophages were found only in the context of cortical demyelination, in all three cortical plaque types (Fig. 2A), and most commonly in leukocortical lesions (25 of 39 lesions). Subpial lesions (4 of 26) and intracortical lesions (3 of 13) also contained myelin-laden macrophages (Fig. 3A), occasionally found in cortical layer 1 at the subpial rim (Fig. 3B) and in the subarachnoid space (Fig. 3C). We detected activated microglia in all 78 cortical lesions.
Figure 2. Inflammatory Features of Cortical Plaques.
Panel A shows the percentage of plaques with myelin-laden macrophages, according to plaque type, among 78 lesions (41 patients) with cortical demyelination. The percentage of myelin-laden macrophages in leukocortical plaques (LK) was significantly higher than the percentages in intracortical plaques (IC) (P = 0.02) and subpial plaques (SP) (P<0.001). The percentages did not differ significantly between IC plaques and SP plaques (P = 0.56). Panel B shows the percentage of plaques with mild T-cell inflammation and the percentage with moderate or severe T-cell inflammation among 71 lesions (38 patients) analyzed on the basis of CD3+ counts. The percentages of plaques that showed any inflammation did not differ significantly across plaque types (P = 0.09). I bars represent 95% confidence intervals based on logistic-regression models. Significance tests were also based on logistic-regression models.
Figure 3. Components of Parenchymal Inflammatory Infiltrates in Cortical Lesions.
In Panel A, myelin-laden macrophages (arrow) indicate the presence of active cortical demyelination in early multiple sclerosis (immunohistochemical staining for proteolipid protein [PLP]). In Panel B, macrophages are present in the molecular layer of the cortex at the subpial rim (immunohistochemical staining for CD68). In Panel C, myelin-laden macrophages (arrow) are also present in the subarachnoid space (PLP staining), and the inset shows the myelin-laden macrophage (arrow) at a higher magnification (PLP staining). In Panel D, marked perivascular inflammation is present in cortical plaques (PLP staining). Components of the perivascular inflammatory infiltrates include CD3+ T cells in Panel E (immunohistochemical staining for CD3) and cytotoxic T cells in Panel F (immunohistochemical staining for CD8).
CORTICAL INFLAMMATION
In 38 patients who had a representative corticalplaque distribution and sufficient tissue available for analysis, we analyzed 71 cortical lesions for CD3+ T cells and 70 for CD8+ T cells. Perivascular CD3+ T-cell inflammation was observed in 58 of 71 cortical plaques (82%) (Fig. 2B, 3D, and 3E), and CD8+ T cells were present in 54 of 70 cortical plaques (77%) (Fig. 3F, and Fig. 2B in the Supplementary Appendix). Leukocortical lesions were highly inflammatory. The majority of intracortical and subpial plaques contained perivascular CD3+ and CD8+ T-cell infiltrates. Furthermore, 23% of subpial lesions contained moderate-to-marked CD3+ T-cell inflammation. We observed B-cell perivascular cortical inflammation in 4 of 15 cortical plaques analyzed (27%). Owing to limited tissue availability, we did not probe for the presence of other lymphocyte subsets.
MENINGEAL INFLAMMATION
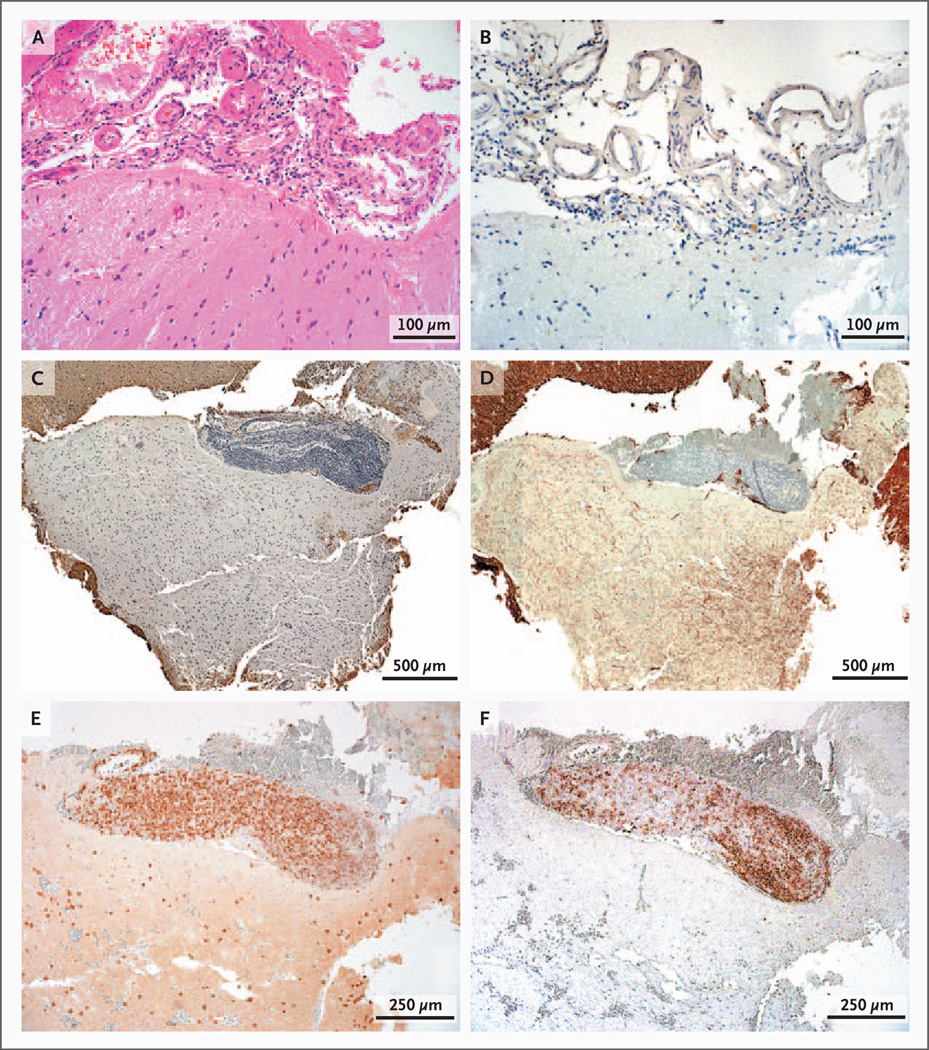
Of the 138 patients with inflammatory demyelinating disease and with cortical tissue available for analysis, 43 (31%) had meningeal tissue available for analysis. Of these 43 patients, 15 (35%) had cortical demyelination — a proportion similar to that in the overall cohort (38%). Tissue specimens from 2 patients were initially excluded from the analysis because of surgical hemorrhage. We assessed the other 41 patients for focal perivascular meningeal inflammation. Surgical hemorrhage impaired the assessment for diffuse meningeal inflammation in 11 patients, who were then also excluded from the analysis. The patients with cortical demyelination were more likely to have diffuse meningeal inflammation (Table 3 and Fig. 3A in the Supplementary Appendix), as well as moderate or marked focal perivascular meningeal inflammation (Table 4 and Fig. 3B in the Supplementary Appendix), than were the patients without cortical demyelination. Diffuse meningeal inflammation (Fig. 4A and 4B) was significantly and strongly associated with cortical demyelination (odds ratio, 45; 95% confidence interval [CI], 4 to 478; P<0.001), whereas focal perivascular meningeal inflammation (Fig. 4C, 4E, and 4F) was less strongly, albeit still significantly, associated with cortical demyelination (odds ratio, 15; 95% CI, 1 to 213; P = 0.002). Among confirmed cases of multiple sclerosis or a clinically isolated syndrome, robust associations between meningeal inflammation and cortical demyelination were noted (Tables 5 through 8 in the Supplementary Appendix). Remarkably, there was a 90% probability that moderate-to-marked meningeal inflammation was topographically associated with cortical demyelination, especially for subpial plaques as compared with intracortical plaques and with leukocortical plaques (P = 0.004 for both comparisons) with respect to diffuse meningeal inflammation, and as compared with intracortical plaques (P = 0.001) and leukocortical plaques (P = 0.03) with respect to focal meningeal inflammation (Fig. 4C). The time from the onset of symptoms to biopsy was not related to the presence or absence of cortical demyelination or meningeal inflammation.
Figure 4. Components of Meningeal Inflammatory Infiltrates.
Panel A shows moderate-to-marked, diffuse meningeal inflammation (hematoxylin and eosin). Panel B shows moderate-to-marked, diffuse meningeal inflammation topographically associated with a subpial plaque (immunohistochemical staining for PLP). Panel C shows marked perivascular meningeal inflammation topographically associated with a subpial plaque (PLP staining). Neuritic loss reflects the destructive nature of the subpial plaque in Panel D (immunohistochemical staining for neurofilament). Components of the perivascular meningeal inflammatory infiltrates include CD3+ T cells in Panel E (immunohistochemical staining for CD3) and B cells in Panel F (immunohistochemical staining for CD20).
NEURODEGENERATION
Neurodegeneration that occurs in multiple sclerosis lesions includes neuronal, neuritic, and oligodendroglial injury, in addition to demyelination. Such changes in the absence of inflammation have been reported in chronic cortical plaques, raising the question of whether inflammation and neurodegeneration are independent processes in multiple sclerosis.4,7,24 Several cortical lesions that we examined showed neuritic swelling, suggesting acute injury, although the majority showed relative preservation of neurites. We observed severe focal neuritic loss in two subpial lesions (one in each of two patients). In one patient, a large focal meningeal infiltrate was topographically associated with a destructive subpial lesion (Fig. 4D). Oligodendrocyte density was reduced (Fig. 5B) in a subset of lesions, as compared with adjacent, nondemyelinated cortex (Fig. 5A). Focal neuronal injury was apparent in several early cortical plaques (Fig. 5C). Neurodegenerative changes occurred on a background of inflammation (Fig. 5D and 5E).
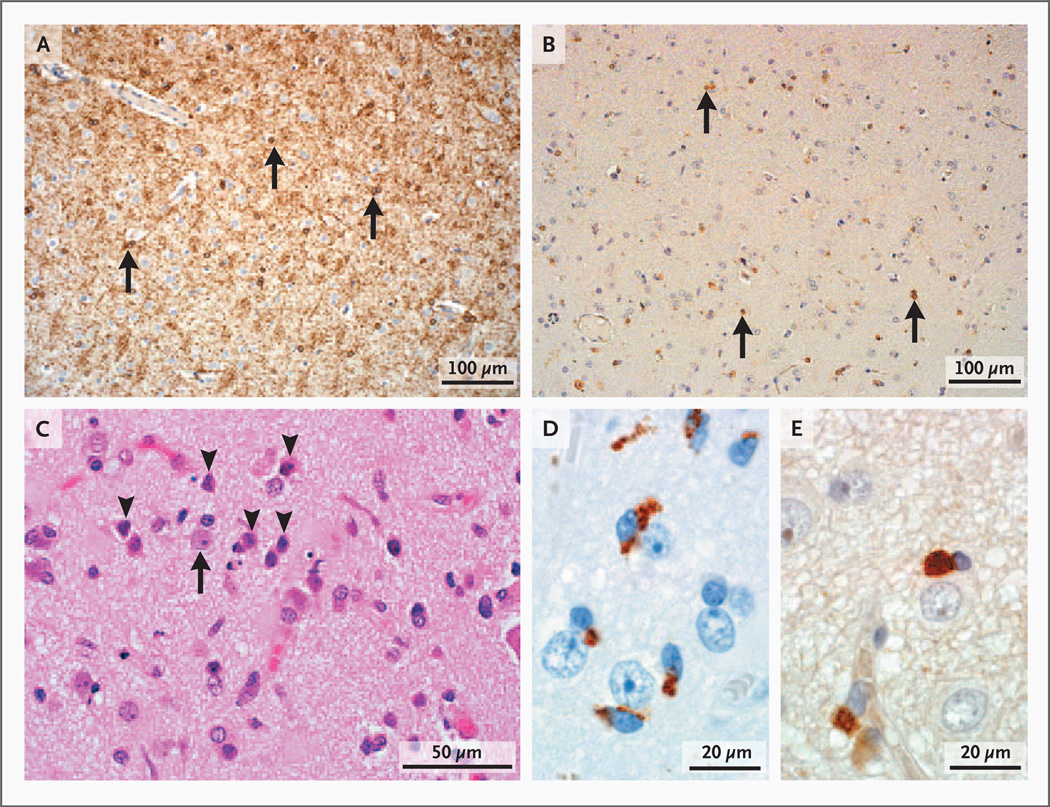
Figure 5. Other Neuropathological Characteristics of Cortical Demyelinating Lesions in Early Multiple Sclerosis.
Panel A shows normal oligodendrocyte density (arrows) in nondemyelinated cortex (immunohistochemical staining for 2′3′-cyclic-nucleotide 3′-phosphodiesterase [CNPase]). Panel B shows reduced oligodendrocyte density (arrows) in demyelinated cortex (CNPase staining). In Panel C, neuronal injury is evidenced by the presence of pyknotic neurons (arrowheads) scattered among healthy neurons (arrow) (hematoxylin and eosin). Microglia in Panel D (immunohistochemical staining for KiM1P) are close to neurons, and T cells in Panel E (immunohistochemical staining for CD3) are close to oligodendrocytes.
DISCUSSION
We characterized demyelinating lesions in cortical-biopsy specimens from patients with early-stage multiple sclerosis, which were obtained in passing during diagnostic procedures targeting white-matter lesions (Fig. 4 in the Supplementary Appendix). Nearly 40% of these patients had cortical demyelination. Indirect evidence that cortical demyelination is common in early-stage multiple sclerosis comes from MRI studies showing cortical lesions in approximately 30% of 119 patients with a clinically isolated syndrome.25 Others have reported cortical lesions and cortical atrophy on MRI in patients with early multiple sclerosis,26–29 although cortical lesions were not observed in a study of children with multiple sclerosis.30
The spatial separation of intracortical and subpial lesions (which together represented about 50% of the lesions detected) from the biopsy specimens of white-matter lesions suggests intrinsic cortical demyelinating disease. The prevalence of intrinsic cortical lesions was remarkably high, given the small tissue samples available for study (core diameter, 1 mm).
We do not believe that the presence of tumefactive white-matter lesions affects the biology of the cortical lesions, hence our interpretation of the presence and appearance of these cortical lesions. We believe that the patients in our study who underwent biopsy are typical of those with multiple sclerosis; among 77 patients for whom long-term clinical follow-up data were available, 58 (75%) had definite multiple sclerosis and 19 (25%) were categorized as having a clinically isolated syndrome at the last follow-up.
Understanding the neuropathophysiology of multiple sclerosis requires analysis of tissue, which carries potential biases. Despite atypical clinical and radiographic presentations, evidence suggests that the results of biopsy studies in multiple sclerosis can be cautiously extrapolated to prototypical multiple sclerosis. In a previous study, we compared our patients with a population-based cohort of 218 persons with definite multiple sclerosis who were matched for age, sex, and disease duration with 91 persons with inflammatory demyelinating disease of the central nervous system who underwent biopsy, with a median clinical follow-up of 4.4 years.31 Multiple sclerosis developed in 82 of the 91 patients who underwent biopsy, and the clinical course and extent of disability in these patients were indistinguishable from those of patients in the prevalence cohort, who did not undergo biopsy. In another report, multiple sclerosis had developed by the time of the last follow-up assessment (median, 3.9 years) in 70% of 168 patients with tumefactive inflammatory demyelinating disease who underwent biopsy.32
The relevance of cortical injury in the diagnosis and prognosis of multiple sclerosis is widely acknowledged.28,33 Cognitive impairment correlates positively with gray-matter atrophy,34 cortical-lesion burden,29 and reduced cortical thickness.35 Cortical-lesion volume is an independent predictor of disability progression at follow-up,35 and cortical lesions are less common in patients with benign multiple sclerosis, in which remission between relapses is almost complete, with little (if any) accumulation of disability 15 to 20 years after the diagnosis.36 Cortical lesions are more common in patients who have relapsing–remitting multiple sclerosis with seizures than in those who have relapsing–remitting multiple sclerosis without seizures. 37 Therefore, an understanding of the prevalence and extent of cortical demyelination in early multiple sclerosis may help inform assessment of the prognosis and treatment decisions.
We found that cortical demyelination is common early in multiple sclerosis, and our characterization of the lesion underscored its inflammatory character. Cortical demyelination that occurs close to the onset of multiple sclerosis differs substantially from that seen in chronic multiple sclerosis. These findings do not support a primary (noninflammatory) neurodegenerative process during early-stage multiple sclerosis. Differences between cortical demyelination in early multiple sclerosis and in long-standing, progressive multiple sclerosis, in which inflammatory cortical demyelination is typically not observed, may relate to efficient clearance of cortical inflammation.38,39 With respect to a potential mechanism of disease progression, we speculate that myelin-laden macrophages may leave the cortex, enter the cerebrospinal fluid (CSF), gain access to deep cervical lymph nodes to promote epitope spreading,40 and thus propagate the disease process (Fig. 5 in the Supplementary Appendix). Antigen-presenting cells injected into the CSF of rodents were found in deep cervical lymph nodes,41 and macrophage-containing myelin debris has been observed in the cervical lymph nodes of patients with multiple sclerosis.42
Meningeal aggregates in the tissues of patients with multiple sclerosis may contribute to cortical demyelination and progression.6,11,43,44 During the prodrome in experimental autoimmune encephalomyelitis, pathogenic T cells enter the CSF,45,46 are restimulated by meningeal antigen-presenting cells,47 undergo clonal expansion, and produce cytokines, promoting T-cell infiltration across pial vessels,48 activation of deeper vasculature,49 parenchymal invasion, and the onset of disease. These mechanisms have been studied mainly in the white matter of the spinal cord in experimental autoimmune encephalomyelitis, adjacent to CSF flow pathways. Cortex is the only common CSF-adjacent tissue obtained on biopsy in the clinical setting, and our findings broadly correspond to observations made in experimental autoimmune encephalomyelitis (Fig. 5 in the Supplementary Appendix). Subpial cortical demyelination showed a strong topographic relation to meningeal inflammation, suggesting that meningeal infiltrates may contribute to early cortical demyelination.
We observed concurrent subpial and leukocortical lesions in individual tissue sections, suggesting that superficial demyelinating disease may contribute to the generation of deeper lesions by means of cytokine diffusion.41 In support of this hypothesis, a recent analysis of autopsy specimens from patients with progressive multiple sclerosis showed antigen-experienced B-cell clones in meningeal aggregates that were identical to those found in parenchymal perivascular spaces near plaques, as indicated by variable-region sequence alignment.50
Our findings of microglial activation, neuritic injury, pyknotic neurons, and reduced oligodendrocyte density in patients with early multiple sclerosis are consonant with the findings in patients with progressive multiple sclerosis,4 underscoring the potential of cortical demyelination to cause irreversible injury, although inflammation may resolve rapidly. The relationship between early cortical demyelination and cognitive impairment, disease progression, and cortical atrophy awaits future research.
Supplementary Material
Acknowledgments
Supported by grants from the National Multiple Sclerosis Society (NMSS RG3185-B-3, to Dr. Lucchinetti) and the National Institutes of Health (1R01NS049577, to Dr. Lucchinetti, and P50NS38667, to Dr. Ransohoff).
We thank Patricia Ziemer for technical assistance, Linda Linbo for assistance in patient recruitment, and Dr. Gabriele DeLuca for editorial input on an earlier draft of the manuscript.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Lumsden C. The neuropathology of multiple sclerosis. In: Vinken P, Bruyn G, editors. Handbook of clinical neurology. New York: Elsevier; 1970. pp. 217–309. [Google Scholar]
- 2.Brownell B, Hughes JT. The distribution of plaques in the cerebrum in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1962;25:315–320. doi: 10.1136/jnnp.25.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kidd D, Barkhof F, McConnell R, Algra PR, Allen IV, Revesz T. Cortical lesions in multiple sclerosis. Brain. 1999;122:17–26. doi: 10.1093/brain/122.1.17. [DOI] [PubMed] [Google Scholar]
- 4.Peterson JW, Bø L, Mørk S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50:389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- 5.Bø L, Vedeler C, Nyland H, Trapp B, Mørk S. Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler. 2003;9:323–331. doi: 10.1191/1352458503ms917oa. [DOI] [PubMed] [Google Scholar]
- 6.Kutzelnigg A, Lucchinetti C, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- 7.Wegner C, Esiri MM, Chance SA, Palace J, Matthews PM. Neocortical neuronal, synaptic, and glial loss in multiple sclerosis. Neurology. 2006;67:960–967. doi: 10.1212/01.wnl.0000237551.26858.39. [DOI] [PubMed] [Google Scholar]
- 8.Bø L, Vedeler C, Nyland H, Trapp B, Mørk S. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol. 2003;62:723–732. doi: 10.1093/jnen/62.7.723. [DOI] [PubMed] [Google Scholar]
- 9.Kutzelnigg A, Lassmann H. Cortical demyelination in multiple sclerosis: a substrate for cognitive deficits. J Neurol Sci. 2006;245:123–126. doi: 10.1016/j.jns.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 10.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14:164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magliozzi R, Howell O, Vora A, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130:1089–1104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- 12.De Stefano N, Matthews PM, Filippi M, et al. Evidence of early cortical atrophy in MS: relevance to white matter changes and disability. Neurology. 2003;60:1157–1162. doi: 10.1212/01.wnl.0000055926.69643.03. [DOI] [PubMed] [Google Scholar]
- 13.Dalton CM, Chard DT, Davies GR, et al. Early development of multiple sclerosis is associated with progressive grey matter atrophy in patients presenting with clinically isolated syndromes. Brain. 2004;127:1101–1107. doi: 10.1093/brain/awh126. [DOI] [PubMed] [Google Scholar]
- 14.Vrenken H, Pouwels PJ, Geurts JJ, et al. Altered diffusion tensor in multiple sclerosis normal-appearing brain tissue: cortical diffusion changes seem related to clinical deterioration. J Magn Reson Imaging. 2006;23:628–636. doi: 10.1002/jmri.20564. [Erratum, J Magn Reson Imaging 2008;28:1309.] [DOI] [PubMed] [Google Scholar]
- 15.Calabrese M, Gallo P. Magnetic resonance evidence of cortical onset of multiple sclerosis. Mult Scler. 2009;15:933–941. doi: 10.1177/1352458509106510. [DOI] [PubMed] [Google Scholar]
- 16.Popescu BF, Bunyan RF, Parisi JE, Ransohoff RM, Lucchinetti CF. A case of multiple sclerosis presenting with inflammatory cortical demyelination. Neurology. 2011;76:1705–1710. doi: 10.1212/WNL.0b013e31821a44f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart MN, Earle KM. Haemorrhagic and perivenous encephalitis: a clinical-pathological review of 38 cases. J Neurol Neurosurg Psychiatry. 1975;38:585–591. doi: 10.1136/jnnp.38.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 19.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 20.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 21.Vass K, Lassmann H, Wekerle H, Wisniewski HM. The distribution of Ia antigen in the lesions of rat acute experimental allergic encephalomyelitis. Acta Neuropathol. 1986;70:149–160. doi: 10.1007/BF00691433. [DOI] [PubMed] [Google Scholar]
- 22.Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 23.Brück W, Porada P, Poser S, et al. Monocyte-macrophage differentiation in early multiple sclerosis lesions. Ann Neurol. 1995;38:788–796. doi: 10.1002/ana.410380514. [DOI] [PubMed] [Google Scholar]
- 24.Vercellino M, Plano F, Votta B, Mutani R, Giordana MT, Cavalla P. Grey matter pathology in multiple sclerosis. J Neuropathol Exp Neurol. 2005;64:1101–1107. doi: 10.1097/01.jnen.0000190067.20935.42. [DOI] [PubMed] [Google Scholar]
- 25.Filippi M, Rocca MA, Calabrese M, et al. Intracortical lesions: relevance for new MRI diagnostic criteria for multiple sclerosis. Neurology. 2010;75:1988–1994. doi: 10.1212/WNL.0b013e3181ff96f6. [DOI] [PubMed] [Google Scholar]
- 26.Tallantyre EC, Morgan PS, Dixon JE, et al. 3 Tesla and 7 Tesla MRI of multiple sclerosis cortical lesions. J Magn Reson Imaging. 2010;32:971–977. doi: 10.1002/jmri.22115. [DOI] [PubMed] [Google Scholar]
- 27.Tardif CL, Collins DL, Eskildsen SF, Richardson JB, Pike GB. Segmentation of cortical MS lesions on MRI using automated laminar profile shape analysis. Med Image Comput Assist Interv. 2010;13:181–188. doi: 10.1007/978-3-642-15711-0_23. [DOI] [PubMed] [Google Scholar]
- 28.Calabrese M, Filippi M, Gallo P. Cortical lesions in multiple sclerosis. Nat Rev Neurol. 2010;6:438–444. doi: 10.1038/nrneurol.2010.93. [DOI] [PubMed] [Google Scholar]
- 29.Calabrese M, De Stefano N, Atzori M, et al. Detection of cortical inflammatory lesions by double inversion recovery magnetic resonance imaging in patients with multiple sclerosis. Arch Neurol. 2007;64:1416–1422. doi: 10.1001/archneur.64.10.1416. [DOI] [PubMed] [Google Scholar]
- 30.Absinta M, Rocca MA, Moiola L, et al. Cortical lesions in children with multiple sclerosis. Neurology. 2011;76:910–913. doi: 10.1212/WNL.0b013e31820f2e69. [DOI] [PubMed] [Google Scholar]
- 31.Pittock SJ, McClelland RL, Achenbach SJ, et al. Clinical course, pathologic correlations, and outcome of biopsy proven inflammatory demyelinating disease. J Neurol Neurosurg Psychiatry. 2005;76:1693–1697. doi: 10.1136/jnnp.2004.060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucchinetti CF, Gavrilova RH, Metz I, et al. Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain. 2008;131:1759–1775. doi: 10.1093/brain/awn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calabrese M, Rinaldi F, Grossi P, Gallo P. Cortical pathology and cognitive impairment in multiple sclerosis. Expert Rev Neurother. 2011;11:425–432. doi: 10.1586/ern.10.155. [DOI] [PubMed] [Google Scholar]
- 34.Rudick RA, Lee JC, Nakamura K, Fisher E. Gray matter atrophy correlates with MS disability progression measured with MSFC but not EDSS. J Neurol Sci. 2009;282:106–111. doi: 10.1016/j.jns.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calabrese M, Rocca MA, Atzori M, et al. Cortical lesions in primary progressive multiple sclerosis: a 2-year longitudinal MR study. Neurology. 2009;72:1330–1336. doi: 10.1212/WNL.0b013e3181a0fee5. [DOI] [PubMed] [Google Scholar]
- 36.Calabrese M, Filippi M, Rovaris M, et al. Evidence for relative cortical sparing in benign multiple sclerosis: a longitudinal magnetic resonance imaging study. Mult Scler. 2009;15:36–41. doi: 10.1177/1352458508096686. [DOI] [PubMed] [Google Scholar]
- 37.Calabrese M, De Stefano N, Atzori M, et al. Extensive cortical inflammation is associated with epilepsy in multiple sclerosis. J Neurol. 2008;255:581–586. doi: 10.1007/s00415-008-0752-7. [DOI] [PubMed] [Google Scholar]
- 38.Merkler D, Ernsting T, Kerschensteiner M, Brück W, Stadelmann C. A new focal EAE model of cortical demyelination: multiple sclerosis-like lesions with rapid resolution of inflammation and extensive remyelination. Brain. 2006;129:1972–1983. doi: 10.1093/brain/awl135. [DOI] [PubMed] [Google Scholar]
- 39.Lassmann H, Kitz K, Wisniewski HM. Histogenesis of demyelinating lesions in the spinal cord of guinea pigs with chronic relapsing experimental allergic encephalomyelitis. J Neurol Sci. 1981;50:109–121. doi: 10.1016/0022-510x(81)90046-0. [DOI] [PubMed] [Google Scholar]
- 40.Scheinecker C, McHugh R, Shevach EM, Germain RN. Constitutive presentation of a natural tissue autoantigen exclusively by dendritic cells in the draining lymph node. J Exp Med. 2002;196:1079–1090. doi: 10.1084/jem.20020991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hatterer E, Davoust N, Didier-Bazes M, et al. How to drain without lymphatics? Dendritic cells migrate from the cerebrospinal fluid to the B-cell follicles of cervical lymph nodes. Blood. 2006;107:806–812. doi: 10.1182/blood-2005-01-0154. [DOI] [PubMed] [Google Scholar]
- 42.van Zwam M, Huizinga R, Melief MJ, et al. Brain antigens in functionally distinct antigen-presenting cell populations in cervical lymph nodes in MS and EAE. J Mol Med. 2009;87:273–286. doi: 10.1007/s00109-008-0421-4. [DOI] [PubMed] [Google Scholar]
- 43.Lassmann H, Wisniewski HM. Chronic relapsing EAE: time course of neurological symptoms and pathology. Acta Neuropathol. 1978;43:35–42. doi: 10.1007/BF00684996. [DOI] [PubMed] [Google Scholar]
- 44.Corcione A, Casazza S, Ferretti E, et al. Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc Natl Acad Sci U S A. 2004;10:11064–11069. doi: 10.1073/pnas.0402455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reboldi A, Coisne C, Baumjohann D, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 46.Kivisäkk P, Mahad DJ, Callahan MK, et al. Human cerebrospinal fluid central memory CD4+ T-cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A. 2003;100:8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kivisäkk P, Imitola J, Rasmussen S, et al. Localizing central nervous system immune surveillance: meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann Neurol. 2009;65:457–469. doi: 10.1002/ana.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bartholomäus I, Kawakami N, Odoardi F, et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- 49.Brown DA, Sawchenko PA. Time course and distribution of inflammatory and neurodegenerative events suggests structural bases for the pathogenesis of experimental autoimmune encephalomyelitis. J Comp Neurol. 2007;502:236–260. doi: 10.1002/cne.21307. [DOI] [PubMed] [Google Scholar]
- 50.Lovato L, Willis SN, Rodig SJ, et al. Related B cell clones populate the meninges and parenchyma of patients with multiple sclerosis. Brain. 2011;134:534–541. doi: 10.1093/brain/awq350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.