Abstract
Ambiguous visual stimuli provide the brain with sensory information that contains conflicting evidence for multiple mutually exclusive interpretations. Two distinct aspects of the phenomenological experience associated with viewing ambiguous visual stimuli are the apparent stability of perception whenever one perceptual interpretation is dominant, and the instability of perception that causes perceptual dominance to alternate between perceptual interpretations upon extended viewing. This review summarizes several ways in which contextual information can help the brain resolve visual ambiguities and construct temporarily stable perceptual experiences. Temporal context through prior stimulation or internal brain states brought about by feedback from higher cortical processing levels may alter the response characteristics of specific neurons involved in rivalry resolution. Furthermore, spatial or crossmodal context may strengthen the neuronal representation of one of the possible perceptual interpretations and consequently bias the rivalry process towards it. We suggest that contextual influences on perceptual choices with ambiguous visual stimuli can be highly informative about the neuronal mechanisms of context-driven inference in the general processes of perceptual decision-making.
Keywords: vision, rivalry, context, spatial, temporal, crossmodal
1. Introduction
Ambiguous or multistable visual stimuli such as the Necker cube (figure 1a) can give rise to multiple, mutually exclusive, perceptual interpretations. In the case of the Necker cube, the brain interprets a two-dimensional line drawing as a three-dimensional cube, but the absence of explicit depth cues renders the cube's three-dimensional orientation ambiguous. Such dissociation between stimulus and percept allows one to study how the brain ‘chooses’ between multiple correct interpretations in establishing a conscious percept. Two hallmark features of ambiguous visual stimuli are the mutual exclusivity of their possible perceptual interpretations and the perceptual alternations that occur between these interpretations when stimuli are continuously viewed [1,2]. Mutual exclusivity entails that, at any given moment, perception commits to one interpretation (the dominant percept) while disregarding or suppressing the other possibility. Upon prolonged exposure, these interpretations will reverse every few seconds in a cycle of perceptual alternations that occur without any physical changes in stimulus composition. Concerning this perceptual cycle, a distinction can be made between the apparent stability of perception during dominance periods and the instability of perception associated with perceptual alternations. Apart from relatively short transition periods when dominance reverses, there is very little (if any) phenomenological difference between percepts evoked by ambiguous stimuli and those resulting from unambiguous stimuli. Factors that influence the temporary stable perception of ambiguous visual stimuli and the associated perceptual choice processes may thus be instrumental in revealing general mechanisms of visual perception.
Figure 1.
(a) A Necker cube. A two-dimensional line drawing of a cube lacks explicit depth structure, rendering its three-dimensional configuration ambiguous. (b) Additional information can resolve the perceptual ambiguity.
A common conceptualization of neuronal mechanisms involved in the resolution of perceptual ambiguities is that of a competition between neuronal populations that each code for a particular perceptual interpretation. The more active of these populations will win the competition and determine perceptual dominance [3]. Ambiguous stimuli that contain equal evidence for the two interpretations1 will theoretically evoke equal levels of activity in the competing neuronal populations. The resulting equilibrium will then be broken in favour of one of the percepts by random fluctuations in neuronal activity. This dependence on neural noise predicts that the choice of the dominant percept will be random. In reality however, there are many factors that influence this perceptual choice process. Objects projected on the retina in natural vision are rarely perceived in isolation from their context. Additional information from this context may help the brain interpret visual patterns that are often ambiguous at the retinal level. Perception of ambiguous stimuli can similarly benefit from information derived from, e.g. a spatial context of simultaneously presented stimuli (figure 1b), or a temporal context stored in recent or long-term memory, another modality or even from concurrent internal brain states and processes. On the basis of some of the ideas developed elsewhere [4], this review summarizes existing human psychophysical evidence for contextual influences on the perceptual choices associated with ambiguous visual stimuli.
2. Temporal context
Choices and decisions in the present are influenced by those made in the past, a notion that also holds for perceptual choices arising from neuronal assemblies in the brain. Recent and long-term history of activity patterns can change both sensitivity [5] and connectivity [6] in the brain. During rivalry, when perceptual interpretations of an ambiguous stimulus are candidates for conscious representation, influences of previous stimulation can roughly be divided into adaptation and priming effects. Complex forms of adaptation may fundamentally alter functional cortical circuits, but even a more simple type of adaptation, known as fatigue, can strongly influence perception by reducing perceptual and neuronal sensitivity for stimulus features that the system was previously exposed to. These sensitivity reductions may occur on multiple timescales [7–9] and in multiple cortical areas simultaneously [10]. It is however also possible that pre-exposure to stimulus aspects increases the system's sensitivity for these particular aspects (priming). Whether a stimulus will have a priming (facilitating) or adaptation (suppressive) effect on the subsequent resolution of perceptual ambiguities crucially depends on its timing and contrast. Short exposures to stimulus features generally have facilitative priming effects, whereas longer exposures cause adaptation [11,12].
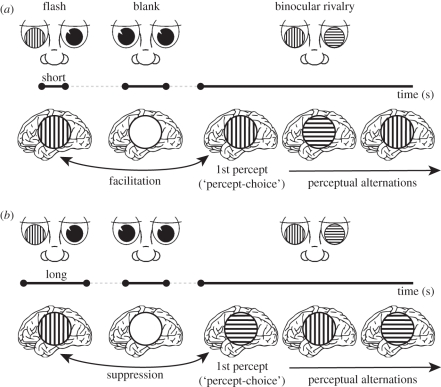
The transition between these opposing influences has been shown in binocular rivalry experiments on ‘flash suppression’ and ‘flash facilitation’ [12]. In binocular rivalry, a perceptual ambiguity arises from conflicting images presented to the individual eyes (e.g. a horizontal grating pattern in one eye and a vertical pattern in the other). Upon prolonged exposure, this perceptual conflict results in alternating perceptual dominance of either eye's image rather than in a mixture of the two images. In flash suppression, one eye's image is initially suppressed during rivalry as a result of pre-exposure to that same image in a non-rivalry situation [13] (figure 2a). The image that is first dominant in the rivalry situation will thus be the one that was not shown during pre-exposure. The general explanation for flash suppression is that monocular pre-exposure will cause adaptation in neurons that encode this stimulus. Consequently, they will respond with reduced strength during subsequent rivalry, causing them to lose the competition and have their corresponding percepts suppressed. In flash facilitation on the other hand, brief pre-exposure to a stimulus results in initial dominance of the corresponding image rather than suppression [12] (figure 2b). Interestingly, the two factors that determined whether pre-exposure had a facilitative or suppressive effect were the stimulus intensity and the pre-exposure duration. If pre-exposure stimuli had low contrast or were presented only briefly, they facilitated dominance of the corresponding image during subsequent rivalry, whereas suppression was observed with pre-exposure stimuli that were either high in contrast or presented for a longer duration [12]. While flash suppression and facilitation seem incompatible at first sight, the authors used a computational model of percept-choice mechanisms [14] to explain both phenomena in a single framework [12]. In this model, the neuronal drive caused by pre-exposure evokes both a decrease of response sensitivity (adaptation) and a small additive facilitation. Which of these effects dominates at rivalry onset depends on the amount of adaptation caused by the pre-exposed stimulus. If pre-exposure effects indeed result from a carryover of neuronal adaptation during the pre-exposure, one would expect an influence of the time interval between pre-exposure and subsequent rivalry, because neurons may recover from adaptation when they are not driven. Such an influence has indeed been found for binocular rivalry [12,13], ambiguous motion [11] and more complex naturalistic ‘morph’ stimuli [15]. If the interval between pre-exposure and rivalry stimuli gets too long, neither suppressive nor facilitative effects are observed.
Figure 2.
(a) The principle of flash facilitation in binocular rivalry. If a vertical grating is briefly flashed to one eye, it will become initially dominant when binocular rivalry is instigated after a short blank. Stimulation is depicted in the top row and perception in brain icons in the bottom row. (b) Flash suppression. If the flashed grating stimulus is presented to one of the eyes for longer durations, it will initially be suppressed at the onset of rivalry. The distinction between initial percept choice at the onset of binocular rivalry and perceptual alternations upon prolonged viewing is also depicted in these panels.
On the basis of the idea of flash suppression, an experimental paradigm called continuous flash suppression (CFS) has been developed that allows stimuli to be suppressed from consciousness for much longer durations than are typically observed in binocular rivalry. In CFS, one eye's image is strongly suppressed by a highly salient image presented to the other eye. The salience of this second image can be established with bright colours and constant changes in composition, for instance by using a rapid iteration of a series of Mondrian patterns that consist of collections of saliently coloured rectangles [16]. While CFS is a valuable tool for the experimental study of visual consciousness [17], the fact that one of the images involved in rivalry must be much more salient than the other image means that the perceptual ambiguity is basically resolved by large stimulus biases, which makes the technique less applicable for investigating influences of temporal context. In classic flash suppression, binocular rivalry targets are balanced in stimulus strength, allowing a much more sensitive investigation of external influences on rivalry resolution.
How the perceptual choice process involved in the (temporary) disambiguation of ambiguous stimuli is influenced by previous exposures and the stimulus-free time interval between exposures has recently been studied with so-called onset-rivalry or percept-choice rivalry experiments [14,18–20]. To explain the concept of percept-choice rivalry, we must return to the two main features of ambiguous stimuli: (i) the mutual exclusivity of possible perceptual interpretations, and (ii) the perceptual alternations with prolonged observation. Most existing rivalry research has dealt with the latter characteristic. Dominance duration distributions and switch rates are typically extracted from minutes of stimulus exposure and compared among conditions [21,22]. One could argue that this approach mainly targets the instability of perception under ambiguous input because it focuses on the disappearance of stable percepts without external changes. The apparent stability of a dominant percept, on the other hand, is first constructed when the brain ‘chooses’ a percept at the onset of an ambiguous stimulus. In percept-choice experiments, a series of short ambiguous stimulus presentations is used to repeatedly probe this percept construction process at the onset of stimulus while avoiding spontaneous perceptual alternations during the presentation of stimulus. Using this paradigm, it has been shown that the interval between stimuli strongly affects which percept will be dominant on subsequent presentation. Short stimulus intervals (less than 0.5 s) cause many perceptual alterations between subsequent presentations [14,19,23,24], whereas long intervals (greater than 1.0 s) stabilize perception into the same dominant percept for many subsequent presentations [14,19,23–25]. While perceptual stabilization with long interruption intervals is sometimes referred to as perceptual memory, it is unclear whether any high-level memory processes are actually required. On the contrary, computational models suggest that the neural correlates of this phenomenon are likely located in low-level sensory neurons [14,19,26]. This notion is further supported by the elimination of perceptual ‘memory’ effects by transcranial magnetic stimulation over early cortical areas [27]. The sensitive interplay between reduced responses and a facilitating component of prior stimulation currently seems the most promising hypothesis for perceptual stabilization. Elevated intraneuronal subthreshold baseline potentials that increase a neuron's readiness to become active could be the neuronal basis for such a facilitatory component. Alternatively, interactions within local cortical networks could also provide a facilitatory signal [14,26].
On a longer timescale, adaptation through sensory experience not only changes the sensitivity of neurons, but it also evokes functional plasticity within the local circuits involved in rivalry resolution. Mutual inhibition between neuronal populations related to competing perceptual interpretations is a key component of many computational models of rivalry [14,28,26]. At a perceptual level, the success of inhibition can be assessed from the completeness of perceptual suppression [29,30]. If inhibition is strong, perceptual suppression will be strong and observers will exclusively perceive the dominant image at any given time. If inhibition is weaker, perceptual suppression will be weaker and cause mixed percepts that contain (elements of) multiple perceptual interpretations. Whereas the initial strength of inhibition depends on stimulus features [29,31,32], recent experiments with binocular rivalry have demonstrated that the efficacy of inhibition is dynamically recalibrated by recent perceptual experience [30]. Continuous exposure to a single binocular rivalry stimulus resulted in increasing occurrences of mixture percepts, an indication of weakened inhibition. This weakened inhibition could only be restored by presenting non-rivalling binocular stimuli with the same features as the rivalry images. Short-term slowing of perceptual switch rates during single binocular rivalry trials and long-term speeding of switch rates over many days are also indicative of some form of structural or functional plasticity in the neuronal circuits involved in rivalry [22,33]. The temporal context of recent perception and sensory processing can thus have clear short- and long-term effects on the neuronal processing and perception of currently observed ambiguous stimuli.
3. Spatial context
The visual brain is specialized in working with relative measures. This specialization can already be observed in the centre-surround structure of retinal ganglion cells' receptive fields, where the neuronal response to a luminance spot in the centre depends on the luminance of the surrounding regions. Centre-surround interactions also occur in binocular rivalry. Surround stimulation both deepens perceptual suppression and reduces overall dominance of a high-contrast rivalling centre stimulus with similar features [34,35]. At lower contrasts, this effect reverses, and surround stimulation instead increases the predominance of its matching centre stimulus [35]. Similar increases in the predominance of a centre stimulus as a result of a congruent surround have been shown for global motion patterns [36,37], biological motion patterns [38] and slant orientation [39].
Surrounds also influence the resolution of more object-based perceptual ambiguities for which perceptual competition is thought to take place at higher processing levels. In ambiguously rotating structure-from-motion spheres or cylinders [40], the impression of a three-dimensional object arises from a two-dimensional projection of dots moving as if they are located on the surface of a rotating transparent sphere or cylinder. Since such a stimulus lacks depth information, the rotation direction cannot be unambiguously derived and the perceived rotation direction alternates between the two possibilities. If an ambiguous rotating sphere is presented against a background of dots that move in the same direction as one of the two dot directions in the sphere itself, the sphere dots moving in the opposite direction from the background are perceived as the near side of the sphere [41]. Further research excluded a top-down disambiguation effect of perceiving the sphere as rolling over a conveyor belt of background dots by showing that stereoscopically defining the ‘belt’ to be either closer to or further away from the observer than the cylinder had no effect on the results. Instead, low-level centre-surround suppression of motion information was suggested to disambiguate the bistable sphere by weakening the representation of dots moving in the same direction as the belt. Consequently, the representation of dots that move in the opposite direction will be stronger than that of dots moving in the same direction. This effect is comparable to an actual physical change in dot intensity that also biases a sphere stimulus to the interpretation with the stronger dots constituting the front or near side [42].
Higher level disambiguating spatial influences do nonetheless exist with rotating cylinders. In general, these cylinder stimuli are highly suitable for investigating spatial influences because their local lack of depth information is usually well complemented by globally distributed depth information in natural scenes. A striking example of an apparently more high-level spatial disambiguation can be seen when an ambiguous cylinder is presented next to a cylinder that does contain stereoscopic depth information and that rotates around a parallel axis [43]. If the two cylinders appear to touch, they are almost always perceived to rotate in opposite directions. Because the effect disappears when there is a small gap between the spheres, it has been interpreted as a manifestation of intrinsic laws of friction embedded in the neuronal underpinnings that construct three-dimensional structure from two-dimensional motion information.
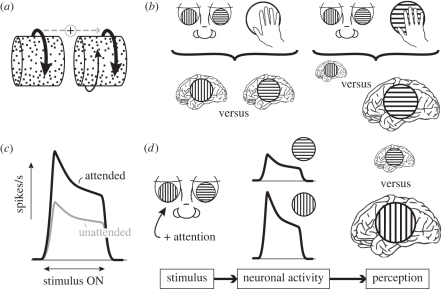
Other spatial contexts have been shown to influence the perception of coaxially oriented cylinders. In this situation, a cylinder with a rotation direction disambiguated by either binocular disparity or a luminance gradient can transfer its rotation direction to an otherwise ambiguous stimulus [44,45] (figure 3a). By presenting only the far or near sides of a context cylinder, the transfer of depth information specifically occurs between the perceived far sides (or backsides) of the stimuli, with the efficacy of information transfer weakening with increasing distance between stimuli [45]. The specificity of information transfer in the far depth field (behind fixation) has led to the idea that this form of spatial disambiguation is related to visual grouping of multiple chunks of visual information belonging to a single, partially occluded object [45]. The visual cortex is well equipped to transfer this kind of information via horizontal connections linking spatially separated columns of similarly tuned neurons [46]. These connections can span several millimetres of cortex representing several degrees of visual space [47]. They are most numerous between columns tuned to similar stimulus features, and their numbers decrease with distance [47]. It is however unknown whether horizontal connectivity is also selective for the depth tuning of the involved neurons, which would be a prerequisite for the interpretation of spatial disambiguation based on occlusion resolution.
Figure 3.
(a) An ambiguous rotating structure-from-motion cylinder (right) can become stabilized by a simultaneously presented coaxial unambiguous cylinder. As a consequence, both cylinders are mostly perceived to rotate in the same direction. (b) Unambiguous tactile orientation information biases perception during binocular rivalry between oriented gratings towards the grating that is congruent with the tactile information. Stimulation is depicted in the top row using eye and hand icons. Perception is indicated with brain icons, where larger icons indicate a greater dominance of the corresponding percept. (c) Schematic effect of attention on the response magnitude of a single neuron. A neuron will produce significantly more action potentials in response to an attended stimulus than to an unattended one. (d) If one of two gratings engaged in binocular rivalry is attended, the neurons representing that grating respond more vigorously than the neurons representing the opposite grating. This difference in activity may cause the attended grating to be dominant for larger proportions of time than the unattended grating.
A certain level of coupling between percepts of multiple simultaneously presented ambiguous stimuli is observed in other situations. Multiple, simultaneously presented Necker cubes, for instance, tend to reverse in perceived orientation together [48,49], independent patches of binocular rivalry gratings are simultaneously dominant when their orientations promote grouping [50] and ambiguous apparent motion dot displays share a perceived direction of motion [51], as do lines ambiguously rotating in depth [52]. All these perceptual grouping phenomena suggest that the brain might make global perceptual choices that effectively treat multiple ambiguities as a single sensory problem when context permits such an interpretation. The spatial coherence in such a perceptual decision may again arise from lateral connectivity in the cortex. Another form of spatial coherence in the resolution of ambiguous stimuli is best noticeable when one uses relatively large binocular rivalry stimuli. When a new perceptual interpretation occurs, it does not instantaneously do so at every location in the visual field at once. Instead, a wave of dominance steadily progresses through visual space, replacing the old percept with a new one [53]. This perceptual wave of dominance is complemented by a similar wave of activation moving across the retinotopic primary visual cortex [54].
It is a favourite topic of debate among binocular rivalry researchers whether binocular rivalry constitutes competition between eyes or between patterns [3,55]. Because it is now abundantly clear that binocular rivalry may involve either type of competition depending on the precise spatiotemporal characteristics of the stimuli [56], we do not reiterate that debate here. Instead, note that the principle of an influence of spatial context on conflicting visual input could even occur entirely within the neuronal binocular rivalry machinery, i.e. in a situation where the rivalling images are not accompanied by any additional context but create a spatial context to the visual conflict by themselves. When complementary patchwork stimuli are presented to the individual eyes, the resulting percepts are based not only on the eye from which visual input originated but also on coherent patterns constructed with multiple spatial patches originating from both eyes [32]. In fact, coherent pattern percepts using input from both eyes occur more often than percepts based on either individual eye's input [32]. Experiments like these illustrate that the visual system uses all the available sensory information it needs to construct ‘sensible’ percepts.
4. Crossmodal context
Disambiguation of sensory input in one modality through crossmodal interactions with information in another modality is a relatively new area of research. Following the same line of reasoning as for the disambiguating capacity of spatiotemporal context, sensory information may likely be integrated over multiple modalities to resolve ambiguities that arise within a unimodal processing stream. The rapidly growing body of research on crossmodal interactions with unambiguous, yet noisy, stimuli supports this view, suggesting that disambiguation of unreliable sensory information is a primary purpose of crossmodal interactions [57].
While perceptual choices for ambiguous stimuli in different sensory modalities can occur independently [2], there have been many reports of crossmodal contextual influences of unambiguous sensory information on the perception of otherwise ambiguous stimuli. One of the earliest reports of crossmodal disambiguation describes how an auditory signal influences the perceptual interpretation of ambiguous visual motion [58]. If two identical visual targets move across each other, they may be perceived to either bounce off or pass through each other. With vision only, the pass percept is more dominant than the bounce percept, but when a sound is played at the moment the two targets coincide, perception is strongly biased towards a bounce interpretation [59].
In binocular rivalry, non-visual input can provide additional evidence for one of the rival targets and bias competition towards the pattern that is congruent with the crossmodal context. If, for instance, one of two rivalling gratings is flickering at a fixed frequency, its predominance is enhanced by a simultaneous sound with amplitude modulation that is synchronous with the visual flicker [60]. This works with motion stimuli as well. Rivalry between motion stimuli with different directions can be biased towards one of the stimuli by a simultaneously presented congruent directional auditory motion signal [61]. The sound-driven boosts in dominance of one visual target in binocular rivalry primarily extend the duration of periods where the congruent image is dominant. Suppression durations, on the other hand, are affected only when the second monocular visual stimulus is incongruent with the sound, not when it is unrelated [61]. This distinction suggests audiovisual interactions in binocular rivalry at two levels of processing: an early crossmodal boost that resolves visual ambiguities is complemented by a high-level strengthening of congruent audiovisual percepts that only occurs for dominant percepts.
Tactile input may also disambiguate ambiguous visual stimuli. When participants touch horizontally or vertically grooved Plexiglas objects while experiencing binocular rivalry between horizontally and vertically oriented grating stimuli, the visual orientation that is congruent with the touched orientation is either released from suppression or remains dominant longer [62] (figure 3b). This crossmodal interaction between vision and touch depends upon the congruence of the spatial frequency of the haptic grooves and the visual gratings. The perceived rotation direction of a structure-from-motion sphere can also be driven by tactile input. When observers touch a real rotating globe while viewing an ambiguous sphere they perceive the visual sphere to rotate in the same direction as the real globe for a much larger proportion of the time than they perceive the opposite direction [63]. Brain imaging has shown that merely touching a rotating sphere evokes weak but reliable activation in visual motion area MT+ (middle temporal complex) [63]. Because the activity of a subset of neurons in this area reflects the perceived rotation of ambiguous rotating spheres [64], the supplementary somatosensory-driven activity could boost the representation of the congruent visual percept and resolve the visual ambiguity. While touching a rotating sphere may passively introduce motion information to the visual conflict, actively controlling the motion of one of two binocular rivalry stimuli increases the predominance of this stimulus as well, suggesting that there is also a role for the motor system in visual perceptual choices [65].
The recent discovery of perceptual rivalry between olfactory stimuli presented to individual nostrils [66] has introduced the olfactory system to the toolbox of rivalry researchers. Olfactory stimuli have also been shown to be capable of influencing visual perception during binocular rivalry. Competition between images of roses and marker pens can be biased towards either image by exposing participants to the smell of roses or marker pens [67]. The increased dominance of congruent visual targets was again found both in increased dominance durations and in decreased suppression durations.
It could be argued that many crossmodal disambiguation effects indirectly reflect attention reallocations. The mere presence of sensory input in a second modality could drive attention away from a visual ambiguity [68,69], while the congruence of secondary information in another modality may attract attention towards this particular interpretation and consequently bias the rivalry. The latter explanation of crossmodal interactions via attention is hard to disentangle from more sensory crossmodal integration mechanisms, especially because it is still relatively unclear what the neuronal basis of attention is and where it plays a role in neural processing. Additionally, recent experiments demonstrate that the extent to which observers are able to voluntarily control their perception of a rivalling stimulus is greatly enhanced by crossmodal congruency [70]. However, when observers viewed stimuli passively without exerting any voluntary control, there was no crossmodal congruence benefit, suggesting a complex interplay between multi-modal congruence and attention.
5. Context of internal state
In this section, we discuss a number of additional ways in which contextual information can influence the perception of ambiguous stimuli. These factors are broadly summarized as ‘internal state’ effects and include attention, emotional value, intention and culture. They have in common that the disambiguating additional information is not extracted from exogenous sensory streams, but comes from endogenous brain states that have either been established over an entire lifespan or fluctuate on shorter timescales.
We briefly touched upon a key candidate for the endogenous disambiguation of visual stimuli in the previous section: attention. Even though centuries of attention research have not clarified what attention is, they have provided a reasonably good idea of what attention does, particularly when it comes to visual perception. At a perceptual level, directing attention towards a visual stimulus improves performance on discrimination and detection tasks [71]. At the neuronal level, attention has been shown to increase neuronal activity and enhance sensitivity [72,73]. It is therefore not surprising that while attention is not strictly necessary for perceptual alternations to occur [74], it does profoundly affect perceptual experience during rivalry. Attracting attention towards or pulling attention away from a continuously presented rivalry stimulus speeds up or slows down the perceptual alternation rate, respectively [68,69]. This effect is comparable to physical manipulations of stimulus contrast [42,75] and is consistent with the idea that attention boosts the apparent contrast by increasing the response of a select group of neurons [71] (figure 3c). Following the same reasoning, attention directed towards one of two binocular rivalry stimuli should boost its apparent contrast and corresponding neuronal activity, giving the attended pattern an advantage in the rivalry process, which would result in its perceptual dominance (figure 3d). These effects have indeed been found with both exogenous and endogenous attention [76–78] and with tasks where observers intentionally steer perception towards a desired percept [19,79,80]. Attention is, however, not omnipotent and perceptual switches do still occur, as is also the case with auditory stimuli [81]. The same is true for physical stimulus manipulations that bias perception of ambiguous stimuli, but do not eliminate perceptual switches. Finally, the effect of voluntary control is considerably stronger for perceptual rivalry stimuli for which both eyes see the same stimulus than for binocular rivalry stimuli [19,79,80]. Because the former form of rivalry is presumably resolved at higher cortical processing levels than the latter, this is consistent with the finding that attention-driven increases of neuronal activity are larger in higher cortical areas [82].
The effects of emotional content on visual rivalry may be closely related to those of attention and could perhaps be summarized as a modulation of stimulus importance, ecological value or salience [83]. Natural images are reported to be dominant over unnatural images in binocular rivalry [84], as are images of emotional over (more) neutral faces or scenes [85–87] and upright faces over inverted ones [88]. The personal preferences or mental condition of the observer [89–91], as well as their cultural background or religion can also influence particular forms of rivalry [92]. All these high-level, cognitive influences may ultimately be related to the sensitivity and connectivity of the neuronal circuits that process the stimuli. They thus essentially bias conscious perception through inhomogeneities in the properties of the involved neurons, making it appropriate to compare them to personal biases that the participants may have for a certain eye of origin in binocular rivalry, or a certain rotation direction in ambiguous structure-from-motion. These biases can be quite large and retinotopic in nature, suggesting that they indeed reflect low-level neuronal inhomogeneities in sensory cortex [18,93–95].
Visual processing already starts when photons hit the retina. Sensory information will pass multiple neurons and processing stages before ambiguities are resolved and a coherent percept is generated. At all processing stages before rivalry resolution, the competition between perceptual interpretations may become biased. Irrespective of whether these biases emerge from inhomogeneous neuronal properties, attentional gain mechanisms or high-level feedback projections, their effects on rivalry resolution are essentially similar and resemble the consequences of simple stimulus strength manipulations (see [2] for a similar argument).
6. Concluding remarks
The range of context-driven perceptual disambiguation mechanisms presented in this review suggests that the resolution of ambiguous sensory information in favour of temporarily stable perception takes place at multiple processing loci along the visual cortical hierarchy. The fact that we are hardly ever aware of ambiguous visual input during natural vision must be attributed to the fact that ambiguous stimulation is truly ambiguous only when it is completely isolated from spatiotemporal and crossmodal context [96]. Even if such an improbable situation ever occurred, pre-existing neural states could quickly drive perception towards one particular interpretation. Using all the available contextual information, the brain is capable both of reaching initially stable percepts at the onset of ambiguous stimuli and of biasing the cycle of perceptual alternations so that more time is spent perceiving interpretations supported by more (circumstantial) sensory evidence.
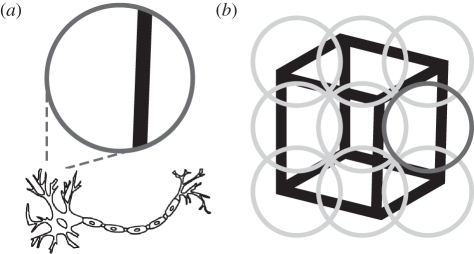
Visual neurons have a spatiotemporal receptive field, meaning that they respond only to events in a particular region of space and with a specific latency [97]. As a consequence, early visual neurons can only encode very limited portions of the scene, which makes sensory information from these single neurons intrinsically ambiguous. This forces the brain to integrate information over many neurons in order to establish percepts (figure 4). Studies on ambiguous stimuli may bridge the gap between studies on perceptual choice processes and neuronal mechanisms of perception by essentially asking the same basic question: how does the brain resolve ambiguous input?
Figure 4.
(a) Sensory information carried by a single neuron is often ambiguous because of the limited amount of information present within the neuron's receptive field (dark grey circle). (b) If the receptive fields of multiple neurons (grey circles) are combined, the ambiguous information of a single neuron (dark grey circle) can be disambiguated and coherent percepts emerge.
Computational models of binocular rivalry usually focus on interneuronal interactions to explain perceptual experience [28,14,26]. Recently, a more conceptual theoretical framework for rivalry has been proposed in which perception is considered a process of largely unconscious inference. In this view, the brain tests its perceptual hypotheses against accumulating sensory information [98], and rivalry merely deviates from normal vision in the sense that current sensory information alone is insufficient to reach definitive perceptual solutions. All contextual influences mentioned in this review fit well with such a Bayesian theory of perception [96,99,100]. Context may actively influence either a prior (temporal context) or the likelihood of current sensory input by adding more information to it. With the incorporation of elements from classic rivalry models such as inhibition, adaptation and noise, this conceptual approach can be related to mechanistic rivalry models while maintaining a broad view of rivalry processes within the context of normal perceptual functioning. Such an approach could facilitate the field's recognition of the particular value of perceptual ambiguities in unravelling more basic mechanisms of perception and neuronal processing.
Acknowledgements
P.C.K. is supported by the NCU Focus and Mass programme of Utrecht University. R.v.E. is supported by a grant from the Flemish Methusalem programme (METH/08/02). R.J.A.v.W. and R.v.E. are jointly supported by a Utrecht University High Potential grant.
Endnote
Perceptual alternations do not require exactly equal evidence for conflicting perceptual interpretations. Mechanisms that are involved in the perception of (moderately) biased ambiguous stimuli can in fact be highly informative about the nature of these perceptual alternations.
References
- 1.Long G., Toppino T. 2004. Enduring interest in perceptual ambiguity: alternating views of reversible figures. Psychol. Bull. 130, 748–768 10.1037/0033-2909.130.5.748 (doi:10.1037/0033-2909.130.5.748) [DOI] [PubMed] [Google Scholar]
- 2.Hupé J. M., Joffo L.-M., Pressnitzer D. 2008. Bistability for audiovisual stimuli: perceptual decision is modality specific. J. Vis. 8, 1. 10.1167/8.7.1 (doi:10.1167/8.7.1) [DOI] [PubMed] [Google Scholar]
- 3.Blake R., Logothetis N. K. 2002. Visual competition. Nat. Rev. Neurosci. 3, 13–21 10.1038/nrn701 (doi:10.1038/nrn701) [DOI] [PubMed] [Google Scholar]
- 4.Klink P. C. 2011. Neural mechanisms of context-driven conscious visual perception. PhD thesis, Utrecht University, Utrecht, The Netherlands [Google Scholar]
- 5.Kohn A. 2007. Visual adaptation: physiology, mechanisms, and functional benefits. J. Neurophysiol. 97, 3155–3164 10.1152/jn.00086.2007 (doi:10.1152/jn.00086.2007) [DOI] [PubMed] [Google Scholar]
- 6.Holtmaat A., Svoboda K. 2009. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 10, 647–658 10.1038/nrn2699 (doi:10.1038/nrn2699) [DOI] [PubMed] [Google Scholar]
- 7.Brascamp J. W., Knapen T. H. J., Kanai R., Noest A. J., van Ee R., van den Berg A. V. 2008. Multi-timescale perceptual history resolves visual ambiguity. PLoS ONE 3, e1497. 10.1371/journal.pone.0001497 (doi:10.1371/journal.pone.0001497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pastukhov A., Braun J. 2008. A short-term memory of multi-stable perception. J. Vis. 8, 7. 10.1167/8.13.7 (doi:10.1167/8.13.7) [DOI] [PubMed] [Google Scholar]
- 9.Wark B., Fairhall A., Rieke F. 2009. Timescales of inference in visual adaptation. Neuron 61, 750–761 10.1016/j.neuron.2009.01.019 (doi:10.1016/j.neuron.2009.01.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Boxtel J. J. A., Alais D., van Ee R. 2008. Retinotopic and non-retinotopic stimulus encoding in binocular rivalry and the involvement of feedback. J. Vis. 8, 17. 10.1167/8.5.17 (doi:10.1167/8.5.17) [DOI] [PubMed] [Google Scholar]
- 11.Kanai R., Verstraten F. A. J. 2005. Perceptual manifestations of fast neural plasticity: motion priming, rapid motion aftereffect and perceptual sensitization. Vision Res. 45, 3109–3116 10.1016/j.visres.2005.05.014 (doi:10.1016/j.visres.2005.05.014) [DOI] [PubMed] [Google Scholar]
- 12.Brascamp J. W., Knapen T. H. J., Kanai R., van Ee R., van den Berg A. V. 2007. Flash suppression and flash facilitation in binocular rivalry. J. Vis. 7, 12. 10.1167/7.12.12 (doi:10.1167/7.12.12) [DOI] [PubMed] [Google Scholar]
- 13.Wolfe J. M. 1984. Reversing ocular dominance and suppression in a single flash. Vision Res. 24, 471–478 10.1016/0042-6989(84)90044-0 (doi:10.1016/0042-6989(84)90044-0) [DOI] [PubMed] [Google Scholar]
- 14.Noest A. J., van Ee R., Nijs M. M., van Wezel R. J. A. 2007. Percept-choice sequences driven by interrupted ambiguous stimuli: a low-level neural model. J. Vis. 7, 10. 10.1167/7.8.10 (doi:10.1167/7.8.10) [DOI] [PubMed] [Google Scholar]
- 15.Daelli V., van Rijsbergen N. J., Treves A. 2010. How recent experience affects the perception of ambiguous objects. Brain Res. 1322, 81–91 10.1016/j.brainres.2010.01.060 (doi:10.1016/j.brainres.2010.01.060) [DOI] [PubMed] [Google Scholar]
- 16.Tsuchiya N., Koch C. 2005. Continuous flash suppression reduces negative afterimages. Nat. Neurosci. 8, 1096–1101 10.1038/nn1500 (doi:10.1038/nn1500) [DOI] [PubMed] [Google Scholar]
- 17.Kanai R., Tsuchiya N., Verstraten F. A. J. 2006. The scope and limits of top-down attention in unconscious visual processing. Curr. Biol. 16, 2332–2336 10.1016/j.cub.2006.10.001 (doi:10.1016/j.cub.2006.10.001) [DOI] [PubMed] [Google Scholar]
- 18.Carter O. L., Cavanagh P. 2007. Onset rivalry: brief presentation isolates an early independent phase of perceptual competition. PLoS ONE 2, e343. 10.1371/journal.pone.0000343 (doi:10.1371/journal.pone.0000343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klink P. C., van Ee R., Nijs M. M., Brouwer G. J., Noest A. J., van Wezel R. J. A. 2008. Early interactions between neuronal adaptation and voluntary control determine perceptual choices in bistable vision. J. Vis. 8, 16. 10.1167/8.5.16 (doi:10.1167/8.5.16) [DOI] [PubMed] [Google Scholar]
- 20.Pearson J., Brascamp J. W. 2008. Sensory memory for ambiguous vision. Trends Cogn. Sci. 12, 334–341 10.1016/j.tics.2008.05.006 (doi:10.1016/j.tics.2008.05.006) [DOI] [PubMed] [Google Scholar]
- 21.Brascamp J. W., van Ee R., Pestman W., van den Berg A. V. 2005. Distributions of alternation rates in various forms of bistable perception. J. Vis. 5, 287–298 10.1167/5.4.1 (doi:10.1167/5.4.1) [DOI] [PubMed] [Google Scholar]
- 22.van Ee R. 2005. Dynamics of perceptual bi-stability for stereoscopic slant rivalry and a comparison with grating, house-face, and Necker cube rivalry. Vision Res. 45, 29–40 10.1016/j.visres.2004.07.039 (doi:10.1016/j.visres.2004.07.039) [DOI] [PubMed] [Google Scholar]
- 23.Orbach J., Zucker E., Olson R. 1966. Reversibility of the Necker Cube. VII. Reversal rate as a function of figure-on and figure-off duration. Percept. Motor Skill 17, 615–618 10.2466/pms.1966.22.2.615 (doi:10.2466/pms.1966.22.2.615) [DOI] [Google Scholar]
- 24.Kornmeier J., Ehm W., Bigalke H., Bach M. 2007. Discontinuous presentation of ambiguous figures: how interstimulus-interval durations affect reversal dynamics and ERPs. Psychophysiology 44, 552–560 10.1111/j.1469-8986.2007.00525.x (doi:10.1111/j.1469-8986.2007.00525.x) [DOI] [PubMed] [Google Scholar]
- 25.Leopold D. A., Wilke M., Maier A., Logothetis N. K. 2002. Stable perception of visually ambiguous patterns. Nat. Neurosci. 5, 605–609 10.1038/nn0602-851 (doi:10.1038/nn0602-851) [DOI] [PubMed] [Google Scholar]
- 26.Wilson H. R. 2007. Minimal physiological conditions for binocular rivalry and rivalry memory. Vision Res. 47, 2741–2750 10.1016/j.visres.2007.07.007 (doi:10.1016/j.visres.2007.07.007) [DOI] [PubMed] [Google Scholar]
- 27.Brascamp J. W., Kanai R., Walsh V., van Ee R. 2010. Human middle temporal cortex, perceptual bias, and perceptual memory for ambiguous three-dimensional motion. J. Neurosci. 30, 760–766 10.1523/JNEUROSCI.4171-09.2010 (doi:10.1523/JNEUROSCI.4171-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lankheet M. J. M. 2006. Unraveling adaptation and mutual inhibition in perceptual rivalry. J. Vis. 6, 304–310 10.1167/6.4.1 (doi:10.1167/6.4.1) [DOI] [PubMed] [Google Scholar]
- 29.Hollins M. 1980. The effect of contrast on the completeness of binocular rivalry suppression. Percept. Psychophys. 27, 550–556 10.3758/BF03198684 (doi:10.3758/BF03198684) [DOI] [PubMed] [Google Scholar]
- 30.Klink P. C., Brascamp J. W., Blake R., van Wezel R. J. A. 2010. Experience-driven plasticity in binocular vision. Curr. Biol. 20, 1464–1469 10.1016/j.cub.2010.06.057 (doi:10.1016/j.cub.2010.06.057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y., Rose D., Blake R. 1992. On the variety of percepts associated with dichoptic viewing of dissimilar monocular stimuli. Perception 21, 47–62 10.1068/p210047 (doi:10.1068/p210047) [DOI] [PubMed] [Google Scholar]
- 32.Kovács I., Papathomas T. V., Yang M., Fehér A. 1996. When the brain changes its mind: interocular grouping during binocular rivalry. Proc. Natl Acad. Sci. USA 93, 15 508–15 511 10.1073/pnas.93.26.15508 (doi:10.1073/pnas.93.26.15508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki S., Grabowecky M. 2007. Long-term speeding in perceptual switches mediated by attention-dependent plasticity in cortical visual processing. Neuron 56, 741–753 10.1016/j.neuron.2007.09.028 (doi:10.1016/j.neuron.2007.09.028) [DOI] [PubMed] [Google Scholar]
- 34.Fukuda H., Blake R. 1992. Spatial interactions in binocular rivalry. J. Exp. Psychol. Hum. 18, 362–370 10.1037/0096-1523.18.2.362 (doi:10.1037/0096-1523.18.2.362) [DOI] [PubMed] [Google Scholar]
- 35.Paffen C., Tadin D., te Pas S., Blake R., Verstraten F. A. J. 2006. Adaptive center-surround interactions in human vision revealed during binocular rivalry. Vision Res. 46, 599–604 10.1016/j.visres.2005.05.013 (doi:10.1016/j.visres.2005.05.013) [DOI] [PubMed] [Google Scholar]
- 36.Alais D., Blake R. 1998. Interactions between global motion and local binocular rivalry. Vision Res. 38, 637–644 10.1016/S0042-6989(97)00190-9 (doi:10.1016/S0042-6989(97)00190-9) [DOI] [PubMed] [Google Scholar]
- 37.Sobel K., Blake R. 2002. How context influences predominance during binocular rivalry. Perception 31, 813–824 10.1068/p3279 (doi:10.1068/p3279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watson T. L., Pearson J., Clifford C. W. G. 2004. Perceptual grouping of biological motion promotes binocular rivalry. Curr. Biol. 14, 1670–1674 10.1016/j.cub.2004.08.064 (doi:10.1016/j.cub.2004.08.064) [DOI] [PubMed] [Google Scholar]
- 39.Graf E. W., Adams W. J. 2008. Surface organization influences bistable vision. J. Exp. Psychol. Hum. 34, 502–508 10.1037/0096-1523.34.2.502 (doi:10.1037/0096-1523.34.2.502) [DOI] [PubMed] [Google Scholar]
- 40.Andersen R. A., Bradley D. C. 1998. Perception of three-dimensional structure from motion. Trends Cogn. Sci. 2, 222–228 10.1016/S1364-6613(98)01181-4 (doi:10.1016/S1364-6613(98)01181-4) [DOI] [PubMed] [Google Scholar]
- 41.Sereno M. E., Sereno M. I. 1999. 2-D center-surround effects on 3-D structure-from-motion. J. Exp. Psychol. Hum. 25, 1834–1854 10.1037/0096-1523.25.6.1834 (doi:10.1037/0096-1523.25.6.1834) [DOI] [PubMed] [Google Scholar]
- 42.Klink P. C., van Ee R., van Wezel R. J. A. 2008. General validity of Levelt's propositions reveals common computational mechanisms for visual rivalry. PLoS ONE 3, e3473. 10.1371/journal.pone.0003473 (doi:10.1371/journal.pone.0003473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilroy L. A., Blake R. 2004. Physics embedded in visual perception of three-dimensional shape from motion. Nat. Neurosci. 7, 921–922 10.1038/nn1297 (doi:10.1038/nn1297) [DOI] [PubMed] [Google Scholar]
- 44.Freeman E. D., Driver J. 2006. Subjective appearance of ambiguous structure-from-motion can be driven by objective switches of a separate less ambiguous context. Vision Res. 46, 4007–4023 10.1016/j.visres.2006.07.008 (doi:10.1016/j.visres.2006.07.008) [DOI] [PubMed] [Google Scholar]
- 45.Klink P. C., Noest A. J., Holten V., van den Berg A. V., van Wezel R. J. A. 2009. Occlusion-related lateral connections stabilize kinetic depth stimuli through perceptual coupling. J. Vis. 9, 20. 10.1167/9.10.20 (doi:10.1167/9.10.20) [DOI] [PubMed] [Google Scholar]
- 46.Gilbert C. D., Wiesel T. N. 1989. Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. J. Neurosci. 9, 2432–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ts'o D. Y., Gilbert C. D., Wiesel T. N. 1986. Relationships between horizontal interactions and functional architecture in cat striate cortex as revealed by cross-correlation analysis. J. Neurosci. 6, 1160–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flügel J. C. 1913. the influence of attention in illusions of reversible perspective. Br. J. Psychol. 5, 357–397 [Google Scholar]
- 49.Adams P. A., Haire M. 1958. Structural and conceptual factors in the perception of double-cube figures. Am. J. Psychol. 71, 548–556 10.2307/1420250 (doi:10.2307/1420250) [DOI] [PubMed] [Google Scholar]
- 50.Alais D., Blake R. 1999. Grouping visual features during binocular rivalry. Vision Res. 39, 4341–4353 10.1016/S0042-6989(99)00146-7 (doi:10.1016/S0042-6989(99)00146-7) [DOI] [PubMed] [Google Scholar]
- 51.Ramachandran V. S., Anstis S. 1983. Perceptual organization in moving patterns. Nature 304, 529–531 10.1038/304529a0 (doi:10.1038/304529a0) [DOI] [PubMed] [Google Scholar]
- 52.Gillam B. 1972. Perceived common rotary motion of ambiguous stimuli as a criterion of perceptual grouping. Percept. Psychophys. 99–101 10.3758/BF03212694 (doi:10.3758/BF03212694) [DOI] [Google Scholar]
- 53.Wilson H. R., Blake R., Lee S.-H. 2001. Dynamics of travelling waves in visual perception. Nature 412, 907–910 10.1038/35091066 (doi:10.1038/35091066) [DOI] [PubMed] [Google Scholar]
- 54.Lee S.-H., Blake R., Heeger D. J. 2005. Traveling waves of activity in primary visual cortex during binocular rivalry. Nat. Neurosci. 8, 22–23 10.1038/nn1365 (doi:10.1038/nn1365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Logothetis N. K., Leopold D. A., Sheinberg D. L. 1996. What is rivalling during binocular rivalry? Nature 380, 621–624 10.1038/380621a0 (doi:10.1038/380621a0) [DOI] [PubMed] [Google Scholar]
- 56.Lee S.-H., Blake R., Heeger D. J. 2007. Hierarchy of cortical responses underlying binocular rivalry. Nat. Neurosci. 10, 1048–1054 10.1038/nn1939 (doi:10.1038/nn1939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ernst M. O., Bülthoff H. H. 2004. Merging the senses into a robust percept. Trends Cogn. Sci. 8, 162–169 10.1016/j.tics.2004.02.002 (doi:10.1016/j.tics.2004.02.002) [DOI] [PubMed] [Google Scholar]
- 58.Sekuler R., Sekuler A. B., Lau R. 1997. Sound alters visual motion perception. Nature 385, 308. 10.1038/385308a0 (doi:10.1038/385308a0) [DOI] [PubMed] [Google Scholar]
- 59.Shimojo S., Shams L. 2001. Sensory modalities are not separate modalities: plasticity and interactions. Curr. Opin. Neurobiol. 11, 505–509 10.1016/S0959-4388(00)00241-5 (doi:10.1016/S0959-4388(00)00241-5) [DOI] [PubMed] [Google Scholar]
- 60.Kang M. S. 2005. Perceptual synergy between seeing and hearing revealed during binocular rivalry. Psichologija 32, 7–15 [Google Scholar]
- 61.Conrad V., Bartels A., Kleiner M., Noppeney U. 2010. Audiovisual interactions in binocular rivalry. J. Vis. 10, 27. 10.1167/10.10.27 (doi:10.1167/10.10.27) [DOI] [PubMed] [Google Scholar]
- 62.Lunghi C., Binda P., Morrone M. C. 2010. Touch disambiguates rivalrous perception at early stages of visual analysis. Curr. Biol. 20, R143–R144 10.1016/j.cub.2009.12.015 (doi:10.1016/j.cub.2009.12.015) [DOI] [PubMed] [Google Scholar]
- 63.Blake R., Sobel K. V., James T. W. 2004. Neural synergy between kinetic vision and touch. Psychol. Sci. 15, 397–402 10.1111/j.0956-7976.2004.00691.x (doi:10.1111/j.0956-7976.2004.00691.x) [DOI] [PubMed] [Google Scholar]
- 64.Bradley D. C., Chang G., Andersen R. A. 1998. Encoding of three-dimensional structure-from-motion by primate area MT neurons. Nature 392, 714–717 10.1038/33688 (doi:10.1038/33688) [DOI] [PubMed] [Google Scholar]
- 65.Maruya K., Yang E., Blake R. 2007. Voluntary action influences visual competition. Psychol. Sci. 18, 1090–1098 10.1111/j.1467-9280.2007.02030.x (doi:10.1111/j.1467-9280.2007.02030.x) [DOI] [PubMed] [Google Scholar]
- 66.Zhou W., Chen D. 2009. Binaral rivalry between the nostrils and in the cortex. Curr. Biol. 19, 1561–1565 10.1016/j.cub.2009.07.052 (doi:10.1016/j.cub.2009.07.052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou W., Jiang Y., He S., Chen D. 2010. Olfaction modulates visual perception in binocular rivalry. Curr. Biol. 20, 1356–1358 10.1016/j.cub.2010.05.059 (doi:10.1016/j.cub.2010.05.059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paffen C., Alais D., Verstraten F. A. J. 2006. Attention speeds binocular rivalry. Psychol. Sci. 17, 752–756 10.1111/j.1467-9280.2006.01777.x (doi:10.1111/j.1467-9280.2006.01777.x) [DOI] [PubMed] [Google Scholar]
- 69.Alais D., van Boxtel J. J., Parker A., van Ee R. 2010. Attending to auditory signals slows visual alternations in binocular rivalry. Vision Res. 50, 929–935 10.1016/j.visres.2010.03.010 (doi:10.1016/j.visres.2010.03.010) [DOI] [PubMed] [Google Scholar]
- 70.van Ee R., van Boxtel J. J. A., Parker A. L., Alais D. 2009. Multisensory congruency as a mechanism for attentional control over perceptual selection. J. Neurosci. 29, 11641–11649 10.1523/JNEUROSCI.0873-09.2009 (doi:10.1523/JNEUROSCI.0873-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carrasco M., Ling S., Read S. 2004. Attention alters appearance. Nat. Neurosci. 7, 308–313 10.1038/nn1194 (doi:10.1038/nn1194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spitzer H., Desimone R., Moran J. 1988. Increased attention enhances both behavioral and neuronal performance. Science 240, 338–340 10.1126/science.3353728 (doi:10.1126/science.3353728) [DOI] [PubMed] [Google Scholar]
- 73.Reynolds J. H., Pasternak T., Desimone R. 2000. Attention increases sensitivity of V4 neurons. Neuron 26, 703–714 10.1016/S0896-6273(00)81206-4 (doi:10.1016/S0896-6273(00)81206-4) [DOI] [PubMed] [Google Scholar]
- 74.Pastukhov A., Braun J. 2007. Perceptual reversals need no prompting by attention. J. Vis. 7, 5. 10.1167/7.10.5 (doi:10.1167/7.10.5) [DOI] [PubMed] [Google Scholar]
- 75.Levelt J. W. M. 1966. The alternation process in binocular rivalry. Br. J. Psychol. 57, 225–238 10.1111/j.2044-8295.1966.tb01023.x (doi:10.1111/j.2044-8295.1966.tb01023.x) [DOI] [Google Scholar]
- 76.Chong S., Tadin D., Blake R. 2005. Endogenous attention prolongs dominance durations in binocular rivalry. J. Vis. 5, 1004–1012 10.1167/5.8.1004 (doi:10.1167/5.8.1004) [DOI] [PubMed] [Google Scholar]
- 77.Mitchell J. F., Stoner G. R., Reynolds J. H. 2004. Object-based attention determines dominance in binocular rivalry. Nature 429, 410–413 10.1038/nature02584 (doi:10.1038/nature02584) [DOI] [PubMed] [Google Scholar]
- 78.Chong S., Blake R. 2006. Exogenous attention and endogenous attention influence initial dominance in binocular rivalry. Vision Res. 46, 1794–1803 10.1016/j.visres.2005.10.031 (doi:10.1016/j.visres.2005.10.031) [DOI] [PubMed] [Google Scholar]
- 79.Meng M., Tong F. 2004. Can attention selectively bias bistable perception? Differences between binocular rivalry and ambiguous figures. J. Vis. 4, 539–551 10.1167/4.8.539 (doi:10.1167/4.8.539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Ee R., van Dam L., Brouwer G. J. 2005. Voluntary control and the dynamics of perceptual bi-stability. Vision Res. 45, 41–55 10.1016/j.visres.2004.07.030 (doi:10.1016/j.visres.2004.07.030) [DOI] [PubMed] [Google Scholar]
- 81.Moore B. C. J., Gockel H. R. 2012. Properties of auditory stream formation. Phil. Trans. R. Soc. B 367, 919–931 10.1098/rstb.2011.0355 (doi:10.1098/rstb.2011.0355). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maunsell J. H. R., Cook E. P. 2002. The role of attention in visual processing. Phil. Trans. R. Soc. Lond. B 357, 1063–1072 10.1098/rstb.2002.1107 (doi:10.1098/rstb.2002.1107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Treue S. 2003. Visual attention: the where, what, how and why of saliency. Curr. Opin. Neurobiol. 13, 428–432 10.1016/S0959-4388(03)00105-3 (doi:10.1016/S0959-4388(03)00105-3) [DOI] [PubMed] [Google Scholar]
- 84.Baker D. H., Graf E. 2009. Natural images dominate in binocular rivalry. Proc. Natl Acad. Sci. USA 106, 5436–5441 10.1073/pnas.0812860106 (doi:10.1073/pnas.0812860106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alpers G. W. 2006. Emotional pictures predominate in binocular rivalry. Cogn. Emotion 20, 596–607 10.1080/02699930500282249 (doi:10.1080/02699930500282249) [DOI] [PubMed] [Google Scholar]
- 86.Yang E., Zald D. H., Blake R. 2007. Fearful expressions gain preferential access to awareness during continuous flash suppression. Emotion 7, 882–886 10.1037/1528-3542.7.4.882 (doi:10.1037/1528-3542.7.4.882) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yoon K. L., Hong S. W., Joormann J., Kang P. 2009. Perception of facial expressions of emotion during binocular rivalry. Emotion 9, 172–182 10.1037/a0014714 (doi:10.1037/a0014714) [DOI] [PubMed] [Google Scholar]
- 88.Jiang Y., Costello P., He S. 2007. Processing of invisible stimuli: advantage of upright faces and recognizable words in overcoming interocular suppression. Psychol. Sci. 18, 349–355 10.1111/j.1467-9280.2007.01902.x (doi:10.1111/j.1467-9280.2007.01902.x) [DOI] [PubMed] [Google Scholar]
- 89.Kohn H. 1960. Some personality variables associated with binocular rivalry. Psychol. Rec. 10, 9–13 [Google Scholar]
- 90.Carter O. L., Presti D., Callistemon C., Ungerer Y., Liu G. B., Pettigrew J. D. 2005. Meditation alters perceptual rivalry in Tibetan Buddhist monks. Curr. Biol. 15, R412–R413 10.1016/j.cub.2005.05.043 (doi:10.1016/j.cub.2005.05.043) [DOI] [PubMed] [Google Scholar]
- 91.Gray K. L. H., Adams W. J., Garner M. 2009. The influence of anxiety on the initial selection of emotional faces presented in binocular rivalry. Cognition 113, 105–110 10.1016/j.cognition.2009.06.009 (doi:10.1016/j.cognition.2009.06.009) [DOI] [PubMed] [Google Scholar]
- 92.LoSciuto L. A., Hartley E. L. 1963. Religious affiliation and open-mindedness in binocular resolution. Percept. Motor Skill 17, 427–430 [DOI] [PubMed] [Google Scholar]
- 93.Knapen T., Brascamp J., Adams W. J., Graf E. W. 2009. The spatial scale of perceptual memory in ambiguous figure perception. J. Vis. 9, 16. 10.1167/9.13.16 (doi:10.1167/9.13.16) [DOI] [PubMed] [Google Scholar]
- 94.van Ee R. 2011. Percept-switch nucleation in binocular rivalry reveals local adaptation characteristics of early visual processing. J. Vis. 11, 13. 10.1167/11.2.13 (doi:10.1167/11.2.13) [DOI] [PubMed] [Google Scholar]
- 95.Raemaekers M., van der Schaaf M., van Ee R., van Wezel R. 2008. Widespread fMRI activity differences between perceptual states in visual rivalry are correlated with differences in observer biases. Brain Res. 1252, 161–171 10.1016/j.brainres.2008.11.078 (doi:10.1016/j.brainres.2008.11.078) [DOI] [PubMed] [Google Scholar]
- 96.Kersten D., Yuille A. 2003. Bayesian models of object perception. Curr. Opin. Neurobiol. 13, 150–158 10.1016/S0959-4388(03)00042-4 (doi:10.1016/S0959-4388(03)00042-4) [DOI] [PubMed] [Google Scholar]
- 97.DeAngelis G. C., Ohzawa I., Freeman R. D. 1995. Receptive-field dynamics in the central visual pathways. Trends Neurosci. 18, 451–458 10.1016/0166-2236(95)94496-R (doi:10.1016/0166-2236(95)94496-R) [DOI] [PubMed] [Google Scholar]
- 98.Hohwy J., Roepstorff A., Friston K. 2008. Predictive coding explains binocular rivalry: an epistemological review. Cognition 108, 687–701 10.1016/j.cognition.2008.05.010 (doi:10.1016/j.cognition.2008.05.010) [DOI] [PubMed] [Google Scholar]
- 99.van Ee R., Adams W. J., Mamassian P. 2003. Bayesian modeling of cue interaction: bistability in stereoscopic slant perception. J. Opt. Soc. Am. A 20, 1398–1406 10.1364/JOSAA.20.001398 (doi:10.1364/JOSAA.20.001398) [DOI] [PubMed] [Google Scholar]
- 100.Knill D. C., Pouget A. 2004. The Bayesian brain: the role of uncertainty in neural coding and computation. Trends Neurosci. 27, 712–719 10.1016/j.tins.2004.10.007 (doi:10.1016/j.tins.2004.10.007) [DOI] [PubMed] [Google Scholar]