Abstract
Cytokinesis in mammalian cells requires actin assembly at the equatorial region. Although functions of RhoA in this process have been well established, additional mechanisms are likely involved. We have examined if Cdc42 is involved in actin assembly during cytokinesis. Depletion of Cdc42 had no apparent effects on the duration of cytokinesis, while overexpression of constitutively active Cdc42 (CACdc42) caused cytokinesis failure in normal rat kidney epithelial cells. Cells depleted of Cdc42 displayed abnormal cell morphology and caused a failure of tight accumulation of actin and RhoA at the equator. In contrast, in cells overexpressing CACdc42, actin formed abnormal bundles and RhoA was largely eliminated from the equator. Our results suggest that accurate regulation of Cdc42 activity is crucial for proper equatorial actin assembly and RhoA localization during cytokinesis. Notably, our observations also suggest that tight actin concentration is not essential for cytokinesis in adherent mammalian cells.
INTRODUCTION
Cell division, which consists of mitosis and cytokinesis, is fundamental to growth and development in all eukaryotes. Cytokinesis is the process that divides the cytoplasm of the mother cell into two daughter cells. In animal cells, actin filaments and myosin II accumulate at the equator after chromosome separation, generating contractile forces to constrict the cortex and divide.
The Rho family of small GTPases including RhoA, Cdc42 and Rac1 has been implicated in the regulation of actin cytoskeleton in a wide range of cellular processes [1]. Rho family GTPases cycle between an active GTP-bound and an inactive GDP-bound states and their cycling is regulated by the upstream regulators, gunanine nucleotide exchange factors (GEFs), and GTPase-activating proteins (GAPs) [2].
A requirement of RhoA in equatorial actin assembly during cytokinesis was previously found [3–5]. Several studies showed that endogenous RhoA accumulated at the equator during cytokinesis [6–8]. Implication of Cdc42 in cytokinesis in animal cells has been controversial [9, 10]. Moreover, the roles of Cdc42 in the regulation of actin dynamics and organization during cell division remain unknown.
A report showed that during wound healing in Xenopus embryos Cdc42 and RhoA concentrated around the wound in distinct zones and had distinct roles in the organization and functions of actomyosin [11]. While RhoA regulated contractility, Cdc42 provided actin filaments to the wound region [11]. During cell division in mammalian cells, FRET-based analyses have revealed that Cdc42 activity is relatively high in the cytoplasm outside the equator, whereas RhoA is active at the plasma membrane including the cleavage furrow region [12]. These observations suggest the possibility that, as seen in wound healing and polar body emission [11, 13], RhoA and Cdc42 may have distinct but complementary functions in the cleavage furrow formation.
In the present study, we have tested if Cdc42 is involved in the regulation of actin cytoskeleton during cytokinesis of adherent mammalian cells.
MATERIAL AND METHODS
Cell culture, microscopy, and image processing
NRK cells (NRK-52E; ATCC) were maintained in Kaighn’s modified F12 (F12K) medium with 1 mM L-glutamine (Sigma-Aldrich) supplemented with 10% FBS (BioWest), 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were grown on glass chamber dishes as previously described [14]. For live-cell imaging, cells were maintained at 37°C in a custom made incubator built on top of an Axiovert 200 M inverted microscope (Carl Zeiss) and viewed with a 100×, NA1.30, Plan-NEOFLUAR lens. All images were acquired with a cooled charge-coupled device camera (CoolSNAPHQ, Roper Scientific) and processed with MetaView imaging software (Universal Imaging). Immunofluorescence staining was analyzed using a LSM 510 Meta confocal microscope system (100×, NA 1.4 Plan-Apochromat lens; Carl Zeiss).
Plasmids, Ttransfection, and RNA interference
GFP and mRFP fused constitutively active Cdc42 (CACdc42) were constructed using the respective HA tagged cDNAs [15] as template and pXJ40 plasmid as an expression vector. GFP-actin was obtained from BD Clontech.
NRK cells grown on glass chamber dishes were transiently transfected with 1–2 μg of plasmids by using Superfect reagent (QIAGEN) or Lipofectamine (Invitrogen) according to manufacturer's instruction. Cells were transiently transfected with 1–2 μg of plasmids by using Superfect reagent (QIAGEN) according to manufacturer's instruction.
The siRNA targeting rat Cdc42 gene (AAAGACTCCTTTCTTGCTTGT) was previously described [16]. Control non-targeted siRNA and siRNA targeting Cdc42 were synthesized using a Silencer siRNA Construction Kit (Ambion). Cells were transfected with 100 nM Cdc42 siRNA using Lipofectamine according to manufacturer's instruction. At 72 h after transfection, the protein level of Cdc42 was analyzed by immunoblotting using antibodies against Cdc42 (Santa Cruz Biotechnology, sc-8401) and GAPDH for a loading control (IMGENEX).
Quantification of fluorescence signals and data analysis
Fluorescence intensity of GFP-actin along the equator or near the polar cortex was measured at 2 different regions (0.9 μm × 0.9 μm square). Statistical analysis was performed using Excel.
RESULTS AND DISCUSSION
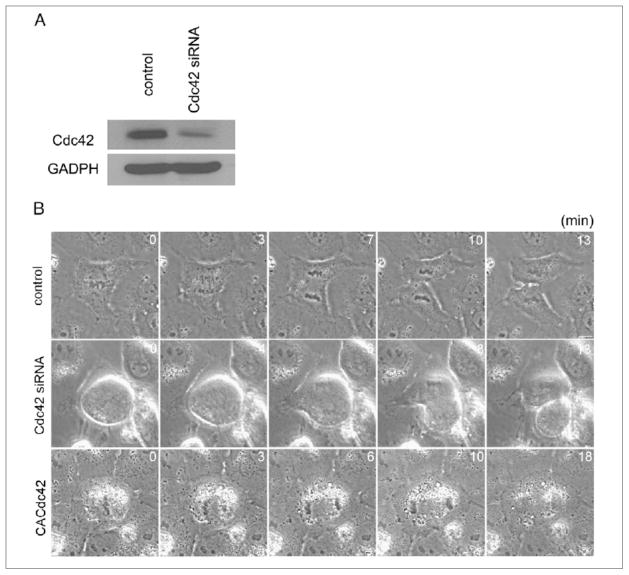
To examine if Cdc42 is required for cytokinesis in NRK cells, cells depleted of Cdc42 or cells overexpressing GFP fused constitutively active Cdc42 (GFP-CACdc42) were monitored by time-lapse phase microscopy. The amount of Cdc42 was strongly reduced in cells transfected with siRNA against Cdc42 but not non-targeted control siRNA (Figure 1A). In contrast to previous observations in HeLa cells [17], no apparent defects were found in chromosome congression and separation in cells depleted of Cdc42 and cells expressing GFP-CACdc42. Furthermore, cells depleted of Cdc42 initiated and completed cytokinesis without any apparent defects (Figure 1B, Cdc42 siRNA). The duration of cytokinesis (from anaphase onset to formation of midbody) in cells depleted of Cdc42 (11.89 ± 0.89 min; n = 9) was similar to that of control cells (10.91 ± 0.65 min; n = 11). The only defect observed was that cells depleted of Cdc42 showed abnormal round-shape morphology during mitosis and cytokinesis (Figure 1B, Cdc42 siRNA). In contrast, GFP-CACdc42 expression caused cytokinesis failure dependently on its expression level. In cells overexpressing GFP-CACdc42 at high level, no apparent furrow ingression was observed (7/18; Figure 1B, CACdc42). In cells overexpressing GFP-CACdc42 at intermediate level, the cleavage furrow ingressed but regressed (4/18). Cells overexpressing GFP-CACdc42 at low level showed normal cytokinesis (7/18). No defects in cytokinesis seen in cells depleted of Cdc42 or overexpressing GFP-CACdc42 observed in control cells.
Figure 1. Effects of depletion of Cdc42 and overexpression of constitutively active Cdc42 on cytokinesis in NRK cells.
(A) Western blot analysis of Cdc42 expression in control cells and cells treated with siRNA against Cdc42. (B) Control non-transfeced cells (control), cells depleted of Cdc42 (Cdc42 siRNA) and cells overexpressing GFP-CACdc42 (CACdc42) were monitored by time-lapse phase-contrast microscopy. Time elapsed in minutes since anaphase onset is shown at the top-right corner of each image. Scale bar represents 10 μm.
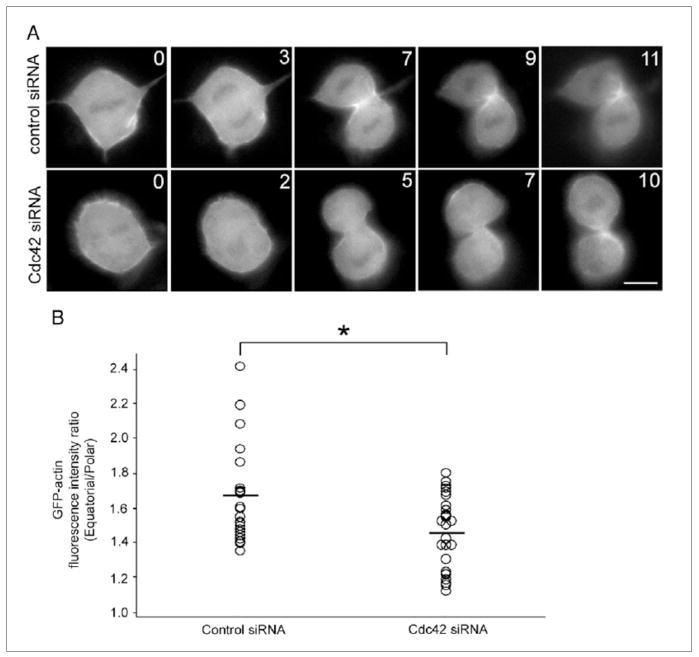
Next, to analyze actin dynamics in cells depleted of Cdc42, cells stably expressing GFP-actin were transfected with siRNA against Cdc42 and then were monitored by time-lapse fluorescence microscopy (Figure 2). In control cells, GFP-actin intensively accumulated at the equatorial region after chromosome separation (Figure 2A, control siRNA). In contrast, equatorial accumulation of GFP-actin was significantly less intense in cells depleted of Cdc42 compared to control cells (Figure 2A and B). These results suggest that the Cdc42 is required for intense accumulation of actin at the equator during cytokinesis and that the formation of tight actin ring is not critical for cytokinesis in NRK cells.
Figure 2. Effects of depletion of Cdc42 on equatorial actin assembly.
(A) Time-lapse fluorescence images of cells stably expressing GFP-actin that are treated with non-target siRNA (Control siRNA) or siRNA against Cdc42 (Cdc42 siRNA). Time elapsed in minutes since anaphase onset is shown at the top-right corner of each image. Scale bar represents 10 μm. (B) Average ratio of GFP-actin fluorescence intensity at the equatorial region to the polar region in control cells (n = 22; Control siRNA) and cells depleted of Cdc42 (n = 27; Cdc42 siRNA). *P < 0.05.
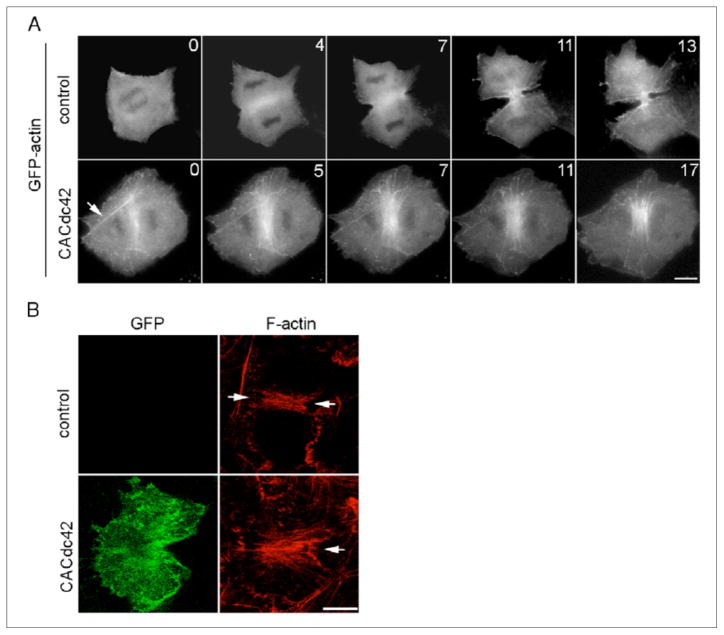
We also analyzed the dynamics of GFP-actin in cells overexpressing mRFP-CACdc42. GFP-actin formed abnormally elongated filaments and thick bundles not only along the equator but also throughout the cytoplasm (Figure 3A, CACdc42, arrow). A similar defect in actin organization was detected in cells expressing GFP-CACdc42 that were stained with rhodamine-phalloidin (Figure 3B, CACdc42). These results suggest that accurate regulation of Cdc42 activity is required for proper actin assembly at the equator during cytokinesis.
Figure 3. Effects of overexpression of constitutively active Cdc42 on equatorial actin assembly.
(A) Cells were transfected with GFP-actin (control) or both GFP-actin and mRFP-CACdc42 (CACdc42) and were monitored by time-lapse fluorescence optics. Thick actin bundles are observed outside the equator in cells transfected with mRFP-CACdc42 (arrow). Time elapsed in minutes since anaphase onset is shown at the top-right corner of each image. Scale bar represents 10 μm. (B) Cells transiently transfected with GFP-CACdc42 (green; GFP) or control non-transfected cells were stained with rhodamine-phalloidin (red; F-actin). Arrows indicate the position of the cleavage furrow. Three-dimensional reconstructed images are presented. The scale bar represents 10 μm.
As RhoA is responsible for equatorial actin assembly, we next tested if depletion of Cdc42 and CACdc42 overexpression affect RhoA localization during cytokinesis. Cells depleted of Cdc42 or overexpressing GFP-CACdc42 were stained for RhoA and were examined under confocal microscopy (Figure 4). In control cells, RhoA was found tightly concentrated to the equatorial region (Figure 4A, control). In contrast, in cells depleted of Cdc42, RhoA failed to concentrate tightly in the spindle midzone (Figure 4A, Cdc42 siRNA). The ratio of the width of the concentrated RhoA to that of the spindle midzone was significantly higher in cells depleted of Cdc42 compared to control cells (Figure 4B). In cells expressing GFP-CACdc42, we frequently observed that RhoA was eliminated from the equator and distributed throughout the cytoplasm (13/17; Figure 4A, CACdc42). These results suggest that the Cdc42 is required for proper accumulation of RhoA at the equator during cytokinesis.
Figure 4. Effects of depletion of Cdc42 and overexpression of constitutively active Cdc42 on RhoA localization during cytokinesis in NRK cells.
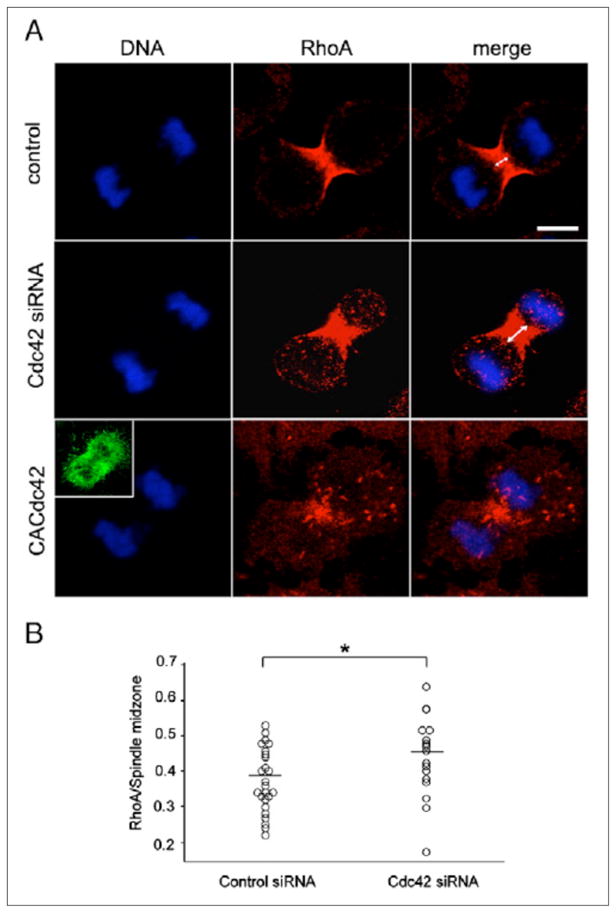
(A) Control non-transfected cells (control), cells depleted of Cdc42 (Cdc42 siRNA) and cells overexpressing GFP-CACdc42 (CACdc42) were fixed and stained for RhoA. The inset shows the image of GFP-CACdc42. Scale bar represents 10 μm. (B) The ratio of the width of the area stained with RhoA antibodies to that of the spindle midzone in control cells (n = 25; Control siRNA) and cells depleted of Cdc42 (n = 25; Cdc42 siRNA). *P < 0.05.
Our results indicate that Cdc42 activity per se is dispensable for cytokinesis in NRK cells, similar to what was seen in fibroblastid cells [18]. However, our observations suggest that precise regulation of Cdc42 activity is required for proper actin assembly and RhoA localization during cytokinesis in adherent mammalian cells.
Cross-talk between different Rho GTPases in the regulation of actin cytoskeleton has been well established in several cellular processes [11, 13, 19, 20]. Cross-talk between RhoA and Cdc42 plays a crucial role in the regulation of actomyosin contractions in wound healing and polar body emission in frog oocytes [11, 13], raising the intriguing possibility that such regulation may be involved in cytokinesis of other cellular systems.
However, Cdc42 depletion caused no apparent defects in cytokinesis, which required RhoA activity. Thus, it is unlikely that depletion of Cdc42 decreases RhoA activity. Consistent with this, loss of Cdc42 does not change RhoA activity in several cell lines [21–23].
Since depletion of Cdc42 caused defects in cell morphology, it is possible that Cdc42 is active throughout the cell during cell division. Consistent with this observation, FRET-based analyses showed that Cdc42 activity was high in the cytoplasm [12]. Thus, depletion of Cdc42 most likely causes defects in actin polymerization throughout the cell. A previous report suggests that actin is required for proper concentration of active RhoA in wound healing [11]. Therefore, it is possible that depletion of Cdc42 significantly impaired actin organization, which in turn caused a failure of tight concentration of RhoA and thus actin filaments at the equator. Conversely, CACdc42 overexpression induced the formation of abnormal actin bundles throughout the cell, which likely leads to dispersed RhoA localization and failure of cytokinesis. All together, our results suggest that Cdc42-dependent actin organization is required for proper RhoA localization during cytokinesis.
Another interesting observation in this study is that the formation of tight actin assembly is dispensable for cytokinesis in NRK cells. Unlike fission yeasts that form the tightly packed actin ring during cytokinesis, such ring structure is hardly detected in cytokinesis of mammalian cells in some situations [24]. Therefore, it is possible that cytokinesis in mammalian cells could be achieved by a mechanism different from that of the purse string-like contractions of tight actomyosin ring. Supporting this idea, cytokinesis in mammalian cells involves tightly-regulated remodeling of actin network mediated by an actin cross-linking protein α-actinin [25].
Acknowledgments
We would like to thank Ms. Keiko Kimura for technical assistance and all lab members of the Murata-Hori laboratory for discussions and/or reading of the manuscript. This study was supported by intramural funds from the Temasek Life Sciences Laboratory.
References
- 1.Etienne M, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 2.Takai Y, Sasaki T, Matozaki T. Small GTP-Binding Proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 3.Kishi K, Sasaki T, Kuroda S, Itoh T, Takai Y. Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) J Cell Biol. 1993;120:1187–1195. doi: 10.1083/jcb.120.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mabuchi I, Hamaguchi Y, Fujimoto H, Morii N, Mishima M, Narumiya S. A rho-like protein is involved in the organisation of the contractile ring in dividing sand dollar eggs. Zygote. 1993;1:325–331. doi: 10.1017/s0967199400001659. [DOI] [PubMed] [Google Scholar]
- 5.O'Connell CB, Wheatley SP, Ahmed S, Wang Y-L. The Small GTP-binding Protein Rho Regulates Cortical Activities in Cultured Cells during Division. J Cell Biol. 1999;144:305–313. doi: 10.1083/jcb.144.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J Cell Biol. 2005;170:571–582. doi: 10.1083/jcb.200501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimura Y, Yonemura S. Centralspindlin regulates ECT2 and RhoA accumulation at the equatorial cortex during cytokinesis. J Cell Sci. 2006;119:104–114. doi: 10.1242/jcs.02737. [DOI] [PubMed] [Google Scholar]
- 8.Kamijo K, Ohara N, Abe M, Uchimura T, Hosoya H, Lee JS, Miki T. Dissecting the Role of Rho-mediated Signaling in Contractile Ring Formation. Mol Biol Cell. 2006;17:43–55. doi: 10.1091/mbc.E05-06-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutartre H, Davoust J, Gorvel JP, Chavrier P. Cytokinesis arrest and redistribution of actin-cytoskeleton regulatory components in cells expressing the Rho GTPase CDC42Hs. J Cell Sci. 1996;109:367–377. doi: 10.1242/jcs.109.2.367. [DOI] [PubMed] [Google Scholar]
- 10.Drechsel DN, Hyman AA, Hall A, Glotzer M. A requirement for Rho and Cdc42 during cytokinesis in Xenopus embryos. Curr Biol. 1997;7:12–23. doi: 10.1016/s0960-9822(06)00023-6. [DOI] [PubMed] [Google Scholar]
- 11.Benink HA, Bement WM. Concentric zones of active RhoA and Cdc42 around single cell wounds. J Cell Biol. 2005;168:429–439. doi: 10.1083/jcb.200411109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshizaki H, Ohba Y, Kurokawa K, Itoh RE, Nakamura T, Mochizuki N, Nagashima K, Matsuda M. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J Cell Biol. 2003;162:223–232. doi: 10.1083/jcb.200212049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Ma C, Miller AL, Katbi HA, Bement WM, Liu XJ. Polar body emission requires a RhoA contractile ring and Cdc42-mediated membrane protrusion. Dev Cell. 2008;15:386–400. doi: 10.1016/j.devcel.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mckenna NM, Wang YL. Culturing cells on the microscope stage. Methods Cell Biol. 1989;29:195–205. doi: 10.1016/s0091-679x(08)60195-8. [DOI] [PubMed] [Google Scholar]
- 15.Govind S, Kozma R, Monfries C, Lim L, Ahmed S. Cdc42Hs Facilitates Cytoskeletal Reorganization and Neurite Outgrowth by Localizing the 58-kD Insulin Receptor Substrate to Filamentous Actin. J Cell Biol. 2001;152:579–594. doi: 10.1083/jcb.152.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, Condeelis J. Molecular mechanisms of invadopodium formation: the role of t he N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oceguera-Yanez F, Kimira K, Yasuda S, Higashi C, Kitamura T, Hiraoka Y, Haraguchi T, Narumiya S. Ect2 and MgcRacGAP regulate the activation and function of Cdc42 in mitosis. J Cell Biol. 2005;168:221–232. doi: 10.1083/jcb.200408085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czuchra A, Wu X, Meyer H, van Hengel J, Schroeder T, Geffers R, Rottner K, Brakebusch C. Cdc42 is not essential for filopodium formation, directed migration, cell polarization and mitosis in fibroblastoid cells. Mol Cell Biol. 2005;16:4473–4484. doi: 10.1091/mbc.E05-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol. 1999;9:640–648. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- 20.Groeger G, Nobes CD. Co-operative Cdc42 and Rho signalling mediates ephrinB-triggered endothelial cell retraction. Biochem J. 2007;404:23–29. doi: 10.1042/BJ20070146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen F, Ka L, Parrini MC, Mao X, Lopez M, Wu C, Marks PW, Davidson L, Kwiatkowski DJ, Kirchhausen T, Orkin SH, Rosen FS, Mayer BJ, Kirshner MW, Alt FW. Cdc42 is required for PIP2-induced actin polymerization and early development but not for cell viability. Curr Biol. 2000;10:758–765. doi: 10.1016/s0960-9822(00)00571-6. [DOI] [PubMed] [Google Scholar]
- 22.Czuchra A, Wu X, Meyer H, van Hengel J, Schroeder T, Geffers R, Rottner K, Brakebusch C. Cdc42 is not essential for filopodium formation, directed migration, cell polarization, and mitotis in fibroblastoid cells. Mol Biol Cell. 2005;16:4473–4484. doi: 10.1091/mbc.E05-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang L, Wang L, Zheng Y. Gene targeting of Cdc42 and Cdc42GAP affirms the critical involvement of Cdc42 in filopodia induction, directed migration, and proliferation in primary mouse embryonic fibroblasts. Mol Biol Cell. 2006;17:4675–4685. doi: 10.1091/mbc.E06-05-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fishkind DJ, Wang YL. Orientation and three-dimensinal organization of actin filaments in dividing cultured cells. J Cell Biol. 1993;123:837–848. doi: 10.1083/jcb.123.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukhina S, Wang YL, Murata-Hori M. Alpha-actinin is required for tightly regulated remodeling of the actin cortical network during cytokinesis. Dev Cell. 2007;13:554–565. doi: 10.1016/j.devcel.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]