Abstract
For the majority of the Early Caenozoic, a remarkable expanse of humid, mesothermal to temperate forests spread across Northern Polar regions that now contain specialized plant and animal communities adapted to life in extreme environments. Little is known on the taxonomic diversity of Arctic floras during greenhouse periods of the Caenozoic. We show for the first time that plant richness in the globally warm Early Eocene (approx. 55–52 Myr) in the Canadian High Arctic (76° N) is comparable with that approximately 3500 km further south at mid-latitudes in the US western interior (44–47° N). Arctic Eocene pollen floras are most comparable in richness with today's forests in the southeastern United States, some 5000 km further south of the Arctic. Nearly half of the Eocene, Arctic plant taxa are endemic and the richness of pollen floras implies significant patchiness to the vegetation type and clear regional richness of angiosperms. The reduced latitudinal diversity gradient in Early Eocene North American plant species demonstrates that extreme photoperiod in the Arctic did not limit taxonomic diversity of plants.
Keywords: palynology, plants, vertebrates, Eocene, Arctic, latitudinal gradient
1. Introduction
For most of the Early Caenozoic Era, a remarkable expanse of humid, warm-temperate to temperate forests spread across Northern Polar regions [1–6]. During this time, the High Arctic experienced mild, equable, non-freezing temperatures [3–6]. Oxygen isotope ratios of mammal teeth and fish scales (δ18O) and fossil plants suggest an Early Eocene mean annual temperature ranging from 8 to 15°C [7–9]. Warm month mean temperatures probably reached 19–20°C or higher, and winter temperatures were above freezing [6,8,9]. Long-standing evidence for a warm Arctic climate was first indicated by the Eocene fauna on Ellesmere Island (Nunavut, Arctic Canada) that included alligators, a varanid lizard, boid snakes, giant tortoises, tapirs and primates [10–14]. The physiological stresses placed on plants growing in warm polar latitudes are unique—these plants, even in greenhouse conditions of the past, tolerated the same extreme photoperiod that exists currently above the Arctic Circle [15] because the fossil-bearing strata in the High Arctic were just a couple of degrees further south than their present-day latitude [16,17]. Many of these polar plants were deciduous [3,4] and dormant during the dark winter months, but physiologically active during the low-intensity 24 h summer light regime [15,18]. Estimated biomass and annual net primary productivity of Metasequoia (dawn redwood) indicate that these forests were comparable with modern old-growth forests of the Pacific northwest, USA [19]. The fossil floras of the Arctic region have received considerable attention, but quantification of the taxonomic richness of the floras growing under greenhouse climates during the Caenozoic is lacking. For the first time, we quantify plant species richness in the High Arctic during the globally warm Early Eocene Epoch (approx. 54–52 Myr) using palynological data (pollen and spores) from Stenkul Fiord (figure 1) on southern Ellesmere Island, Nunavut, Canada. Fossils found at Stenkul Fiord (ca 77° N; 83° W) include mammals and reptiles [11,20,21], tree stumps and leaves [2] and pollen [22]. The sediments are mapped as the Late Palaeocene–Early Eocene Margaret Formation [23] (=Iceberg Bay Formation [22]), a division of the Eureka Sound Group [22,23], with Eocene sediments cropping out on the southwestern side of the fiord. The coal, sand and silt are part of an ancient swamp, floodplain and upper deltaic mosaic that includes palaeosols, channels and channel fill deposits. These strata at Stenkul Fiord are an exemplar for understanding the Early Eocene fauna and flora of the High Arctic.
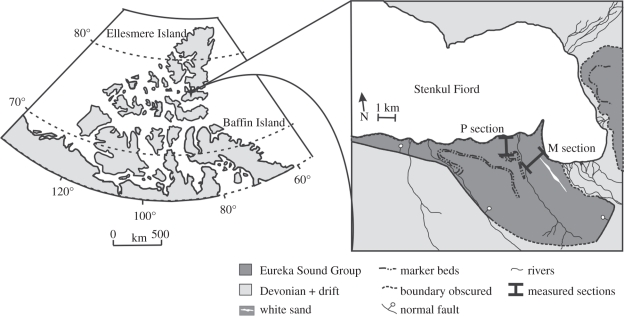
Figure 1.
Location map of Stenkul Fiord, Nunavat, Canada indicating study sections on the southwest of the fiord.
2. Material and methods
Fieldwork at Stenkul Fiord made by subsets of the authors over three decades has resulted in a collection of fossils tied to stratigraphic sections identified principally by clearly defined white sand marker beds (figures 1 and 2). The stratigraphic sections that are used here overlap and are designated M and P sections. Palynological samples from Stenkul Fiord were collected from the coals and provide vegetation information from the predominantly local to regional area. Vertebrate collections map directly into these measured sections from surrounding channels and floodplain environments, and we use vertebrate biostratigraphy to constrain the age of the sediments.
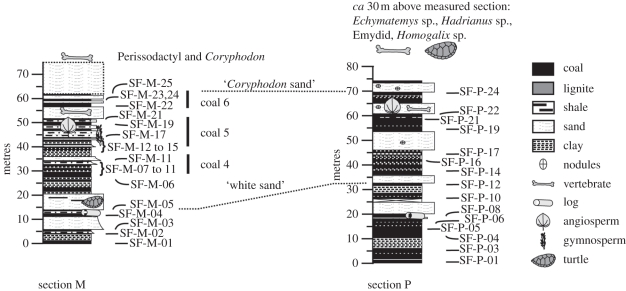
Figure 2.
Stratigraphic measured sections from Stenkul Fiord, Nunavat, Canada indicating position of pollen samples. Important vertebrate and plant megafossil horizons are indicated within sections. Coal bed numbers are adapted from Reidiger & Bustin [23].
Thirty-seven pollen samples were processed as detailed by Harrington et al. [24] and raw count data are presented in the electronic supplementary material. Palynological data from the Early Eocene for comparison with Stenkul Fiord were taken from multiple sources including published monographs. Samples from the Bighorn Basin, Powder River Basin (both Wyoming) and Williston Basin (North Dakota) are from published accounts [24–27] and represent relative abundance count data for the US western interior. Other datasets from the Arctic, North America and Europe (electronic supplementary material) are all dated to the Early Eocene [24–26,28–41]. In most cases, the dating is just to the Early Eocene based on bio- and lithostratigraphy. Comparisons with Asian pollen floras are problematic and the available floras documented in the literature are not strongly representative of European or American records with the exception of cosmopolitan families that are common throughout the holarctic region [42,43]. Because the published data sources represent the taxonomy of multiple palynologists, the taxa lists were standardized to one another before data analysis took place. All data sources had photographic plates that allowed comparison between form-genera and form-species. Presence in a particular area was scored as ‘1’ and absence as ‘0’. In some cases where there is reasonable evidence that a taxon should be present in a dataset but was lacking, presence was scored as ‘?’. Taxa were only scored as ‘?’ where a close region (or regions) contained a taxon but it was missing from the target locality.
In an attempt to contextualize the richness of the Eocene datasets in terms of modern vegetation types, North American pollen data from 46 Late Holocene (less than 3000 Ka) localities were selected from the North American Pollen Database (http://www.ncdc.noaa.gov/paleo/napd.html; electronic supplementary material, figure S1 and table S1). Localities were chosen following the criteria described by Harrington [44]. These pollen records predominantly represent regional pollen floras. The classification and geographical boundaries of the modern vegetation types are taken from Jackson & Overpeck [45].
Relative abundance data were used from Stenkul Fiord, the western interior (Bighorn, Powder River and Williston basins) and from the Holocene vegetation types to determine species accumulation curves (SACs). Within-sample rarefaction was run using relative abundance data. The Bray–Curtis similarity metric was run on the Palaeogene datasets and bootstrapped confidence intervals were calculated through 1000 reiterations of the dataset. In order to demonstrate the distance decay between Palaeogene floras, the distance in kilometres between each locality was obtained. To compensate for the widening of the North Atlantic over the past 53 Myr, the distance that North America and Europe have drifted from one another was estimated using the average spreading rate of the North Atlantic [46]. As a rough estimate, the European and North American localities were approximately 1000 km nearer to one another than their modern distribution. Distance decay in composition between floras was revealed using the Bray–Curtis metric.
3. Results
(a). Age model
We assign ages to the measured sections at Stenkul Fiord primarily based on composition of the vertebrate fauna, including Perissodactyla, Creodonta, turtles and less significantly, alligators. Taxa from the Early Eocene (Wasatchian, North American Land Mammal ‘Age’) are known from the westernmost sections that are correlative with the P-section on the southern shore of Stenkul Fiord. Fossils include the turtle Echmatemys, one of the most common turtles in the North American Eocene appearing in mid-latitudes at the Palaeocene–Eocene boundary, the large tortoise Hadrianus that first appeared at mid-latitudes in the Middle Wasatchian (Wa3), and a turtle belonging to Emydidae, a family that appeared in mid-latitude North America in the Early Wasatchian (Wa1) [47]. The Echmatemys fossils are inseparable from Echmatemys testudinea, the earliest known species of this genus (ranging from Wa0 to Wa5). The turtles from Stenkul Fiord suggest a Middle Wasatchian (Graybullian; approx. 53–54.5 Ma [48]) age that is consistent with the occurrence of the perissodactyl Homogalax, which first appeared at mid-latitudes in Graybullian time [49], but survived into Late Wasatchian time in the Bighorn Basin, Wyoming [50]. East of the P-section but still along the southern shore of Stenkul Fiord, fossils of an emydid turtle are known from the ‘White sand’ level in (figure 2). Stratigraphically higher, at least two perissodactyl taxa and a partial skull of Coryphodon have been recovered. Perissodactyls first appeared in North America at the onset of the Eocene [51]. The creodont Palaeonictis found within the Stenkul sections has a range in the US western interior from Late Palaeocene to Wa-4 or Wa-5 in the Early Eocene [52,53]. A zircon recovered from volcanic ash located at the base of the stratigraphic sections on the southern shore of Stenkul Fiord yields a preliminary date by sensitive high resolution ion microprobe (SHRIMP) analysis of 52.6 ± 1.9 Ma [54] that is clearly Eocene. Pollen assemblages have been dated by planktonic dinocysts in other parts of the Arctic, and pollen floras from the sections at Stenkul are consistent with an Early Eocene age (NP10–NP11), based on the composition and presence of some taxa that are also shared with northern Alaska [28]. While the available data cannot discount the possibility that the Stenkul Fiord sections capture the early part of the Early Eocene climatic optimum (EECO), vertebrate fossils and pollen assemblages suggest an Early Eocene age after the Palaeocene–Eocene boundary but before the EECO.
(b). Floral composition
The megaflora contains long-ranging, predominantly gymnosperm taxa in the coal-forming environments. These include Glyptostrobus (swamp cypress), Metasequoia (dawn redwood) and a yet undescribed species of Picea (spruce) but angiosperms, such as Cercidiphyllum (katsura tree) and Nordenskioldia, are also encountered. The megaflora from Stenkul Fiord is typical for the Early Eocene in the Arctic [2]. Although angiosperm leaves are more common in the mudstone–siltstone and indurated sandstone layers between the coal seams [2], these facies are frost-shattered and intact leaves are generally not preserved. In contrast to the low-diversity megaflora, pollen samples collected from coals contain 86 morphotypes from 37 different samples. Taxodiaceous pollen accounts for 48 per cent of all grains and the remaining most abundant morphotypes are predominantly wind-pollinated and spore-producing groups, such as Betulaceae–Myricaceae (birch/bayberry), Pinaceae (pines), Juglandaceae (hickory), Ulmaceae (elm) and pteridophytes (ferns; electronic supplementary material, figure S2). Systematic affinities of the pollen indicate the presence of families that are typically found growing in the present-day eastern United States, such as Nyssaceae (dogwoods), Fagaceae (beeches), Myricaceae and Betulaceae, together with Anacardiaceae (cashews) and Icacinaceae. Palynomorph groups include pteridophytes (n = 13), gymnosperms (n = 3) monocots (n = 8), eudicot angiosperms (n = 58) and the extinct Normapolles group (n = 4). The pollen floras indicate presence of evergreen taxa, including Pinus (pines) and Picea (spruce) as well as putative palm pollen (e.g. Monocolpopollenites reticulatus).
The pollen floras contain notable Arctic Eocene taxa (electronic supplementary material, figure S3) such as Carya (hickory) that are similar to modern species, several different types of Intratriporopollenites that are related to Bombacaceae, Sterculiaceae or Tiliaceae (cottonwood, cocoa and lindens), Ailanthipites fluens (tree-of-heaven), Aesculiidites sp. B (?Anacardiaceae), Mediocolpopollis sp. and Diervilla (Bush honeysuckle). The estimated richness of Arctic megafloras during the Late Palaeocene–Early Eocene is approximately 28–30 species [3], but these numbers are based only on inventories of megafossils. Such a discrepancy between richness in pollen floras and megafloras, however, is not unique because the same pattern is observed in well-sampled regions, such as the Bighorn Basin, Wyoming that records less than 40 megafossil morphotypes in the Early Eocene [55] but more than 80 pollen and spore morphotypes [25]. The presence of so many morphologically distinctive sporomorphs in the Arctic, and especially numbers of angiosperms that belong to groups not recorded in the megaflora still indicates some significant spatial heterogeneity of the vegetation over the ancient landscape.
(c). Floral richness
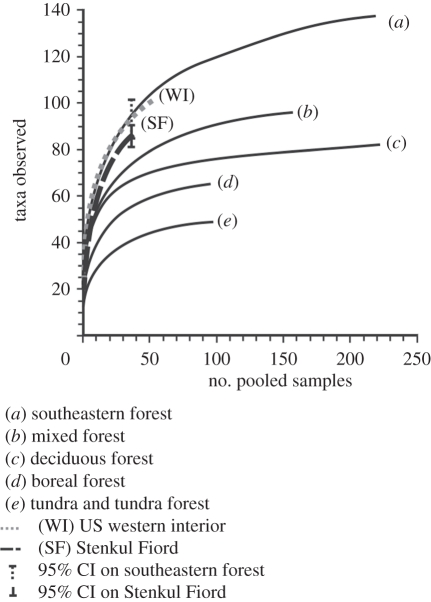
When the pollen samples from Stenkul Fiord are pooled, they have a SAC that rises steeply with increased sampling effort (figure 3). The SAC for the Stenkul Fiord samples is comparable with that of pooled pollen samples from localities of similar age from the US western interior collected from the Early Eocene and, with the exception of one sample from the Bighorn Basin, from before the EECO. These demonstrate a much reduced latitudinal gradient in plant richness during the Early Eocene. When these results are compared with Late Holocene pollen samples, collected from modern vegetation types in North America (figure 3), they indicate that the Early Eocene sections demonstrate greatest similarity of species accumulation with modern US southeastern forests that extend from Florida to Virginia [45] than with any other vegetation type. If 291 pollen and spore grains are counted from each sample from Stenkul Fiord, then a mean alpha (α) within-sample richness of 19 taxa would be expected. Late Holocene pollen samples from the southeastern United States also expect a mean richness of 19 taxa within-sample for the same sampling intensity.
Figure 3.
Species accumulation curves for Early Eocene pollen assemblages from Stenkul Fiord, the US western interior and the Late Holocene from modern vegetation types of eastern North America. The mixed forest is spread over a wider geographical area than the deciduous forest (electronic supplementary material, figure S1). Therefore, the apparently lower richness of the deciduous forest is an area/geographical artefact only.
(d). Comparisons with other regions
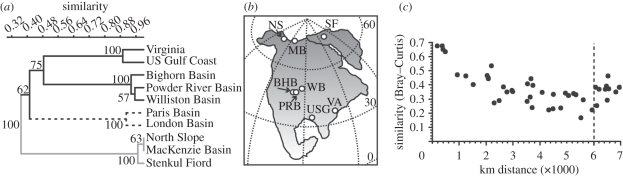
Surprisingly, an estimated 46 per cent of the morphotypes found in the Early Eocene Arctic flora at Stenkul Fiord are not found in correlative strata from North America, Asia or Europe. They also never appear in these or other regions later in the Caenozoic. Either the Arctic region is a source of some evolutionary novelty, or it recruited plants from eastern Siberia, where pollen data are lacking, rather than from North America or Europe. Statistical analyses of the floral composition from different regions in North America and Europe demonstrate that Arctic floras are taxonomically unique (figure 4a,b). The Bray–Curtis metric indicates the greatest differences occur between the Arctic floras and those from the rest of North America and Europe (figure 4a). There is strong spatial auto-correlation between floras because sampling localities are more dissimilar compositionally to one another the further apart they lie (figure 4c). All Arctic sites are therefore most similar to one another and results from Stenkul are not an anomaly. This geographical pattern is statistically significant up to 6000 km apart using permutation (rs = 0.79, p < 0.001).
Figure 4.
(a) Bray–Curtis similarity of Early Eocene pollen floras from different North American and European sites. Numbers on the cluster nodes indicate bootstrap support through 1000 random replicates. (b) Location of North American Early Eocene sites used in panel (a). SF, Stenkul Fiord; MB, MacKenzie Basin; NS, North Slope; BHB, Bighorn Basin; PRB, Powder River Basin; WB, Williston Basin; VA, Virginia; USG, US Gulf Coast. European sites are off the map. (c) Distance decay of Early Eocene pollen flora composition with increasing sampling distance using the Bray–Curtis metric. Dashed line at 6000 km indicates the breakpoint.
4. Conclusions
The pollen flora from Stenkul Fiord demonstrates that Early Eocene High Arctic ecosystems on Ellesmere Island contained floras with similarly high richness to modern southeastern forests of the United States. As many taxa are angiosperms, and this group has a strong modifying impact on climate [56,57], the heterogeneous ancient Arctic vegetation type may have had a significant impact on regional hydrology and climate of the High Arctic during the Early Eocene. Angiosperms, if more abundant than thought previously, may be key to understanding the warm Arctic climates because the maintenance of such conditions are still unclear [6,58]. Pollen data confirm observations on fossil insects and plants from high latitudes in the Early Eocene that show significant variance in the latitudinal gradient in species richness compared with the modern pattern [59,60]. Significantly, the Arctic floral records demonstrate that photoperiod is not a significant obstacle to capping biodiversity in the Arctic, and that richness can approximate that in present-day mid-latitudes. Hence, model predictions of future global warming scenarios that indicate the expansion of temperate vegetation to higher latitudes [58,61] represent only a starting point for changing plant communities, if the duration of warming is prolonged and not associated with freezing temperatures.
Acknowledgements
J.E. was supported by NSF ARC0454906 and ARC0804627. Arctic vertebrate fossils were collected in 1970s–2002 by M. Dawson, R. West, J. H. Hutchison, the late M. McKenna and J. Eberle, and are on loan to Dawson, Hutchison and Eberle from the Canadian Museum of Nature (K. Shepherd, M. Feuerstack) and the Nunavut Government (D. Stenton, J. Ross and J. Kotierk). Logistical support for Arctic fieldwork (which spanned decades) was provided by the Polar Continental Shelf Project, a division of Natural Resources Canada. This is contribution number 254 of the Smithsonian Institution NMNH Evolution of Terrestrial Ecosystems Programme.
References
- 1.Wolfe J. A. 1977. Paleogene floras from the Gulf of Alaska region. Geol. Surv. Prof. Pap. 997, 1–108 [Google Scholar]
- 2.Hickey L. J., West R. M., Dawson M. R., Choi D. K. 1983. Arctic terrestrial biota: paleomagnetic evidence of age disparity with mid-northern latitudes during the late Cretaceous and early Tertiary. Science 221, 1153–1156 10.1126/science.221.4616.1153 (doi:10.1126/science.221.4616.1153) [DOI] [PubMed] [Google Scholar]
- 3.McIver E. E., Basinger J. F. 1999. Early Tertiary floral evolution in the Canadian high arctic. Ann. Missouri Bot. Gard. 86, 523–545 10.2307/2666184 (doi:10.2307/2666184) [DOI] [Google Scholar]
- 4.Basinger J. F., Greenwood D. R., Sweda T. 1994. Early Tertiary vegetation of Arctic Canada and its relevance to palaeoclimatic interpretations. In Arctic plants and climates: 65 million years of change (ed. Boulter M. C.), pp. 175–198 Berlin, Germany: Springer [Google Scholar]
- 5.Eldrett J. S., Greenwood D. R., Harding I. C., Huber M. 2009. Increased seasonality through the Eocene to Oligocene transition in northern high latitudes. Nature 459, 969–973 10.1038/nature08069 (doi:10.1038/nature08069) [DOI] [PubMed] [Google Scholar]
- 6.Sluijs A., et al. 2008. Warm and wet conditions in the Arctic region during Eocene thermal maximum 2. Nat. Geosci. 2, 777–780 10.1038/ngeo668 (doi:10.1038/ngeo668) [DOI] [Google Scholar]
- 7.Greenwood D. R., Wing S. L. 1995. Eocene continental climates and latitudinal temperature gradients. Geology 23, 1044–1048 (doi:10.1130/0091-7613(1995)023<1044:ECCALT>2.3.CO;2) [DOI] [Google Scholar]
- 8.Weijers J. W. H., Schouten S., Sluijs A., Brinkhuis H., Sinninghe Damsté J. S. 2007. Warm arctic continents during the Palaeocene–Eocene thermal maximum. Earth Planet. Sci. Lett. 261, 581–592 10.1016/j.epsl.2007.06.033 (doi:10.1016/j.epsl.2007.06.033) [DOI] [Google Scholar]
- 9.Eberle J. J., Fricke H. C., Humphrey J. D., Hackett L., Newbrey M. G., Hutchison J. H. 2010. Seasonal variability in Arctic temperatures during early Eocene time. Earth Planet. Sci. Lett. 296, 481–486 10.1016/j.epsl.2010.06.005 (doi:10.1016/j.epsl.2010.06.005) [DOI] [Google Scholar]
- 10.Dawson M. R., West R. M., Hutchison J. H. 1976. Paleogene terrestrial vertebrates: northernmost occurrence, Ellesmere Island, Canada. Science 192, 781–782 10.1126/science.192.4241.781 (doi:10.1126/science.192.4241.781) [DOI] [PubMed] [Google Scholar]
- 11.Estes R., Hutchison J. H. 1980. Eocene lower vertebrates from Ellesmere Island, Canadian Arctic Archipelago. Palaeogeogr. Palaeoclimatol. Palaeoecol. 30, 325–347 10.1016/0031-0182(80)90064-4 (doi:10.1016/0031-0182(80)90064-4) [DOI] [Google Scholar]
- 12.Dawson M. R., McKenna M. C., Beard K. C., Hutchison J. H. 1993. An Early Eocene Plagiomenid mammal from Ellesmere and Axel Heiberg Islands, Arctic Canada. Kaupia 3, 179–192 [Google Scholar]
- 13.Markwick P. J. 1994. ‘Equability’, continentality, and Tertiary ‘climate’: the Crocodilian perspective. Geology 22, 613–616 (doi:10.1130/0091-7613(1994)022<0613:ECATCT>2.3.CO;2) [DOI] [Google Scholar]
- 14.Eberle J. J. 2005. A new ‘tapir’ from Ellesmere Island, Arctic Canada: implications for northern high-latitude palaeobiogeography and Tapir palaeobiology. Palaeogeogr. Palaeoclimatol. Palaeoecol. 227, 311–322 10.1016/j.palaeo.2005.06.008 (doi:10.1016/j.palaeo.2005.06.008) [DOI] [Google Scholar]
- 15.Royer D. L., Osborne C. P., Beerling D. J. 2003. Carbon lose by deciduous trees in a CO2-rich ancient polar environment. Nature 424, 60–62 10.1038/nature01737 (doi:10.1038/nature01737) [DOI] [PubMed] [Google Scholar]
- 16.McKenna M. C. 1980. Eocene paleolatitude, climate and mammals of Ellesmere Island. Palaeogeogr. Palaeoclimatol. Palaeoecol. 30, 349–362 10.1016/0031-0182(80)90065-6 (doi:10.1016/0031-0182(80)90065-6) [DOI] [Google Scholar]
- 17.Irving E., Wynne P. J. 1991. The paleolatitude of the Eocene fossil forests of Arctic Canada. Geol. Surv. Can. Bull. 403, 209–211 [Google Scholar]
- 18.Jahren A. H., Sternberg L. S. 2008. Annual patterns within tree rings of the Arctic middle Eocene (ca. 45 Ma): isotopic signatures of precipitation, relative humidity, and deciduousness. Geology 36, 99–102 10.1130/G23876A.1 (doi:10.1130/G23876A.1) [DOI] [Google Scholar]
- 19.Williams C. J., LePage B. A., Johnson A. H., Vann D. R. 2009. Structure, biomass, and productivity of a late Paleocene arctic forest. Proc. Acad. Nat. Sci. (Philadelphia) 158, 107–127 10.1635/053.158.0106 (doi:10.1635/053.158.0106) [DOI] [Google Scholar]
- 20.Eberle J. J., McKenna M. C. 2002. Early Eocene Leptictida, Pantolesta, Creodonta, Carnivora, and Mesonychidae (Mammalia) from the Eureka Sound Group, Ellesmere Island, Nunavut. Can. J. Earth Sci. 39, 899–910 10.1139/e02-001 (doi:10.1139/e02-001) [DOI] [Google Scholar]
- 21.Dawson M. R. 1990. Terrestrial vertebrates from the Tertiary of Canada's arctic islands. Can. Mus. Nat. 1, 91–104 [Google Scholar]
- 22.Kalkreuth W. D., Riediger C., Mcintyre D., Richardson R., Fowler M., Marchioni D. 1996. Petrological, palynological and geochemical characteristics of Eureka Sound Group coals (Stenkul Fiord, southern Ellesmere Island, Arctic Canada). Int. J. Coal Geol. 30, 151–182 10.1016/0166-5162(96)00005-5 (doi:10.1016/0166-5162(96)00005-5) [DOI] [Google Scholar]
- 23.Reidiger C. L., Bustin R. M. 1987. The Eureka sound formation, southern Ellesmere Island. Bull. Can. Petrol. Geol. 35, 123–142 [Google Scholar]
- 24.Harrington G. J., Clechenko E. R., Kelly D. C. 2005. Palynology and organic-carbon isotopes ratios across a terrestrial Palaeocene–Eocene boundary section in the Williston Basin, North Dakota, USA. Palaeogeogr. Palaeoclimatol. Palaeoecol. 226, 214–232 10.1016/j.palaeo.2005.05.013 (doi:10.1016/j.palaeo.2005.05.013) [DOI] [Google Scholar]
- 25.Wing S. L., Harrington G. J. 2001. Floral response to rapid warming in the earliest Eocene and implications for concurrent faunal change. Paleobiology 27, 539–563 (doi:10.1666/0094-8373(2001)027<0539:FRTRWI>2.0.CO;2) [DOI] [Google Scholar]
- 26.Wing S. L., Harrington G. J., Bowen G. J., Koch P. L. 2003. Floral change during the Initial Eocene Thermal Maximum in the Powder River Basin, Wyoming. Geol. Soc. Am. Special Pap. 369, 425–440 10.1130/0-8137-2369-8.425 (doi:10.1130/0-8137-2369-8.425) [DOI] [Google Scholar]
- 27.Clechenko E. R., Kelly D. C., Harrington G. J., Stiles C. A. 2007. Terrestrial records of a regional weathering profile at the Paleocene–Eocene boundary in the Williston Basin of North Dakota. Geol. Soc. Am. Bull. 119, 428–442 10.1130/B26010.1 (doi:10.1130/B26010.1) [DOI] [Google Scholar]
- 28.Frederiksen N. O., Edwards L. E., Ager T. A., Sheehan T. P. 2002. Palynology of Eocene strata in the Sagavanirktok and Canning formations of the North slope of Alaska. Palynology 26, 59–94 [Google Scholar]
- 29.Norris G. 1997. Paleocene–Pliocene deltaic to inner shelf palynostratigraphic zonation, depositional environments and paleoclimates in the Imperial Adgo F-28 Well, Beaufort-Mackenzie Basin. Geol. Surv. Can. Bul. 523, 1–71 [Google Scholar]
- 30.Frederiksen N. O. 1979. Paleogene sporomorph biostratigraphy, Northeastern Virginia. Palynology 3, 130–167 10.1080/01916122.1979.9989187 (doi:10.1080/01916122.1979.9989187) [DOI] [Google Scholar]
- 31.Pocknall D. T. 1987. Paleoenvironments and age of the Wasatch Formation (Eocene), Powder River Basin, Wyoming. Palaios 2, 368–376 10.2307/3514762 (doi:10.2307/3514762) [DOI] [Google Scholar]
- 32.Pocknall D. T., Nichols D. J. 1996. Palynology of coal zones of the Tongue River Member (upper Paleocene) of the Fort Union Formation, Powder River Basin, Montana and Wyoming. AASP Contrib. Ser. 32, 1–58 [Google Scholar]
- 33.Frederiksen N. O. 1980. Paleogene sporomorphs from South Carolina and quantitative correlations with the Gulf Coast. Palynology 4, 125–179 10.1080/01916122.1980.9989205 (doi:10.1080/01916122.1980.9989205) [DOI] [Google Scholar]
- 34.Harrington G. J. 2001. Impact of Paleocene/Eocene greenhouse warming on North American paratropical forests. Palaios 16, 266–278 [Google Scholar]
- 35.Tschudy R. H. 1973. Stratigraphic distribution of significant Eocene palynomorphs of the Mississippi Embayment. Geol. Surv. Prof. Pap. 743B, 1–24 [Google Scholar]
- 36.Gruas-Cavagnetto C. 1968. Étude palynologique des divers gisements du Sparnacien du Bassin de Paris. Mém. Soc. Géol. France 110, 1–144 [Google Scholar]
- 37.Roche E. 1973. Étude des sporomorphes du Landénien de Belgique et de quelques gisements du Sparnacien français. Mém. expl. Cartes géol. Min. Belgique 13, 1–138 [Google Scholar]
- 38.Gruas-Cavagnetto C. 1978. Étude Palynologique de l'Éocène du Bassin Anglo-Parisien. Mém. Soc. Géol. France 131, 1–63 [Google Scholar]
- 39.Gruas-Cavagnetto C. 1970. Microflore et microplancton des Woolwich Beds (Swanscombe, Kent). Pollen et Spores 12, 71–82 [Google Scholar]
- 40.Gruas-Cavagnetto C. 1976. Étude palynologique du Paléogène du Sud de l'Angleterre. Cah. Micropal. 1, 1–49 [Google Scholar]
- 41.Collinson M. E., Steart D. C., Harrington G. J., Hooker J. J., Scott A. C., Allen L. O., Glasspool I. J., Gibbons S. J. 2009. Palynological evidence of vegetation dynamics in response to palaeoenvironmental change across the onset of the Paleocene–Eocene thermal maximum at Cobham, Southern England. Grana 48, 38–66 10.1080/00173130802707980 (doi:10.1080/00173130802707980) [DOI] [Google Scholar]
- 42.Ruiqi G., et al. 2000. Palynology of Petroliferous basins in China. Beijing, China: Petroleum Industry Press [Google Scholar]
- 43.Li H., Zheng Y. 1995. Palaeogene floras. In Fossil floras of China through the geological ages (eds Li X., et al.), pp. 455–505 Guangzhou, China: Guandong Science and Technology Press [Google Scholar]
- 44.Harrington G. J. 2004. Structure of the North American vegetation gradient during the late Paleocene/early Eocene warm climate. Evol. Ecol. Res. 6, 33–48 [Google Scholar]
- 45.Jackson S. T., Overpeck J. T. 2000. Responses of plant populations and communities to environmental changes of the late Quaternary. Paleobiol. Suppl. 26, 94–220 [Google Scholar]
- 46.Pitman W. C., III, Talwani M. 1972. Sea-floor spreading in the North Atlantic. Geol. Soc. Am. Bull. 83, 619–646 10.1130/0016-7606(1972)83[619:SSITNA]2.0.CO;2 (doi:10.1130/0016-7606(1972)83[619:SSITNA]2.0.CO;2) [DOI] [Google Scholar]
- 47.Holroyd P. A., Hutchison J. H., Strait S. G. 2001. Turtle diversity and abundance through the lower Eocene Willwood Formation of the southern Bighorn Basin. Univ. Michigan Pap. Paleontol. 33, 97–107 [Google Scholar]
- 48.Koch P. L., et al. 2003. Carbon and oxygen isotope records from paleosols spanning the Paleocene–Eocene boundary, Bighorn Basin, Wyoming. Geol. Soc. Am. Speical Pap. 369, 49–64 [Google Scholar]
- 49.Robinson P., et al. 2004. Wasatchian through Duchesnean biochronology. In Late Cretaceous and Cenozoic mammals of North America. (ed. Woodburne M. O.), pp. 106–155 Columbia University Press [Google Scholar]
- 50.Chew A. E. 2009. Paleoecology of the early Eocene Willwood mammal fauna from the central Bighorn Basin, Wyoming. Paleobiology 35, 13–31 10.1666/07072.1 (doi:10.1666/07072.1) [DOI] [Google Scholar]
- 51.Gingerich P. D. 2006. Environment and evolution through the Paleocene–Eocene thermal maximum. Trends Ecol. Evol. 21, 246–253 10.1016/j.tree.2006.03.006 (doi:10.1016/j.tree.2006.03.006) [DOI] [PubMed] [Google Scholar]
- 52.Gunnell G. F. 1998. Creodonta. In Evolution of Tertiary mammals of North America (eds Janis C. M., Scott K. M., Jacobs L. L.), pp. 91–105 Cambridge: Cambridge University Press [Google Scholar]
- 53.Gingerich P. D., Clyde W. C. 2001. Overview of mammalian biostratigraphy in the Paleocene–Eocene Fort Union and Willwood formations of the Bighorn and Clarks Fork basins. Univ. Michigan Pap. Paleontol. 33, 1–14 [Google Scholar]
- 54.Reinhardt L., et al. 2010. Altered volcanic ashes in Paleocene–Eocene Eureka Sound Group sediments (Ellesmere Island, Arctic Canada): new stratigraphic tie-points? GeoCanada Abstr. http://www.geocanada2010.ca/program/program-schedule/posters.html [Google Scholar]
- 55.Wing S. L. 1998. Late Paleocene–Early Eocene floral and climatic changes in the Bighorn Basin, Wyoming. In Late Paleocene–Early Eocene climatic and biotic events in the marine and terrestrial records (eds Aubry M.-P., Lucas S. G., Berggren W. A.), pp. 380–400 Columbia University Press [Google Scholar]
- 56.Boyce C. K., Lee E. 2010. An exceptional role for flowering plant physiology in the expansion of tropical rainforests and biodiversity. Proc. R. Soc. B 277, 3437–3443. (doi:10.1098/rspb.2010.0485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swann A. L., Fung I. Y., Levis S., Bonan G. B., Doney S. C. 2010. Changes in Arctic vegetation amplify high-latitude warming through the greenhouse effect. Proc. Natl Acad. Sci. USA 107, 1295–1300 10.1073/pnas.0913846107 (doi:10.1073/pnas.0913846107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sluijs A., et al. 2006. Subtropical Arctic Ocean temperatures during the Palaeocene–Eocene thermal maximum. Nature 441, 610–613 10.1038/nature04668 (doi:10.1038/nature04668) [DOI] [PubMed] [Google Scholar]
- 59.Archibald S. B., Bossert W. H., Greenwood D. R., Farrell B. D. 2010. Seasonality, the latitudinal gradient of diversity, and Eocene insects. Paleobiology 36, 374–398 10.1666/09021.1 (doi:10.1666/09021.1) [DOI] [Google Scholar]
- 60.Wilf P., Johnson K. R., Cúneo N. R., Smith M. E., Singer B. S., Gandolfo M. A. 2005. Eocene plant diversity at Laguna del Hunco and Río Pichileufú, Patagonia, Argentina. Am. Nat. 165, 634–650 10.1086/430055 (doi:10.1086/430055) [DOI] [PubMed] [Google Scholar]
- 61.Wolf A., Callaghan T. V., Larson K. 2008. Future changes in vegetation and ecosystem function of the Barents Region. Clim. Change 87, 51–73 10.1007/s10584-007-9342-4 (doi:10.1007/s10584-007-9342-4) [DOI] [Google Scholar]