Abstract
The hormones leptin and ghrelin act in apposition to one another in the regulation of body weight homeostasis. Interestingly, both leptin receptor expression and ghrelin receptor expression have been observed within many of the same nuclei of the central nervous system (CNS), suggesting that these hormones may act on a common population of neurons to produce changes in food intake and energy expenditure. In the present study we explored the extent of this putative direct leptin and ghrelin interaction in the CNS and addressed the question of whether a loss of ghrelin signaling would affect sensitivity to leptin. Using histological mapping of leptin receptor and ghrelin receptor expression, we found that cells containing both leptin receptors and ghrelin receptors are mainly located in the medial part of the hypothalamic arcuate nucleus. In contrast, coexpression was much less extensive elsewhere in the brain. To assess the functional consequences of this observed receptor distribution, we explored the effect of ghrelin receptor deletion on leptin sensitivity. In particular, the responses of ad libitum-fed, diet-induced obese and fasted mice to the anorectic actions of leptin were examined. Surprisingly, we found that deletion of the ghrelin receptor did not affect the sensitivity to exogenously administrated leptin. Thus, we conclude that ghrelin and leptin act largely on distinct neuronal populations and that ghrelin receptor deficiency does not affect sensitivity to the anorexigenic and body weight-lowering actions of leptin.
Keywords: GHSR, LepRb, obesity, food intake, arcuate nucleus
The hormones leptin and ghrelin are key players in the normal regulation of body weight homeostasis. Accumulating evidence indicates that a main role of these two hormones is to signal energy sufficiency status to the brain (Coppari et al., 2005). Leptin is produced by white adipose tissue and plasma leptin concentrations increase in conditions of positive energy balance, particularly in the setting of increased adiposity (Halaas et al., 1995; Friedman, 2009). A fasting-induced decrease in leptin levels is one of the key signals driving the neuroendocrine, metabolic, and behavioral adaptations that promote a decrease in energy expenditure and increase food intake (Ahima et al., 1996; Myers et al., 2008). Ghrelin is an octanoylated peptide hormone, synthesized mainly by cells of the stomach and intestine (Kojima et al., 1999). In contrast to leptin, plasma ghrelin concentrations increase in conditions of negative energy balance, such as prior to meals and in the setting of caloric restriction or cachexia, with ghrelin potently stimulating feeding and lowering energy expenditure (Tschop et al., 2000, 2001; Cummings et al., 2001; Otto et al., 2001). Similar to leptin, ghrelin acts within the central nervous system (CNS) to exert many of its metabolic effects (Guan et al., 1997; Mitchell et al., 2001; Zigman et al., 2006). Thus, determining the interaction between leptin and ghrelin signaling within the CNS is critical for our understanding of the mechanisms governing energy balance.
Previous studies that have examined either leptin-responsive or ghrelin-responsive pathways in the brain indicate that many of the central targets of these hormones overlap (Zigman and Elmquist, 2003; Nogueiras et al., 2008). Leptin-responsive neurons express the long-form of leptin receptor (LepRb) mRNA, which is a single-trans-membrane-domain protein of the cytokine receptor family. This splice variant of the leptin receptor is required for leptin's biologic effects and is highly expressed within several CNS sites, including hypothalamic and extrahypothalamic nuclei (Elmquist et al., 1998, 2005; Scott et al., 2009). Neurons directly activated by octanoylated ghrelin express the ghrelin receptor or growth hormone secretagogue receptor (GHSR) (Kojima et al., 1999). The functional type 1a variant of GHSR, a G-protein-coupled receptor, is also highly expressed within the CNS (Guan et al., 1997; Mitchell et al., 2001; Zigman et al., 2006). Interestingly, it has been shown that GHSRs are localized in many of the same central sites where LepRbs are found. Thus, given their opposite effects on eating and body weight, yet similar CNS receptor expression sites, it can be hypothesized that leptin and ghrelin regulate the same neurons in an opposite fashion, and that such regulation would define net food intake and energy expenditure. In support of this hypothesis, it has been shown that activation of central leptin signaling reduces ghrelin's effects (Kohno et al., 2007; Nakazato et al., 2001). These studies have shown that the orexigenic effect of intracerebroventricular ghrelin is suppressed by leptin pretreatment, and it has been proposed that this is due, at least in part, to a transient inhibition by leptin of the ghrelin-induced increase of cytosolic calcium concentration in neuropeptide Y neurons. In contrast, the modulation of leptin sensitivity by ghrelin has not yet been fully examined.
In the present study we systematically examined the distribution of LepRb and GHSR and the potential coexpression of both receptors throughout the adult mouse brain and cervical spinal cord. To map LepRb expression we used animals derived from a cross of LepRb-IRES-Cre mice, which express Cre recombinase under the control of the LepRb gene, with a Cre reporter mouse line, in which exposure to Cre recombinase results in excision of a transcriptional blocking sequence and subsequent EYFP synthesis only in Cre-expressing cells (DeFalco et al., 2001; Scott et al., 2009). In other words, the mice derived from this genetic cross express EYFP in LepRb-containing cells. We assessed double labeling for ghrelin and leptin receptors by performing in situ hybridization histochemistry (ISHH) for GHSR and immunohistochemistry (IHC) for EYFP on the same coronal brain and cervical spinal cord sections. To further assess the functional consequence of GHSR and LepRb colocalization, we performed a series of studies in which responses to leptin in GHSR-null mice, which genetically lack expression of GHSRs, were evaluated.
MATERIALS AND METHODS
Animals
Male mice were housed on a 12-hour light/dark cycle with regular chow (4 g% fat, diet #7001, Harlan-Teklad, Madison, WI), which provides 2.9 kcal/g of energy, and water available ad libitum, except when indicated. All animal procedures were carried out in accordance with National Institutes of Health (NIH) guidelines and University of Texas Southwestern (UTSW) Institutional Animal Care and Use Committee guidelines. In this study we used adult (8–10 weeks old) C57BL6/J wildtype, LepRb-IRES-Cre mice (gift from Dr. Jeffrey Friedman, Rockefeller University, NY), Gt(ROSA)26Sortm1.1(EYFP)Cosreporter mice (Jackson Laboratory, Bar Harbor, ME) (Srinivas et al., 2001) and GHSR-null mice. Study animals of genetic models were derived from crosses between heterozygous animals backcrossed > 10 generations onto a C57BL6/J genetic background.
LepRb-IRES-Cre mice express Cre recombinase selectively in cells that produce LepRb (DeFalco et al., 2001; Scott et al., 2009). In order to visualize LepRb-containing cells, we crossed the LepRb-IRES-Cre mice with reporter mice that express EYFP in a Cre-dependent manner. The reporter mouse was designed to contain a construct with a loxP-flanked transcriptional stop sequence preceding the EYFP gene, inserted into the ROSA26 locus. In the Cre recombinase-producing cells, the transcriptional termination sequence is excised, allowing EYFP production from the ROSA26 locus in LepRb-expressing cells (Scott et al., 2009). Hereafter, we will refer to the LepRb-IRES-Cre × Gt(ROSA)26Sortm1.1(EYFP)Cos mice as LepRb-EYFP mice.
We have previously validated this LepRb-IRES-Cre mouse line (Scott et al., 2009). The fidelity of the LepRb-Cre line was validated by performing a dual ISHH/IHC analysis for LepRb mRNA and LacZ in mice derived from a cross of LepRb-Cre mice with LacZ reporter mice. We found coexpression of LepRb mRNA in nearly all of the LacZ-immunoreactive (LacZ-IR) cells throughout the mouse brain. False-positive cells with LacZ-IR and no LepRb mRNA coexpression were restricted to very limited areas, such as the bed nucleus of the stria terminalis and the paraventricular hypothalamic nucleus. Given that these areas had inconsistent labeling for LepRb mRNA in the wildtype brain and that LacZ-IR cells were sparse, it is likely that this discrepancy is due to levels of LepRb mRNA below the detection threshold of our ISHH technique. Another possibility is that Cre-dependent activation of LacZ expression occurred during a restricted period of development, causing a detection of LacZ-IR in the absence of adult leptin receptor expression. In other regions, particularly in hippocampal, thalamic, and cerebral cortex nuclei, some false-negative cells, with LepRb mRNA but no detectable LacZ-IR, were found. These areas were regions with low to moderate LepRb expression, as detected by ISHH. Thus, the lack of LacZ-IR in those areas may be due to reduced level of the Cre recombinase and, as a consequence, reduced ability to induce recombination. Importantly, these few discrepancies were located in areas with very limited GHSR gene expression. Thus, it is unlikely that the use of the LepRb-EYFP mice in the current study has resulted in an underestimation of the actual number of LepRb and GHSR coexpressing cells.
The generation of GHSR-null mice has been fully explained in the past (Zigman et al., 2005). Briefly, the targeting construct was generated using ET cloning and related technologies within EL250 cells. The genetic construct of the GHSR-null mice was created by inserting a loxP-flanked transcriptional blocking cassette into a putative intron located downstream of the transcriptional start site and upstream of the translational start site of the murine Ghsr gene. This mouse model is a nonstandard “knockout” animal, in which the Ghsr locus was modified in a way that GHSR expression is inhibited but can be reactivated in a Cre-dependent manner. (We did not take advantage of the loxP sites within the modified Ghsr gene in the current study.) GHSR-null mice with their respective wildtype littermates were generated as reported previously (Zigman et al., 2005).
Histology
For procurement of tissue for histological examination, LepRb-EYFP adult male mice (20–30 g, 4–8 weeks old, n = 4) were housed with ad libitum access to both food and water in a light (12 hours on, 12 hours off) and temperature (21.5–22.5°C)-controlled environment. For the experiments investigating the LepRb-expressing cells responsive to peripherally administered ghrelin, ad libitumfed mice were injected with ghrelin (2 μg/g body weight, s.c.) or saline and perfused 2 hours later, as follows. Mice were deeply anesthetized with an intraperitoneal injection of chloral hydrate (350 mg/kg) and perfused transcardially with diethylpyrocarbonate (DEPC)-treated phosphate-buffered saline (PBS) followed by 10% neutral buffered formalin. Brains were removed, postfixed in the same fixative for 4–6 hours at 4°C, immersed in 20% sucrose in DEPC-treated PBS, pH 7.0 at 4°C, and cut coronally at 25 μm into five equal series on a sliding microtome. Tissue was stored at 20°C in antifreeze solution (Elias et al., 1998) until processed.
Generation of GHSR cRNA probes
We generated the GHSR cRNA probes as previously explained in detail (Zigman et al., 2006), although here a 916-bp fragment of cDNA amplified with GHSR-specific primers (mGHSR1047, 5′-GTGGTGTTTGCTTTCATCCTC-3′ and mGHSR1962, 5′-CATGCTCAAATTAAATGCATCC-3′) was used as template. The amplified PCR products were gel-purified and then subcloned into PCR4-TOPO vector (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. The sequences and directionalities of the inserts were confirmed by DNA sequencing at the core DNA sequencing facility at UTSW Medical Center. To generate antisense 35S-labeled cRNA to use as probes, the plasmid was linearized by restriction digestion and then subjected to in vitro transcription with either T3 or T7 RNA polymerases according to the manufacturer's protocol (Ambion, Austin, TX). Control sense riboprobes were similarly generated.
Dual-label ISHH/IHC
Free-floating sections of mouse brains were processed sequentially by ISHH and then IHC using a protocol reported previously by our laboratories (Elias et al., 1998). Series of three different mouse brains were processed for GHSR-LepRb coexpression. Briefly, brain sections were first rinsed in DEPC-treated PBS, pH 7.0, and were pretreated with 1% sodium borohydride (Sigma, St. Louis, MO) in DEPC-treated PBS for 15 minutes at room temperature. After thorough washing in DEPC-treated PBS, the sections were rinsed in 0.1 M triethanolamine (TEA, pH 8.0), incubated in 0.25% acetic anhydride in 0.1 M TEA for 10 minutes, then washed again in 2× saline-sodium citrate (SSC). The 35S-labeled GHSR mouse cRNA riboprobe was diluted to 106 cpm/ml in a hybridization solution containing 50% formamide, 10 mM Tris-HCl, pH 8.0, 5.0 mg tRNA (Invitrogen), 10 mM dithiothreitol (DTT), 10% dextran sulfate, 0.3 M NaCl, 1 mM EDTA, pH 8.0, and 1× Denhardt's solution. Next the sections were incubated at 57°C for 12–16 hours in the hybridization solution. Subsequently, sections were rinsed in 4× SSC and incubated in 0.002% RNase solution A (Roche Molecular Biochemicals, Indianapolis, IN) with 0.5 M NaCl, 10 mM Tris-HCl, pH 8.0, and 1 mM EDTA for 30 minutes, followed by a 30-minute incubation in the same buffer minus the RNase. Sections were rinsed with 2× SSC and then with 50% formamide in 0.2× SSC at 50°C. The sections were then submitted to stringency washes as follows: 2× SSC at 50°C for 1 hour; 0.2× SSC at 55°C for 1 hour; 0.2× SSC at 60°C for 1 hour. IHC was begun on the same ISHH-processed sections after first washing them in PBS, pH 7.4. Sections were pretreated with 0.3% hydrogen peroxide in PBS, pH 7.4, for 30 minutes at room temperature and then were incubated in 3% normal donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA) with 0.25% Triton X-100 in PBS (PBT) for 1 hour. Next, the slides were incubated overnight at room temperature in polyclonal anti-GFP antiserum made in rabbit (Molecular Probes/Invitrogen, Eugene, OR; cat. no. A-6455, lot 71B1, 1:20,000 in PBT-azide). As described previously, this antiserum crossreacts with EYFP (Scott et al., 2009). Furthermore, according to our prior report (Scott et al., 2009), the GFP antiserum does not show reactivity in the absence of EYFP transgene expression. After washing in PBS, sections were incubated in biotinylated donkey antirabbit IgG (Jackson ImmunoResearch Laboratories; 1:1,000) for 1 hour at room temperature, followed by incubation for 1 hour in a solution of avidin-biotin complex (Vectastain Elite ABC Kit, Vector Laboratories, Burlingame, CA; 1:500) diluted in PBS. The sections were next washed in PBS and incubated in a solution of 0.04% diaminobenzidine tetrahydrochloride (DAB; Sigma) and 0.01% hydrogen peroxide in PBS. Brain sections were mounted onto SuperFrost Plus slides (Fisher Scientific, Pittsburgh, PA), and slides were placed in x-ray film cassettes with BMR-2 film (Kodak, Rochester, NY) for 2 days. Slides were then dipped in NTB2 photographic emulsion (Kodak), dried, and stored in desiccant-containing, foil-wrapped slide boxes at 4°C for 4 weeks. Control experiments to confirm the specificity of this protocol involved hybridization with a sense GHSR riboprobe, which showed no evidence of nonspecific labeling. Also, we performed control experiments to test the specificity of the IHC reactions; they included IHC in wildtype brain samples and LepRb-YFP brain samples with omission of primary antiserum. In neither of these controls was staining observed.
Data analysis, estimates of cell counts, and production of photomicrographs
ISHH patterns were visualized first on autoradiographic film and then by observing slides dipped in photographic emulsion for direct, cellular visualization. Brain sections were viewed with both a Zeiss Axioskop and a Zeiss Stemi 2000-C dissecting microscope using both brightfield and darkfield optics. Photomicrographs were produced with a Zeiss digital camera attached to the microscopes and a Dell desktop computer. Criteria used to determine whether a GFP-IR cell coexpressed GHSR mRNA included both 1) brightfield visualization of silver granules overlying the DAB-stained cell at 5× the background density of silver granule deposition, and 2) conformation of the overlying silver granules to the shape of the DAB-stained cell. Cell counts were performed on every fifth section of each mouse brain then multiplied by 5 to obtain an estimate of the absolute cell number for each CNS location, as done in the past (Scott et al., 2009). Estimates of cell counts were performed using a 10× objective. The data were corrected for double counting, according to the method of Abercrombie (1946), whereby the ratio of the actual number of neurons to the observed number is represented by T/T+h where T = section thickness, and h = the mean diameter of the neuron along the axis perpendicular to the plane of section. It is important to note that the double-label studies are inherently qualitative. Thus, our results provide data for relative comparisons of cell numbers between brain nuclei and are not accurate counts of absolute cell numbers. Data are presented as average ± standard error of the mean (SEM) (of three mice). An image editing software program, Adobe Photo-Shop 7.0 (San Jose, CA), was used to combine the photo-micrographs into plates, adjust sharpness, contrast and brightness, and remove any obvious dust artifacts from the darkfield images.
Assessment of responsiveness to leptin
GHSR-null and wildtype littermate study animals were derived from crosses of animals heterozygotic for the recombinant, GHSR-null allele. Animals were weaned at 3 weeks of age. One set of GHSR-null and wildtype littermates was provided with ad libitum regular chow and water. Food intake in response to leptin was evaluated when these mice were 10–12 weeks of age. An independent set of GHSR-null and wildtype mice was provided with ad libitum water and high-fat diet (HFD, 88137 Western diet; Harlan Teklad, Houston, TX, which provides 5.3 Kcal/g and 42% Kcal from fat) beginning at age 4 weeks. Responses to leptin were evaluated in these mice after 16 weeks exposure to HFD. All study animals were initially group housed and then were moved into individual housing (one mouse per cage) 3 days before the below leptin administration experiments to acclimate them to being alone and to allow food intake measurements. Mouse leptin was obtained from Dr. E. Parlow (National Institute of Diabetes and Digestive and Kidney Diseases and the National Hormone and Pituitary Program, Torrance, CA). We assessed the response to leptin administration using three different experimental conditions in three different cohorts of mice: 1) acute leptin administration to ad libitum-fed mice. For this study, mice fed with either regular chow or HFD were used. Each mouse was used for both leptin and vehicle treatments on 2 independent days. On the first experimental day, food was removed at 4 pm and animals (n = 6–7 per group per treatment) were weighed and injected with either leptin (at 2 μg/g BW, intraperitoneal [i.p.]) or PBS (total volume = 150 μl). Immediately before the dark cycle started, at 6 pm, a weighed amount of food was reintroduced in the cages. Sixteen hours later the animals and the remaining food were weighed. Five days later the same protocol was repeated in a cross-over fashion in which mice previously injected with leptin were injected with PBS and vice versa. We have successfully studied the actions of leptin on food intake and body weight with this protocol previously (Enriori et al., 2007). 2) Chronic leptin treatment in ad libitum-fed mice. For this study, mice fed with either regular chow or HFD were used. On the experimental day, animals (n = 6–8 per group) were injected twice daily at 8:00 am and 4:30 pm with leptin (1 μg/g BW, i.p.) or PBS (total volume = 150 μl) according to the following scheme: PBS injections for 3 days, followed by leptin injections for 3 days, followed by PBS injections for 2 days. Animals and food were weighed daily during the injection period at 10:00 am. This protocol has previously been established by others (Bjornholm et al., 2007). 3) Acute leptin treatment in fasted-refed mice. This study was performed with mice maintained on regular chow. On the experimental day, animals (n = 6–8 per group) were weighed and regular chow was removed. Twenty-four hours later the animals were weighed and injected with either leptin (at 2 μg/g BW, i.p.) or PBS (total volume = 150 μl). One hour later mice were provided with a weighed amount of chow and food intake was measured after 24 hours. Body weights were also measured at 24 hours. Plasma acyl-ghrelin levels of wildtype and GHSR-null mice in ad libitum-fed and 24-hour fasting conditions were measured in EDTA- and protease inhibitor-treated, acid-stabilized plasma samples using an EIA kit according to the manufacturer's instructions (#10006307, Cayman Chemical, Ann Arbor, MI), as previously described (Sakata et al., 2009).
Statistics
Data are expressed as mean ± SEM. Comparisons were carried out by repeated-measures analysis of variance (ANOVA) when assessing the acute food intake responses (with genotype as “between factor” and treatment as “within factor” variables). Tukey–Kramer post-hoc analysis was used for all comparisons with significant P values. The program NCSS 2004 (Number Cruncher Statistical Systems) was used for all statistical analyses.
RESULTS
Sites of colocalization of GHSR mRNA expression and GFP-immunoreactivity in LepRb-EYFP mice
To determine the potential neuronal groups where ghrelin and leptin could converge, we performed ISSH for GHSR and IHC for GFP on the same coronal brain sections of LepRb-EYFP mice. We used an antibody raised against full-length GFP to detect EYFP, taking advantage of the crossreactivity of the anti-GFP antibody with EYFP (which differs from GFP in only 4 out of 238 amino acid residues). Brain sections analyzed included those from the level of the olfactory bulbs down to the cervical spinal cord. GFP-IR and GHSR mRNA-positive cells were localized in the same brain regions as previously shown (Zigman et al., 2006; Scott et al., 2009). The nomenclature used to describe the mouse brain nuclei corresponds, for the most part, to the descriptions in the mouse brain atlas of Paxinos and Franklin (2001). The observed coexpression patterns are outlined in Table 1 and are visually summarized in representative photomicrographs in Figure 1. Only areas that demonstrated LepRb-IRES-Cre reporter expression (GFP-IR) were examined for colocalization with GHSR mRNA.
TABLE 1.
Relative Densities of GHSR mRNA Expression, Localization of GFP- and Estimates of Coexpression of GHSR mRNA in GFP-IR Cells in LepRb-EYFP Mouse Brain
| GHSR1 | GFP-IR2 | GFP-IR cells expressing GHSR (%)3 | |
|---|---|---|---|
| Hippocampus and septum | |||
| Ammon's horn, CA3 | +/– | + | no |
| Dentate gyrus | +/– | ++ | no |
| Hypothalamus | |||
| Anterior hypothalamic area-AHA | + | + | no |
| Arcuate nucleus-Arc (lateral part) | + | +++ | 10 ± 4 |
| Arcuate nucleus-Arc (medial part) | ++++ | +++ | 90 ± 5 |
| Dorsomedial nucleus-DMH | ++ | +++ | 2 ± 1 |
| Paraventricular nucleus-PVH | ++ | +/– | no |
| Premamillary nucleus, ventral-PMV | ++ | +++ | 4 ± 4 |
| Retrochiasmatic area-RCA | + | ++++ | no |
| Ventromedial nucleus, central-cVMH | ++ | + | 16 ± 7 |
| Midbrain, pons, and medulla oblongata | |||
| Dorsal motor nucleus of the vagus-DMNV | ++ | +/– | no |
| Dorsal raphe nucleus-DR | + | +++ | no |
| Edinger Westphal nucleus-EW | +++ | + | no |
| Lateral parabrachial nucleus, LPB | ++ | ++ | no |
| Nucleus of the solitary tract-NTS | ++ | ++ | 1 ± 1 |
| Substantia nigra, pars compacta-SNC | +++ | + | 3 ± 3 |
| Ventral tegmental area-VTA | ++ | ++ | 5 ± 4 |
Only areas demonstrating LepRb reporter expression were analyzed.
Qualitative estimates of GHSR mRNA expression were made by considering both signal strength and the number of labeled cells: ++++, highest density; +++, high density; ++, moderate density; +, low density; +/–, inconsistent visualization
Qualitative estimates of GFP-IR were based on the number of labeled cells: ++++, highest density; +++, high density; ++, moderate density; +, low density; +/–, inconsistent visualization
The percentage of GFP-IR neurons coexpressing GHSR mRNA was determined at all positive levels through the brain for each nucleus. Strict criteria (described in Materials and Methods) were used to make these estimates. The data were corrected for overcounting as per Abercrombie (Abercrombie, 1946) and are reported as the mean percentage ± SEM for three different brains. no, indicates regions without GFP-IR cells expressing GHSR.
Figure 1.
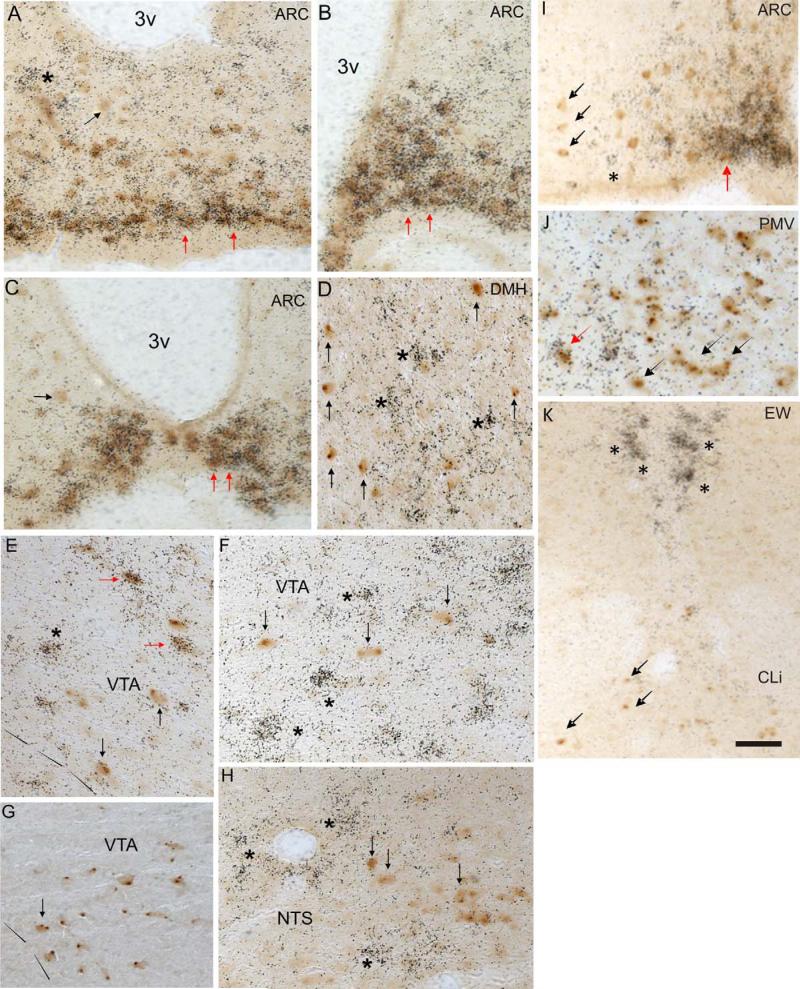
GHSR and LepRb coexpressing neurons are mainly localized to the medial aspect of the hypothalamic Arc. Dual-label histochemistry was performed on coronal sections of mouse brains. Neurons with GFP-IR, which reports on LepRb expression, are stained brown. Neurons expressing GHSR mRNA have overlying punctuate black sliver granules. Strong coexpression of LepRb and GHSR is observed in the medial basal Arc (A–C, examples indicated by red arrows). Medially, few cells were singly labeled for GHSR mRNA (example indicated by asterisk) or GFP-IR (example indicated by black arrow), while laterally a significant number of LepRb singly labeled neurons predominated (C, black arrow). Panel I further demonstrates the segregation of coexpression, with singly labeled cells laterally (asterisk, black arrows) and significant coexpression medially (red arrow). Coexpression in other regions of the hypothalamus was minimal, as in the DMH, with the majority of cells showing segregated expression of LepRb and GHSR (D, black arrows, asterisks). Scattered LepRb neurons exhibited coexpression in the PMV (J, red arrow), yet most were singly labeled (black arrow and asterisk). Distribution of LepRb and GHSR expression in the VTA also exhibited minimal overlap (E–G, red arrows). In the rostral VTA, occasional double-positive cells (E, red arrows) were observed. Large numbers of singly labeled GHSR neurons intermixed with scattered LepRb-positive cells (F, asterisks and black arrows). Caudally in the VTA, few GHSR-positive cells were seen, and LepRb-expressing neurons predominated (G, black arrow). Elsewhere in the caudal midbrain, segregation of GHSR and LepRb expression was observed in the Edinger Westphal (EW) and caudal linear nucleus (Cli) (K). Lastly, similar to other sites showing expression of both LepRb and GHSR, hindbrain expression of the two receptors showed minimal overlap (H, asterisks and arrows). Scale bar = 50 μm.
Hippocampus and septum
Both GFP-IR and GHSR mRNA expression were found in Ammon's horn (CA3) and the dentate gyrus of LepRb-EYFP mice. However, we did not find cells with coexpression of both GFP-IR and GHSR mRNA signals.
Hypothalamus
The sites with GFP-IR and GHSR mRNA expression in hypothalamus of the LepRb-EYFP mice included the arcuate (Arc), dorsomedial (DMH), paraventricular (PVH), premamillary (PMV), and ventromedial (VMH) nuclei, and also the anterior hypothalamic (AHA) and retrochiasmatic (RCA) areas. However, the percentage of dual-labeled cells for both GFP-IR and GHSR mRNA expression varied among the different nuclei. Within the Arc, we found the most abundant number of cells positive for both GFP-IR and GHSR mRNA expression. The Arc was one of the areas with the most GFP staining, with GFP-positive cells located across the entire rostral-caudal and medial-lateral extent of the nucleus. The Arc also contained dense, abundant GHSR mRNA message, which also spanned the rostral-caudal expanse of the nucleus, although it was more concentrated in the medial part of the Arc's medial-lateral axis (Fig. 1A–C,I). Cells positive for both GFP-IR and GHSR mRNA expression were mainly located in the medial part of the Arc, where 90 ± 5% of GFP-IR neurons coexpressed GHSR mRNA. In contrast, only 10 ± 4% of GFP-IR neurons coexpressed GHSR mRNA in the lateral part of the Arc (I). The VMH showed moderate expression of both GFP-IR and GHSR mRNA. However, the signals for each of them were differentially distributed within this nucleus. GFP-IR was relatively sparse within the VMH, localized mostly to the central division (cVMH). In contrast, GHSR expression was limited to the ventrolateral subdivision and the capsule of the VMH (vlVMH and capVMH, respectively). Cells positive for both GFP-IR and GHSR mRNA expression were exclusively located in the vlVMH, where 16 ± 7% of the GFP-IR neurons coexpressed GHSR mRNA. In other nuclei, such as the DMH and PMV, we found only a few cells positive for both signals (Fig. 1D,J). In the AHA, RCA, and PVH we did not find cells coexpressing GFP-IR and GHSR mRNA expression.
Midbrain, pons, and medulla oblongata
Many nuclei within the midbrain and brainstem of LepRb-EYFP mice contained GFP-IR and/or GHSR mRNA expression. These areas include the dorsal raphe (DR), Edinger Westphal (EW), and lateral parabrachial (LPB) nuclei, as well as the dorsal motor nucleus of the vagus (DMNV), nucleus of the solitary tract (NTS), substantia nigra pars compacta (SNC), and ventral tegmental area (VTA). Within these sites we found the most abundant number of cells positive for both GFP-IR and GHSR mRNA expression in the VTA (Fig. 1E–G). Interestingly, most ghrelin- and leptin-responsive cells were segregated within the VTA. GHSR mRNA expression was more concentrated in the rostral part of the VTA (Fig. 1E,F), while GFP-positive cells were located preferentially in the caudal part of the VTA (Fig. 1G). Cells positive for both GFPIR and GHSR mRNA expression were mainly located in the central part of the VTA, where 5 ± 4% of GFP-IR neurons coexpressed GHSR mRNA. Regarding the other midbrain and brainstem nuclei, we found only a few cells positive for GFP-IR and GHSR mRNA expression in the SNC and the NTS (Fig. 1H). In the DR, EW, and LPB we did not find any cells coexpressing GFP-IR and GHSR mRNA (Fig. 1K). Similarly, in the dorsal hindbrain minimal colocalization was observed in the NTS, DMNV, or AP. Within the NTS, Lepr-expressing neurons were located within the ventrolateral NTS, while GHSR neurons populated the dorsolateral NTS (Fig. 1H).
Acute and chronic leptin administration to GHSR-null and wildtype mice maintained on regular chow
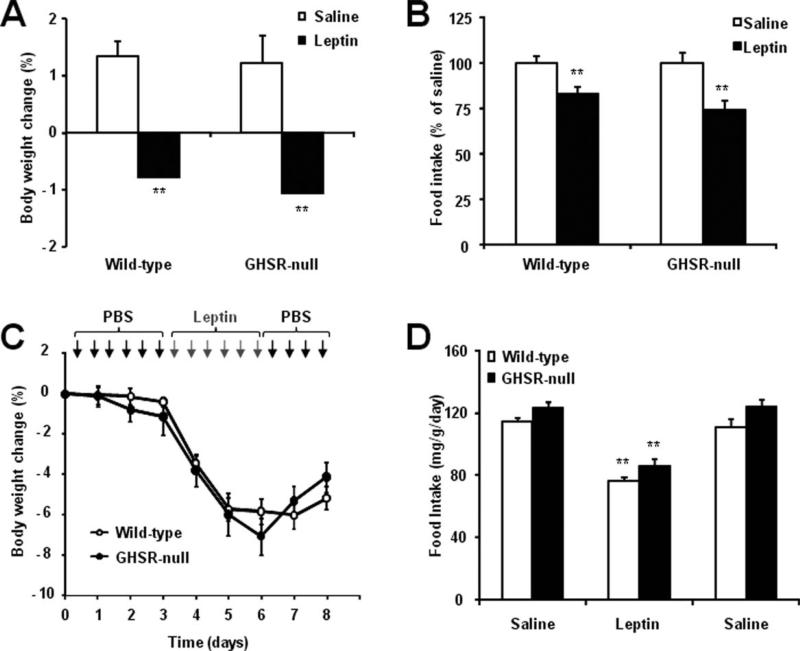
The level of colocalization of GFP-IR and GHSR mRNA expression was particularly high in the Arc nucleus, a key player in body weight and food intake regulation. It has been shown that leptin and ghrelin have opposite roles in the regulation of the food intake. Also, it has been hypothesized that ghrelin might affect leptin sensitivity (and vice versa). Thus, we decided to evaluate the body weight and food intake responses of GHSR-null mice to peripherally administered leptin. The experiment was performed with ad libitum-fed GHSR-null and wildtype littermates, which had similar body weights (24.9 ± 0.6 and 23.9 ± 0.5 g, respectively; P = NS). A single injection of leptin (2 μg/g BW, i.p.) significantly reduced body weight and overnight food intake in wildtype mice, as expected from previous studies using this same protocol (Enriori et al., 2007) (Fig. 2A,B). Leptin had the same effect in GHSR-null mice and no significant differences were found between genotypes (Fig. 2A,B). Next, we tested the body weight and food intake response of ad libitum-fed GHSR-null and wildtype littermates to a more chronic treatment with leptin (1 μg/g BW, i.p.; twice daily injections for 3 successive days). This latter treatment schedule significantly reduced body weight and food intake in wildtype mice, as previously shown using this same protocol (Bjornholm et al., 2007), and it had the same effect in GHSR-null mice (Fig. 2C,D). No significant differences between genotypes were found.
Figure 2.
Wildtype and GHSR-null mice maintained on regular chow are equally sensitive to the anorectic actions of acute or chronic leptin treatment. A single injection of leptin (2 μg/g BW, i.p.) reduced body weight A) and overnight food intake (B) similarly in both ad libitum-fed GHSR-null and wildtype littermates. A chronic treatment with twice daily injections of leptin (1 μg/g BW, i.p.) for 3 successive days reduced body weight (C) and average daily food intake during the injection periods (D) similarly in both ad libitum-fed GHSR-null and wildtype littermates. Data represent the mean ± SEM. **P < 0.01.
Acute and chronic leptin administration to wildtype and GHSR-null and wildtype mice maintained on HFD
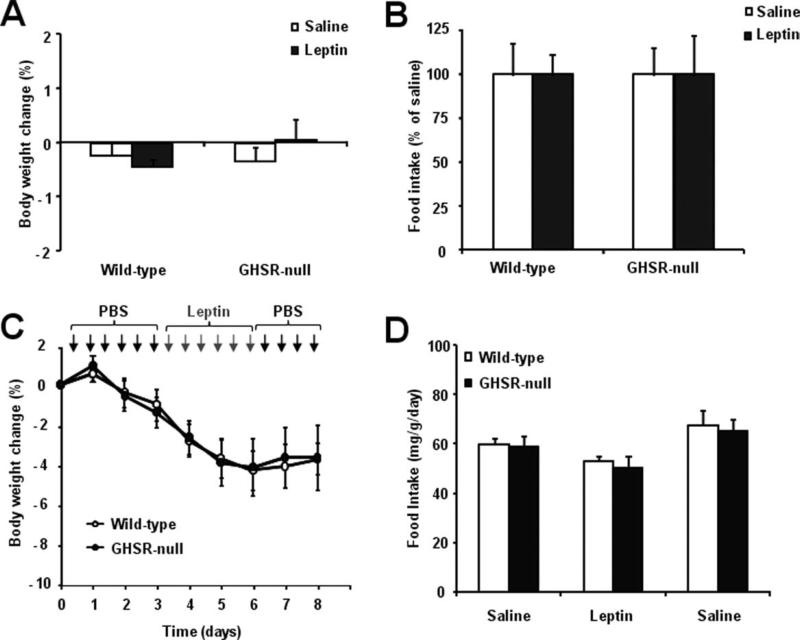
We previously reported that GHSR-null mice on a mixed genetic background fed HFD gain less body weight than do wildtype littermates. Chronic maintenance of wildtype C57Bl6/J mice on an HFD results in diet-induced obesity and leptin resistance (Collins et al., 2004). Thus, we decided to test if GHSR-null mice (which in the current study are on a pure C57Bl6/J genetic background) and wildtype littermates fed with HFD have a differential sensitivity to the anorectic actions of leptin. These studies were performed in mice exposed to HFD for 16 weeks. As reported previously, GHSR-null mice fed on HFD were leaner than wildtype mice (35.5 ± 1.2 and 38.3 ± 1.2 g, respectively; P = 0.05), although the magnitude of the body weight difference was no longer as great as that observed previously using mice on a mixed genetic background (Zigman et al., 2005). A single injection of leptin (2 μg/g BW, i.p.) failed to reduce either body weight or overnight food intake in either genotype (Fig. 3A,B, respectively). Next, we exposed the HFD-treated GHSR-null and wildtype littermates to the previously described chronic leptin protocol. Three days of twice daily leptin administration (1 μg/g BW, i.p.) slightly reduced body weight in both groups of mice (Fig. 3C). Food intake was not affected by chronic leptin treatment (Fig. 3D). No significant differences between genotypes were found.
Figure 3.
GHSR-null mice maintained on HFD are insensitive to the anorectic actions of acute or chronic leptin treatment. A single injection of leptin (2 μg/g BW, i.p.) failed to reduce body weight (A) and overnight food intake (B) in both GHSR-null and wildtype littermates. A chronic treatment with twice-daily injections of leptin (1 μg/g BW, i.p.) for 3 successive days also failed to reduce body weight (C) and overnight food intake (D) in GHSR-null and wildtype littermates. Data represent the mean ± SEM.
Acute leptin treatment to fasted wildtype and GHSR null mice
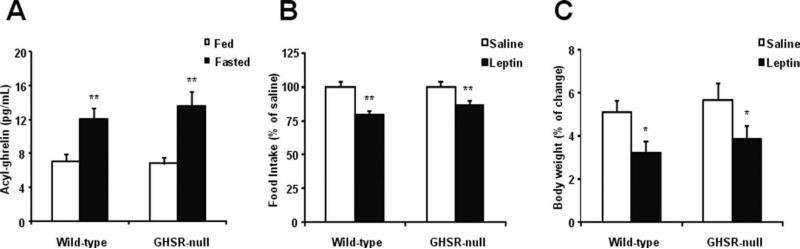
The physiological role of ghrelin may be more relevant under fasting conditions, when plasma ghrelin levels normally increase. Thus, we tested if differences in the sensitivity to the anorectic actions of leptin between GHSR-null and wildtype littermates would become more obvious upon fasting. GHSR-null mice and wildtype littermates had similar reductions in body weight in response to overnight fasting (2.0 ± 0.1 and 2.0 ± 0.2 g, respectively). In addition, overnight fasting produced a significant increase in plasma ghrelin in both groups of mice as compared to ad libitum-fed mice (Fig. 4A). When we evaluated the effect of leptin on the intake of food provided after the fast (fast/refeeding response), we found that leptin administration (2 μg/g BW, i.p.) significantly reduced the food intake in both groups of mice (Fig. 4B). Also, leptin reduced the refeeding-induced increase of body weight in both groups of mice (Fig. 4C). No significant differences were found between food intake and body weight responses of either genotypes.
Figure 4.
Fasted wildtype and GHSR-null mice are equally sensitive to the anorectic actions of leptin. Overnight fasted GHSR-null and wildtype littermates have a similar increase in plasma ghrelin, as compared to ad libitum-fed mice (A). A single injection of leptin (2 μg/g BW, i.p.) to overnight fasted GHSR-null and wildtype littermates significantly reduced the refeeding response (B) and the refeeding-induced increase of body weight in both groups of mice (C). Data represent the mean ± SEM. *P < 0.05; **P < 0.01.
DISCUSSION
In the present study we found that neurons expressing ghrelin and leptin receptors are largely segregated. One exception was the arcuate nucleus of the hypothalamus, where a subpopulation of neurons that expressed both receptors was detected within the medial aspect of the nucleus. Although coexpressing neurons were found elsewhere in the hypothalamus (including the lateral aspect of the Arc, vlVMH, DMH, and PMV), the midbrain (VTA and SNC), and caudal brainstem (NTS), the relative number of coexpressing neurons in those areas was low compared to those expressing either receptor alone. In addition, we were unable to demonstrate an effect of GHSR deletion on sensitivity to the anorexigenic or body weight-reducing actions of exogenously administered leptin. In particular, lean GHSR-null and wild-type littermates demonstrated equivalent decreases in food intake and body weight in response to single peripheral injections of leptin and in response to a more chronic leptin administration protocol. Such was the case in ad libitum-fed animals and fasted-refed animals. Furthermore, GHSR-null and wildtype littermates maintained on HFD for 16 weeks both showed equivalent degrees of leptin resistance as assessed in the same acute and chronic leptin administration models. Thus, our data suggest that ghrelin and leptin act largely on distinct groups of neurons to regulate food intake and body weight, and that ghrelin receptor deficiency does not affect sensitivity to the anorectic actions of leptin
Coexpression within the Arc
In this report we identified a population of medial Arc neurons that highly express both leptin receptors and ghrelin receptors and are well positioned to integrate these physiologically antagonistic signals. The exact chemical identity or identities, however, of these Arc ghrelin- and leptin-coresponsive neurons remains to be determined. Multiple lines of evidence suggest that these cells likely are NPY (neuropeptide Y)- and AgRP (agouti-related gene product)-producing neurons. NPY/AgRP neurons are known to predominate in the more medial aspects of the mouse Arc, whereas POMC/CART neurons localize more to the lateral aspects (Elias et al., 1998, 1999). Within the various subpopulations of Arc neurons, GHSR is primarily expressed in NPY/AgRP neurons (Willesen et al., 1999). It has been shown that ghrelin directly depolarizes and stimulates transcription of c-Fos, along with NPY and AgRP in these neurons (Dickson and Luckman, 1997; Kamegai et al., 2000; Seoane et al., 2003; Cowley et al., 2003). Consistent with ghrelin action in the Arc, blockade of both NPY's and AgRP's actions abolishes ghrelin-induced feeding (Nakazato et al., 2001). Furthermore, ablation of the Arc significantly blunts the orexigenic activity of ghrelin (Tamura et al., 2002). With respect to leptin sensing, most NPY/AgRP neurons also express LepRb (Cheung et al., 1997). It has been shown that leptin directly hyperpolarizes NPY/AgRP neurons and inhibits NPY transcription, AgRP transcription, and release of NPY and AgRP from these neurons (Spanswick et al., 1997; Elias et al., 1999; Enriori et al., 2007). Thus, the current finding of a high degree of ghrelin receptor and leptin receptor coexpression within medial Arc neurons is not unexpected. In contrast, the finding of only a few other extra-Arc brain sites containing only a small number of ghrelin receptor- and leptin receptor-coex-pressing cells is surprising.
Functional implications of ghrelin and leptin receptor coexpression in the Arc
Previous studies have clearly demonstrated an important role for NPY/AgRP neurons in body weight and feeding regulation. Also, previous studies have demonstrated a role for these neurons in both ghrelin's effects and leptin's effects on body weight and feeding. Thus, it seems logical to attribute at least some of the effects of ghrelin and leptin on body weight and feeding to opposing effects by these hormones on the same neurons within the medial Arc. Or rather, it is possible that ghrelin and leptin each partly acts by reducing the actions of the other on this medial group of Arc neurons. Supporting the interaction of leptin and ghrelin signaling in the Arc, leptin receptor-IR previously was found in slightly more than half the ventromedial Arc neurons in which ghrelin induced c-Fos, a marker of neuronal activation (Traebert et al., 2002). In slice preparations, ghrelin depolarized the majority of the neurons of this nucleus that were inhibited by leptin (Traebert et al., 2002). Also, in dispersed Arc neurons, the majority of which contained NPY, ghrelin's ability to increase cytosolic calcium was suppressed by subsequent administration of leptin (Kohno et al., 2007). In agreement with the possibility that leptin and ghrelin signaling are reciprocally regulated, it has been shown that chronic central infusion of leptin to lean, fasted Wistar rats suppresses GHSR agonist-mediated c-fos induction in the Arc (Hewson et al., 2002). Conversely, leptin-resistant fa/fa Zucker rats are more sensitive to GHSR agonist-mediated c-fos induction in the Arc (Hewson et al., 2002). In addition, the ghrelin-induced increase of food intake in leptin-resistant fa/fa Zucker rats is significantly greater than that in lean rats (Brown et al., 2007). These studies are in contrast to a mouse study in which leptin-resistant db/db mice were shown to have less of an orexigenic response to ghrelin than did wildtype mice (Iwakura et al., 2007). Similarly, exposure of wildtype mice to 12 weeks of HFD, which led to diet-induced obesity (a state of known leptin resistance), inhibited ghrelin's ability to induce c-fos in the Arc and its ability to promote NPY and AgRP gene expression in or secretion from the Arc (Briggs et al., 2010). This latter result suggested that diet-induced obesity was a state of not only leptin resistance, but also ghrelin resistance (Briggs et al., 2010). Thus, while certain in vitro and in situ laboratory preparations have suggested that leptin and ghrelin can have opposite physiologic effects on the same Arc neurons, in vivo studies using leptin-resistant models have demonstrated either that leptin resistance is associated with hypersensitivity to ghrelin-induced changes in the Arc and ghrelin's orexigenic effects (in fa/fa rats) or, alternatively, that leptin resistance inhibits ghrelin-induced changes in the Arc and ghrelin's orexigenic effects (in db/db mice and diet-induced obese mice).
To further complicate the manner in which we view the integrated control of food intake and body weight by ghrelin and leptin, a transgenic model of bioactive ghrelin overexpression demonstrates reduced sensitivity to the anorectic actions of leptin (Bewick et al., 2009). However, ablation of ghrelin in leptin-deficient ob/ob mice fails to reduce the obese hyperphagic phenotype of the ob/ob mice, although it does markedly improve the hyperglycemic phenotype of the ob/ob mice (Sun et al., 2006). This last study is in agreement with our current findings demonstrating a lack of improvement in sensitivity to leptin's food intake-reducing and body weight-lowering effects in ghrelin resistant (GHSR-null) animals. Thus, although the current study detected a significant coexpression of leptin receptors and ghrelin receptors within the medial Arc, it is not apparent from our physiological studies that deletion of ghrelin receptors from these neurons has any effect on leptin's anorectic actions.
Functional Implications of disparate expression of ghrelin and leptin receptors in the VTA
The lack of colocalization of ghrelin and leptin receptors within the vast majority of VTA neurons suggests that ghrelin- and leptin-mediated actions on food intake via direct actions in the VTA likely involve different pathways. Infusion of leptin directly into the VTA of rats results in a significant reduction of food intake and body weight acutely (Morton et al., 2009). Correspondingly, leptin has been shown to reduce action potential frequency in in vivo recordings of putative dopaminergic neurons, implying that a direct action of leptin inhibiting dopamine neuron activity underlies its effect on feeding (Hommel et al., 2006). Furthermore, knockdown of VTA leptin receptor expression has been shown to increase feeding along with the rewarding value of sucrose in a sucrose preference test (Hommel et al., 2006). Ghrelin, meanwhile, has also been shown to directly affect dopamine neuronal function, increasing dopamine release in the nucleus accumbens and enhancing action potential generation in VTA dopamine neurons (Abizaid et al., 2006; Skibicka et al., 2011; Jerlhag et al., 2006, 2007). Injection of ghrelin into the VTA increases food intake acutely, intake of a rewarding diet over regular chow, and operant lever pressing for a sucrose reward (Naleid et al., 2005; Abizaid et al., 2006; Egecioglu et al., 2010; Skibicka et al., 2011), while antagonism of GHSR activity in the VTA blunts the orexigenic capacity of peripherally administered ghrelin and also decreases operant lever pressing for a sucrose reward normally induced by an overnight fast (Abizaid et al., 2006; Skibicka et al., 2011). Also, HFD food reward, as measured in calorically restricted mice by conditioned place preference (CPP), has been shown to be dependent on ghrelin receptor signaling, as GHSR-null mice and GHSR antagonist-administered wildtype mice demonstrate a lack of conditioning that is likely VTA-dependent (Perello et al., 2010). Interestingly, leptin and ghrelin appear to regulate different aspects of rewarding behaviors when evaluated by CPP. In particular, we have shown that the physiological increases in ghrelin associated with caloric restriction enhance mainly the associative learning of the reward value of HFD (or rather, the acquisition of CPP for HFD), but are not required for its retrieval (or rather, expression of the learned association) (Perello et al., 2010). In contrast, leptin specifically blocks the expression of food CPP but not the acquisition of food CPP (Figlewicz et al., 2004). In light of these studies, we propose that the physiologically antagonistic actions of leptin and ghrelin in the VTA on food intake and food reward involve the modulation of distinct populations of neurons. Segregation of receptor expression in the VTA, as demonstrated in this report, implies that the circuits and dopamine neurons that effect the actions of leptin and ghrelin are fundamentally different. The investigation of the projection patterns of both ghrelin and leptin receptor expressing cells in the VTA will be crucial to understanding how leptin and ghrelin signals converge on circuits regulating feeding and food reward. For example, prior work has demonstrated leptin receptor expressing VTA neurons project to the amygdala (Leshan et al., 2010) while the projection pattern of GHSR VTA neurons remains to be explored thoroughly.
Functional Implications of disparate expression of ghrelin and leptin receptors in the dorsal vagal complex
Similar to the expression of leptin receptors and ghrelin receptors in the VTA, the current study demonstrates a segregation of receptor expression in the dorsal hindbrain. While leptin receptor expression was observed in the medial part of the NTS, ghrelin receptor expression was visualized dorsolateral to this area. It is thus likely that the populations of neurons that express leptin receptors and ghrelin receptors differ both functionally and neurochemically. For example, previous reports have shown that a significant number of leptin-responsive neurons in the hindbrain also coexpress the neuropeptide glucagon-like peptide (GLP-1) (Huo et al., 2008). These leptin-responsive, GLP-1-expressing neurons were absent from the dorsolateral NTS (Huo et al., 2008), which is the population of neurons that express GHSR. Thus, similar to that observed in other areas of the CNS, leptin and ghrelin appear to activate distinct circuits within the hindbrain.
Ghrelin and diet-induced obesity
We have previously shown that GHSR-null mice are less sensitive to the obesogenic effects of prolonged HFD exposure than wildtype mice (Zigman et al., 2005). In the current study this finding was confirmed. However, the body weight difference observed between GHSR-null and wildtype mice fed HFD was less evident than previously reported (Zigman et al., 2005). The discrepancy may be due to differences in genetic background. The mice used in the current study were backcrossed with C57BL/6J mice for more than 10 generations. In the previous report, we used GHSR-null mice that still contained a small proportion of the parental 129Sv genetic background, which is less susceptible to diet-induced obesity (Zigman et al., 2005). Thus, it is possible that the previous reported difference was enhanced by the 129Sv background present in the GHSR-null mice. A recent study has reported that GHSR knockout mice have similar susceptibility to diet-induced obesity as wildtype mice (Sun et al., 2008). However, in our studies wildtype and GHSR-null mice were exposed to HFD early in their life, whereas in the cited study mice were exposed to HFD when they reached adulthood (Sun et al., 2008). Therefore, differences between the experimental paradigms may be the reason for this discrepancy. Other rodent models support a role for ghrelin in long-term body weight regulation. For example, ghrelin knockout mice exposed to HFD early in life are less sensitive to diet-induced obesity (Wortley et al., 2005), while ghrelin/GHSR double knockout mice exhibit decreased body weight when placed on a standard chow diet (Pfluger et al., 2008). Thus, we can conclude that current evidence suggests that intact ghrelin signaling is required for diet-induced obesity when exposure to HFD starts early in life. Interestingly, we previously showed that circulating, nonfasted leptin levels in GHSR-null mice maintained on HFD were statistically similar to those in heavier wildtype littermates with more adiposity, and not lower as might be expected in leaner animals. However, the current physiologic data indicate that a change in leptin sensitivity does not contribute to the protective effects of GHSR deletion on the development of diet-induced obesity.
CONCLUSION
In summary, we mapped the distribution of LepRb and GHSR coexpressing neurons throughout the mouse CNS. Surprisingly, significant coexpression was observed only in the medial basal arcuate nucleus of the hypothalamus. A few other hypothalamic, midbrain, and caudal brainstem nuclei contained neurons coexpressing both receptors; however, these included by far the minority of the LepRb-containing neurons in those regions. Our physiologic data suggest that sensitivity to leptin's effects on body weight and food intake does not depend on ghrelin signaling. Further studies will be needed to assess whether this observation extends to others of ghrelin's and leptin's shared arenas of action.
ACKNOWLEDGMENT
We thank Dr. Jeffrey Friedman (Rockefeller University, NY) for the gift of the LepRb-IRES-cre mice.
Grant sponsor: National Institutes of Health; Grant number: R01DA024680; Grant number: K08DK068069; Grant number: R01MH085298 (to J.M.Z.); Grant number: K99DA024719-02 (to M.M.S.); Grant number: R01DK71320, RL1DK081185 (to J.K.E.); Grant number: PL1 DK081182; Grant number: UL1RR024923 (which support the UTSW Mouse Metabolic Phenotyping Core); Grant sponsor: Florencio Fiorini Foundation and International Brain Research Organization (grants to M.P.).
Footnotes
The first two authors contributed equally to this work.
Current address for M. Perello: Laboratory of Neurophysiology, Multidisciplinary Institute of Cell Biology (IMBICE-CONICET/CICPBA), La Plata, Buenos Aires, 1900, Argentina.
LITERATURE CITED
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Els-worth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- Bewick GA, Kent A, Campbell D, Patterson M, Ghatei MA, Bloom SR, Gardiner JV. Mice with hyperghrelinemia are hyperphagic and glucose intolerant and have reduced leptin sensitivity. Diabetes. 2009;58:840–846. doi: 10.2337/db08-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornholm M, Munzberg H, Leshan RL, Villanueva EC, Bates SH, Louis GW, Jones JC, Ishida-Takahashi R, Bjorbaek C, Myers MG., Jr Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest. 2007;117:1354–1360. doi: 10.1172/JCI30688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology. 2010;151:4745–4755. doi: 10.1210/en.2010-0556. [DOI] [PubMed] [Google Scholar]
- Brown LM, Benoit SC, Woods SC, Clegg DJ. Intraventricular (i3vt) ghrelin increases food intake in fatty Zucker rats. Peptides. 2007;28:612–616. doi: 10.1016/j.peptides.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138:4489–4492. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav. 2004;81:243–248. doi: 10.1016/j.physbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC, Jr, Lowell BB, Elmquist JK. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1:63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, Enquist L, Friedman JM. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- Dickson SL, Luckman SM. Induction of c-fos messenger ribonucleic acid in neuropeptide Y and growth hormone (GH)-releasing factor neurons in the rat arcuate nucleus following systemic injection of the GH secretagogue, GH-releasing peptide-6. Endocrinology. 1997;138:771–777. doi: 10.1210/endo.138.2.4907. [DOI] [PubMed] [Google Scholar]
- Egecioglu E, Jerlhag E, Salome N, Skibicka KP, Haage D, Bohlooly YM, Andersson D, Bjursell M, Perrissoud D, Engel JA, Dickson SL. Ghrelin increases intake of rewarding food in rodents. Addict Biol. 2010;15:304–311. doi: 10.1111/j.1369-1600.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 2005;493:63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett J, Evans SB, Kaiyala K, Sipols AJ, Benoit SC. Intraventricular insulin and leptin reverse place preference conditioned with high-fat diet in rats. Behav Neurosci. 2004;118:479–487. doi: 10.1037/0735-7044.118.3.479. [DOI] [PubMed] [Google Scholar]
- Friedman JM. Leptin at 14 y of age: an ongoing story. Am J Clin Nutr. 2009;89:973S–979S. doi: 10.3945/ajcn.2008.26788B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Hewson AK, Tung LY, Connell DW, Tookman L, Dickson SL. The rat arcuate nucleus integrates peripheral signals provided by leptin, insulin, and a ghrelin mimetic. Diabetes. 2002;51:3412–3419. doi: 10.2337/diabetes.51.12.3412. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Huo L, Gamber KM, Grill HJ, Bjorbaek C. Divergent leptin signaling in proglucagon neurons of the nucleus of the solitary tract in mice and rats. Endocrinology. 2008;149:492–497. doi: 10.1210/en.2007-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura H, Akamizu T, Ariyasu H, Irako T, Hosoda K, Nakao K, Kangawa K. Effects of ghrelin administration on decreased growth hormone status in obese animals. Am J Physiol Endocrinol Metab. 2007;293:E819–825. doi: 10.1152/ajpendo.00681.2006. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addict Biol. 2006;11:45–54. doi: 10.1111/j.1369-1600.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, Engel JA. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol. 2007;12:6–16. doi: 10.1111/j.1369-1600.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology. 2000;141:4797–4800. doi: 10.1210/endo.141.12.7920. [DOI] [PubMed] [Google Scholar]
- Kohno D, Nakata M, Maekawa F, Fujiwara K, Maejima Y, Kuramochi M, Shimazaki T, Okano H, Onaka T, Yada T. Leptin suppresses ghrelin-induced activation of neuropep-tide Y neurons in the arcuate nucleus via phosphatidylinositol 3-kinase- and phosphodiesterase 3-mediated pathway. Endocrinology. 2007;148:2251–2263. doi: 10.1210/en.2006-1240. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Leshan RL, Opland DM, Louis GW, Leinninger GM, Patterson CM, Rhodes CJ, Munzberg H, Myers MG., Jr Ventral tegmental area leptin receptor neurons specifically project to and regulate cocaine- and amphetamine-regulated transcript neurons of the extended central amygdala. J Neurosci. 2010;30:5713–5723. doi: 10.1523/JNEUROSCI.1001-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell V, Bouret S, Beauvillain JC, Schilling A, Perret M, Kordon C, Epelbaum J. Comparative distribution of mRNA encoding the growth hormone secretagogue-receptor (GHS-R) in Microcebus murinus (Primate, lemurian) and rat forebrain and pituitary. J Comp Neurol. 2001;429:469–489. doi: 10.1002/1096-9861(20010115)429:3<469::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Blevins JE, Kim F, Matsen M, Figlewicz DP. The action of leptin in the ventral tegmental area to decrease food intake is dependent on Jak-2 signaling. Am J Physiol Endocrinol Metab. 2009;297:E202–210. doi: 10.1152/ajpendo.90865.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26:2274–2279. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Tschop MH, Zigman JM. Central nervous system regulation of energy metabolism: ghrelin versus leptin. Ann N Y Acad Sci. 2008;1126:14–19. doi: 10.1196/annals.1433.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto B, Cuntz U, Fruehauf E, Wawarta R, Folwaczny C, Riepl RL, Heiman ML, Lehnert P, Fichter M, Tschop M. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol. 2001;145:669–673. [PubMed] [Google Scholar]
- Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, Rovinsky SA, Woloszyn J, Yanagisawa M, Lutter M, Zigman JM. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol Psychiatry. 2010;67:880–886. doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfluger PT, Kirchner H, Gunnel S, Schrott B, Perez-Tilve D, Fu S, Benoit SC, Horvath T, Joost HG, Wortley KE, Sleeman MW, Tschop MH. Simultaneous deletion of ghrelin and its receptor increases motor activity and energy expenditure. Am J Physiol Gastrointest Liver Physiol. 2008;294:G610–618. doi: 10.1152/ajpgi.00321.2007. [DOI] [PubMed] [Google Scholar]
- Sakata I, Nakano Y, Osborne-Lawrence S, Rovinsky SA, Lee CE, Perello M, Anderson JG, Coppari R, Xiao G, Lowell BB, Elmquist JK, Zigman JM. Characterization of a novel ghrelin cell reporter mouse. Regul Pept. 2009;155:91–98. doi: 10.1016/j.regpep.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoane LM, Lopez M, Tovar S, Casanueva FF, Senaris R, Dieguez C. Agouti-related peptide, neuropeptide Y, and somatostatin-producing neurons are targets for ghrelin actions in the rat hypothalamus. Endocrinology. 2003;144:544–551. doi: 10.1210/en.2002-220795. [DOI] [PubMed] [Google Scholar]
- Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011;180:129–137. doi: 10.1016/j.neuroscience.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390:521–525. doi: 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Asnicar M, Saha PK, Chan L, Smith RG. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab. 2006;3:379–386. doi: 10.1016/j.cmet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Sun Y, Butte NF, Garcia JM, Smith RG. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology. 2008;149:843–850. doi: 10.1210/en.2007-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura H, Kamegai J, Shimizu T, Ishii S, Sugihara H, Oikawa S. Ghrelin stimulates GH but not food intake in arcuate nucleus ablated rats. Endocrinology. 2002;143:3268–3275. doi: 10.1210/en.2002-220268. [DOI] [PubMed] [Google Scholar]
- Traebert M, Riediger T, Whitebread S, Scharrer E, Schmid HA. Ghrelin acts on leptin-responsive neurones in the rat arcuate nucleus. J Neuroendocrinol. 2002;14:580–586. doi: 10.1046/j.1365-2826.2002.00810.x. [DOI] [PubMed] [Google Scholar]
- Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Tschop M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, Folwaczny C. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest. 2001;24:RC19–21. doi: 10.1007/BF03351037. [DOI] [PubMed] [Google Scholar]
- Willesen MG, Kristensen P, Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306–316. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- Wortley KE, del Rincon JP, Murray JD, Garcia K, Iida K, Thorner MO, Sleeman MW. Absence of ghrelin protects against early-onset obesity. J Clin Invest. 2005;115:3573–3578. doi: 10.1172/JCI26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Elmquist JK. Minireview: from anorexia to obesity—the yin and yang of body weight control. Endocrinology. 2003;144:3749–3756. doi: 10.1210/en.2003-0241. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]