Abstract
IL-33 activates eosinophils directly via the ST2 receptor. Like IL-5, IL-33 induces eosinophilia and eosinophilic airway inflammation in mouse models and primes human eosinophil responses. Previously, we reported that IL-5 priming enhances Siglec-8 mediated mitochondrial and reactive oxygen species (ROS)-dependent eosinophilic apoptosis and eliminates caspase dependence of this cell death process. Whether IL-33, like IL-5, augments pro-apoptotic pathways involving receptors such as Siglec-8 and in a similar manner has not been explored. Annexin-V labeling was performed to detect apoptosis in human eosinophils pre-incubated with or without a range of concentrations of IL-33 and/or IL-5 in the presence or absence of Siglec-8 monoclonal antibody (mAb) 2C4 and inhibitors of caspases. Tetramethyl-rhoda-mine staining was used as a marker of mitochondrial membrane potential loss and injury. ROS production was determined by measuring the superoxide dismutase-inhibitable reduction of cytochrome c. Cleavage of poly(ADP-ribose) polymerase (PARP) was assessed using Western blotting. Eosinophils cultured alone or with mAb 2C4 underwent low levels of apoptosis at 24 h. 2C4-induced eosinophil apoptosis was markedly and equally enhanced after culture for 24 h with either IL-33 or IL-5, although IL-5 was more potent. Effects on apoptosis with IL-33 and IL-5 were synergistic. In contrast, percentages of cells exhibiting reduced mitochondrial membrane potential were greater with IL-33 than IL-5 and effects of these cytokines were also synergistic. Antimycin, an inhibitor of mitochondrial electron transport, almost completely inhibited 2C4-induced apoptosis with either IL-33 or IL-5. Surprisingly, 2C4-induced eosinophil ROS production was significantly enhanced with IL-5 but not IL-33. Siglec-8-mediated apoptosis in the presence of IL-33 was more sensitive in magnitude than IL-5 to inhibition by the pan-caspase inhibitor Z-VAD-FMK, yet both cytokine conditions were associated with PARP cleavage. These data demonstrate that IL-33 is as effective but less potent than IL-5 in enhancing Siglec-8-mediated eosinophil apoptosis, and can synergize with IL-5. Eosinophils primed by IL-33 and/or IL-5 in vivo would be expected to display enhanced susceptibility to undergoing Siglec-8-induced apoptosis.
Keywords: Siglec-8, IL-33, Eosinophils, Apoptosis, Reactive oxygen species
1. Introduction
Sialic acid binding immunoglobulin-like lectins (Siglecs) are single-pass transmembrane cell surface proteins found predominantly on leukocytes [1–3]. Among them, Siglec-8 was initially thought to be an eosinophil-specific cell surface protein, but it is also selectively expressed by mast cells and weakly by basophils [4,5]. Siglec-8 recognizes the glycan 6′-sulfo-sialyl Lewis X and its cytoplasmic domain contains an intracellular immunoreceptor tyrosine-based inhibitory motif (ITIM) and an immunoreceptor tyrosine-based switch motif (ITSM) [6,7]. Engagement of Siglec-8 on eosinophils results in caspase- and mitochondrial-dependent apoptosis [8,9]. In addition, previous studies have shown that IL-5 priming potently enhances Siglec-8-mediated mitochondrial and ROS-dependent apoptosis with reduced caspase dependence [10]. Clinical use of IL-5 targeting therapies partially reduces eosinophilia in bronchial tissues and esophageal tissues, suggesting a role for other inflammatory molecules besides IL-5 in these disorders [11].
IL-33 is a newly identified cytokine that shares many biological properties as IL-5 including direct activating effects on eosinophils [12–15]. IL-33 has its own receptor, namely ST2, and stimulation through ST2 activates airway eosinophils and results in exacerbated eosinophilic airway inflammation. IL-33 drives the production of additional cytokines and IgE, and IL-33 administration induces eosinophilia and hypertrophy of bronchial epithelial cells as well as mucus secretion in animal models in vivo. Such changes resemble pathologic findings in bronchial asthma. IL-33 also directly enhances eosinophil survival and primes a numbers of eosinophil responses via activation of NF-κB pathways [13].
Given the scope of IL-33 biology, much of which overlaps with IL-5, we hypothesize that IL-33 treatment of human eosinophils, like IL-5, will enhance Siglec-8-induced apoptosis, although their comparative efficacy, potency and mechanisms of enhancement may differ. In this paper, we demonstrate that IL-33 is as effective, but not as potent, as IL-5 in enhancing Siglec-8-mediated apoptosis. Effects of IL-33 and IL-5 on apoptosis are synergistic, and enhanced apoptosis mediated by both cytokines can be blocked with inhibitors of the mitochondrial respiratory chain or caspases. Surprisingly, IL-33 is more effective than IL-5 in enhancing Siglec-8-mediated mitochondrial injury yet less effective than IL-5 in enhancing Siglec-8-mediated ROS production.
2. Materials and methods
2.1. Antibodies, reagents, and recombinant proteins
Murine monoclonal IgG1 mAb recognizing Siglec-8 (2C4) was generated as previously described [5]. Recombinant human IL-33 and IL-5 were from R&D Systems (Minneapolis, MN). Tetramethylrhodamine ethyl ester perchlorate (TMRE) was purchased from Molecular Probes (Eugene, OR). Mouse anti-human CD44 mAb (clone J-173, IgG1) and mouse anti-human Fas mAb (clone 7C11, IgM) were purchased from Beckman–Coulter (Hialeah, FL). Cyto-chrome c, antimycin, phorbol myristate acetate (PMA) and erythrosin-B were from Sigma–Aldrich (St. Louis, MO). A pan-caspase inhibitor was also tested (Z-VAD-FMK from EMD Chemicals, San Diego, CA).
2.2. Eosinophil purification and culture
Written informed consent for blood donation using an IRB-approved protocol was obtained before enrollment. Eosinophils were purified from peripheral blood after density-gradient centrifugation using Percoll (Pharmacia, Uppsala, Sweden) for separation of mononuclear cells from granulocytes, followed by erythrocyte hypotonic lysis and immunomagnetic negative selection with CD16 antibody Miltenyi microbeads (Auburn, CA). Eosinophil purity and viability were consistently higher than 98%, with neutrophils being the only contaminating cells. Purified peripheral blood eosinophils were cultured in RPMI 1640 medium (Life Technologies, Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (High Clone Laboratories, Logan, UT), 100 U/ml penicillin G and 0.1 mg/ml streptomycin sulfate (Life Technologies, Invitrogen). After pre-incubation for 30 min with or without various cytokines or inhibitors, eosinophils were harvested at different time points over 3–24 h of co-culture with saturating concentrations of 2C4 mAb to Siglec-8 (10 μg/ml) or other mAbs as indicated in the text.
2.3. Assessment of apoptosis and mitochondrial membrane potential
Eosinophil apoptosis and changes in mitochondrial membrane potential were quantified as previously described [9,10]. Briefly, FITC-Annexin-V labeling was performed to detect apoptosis in eosinophils. To measure changes in mitochondrial membrane potential, cells were loaded for 30 min at 37 °C with 100 nM TMRE, a lipid-soluble calcein ester that accumulates in mitochondria and provides an index of the inner membrane potential [16]. Reduction in TMRE staining was used as a marker of mitochondrial membrane potential loss (Δψm) [9]. Separate aliquots of eosinophils in each culture were incubated with the mitochondrial membrane uncoupler carbonylcyanide m-chlorophenyl hydrazone (mCCP, Sigma–Aldrich, at 10 μM, 15 min, 37 °C) as a positive control to induce maximal Δψm, and then stained cells were analyzed by flow cytometry as described [9].
2.4. Assay for ROS production
Using a modification of previously reported methods [17], 0.5 mg/ml fibronectin (Sigma–Aldrich) was used to pre-coat the wells (to minimize background eosinophil activation and ROS production) and 200 μl eosinophil suspension (0.5 × 106 cells/ml) in HBSS (pH 7.4) containing 10 mM HEPES, and 10 mg/ml cytochrome c were seeded into the wells of 96-well tissue culture plates. After stimulation with IL-33 or IL-5 and 2C4 mAb (or PMA at 30 ng/ml as a positive control), the absorbance of reaction wells was repeatedly determined at 550 nm in a microplate reader for up to 3 h. Between absorbance measurements, plates were kept at 37 °C. Super-oxide anion production was calculated using an extinction coefficient of 21.1 × 10–3 M/cm for reduced cytochrome c, and was expressed as nanomoles of cytochrome c reduced per 105 cells.
2.5. Western blot assays
Antibody to poly(ADP-ribose) polymerase (PARP) (Cell Signaling Technologies, Beverly, MA) was used to detect endogenous levels of the full-length PARP-1 (116 kDa), as well as the large fragment of PARP-1 resulting from caspase cleavage (89 kDa). After electrophoresis of eosinophil lysates, proteins were transferred to PVDF membranes and then incubated for 1 h at RT with 1 × TBS, 0.1% Tween-20 with 5% w/v nonfat dry milk. The membranes were incubated overnight at 4 °C with PARP Ab (1:1000). After washing, the membranes were incubated with horseradish peroxidase-linked anti-rabbit IgG (1:2500) for 45 min at RT. Bands were visualized using the ECL western blotting detection system (GE Healthcare, Piscataway, NJ).
2.6. Statistical analysis
Data are presented as the means ± standard error of the mean (SEM) from three independent experiments. Statistical significance between treatment and control group was assessed either by using Student's t test or analysis of variance (ANOVA), as appropriate. P values <0.05 were considered significant.
3. Results
3.1. IL-33 enhances Siglec-8-induced eosinophil apoptosis and alters mitochondrial membrane potential
We have previously shown that IL-5 and GM-CSF enhance Siglec-8-medicated eosinophil apoptosis [8]. Therefore the ability of IL-33 to alter Siglec-8-mediated apoptosis was compared to IL-5. As shown in Fig. 1, both IL-33 and IL-5 enhanced Siglec-8-induced apoptosis in a concentration-dependent manner. While IL-33 and IL-5 had comparable efficacy, IL-5 was approximately tenfold more potent in inducing enhanced apoptosis (left portion of Fig. 1). To determine whether there was any additivity or synergy between IL-33 and IL-5, coincubation with a range of concentrations of each cytokine was compared, as shown in the right portion of Fig. 1. Under some experimental conditions, synergy was seen. For example, synergy was observed with a range of IL-5 concentrations plus 30– 300 pM concentrations of IL-33, since 3–30 pM IL-33 by itself did not significantly enhance apoptosis.
Fig. 1.
Siglec-8-induced human eosinophil apoptosis at 24 h of culture is enhanced by both IL-5 and IL-33. Eosinophils were co-incubated with the indicated concentrations of IL-33 or/and IL-5 with or without anti-Siglec-8 mAb 2C4 for 24 h. Cells were then harvested and apoptosis was analyzed using annexin-V staining. n = 3–6; *P < 0.05; **P < 0.005 comparing indicated conditions.
To explore mechanisms by which IL-33 enhanced Siglec-8-mediated apoptosis, changes in mitochondrial membrane potential (Δψm) were examined under similar cytokine conditions as in Fig. 1, except assays were performed after only 3 h of coincubation. As shown in Fig. 2A, IL-5 at 30 and 300 pM concentrations significantly enhanced Δψm whereas significant changes for IL-33 were only seen at 300 pM concentrations, a pattern identical to apoptosis data shown in Fig. 1. However, these results differed somewhat from those in Fig. 1 in that significant enhancement of IL-5-induced Δψm changes could be seen with even 3 pM IL-33. To further explore mechanisms of IL-33 and IL-5-induced apoptosis, antimycin was used to inhibit mitochondrial electron transport. As shown in Fig. 2B, Siglec-8-induced apoptosis under these experimental conditions was greater with IL-5 than with IL-33, but in the presence of either IL-33 or IL-5, apoptosis was significantly inhibited by antimycin, demonstrating that mitochondrial electron transport was involved in the enhanced apoptosis induced by either of these two cytokines.
Fig. 2.
IL-5 and IL-33 exposure of human eosinophils results in exaggerated Siglec-8-mediated changes in mitochondrial membrane potential and cell death that is dependent on mitochondrial electron transport. Panel A: Eosinophils were co-incubated with the indicated concentrations of IL-33 or/and IL-5 with or without 2C4 for 3 h. Cells were then harvested, and mitochondrial membrane potential (Δψm) was assessed. n = 3; *P < 0.05; **P < 0.005 comparing indicated conditions. Panel B: Eosinophils were co-incubated with or without mAb 2C4 and IL-33 or IL-5 (each at 300 pM) in the presence of antimycin (45 μM), a complex-III inhibitor of the mitochondrial electron transport chain. After 24 h, apoptosis was analyzed using annexin-V staining. n = 3, *P < 0.05.
3.2. Effects of IL-33 and IL-5 on eosinophil ROS generation
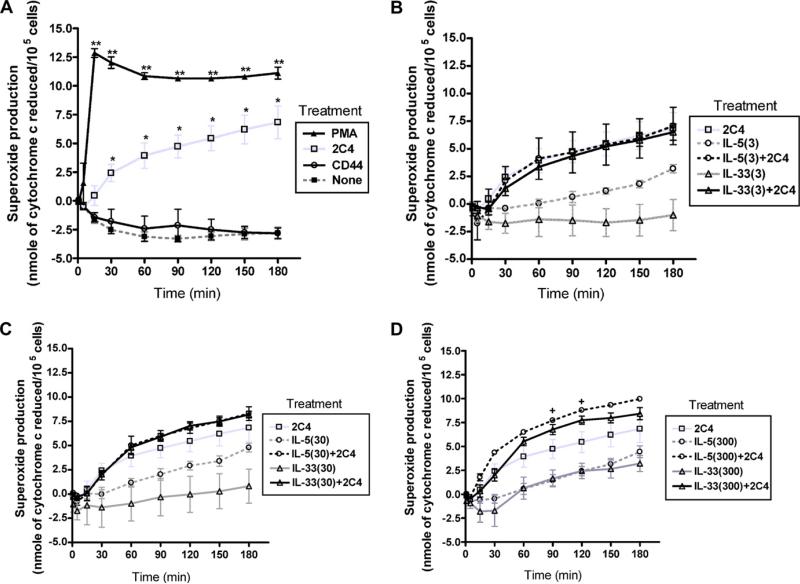
The next series of experiments were performed to directly measure ROS production by human eosinophils in response to activation with 2C4 mAb as well as effects of IL-33 and IL-5 on ROS production. As shown in Fig. 3A, the 2C4 mAb against Siglec-8, but not an isotype-matched control binding CD44 mAb, induced rapid ROS production by human eosinophils, albeit not as effectively as PMA, used as a positive control. In Fig. 3B–D, eosinophils were pre-incubated with increasing concentrations of either IL-33 or IL-5 to determine whether they enhanced 2C4 mAb-induced ROS production. As shown in these three figures, IL-5 alone, even at 3 pM concentrations, enhanced spontaneous ROS production by eosinophils, whereas this was not seen with IL-33 until the 300 pM concentration. However, IL-5 and IL-33, at either 3 or 30 pM concentrations, did not significantly enhance production of ROS beyond that of the 2C4 mAb alone. Indeed, significant enhancement was not seen under any condition except for the 300 pM IL-5 priming condition (Fig. 3D). There was a trend for 300 pM concentrations of IL-33 to enhance ROS production induced by the 2C4 mAb, but this did not reach statistical significance.
Fig. 3.
Effect of IL-5 and IL-33 on spontaneous and Siglec-8-mediated superoxide production by human eosinophils. Eosinophils were incubated without cytokines (Panel A) or 3, 30 or 300 pM concentrations of IL-5 or IL-33 (Panels B–D) for up to 3 h at 37 °C. In Panel A, PMA was used as a positive control, and a mAb to CD44 was used as a negative control. n = 3; *P < 0.05; **P < 0.005 compared to no treatment for Panel A; +P < 0.05 comparing IL-5 at 300 pM to IL-5 at 300 pM plus 2C4 mAb for Panel D.
3.3. Role of caspases in Siglec-8-mediated apoptosis enhanced by IL-33 and IL-5
We next tested the hypothesis that caspases may be playing an important role in Siglec-8 mediated apoptosis as augmented by these cytokines. Previously, we demonstrated that the general caspase inhibitor, Z-VAD-FMK, partially blocked eosinophil apoptosis induced by Siglec-8 antibody crosslinking in the absence of IL-5 priming [8], suggesting the functional association of caspases with Siglec-8 signaling. We thus examined the contribution of caspases in Siglec-8 mediated apoptosis before and after preincubation with either IL-33 or IL-5. Human eosinophils were pretreated with or without Z-VAD-FMK and then incubated with Fas antibody as a positive control [18], or 300 pM of IL-33 or IL-5 plus 2C4 mAb for 24 h. Apoptosis was assessed by Annexin-V positivity. As shown in Fig. 4A, apoptosis induced by Fas antibody was unaffected by either IL-33 or IL-5, and was completely inhibited by Z-VAD-FMK. Apoptosis in the presence of IL-5 was significantly, but incompletely, inhibited by Z-VAD-FMK, whereas for IL-33 preincubation conditions, apoptosis was completely inhibited by Z-VADFMK.
Fig. 4.
Involvement of caspases in IL-5 and IL-33-enhanced Siglec-8-induced human eosinophil apoptosis. Panel A: Human eosinophils were pretreated with or without ZVAD-FMK (ZVAD, 90 μM) and then incubated with anti-Fas mAb as a positive control for caspase-mediated death, or with or without 300 pM of either IL-5 or IL-33 plus or minus 2C4 mAb for 24 h, and apoptosis was assessed as in Fig. 1. Use of the caspase inhibitor completely blocked apoptosis in all conditions. n = 2–4; *P < 0.05 comparing indicated conditions. Panel B: Human eosinophils were incubated with anti-Fas mAb as a positive control for caspase-mediated death, or with or without 300 pM of either IL-5 or IL-33 plus 2C4 mAb for 24 h, and PARP cleavage was assessed. Shown is a representative western blot from three separate experiments with similar results. β-actin served as a loading control.
3.4. Siglec-8 mediated apoptosis in the presence of IL-33 or IL-5 involves PARP cleavage
Eosinophil lysates from non-stimulated conditions, or in the presence of either anti-Fas antibody, 2C4 mAb alone or 2C4 plus 300 pM IL-33 or IL-5, were generated and probed via Western blotting for PARP cleavage, using β-actin as a control. As shown in Fig. 4B, antibody to Fas or Siglec-8 induced partial PARP cleavage, while the addition of IL-33 or IL-5 resulted in a marked, identical enhancement of 2C4-induced PARP cleavage. Thus, Siglec-8-induced eosinophil apoptosis is associated with PARP cleavage and is markedly augmented by preincubation with IL-33 or IL-5.
4. Discussion
In the present study, Siglec-8-mediated eosinophil apoptosis was enhanced by both IL-33 and IL-5. IL-5 was more potent than IL-33 in enhancing eosinophil apoptosis, but both cytokines were equally effective at maximally active concentrations; some synergy was seen between these two cytokines. These two cytokines also significantly enhanced Δψm, but this time with similar potency and efficacy (Fig. 2A). Unlike what was seen for apoptosis, lower concentrations of these cytokines were effective at enhancing Δψm. Inhibition by antimycin suggested that mitochondrial electron transport was involved in the enhanced apoptosis induced by either of these two cytokines (Fig. 2B). While significantly enhanced ROS production following Siglec-8 engagement could be demonstrated without cytokines, only IL-5, not IL-33, significantly enhanced ROS production in response to 2C4 mAb (Fig. 3A–D), which may suggest that ROS generation is not always necessary for the observed effects of IL-33 on apoptosis. Instead, based in part on the use of a pan-caspase inhibitor, and by detection of PARP cleavage (Fig. 4A and B), our findings best support a role for the intrinsic mitochondrial response pathway in Siglec-8-mediated eosinophil apoptosis, especially in the presence of IL-33. The intrinsic mitochondrial response is one of the pathways mediating caspase-dependent apoptosis. In this pathway, death signals induce the opening of mitochondrial permeability transition pores, which cause mitochondrial-membrane depolarization and the release of apoptosis inducing proteins, such as cytochrome c and apoptosis inducing factor [19].
Mitochondria are a major source of production of ROS, but they can also serve as its target during the apoptosis process [20]. In this study, IL-5, but not IL-33, enhanced Siglec-8-mediated ROS production from human eosinophils. Release of apoptogenic factors from mitochondria, the best known of which is cytochrome c, leads to assembly of a large apoptosis-inducing complex called the apopto-some. Cysteine proteases (caspases) are recruited to this complex and, following their activation by proteolytic cleavage, activate other caspases, which in turn target for specific cleavage a large number of cellular proteins. ROS are potent inducers of breaks in DNA, which in turn activate PARP-1, which then ADP-ribosylates histones, transcription factors, and PARP-1 itself, thus promoting an inflammatory response or even death [21–24]. The finding that both IL-5 and IL-33 preincubation markedly and equally enhanced Siglec-8-mediated PARP cleavage despite their observed differences on ROS generation makes it difficult to directly or sequentially connect ROS and PARP cleavage in the mechanism of IL-33-enhanced Siglec-8-mediated eosinophil death.
There are many pro- and anti-apoptotic pathways potentially at work in human eosinophils [25]. For Siglec-8, we previously demonstrated that IL-5 priming enhances Siglec-8-mediated mitochondrial and ROS-dependent eosinophil apoptosis and eliminates caspase dependence [10]. These data are consistent with enhanced ROS production by eosinophils following preincubation with IL-5 and exposure to 2C4 mAb as seen in Fig. 3D. The lack of detectable enhancement of ROS with IL-33 preincubation in this same assay may explain, in part, the persistence of essentially complete caspase-dependency of IL-33-enhnaced Siglec-8-mediated apoptosis seen in Fig. 4A.
In previous papers, both IL-33 and IL-5 were shown to be potent activators of human eosinophils, enhancing their surface CD11b expression and adhesion and prolonging their life span [12,14]. Interestingly, the concentrations optimal for enhancing effects of IL-33 on survival, adhesion and CD11b expression were comparable to, or even greater than, the effects of IL-5, unlike the findings in the present paper, where IL-5 tended to be more potent that IL-33 in most of the assays employed. The greatest exception was seen when we investigated the participation of mitochondrial pathways during Siglec-8-induced apoptosis in the presence of IL-33 or IL-5. It was here that we found that IL-33 was as effective as IL-5 in enhancing Siglec-8-mediated mitochondrial injury. Given the different patterns of concentration dependence between mitochondrial injury, ROS production and apoptosis, mitochondrial injury and ROS generation, by themselves, may not be sufficient to explain the mechanisms responsible for cytokine-enhanced Siglec-8-mediated eosinophil apoptosis.
While both similarities and differences between IL-33 and IL-5 are reported, the unequivocal implication of the present findings is that exposure of eosinophils to IL-33, like IL-5, can result in a similar magnitude of enhancement of Siglec-8-induced apoptosis. Given the growing interest and potential role of IL-33 in asthma and other allergic diseases [13,15], it is expected that exposure to IL-33 in vivo, just as previously proposed for IL-5 [26], may render eosinophils from such patients more susceptible to the proapoptotic effects of a Siglec-8-engaging therapeutic agent.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (AI41472 and AI72265 to BSB). Dr. Bochner also received support as a Cosner Scholar in Translational Research from The Johns Hopkins University School of Medicine and is a co-author on existing and pending Siglec-8-related patents. If Siglec-8-related products are developed in the future, Dr. Bochner may be entitled to a share of royalties received by The Johns Hopkins University on the potential sale of such products. The terms of this arrangement are being managed by The Johns Hopkins University in accordance with its conflict of interest policies.
Abbreviations
- ROS
reactive oxygen species
- mAb
monoclonal antibody
- TMRE
tetramethyl-rhodamine
- PARP
poly(ADP-ribose) polymerase
- Siglec
sialic acid binding immunoglobulin-like lectin
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- ITSM
immunoreceptor tyrosine-based switch motif
- PMA
phorbol myristate acetate
- Δψm
mitochondrial membrane potential loss
- mCCP
carbon-ylcyanide m-chlorophenyl hydrazone
References
- 1.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–66. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 2.Varki A, Angata T. Siglecs – the major sub-family of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- 3.von Gunten S, Bochner BS. Basic and clinical immunology of Siglecs. Ann NY Acad Sci. 2008;1143:61–82. doi: 10.1196/annals.1443.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Floyd H, Ni J, Cornish AL, Zeng Z, Liu D, Carter KC, et al. Siglec-8: a novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem. 2000;275:861–6. doi: 10.1074/jbc.275.2.861. [DOI] [PubMed] [Google Scholar]
- 5.Kikly KK, Bochner BS, Freeman S, Tan KB, Gallagher KT, D'Alessio K, et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells and basophils. J Allergy Clin Immunol. 2000;105:1093–100. doi: 10.1067/mai.2000.107127. [DOI] [PubMed] [Google Scholar]
- 6.Bochner BS, Alvarez RA, Mehta P, Bovin NV, Blixt O, White JR, et al. Glycan array screening reveals a candidate ligand for Siglec-8. J Biol Chem. 2005;280:4307–12. doi: 10.1074/jbc.M412378200. [DOI] [PubMed] [Google Scholar]
- 7.Bochner BS. Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clin Exp Allergy. 2009;39:317–24. doi: 10.1111/j.1365-2222.2008.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–20. doi: 10.1182/blood-2002-10-3058. [DOI] [PubMed] [Google Scholar]
- 9.Nutku E, Hudson SA, Bochner BS. Mechanism of Siglec-8-induced human eosinophil apoptosis: role of caspases and mitochondrial injury. Biochem Biophys Res Commun. 2005;336:918–24. doi: 10.1016/j.bbrc.2005.08.202. [DOI] [PubMed] [Google Scholar]
- 10.Nutku-Bilir E, Hudson SA, Bochner BS. Interleukin-5 priming of human eosinophils alters Siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol. 2008;38:121–4. doi: 10.1165/rcmb.2007-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bochner BS, Gleich GJ. What targeting eosinophils has taught us about their role in diseases. J Allergy Clin Immunol. 2010;126:16–25. doi: 10.1016/j.jaci.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol. 2008;121:1484–90. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Suzukawa M, Koketsu R, Iikura M, Nakae S, Matsumoto K, Nagase H, et al. Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab Invest. 2008;88:1245–53. doi: 10.1038/labinvest.2008.82. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd CM. IL-33 family members and asthma – bridging innate and adaptive immune responses. Curr Opin Immunol. 2010;22:800–6. doi: 10.1016/j.coi.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farkas DL, Wei MD, Febbroriello P, Carson JH, Loew LM. Simultaneous imaging of cell and mitochondrial membrane potentials. Biophys J. 1989;56:1053–69. doi: 10.1016/S0006-3495(89)82754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagata M, Sedgwick JB, Bates ME, Kita H, Busse WW. Eosinophil adhesion to vascular cell adhesion molecule-1 activates superoxide anion generation. J Immunol. 1995;155:2194–202. [PubMed] [Google Scholar]
- 18.Matsumoto K, Schleimer RP, Saito H, Iikura Y, Bochner BS. Induction of apoptosis in human eosinophils by anti-fas antibody treatment in vitro. Blood. 1995;86:1437–43. [PubMed] [Google Scholar]
- 19.Saelens X, Festjens N, Vande Walle L, van Gurp M, van Loo G, Vandenabeele P. Toxic proteins released from mitochondria in cell death. Oncogene. 2004;23:2861–74. doi: 10.1038/sj.onc.1207523. [DOI] [PubMed] [Google Scholar]
- 20.Zamzami N, Kroemer G. The mitochondrion in apoptosis: how Pandora's box opens. Nat Rev Mol Cell Biol. 2001;2:67–71. doi: 10.1038/35048073. [DOI] [PubMed] [Google Scholar]
- 21.Simbulan-Rosenthal CM, Rosenthal DS, Boulares AH, Hickey RJ, Malkas LH, Coll JM, et al. Regulation of the expression or recruitment of components of the DNA synthesome by poly(ADP-ribose) polymerase. Biochemistry. 1998;37:9363–70. doi: 10.1021/bi9731089. [DOI] [PubMed] [Google Scholar]
- 22.Bhatia M, Kirkland JB, Meckling-Gill KA. Overexpression of poly(ADP-ribose) polymerase promotes cell cycle arrest and inhibits neutrophilic differentiation of NB4 acute promyelocytic leukemia cells. Cell Growth Differ. 1996;7:91–100. [PubMed] [Google Scholar]
- 23.Ha HC, Snyder SH. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci USA. 1999;96:13978–82. doi: 10.1073/pnas.96.24.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun XM, MacFarlane M, Zhuang J, Wolf BB, Green DR, Cohen GM. Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J Biol Chem. 1999;274:5053–60. doi: 10.1074/jbc.274.8.5053. [DOI] [PubMed] [Google Scholar]
- 25.Simon HU. Molecules involved in the regulation of eosinophil apoptosis. Chem Immunol Allergy. 2006;91:49–58. doi: 10.1159/000090229. [DOI] [PubMed] [Google Scholar]
- 26.von Gunten S, Bochner BS. Expression and function of Siglec-8 in human eosinophils, basophils and mast cells. In: Pawankar R, Holgate S, Rosenwasser LJ, editors. Allergy frontiers: classification and pathomechanisms. Springer; Tokyo: 2009. pp. 297–313. [Google Scholar]