Abstract
The causes of variation in individual reproductive success over a lifetime are not well understood. In long-lived vertebrates, reproductive output usually increases during early adulthood, but it is difficult to disentangle the roles of development and learning on this gain of reproductive success. Lekking lance-tailed manakins provide an opportunity to separate these processes, as the vast majority of male reproduction occurs after a bird obtains alpha status and maintains a display area in the lek, but the age at which males achieve alpha status varies widely. Using 11 years of longitudinal data on age, social status and genetic siring success, I assessed the factors influencing variation in siring success by individuals over their lifetimes. The data show increases in annual reproductive success with both age and alpha experience. At advanced ages, these gains were offset by senescence in fecundity. Individual ontogeny, rather than compositional change of the population, generated a nonlinear relationship of breeding tenure with lifetime success; age of assuming alpha status was unrelated to tenure as a breeder, or success in the alpha role. Importantly, these findings suggest that social experience can mitigate the negative effects of senescence in older breeders.
Keywords: sexual selection, longevity, reproductive tenure, senescence, Chiroxiphia, cooperative breeding
1. Introduction
In long-lived iteroparous organisms, few variables are as important in determining lifetime reproductive success as longevity as a breeder [1–4]. Though fundamental to an understanding of individual fitness, the mechanisms by which breeding tenure translates into reproductive success are complex and remain incompletely understood. The simplest scenario, in which individuals accumulate an average level of reproductive success in each breeding season, predicts a linear increase in success with each year as a breeder. By contrast, heterogeneity in reproduction across breeding tenure is expected to produce a nonlinear relationship between tenure and lifetime reproductive output. Such heterogeneity may result from changes in the composition of the breeding population, for example through selective disappearance [5] or selective appearance (e.g. delayed breeding) of individuals that vary in quality; or from ontogenetic change that occurs within individual lifetimes [6,7]. Understanding the life-history variables that generate heterogeneity in annual reproductive success (ARS) is critical for correctly identifying and interpreting the influence of selection on key life events and individual traits.
Reproductive rates vary with age in many species [8–11], but factors underlying reproductive changes within the lifetimes of breeders have proved difficult to identify in finer detail. Ontogenetic increases in success may result from learning or experience [12], from physiological development [13] or from increased investment in reproduction as residual reproductive value declines [14]. Individuals also may decline in success at advanced ages owing to senescence [15]. These ontogenetic processes may occur simultaneously or interact in complex ways. For example, it has been proposed that acquired experience can mitigate the negative effects of senescence on individual reproductive performance [16], but empirical support for this hypothesis is scarce in wild populations.
Attempts to assess how ontogeny in success is influenced by both age and experience have been complicated by close correlation of these variables, as individuals necessarily increase in age with each additional year of breeding experience. Lance-tailed manakins (Aves, Pipridae: Chiropxiphia lanceolata) therefore offer an excellent model for investigating the influences of age and experience on annual siring success. Males of this lekking species generally do not reproduce until attaining alpha status [17], and vary considerably in both the age at which they first become alpha and the length of time they maintain alpha status. Age-specific plumage stages in the first three years of life allow precise assessment of male ages [18]. Though lek mating systems are models for the study of sexual selection [19], and age has been suggested as a contributing factor to reproductive success in a number of lekking species [20,21], the influence of life-history heterogeneity on measures of fitness is largely unexplored for lekking species.
With the goal of assessing the factors underlying the relationship between breeding tenure and lifetime reproductive success (LRS) among breeding (alpha) male lance-tailed manakins, I first characterized the relationship of LRS with male tenure as a breeder using 11 years of data on individual social status and genetic siring success. I then quantified the role of ontogeny, selective disappearance and selective appearance in generating age-related patterns of reproductive success, using both specific hypothesis tests and model selection in a generalized linear mixed model (GLMM) approach [22]. Finally, I examined the combined effects of age and experience (years of alpha tenure) on individuals' ARS.
2. Methods
(a). Study site
The study was conducted in 46 ha of secondary-growth tropical forest located at the eastern end of Isla Boca Brava, a ca 3000 ha island in Chiriquí, Panama (8°12′45″ N, 82°12′54″ W). In each year, post-fledging birds were captured in mist nets, marked with a unique combination of one numbered aluminium and three coloured leg bands (A. C. Hughes Ltd), and genetically sampled by collecting a small blood sample from the brachial vein. The data presented here comprise 11 years of monitoring effort from 2000 to 2010, with data on presence in 2011 used to identify 2010 as the end of breeding tenure for one male. Observations were generally conducted from late February or early March until late June, which corresponds to the peak of the lance-tailed manakin breeding season in this area, and shorter field seasons in three years of the study (2003, 2004, 2007) were timed to include peaks of male display activity [23].
(b). Study species and behavioural observations
Male lance-tailed manakins perform elaborate courtship displays at traditional display sites in an exploded lek mating system. Male display areas are at least 50 m apart, and are in auditory but not visual contact with neighbouring territories [23]. Females fly among male display areas to observe courtship displays, and nest outside of mates' territories.
During 1 h periods of continuous monitoring at display areas, observers recorded the colour band combinations of all birds present and all instances of duet songs and dance displays. Duets were overlapping ‘querico’ vocalizations by males perched adjacently, and dances involved up to 11 types of coordinated leaps or flights on a low display perch, often in synchrony with a subordinate partner [23]. All active display areas were observed for at least 10 h per year to allow accurate assessment of male social status, and a subset of 12–18 ‘core’ areas were observed three times per week (2004 to present; 2 h sessions twice weekly 2000–2003; 9063 h of observations in 11 years [24]). In each year, the study area included approximately 27 active display areas, each occupied by one alpha male and associates (see below). Males in this species are site-faithful, allowing displaying males in particular to be resighted reliably across consecutive years and age classes.
Three age-specific predefinitive plumages allow unambigous age determination for males captured before their fourth year after hatching [18]. This study therefore allows assessment of exact age of males up to 14 years after hatching, and minimum age of ≥15 years after hatching; males captured as adult-plumaged individuals in 1999 would be in ≥15th year after hatching in 2010. Only one of 89 adult-plumaged males alive in 2010 was known to be in ≥15th year. Following standard terminology for avian studies, ages are given as time elapsed since hatching, such that a young bird is classified sequentially as hatch year, second year and third year in its first three years of life.
(c). Social status
Male status was determined independent of copulation success, and was usually established months or years before females arrived at a display area [17]. In short, alpha males were the most consistently present males at each display area, participated in the vast majority of duet songs and performed alpha-specific components of paired courtship behaviours, including the distinctive ‘eek’ which ends cooperative dancing bouts. Only alphas performed solo displays for females. Non-alpha males included betas, which formed long-term alliances with alphas and participated in displays with females present; and non-beta adults which formed no clear alliances and displayed only with no females present [24].
Duration of alpha tenure was determined by direct observation of both the first and last breeding seasons in which a male was an alpha. The first year of alpha status therefore followed at least one breeding season in which that individual was present as a non-alpha male, and the last year was followed by a year in which that male was not seen in any observation sessions (and presumed dead).
This study specifically addresses variation in reproduction among breeding or potentially breeding birds, and so analyses are restricted to alpha males. Previous quantification of the relative opportunity for selection showed that attaining alpha status is critical in male life-histories [17]. Because alpha males are generally older than other males in the population [24] and non-alphas very rarely breed [17], including non-alpha adults in the current analyses reveals strong age effects, but confounds assessment of the influence of age on reproductive success among alpha-status individuals. Not all alpha males sire chicks in a given year, and analyses here include both successful and unsuccessful alphas.
Analyses of ARS considered all alpha males of known age for which the current year of alpha tenure was known (102 observed alpha-years for 43 individuals). Analyses of LRS considered all individuals for which both the beginning and end of alpha tenure were directly observed (52 individuals, 30 of which were also of known age). Alpha males included in this study were never observed to regress in social status (i.e. alphas never became a beta or floater after they were identified as alpha), with one exception. One male was identified as an alpha in two years, then moved to become a beta partner at a different display site in the third observation year. This male was included in ARS data for his two years of alpha status, but did not appear in the LRS dataset as he was still alive at the conclusion of this study.
Although the sampling limits of this study preclude inferences about reproductive patterns unique to males with very long alpha tenures, males excluded because of incompletely observed tenures did not differ greatly from others in the analyses. Because behavioural observations were conducted starting in the year 2000, the maximum alpha tenure that could have been observed was 10 breeding seasons. LRS analysis excluded 11 known-age alpha males that were alive and in their first through to their seventh year as alpha in 2010 (2.9 ± 1.8 years observed as alpha). Both ARS and LRS analyses excluded 23 males for which the beginning of alpha tenure was not observed (i.e. most were alphas at the start of the study). These males were alphas for 1–9 observed years (3.4 ± 2.3 years; three males with alpha tenure known to be longer than six years), and had all disappeared from the study population by the end of the current study.
(d). Reproductive success
Reproductive success was quantified as the number of chicks sired, determined by genetic testing and described in detail in the electronic supplementary material. ARS was standardized as the proportion of assigned chicks sired by an individual in that year, multiplied by 95.6 and rounded to the nearest integer value. This value represents the average number of chicks with paternity assigned per year in 2005–2009 during which sampling success was fairly constant (95.6 ± 5.6 chicks sampled in 2005–2009; range 88–103 chicks). This standardization corrects for an improvement in researcher success at finding and sampling manakin nests over the course of this study, which would otherwise overestimate relative success of individuals breeding later in the study, yet maintains the underlying distribution of reproductive success data. A particularly short field season in the year 2004 resulted in reduced sampling of chicks in that year; so applying this standardization probably overestimates success of individuals for which paternity was detected in that year. Values for siring success for the four alphas observed in 2004 therefore were further corrected by subtracting three chicks from the standardized siring success of each successful male, so that the mean siring success of alphas that sired chicks was comparable with that of other years of the study. LRS was quantified as the sum of standardized annual siring success for an individual across its full breeding tenure.
(e). Genetic techniques and paternity assignment
Paternity was assessed for 925 chicks from 570 nests and 237 unique females using microsatellite genotypes at 20 variable loci and considering all adult males (including non-alphas) as candidate sires. Genetic techniques and assignment criteria are described in detail in the electronic supplementary material. Using these criteria, 86.6 per cent of 925 genotyped chicks (801 offspring) were assigned to a known male from the study population. Paternity assignment success for 2000–2006 is reported in DuVal & Kempenaers [17]; assignment success in other years of the study was 91 (93.8%), 89 (81%), 78 (69%) and 130 (82.2%) chicks assigned to identified sires in 2007, 2008, 2009 and 2010, respectively (with the annual percentage of genotyped chicks assigned given in parentheses).
(f). Statistical analyses
The functional form of the relationship between male tenure as an alpha and LRS was characterized using a generalized additive model (GAM) with quasi-Poisson error, and user-defined k of 6 to correspond with the number of discrete tenure lengths observed (package mgcv 1.7–2 in the program R, v. 2.11.1 [25]). GAM is a non-parametric regression which fits a smoothed spline to the data to describe the observed relationship without assuming a specific functional form, with confidence estimates produced through Bayesian likelihood procedures [26]. The GAM model was fitted by penalized quasi-likelihood owing to overdispersion of lifetime success data [27].
Discrete predictions of the ontogeny, selective appearance and selective disappearance hypotheses were first tested using Spearman's rank correlations, generalized linear models (GLMs), and GLMMs. The selective disappearance hypothesis critically predicts a positive correlation between early reproductive success and breeding tenure [28], whereas the selective appearance hypothesis predicts that breeding tenure or success is higher for individuals that become alphas at later ages [6]. By contrast, the individual ontogeny hypothesis predicts that age will be a key predictor of success within individuals' lifetimes.
To consider the potential for simultaneous or interactive effects of life-history variables on reproductive success, I used GLMMs to examine the relationship between ARS and five key variables suggested by the three major hypotheses above. These were individual age, the quadratic term for age (age2), year of tenure as an alpha male, initial year as alpha (a binary variable), and final year as alpha (a binary variable). The binary variables were included to test for the possibility of effects at the beginning or end of an alpha's tenure that were not captured by a continuous measure of years as an alpha (for example, terminal investment before death). I examined interactions of year of alpha tenure, initial year as alpha, and final year as alpha with age and age2 to assess whether short- and long-tenured males experienced different patterns of age-linked reproductive success. This approach was analogous to that of Van de Pol & Verhulst [22], but with binary variables specifying individual appearance and disappearance from the breeding population. Because the mean siring success of alpha males was less than five chicks per year, I used a Poisson model (log link) with Laplace approximation in LMER, package LME4 in the program R [27]. Individual identity was included as a random effect in each model to account for the non-independence of repeated measures of the same individual across its lifetime [29]. Parameter estimates for mixed models are reported as ±1 s.e. The overdispersion scale parameter was estimated as 1.4 from the Pearson's residuals and degrees of freedom (d.f.) from the global model and so no additional correction for overdispersion was included [27]. The global model included all predictors (tenure, age, age2, initial year as alpha and final year as alpha) as well as interactions of age, initial year as alpha and final year as alpha.
Model selection was conducted using corrected Akaike's Information Criterion (AICc), including 1 d.f. for random effects in mixed models. AICc is a small-sample modification of AIC which is appropriate for investigating the effects of hypothesized parameters in mixed models [30,31]. Strong support for a particular model was inferred when the AICc was at least two units lower than the next best model. Confidence intervals for parameter estimates from the best-fit model were developed from the 2.5th and 97.5th percentiles of 10 000 non-parametric bootstraps, resampling cases in the repeated-measures model [32].
3. Results
(a). Alpha tenure and lifetime reproductive success
On average, alpha tenures lasted 2.3 ± 1.5 breeding seasons (mean ± s.d., n = 52 alphas with completely observed tenures, range 1–6 breeding seasons as alpha). Males first assumed alpha status at the age of 7.1 ± 1.7 years after hatching (mean ± s.d.; range: 4th–10th year after hatching, mode: 8th year after hatching, n = 30 males of known age with complete alpha tenure observed).
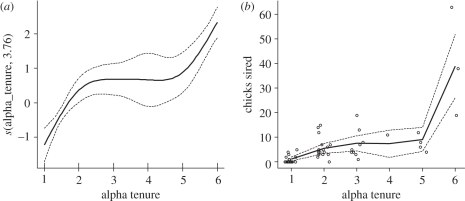
LRS was strongly correlated with tenure as an alpha male (rs = 0.76, p < 0.0001, n = 52 alphas). Curve-fitting in a GAM indicated that the relationship between siring success and years of alpha tenure was significantly nonlinear (GAM: intercept 1.33 ± 0.16, t = 8.40, p < 0.0001; years as alpha: d.f. = 3.76, F4.27 = 19.77, p < 0.0001, 71.1% of deviance explained; figure 1). Female mate fidelity across successive years of alpha tenure may contribute to the high success rates of long-tenure males, but the number of unique females with which a male sired chicks was also strongly correlated with years as an alpha (rs = 0.78, p < 0.0001). The relationship between the number of unique mates and years as an alpha male was nonlinear and comparable with the pattern of siring success described above (GAM: intercept 0.66 ± 1.15, t = 4.35, p < 0.0001; years as alpha: estimated degrees of freedom = 3.73, F4.25 = 20.11, p < 0.001, 69.2% of deviance explained).
Figure 1.
Tenure as an alpha male lance-tailed manakin showed a positive but nonlinear relationship with LRS. (a) The smooth function in a generalized additive model (GAM) describing the relationship of final alpha tenure (total years in the alpha role) to chicks sired in a male's lifetime. (b) Predicted values of LRS from this model for males of varying alpha tenure derived. Solid lines indicate the main estimated values of the smoothing parameter in (a) and of predicted success in (b), and dashed lines show ±1 s.e. of these values. Open circles in (b) represent observed LRS values for males with completely observed alpha tenure (n = 50), and are jittered to visualize overlapping points.
(b). Selective appearance and disappearance
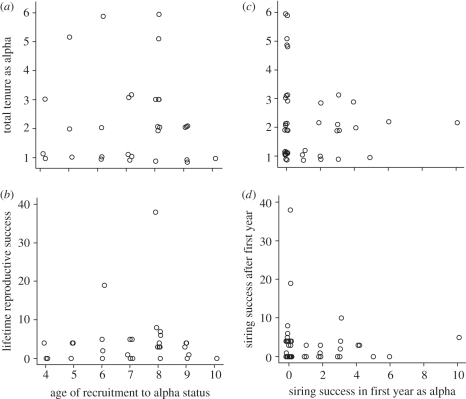
Counter to the predictions of the selective appearance and disappearance hypotheses, I detected no correlation of male age in the first year of alpha status with the number of chicks sired in a male's lifetime (Spearman's rs = 0.07, p = 0.72, n = 30 alphas of exactly known age with complete tenures observed) nor with the length of the subsequent alpha tenure (rs = 0.02, p = 0.93, n = 30; figure 2). Age of becoming alpha was positively correlated with longevity (rs = 0.72, p < 0.0001, n = 30), but the lack of relationship with tenure indicates that this correlation reflected the tautology that males becoming alpha when relatively old cannot also die young and was not indicative of benefits from delayed reproduction.
Figure 2.
Contrary to the predictions of the selective appearance and disappearance hypotheses, age at which male lance-tailed manakins recruited to alpha stats was uncorrelated with (a) total years of alpha tenure or (b) LRS. Siring success in males' first year of alpha status was uncorrelated with (c) total years of alpha tenure or (d) total siring success after the first year of alpha status. Points are jittered to visualize overlapping data.
There was no correlation between success in the first year as alpha and later siring success (rs = −0.07, p = 0.66, n = 43 alphas), nor between success in the first year as alpha and eventual alpha tenure (rs = 0.001, p = 0.99, n = 43; figure 2). Finally, siring success in the first year of alpha status was unrelated to individual longevity (rs = 0.04, p = 0.82, n = 30 alphas of known age).
(c). Individual ontogeny
As predicted by the ontogeny hypothesis, annual siring success during alpha tenure was closely related to individual age (GLMM in LMER—intercept: −1.97 ± 0.42, z = −4.74, p < 0.0001; individual age: 0.29 ± 0.05, z = 6.39, p < 0.0001). All alpha males had relatively low reproductive success in their first year of alpha status (GLMM in LMER—intercept: 0.97 ± 0.13, z = 7.41, p < 0.0001; initial year of alpha status: −1.05 ± 0.18, z = −5.78, p < 0.0001; status as a binary variable with males not in their initial year as the baseline group). An effect of age on ARS within an individual's lifespan may therefore complicate the interpretation of this result, as alphas are always youngest in their first year of alpha tenure. Importantly, ontogenetic changes continued beyond a males' first year of alpha status: when males in their initial year as alphas were excluded from the analysis of age in relation to ARS, the effect of alpha age remained significant (GLMM in LMER—intercept: −0.76 ± 0.52, z = −1.46, p = 0.14; age: 0.19 ± 0.05, z = 3.409, p < 0.001, n = 59 observations on 28 individuals). A non-parametric signed rank test further confirmed that individual males increased in siring success between successive years of alpha status (Wilcoxon-signed rank test: mean change in siring success between first and second years of alpha status = 1.11 ± 3 chicks, signed rank = 67, p = 0.05).
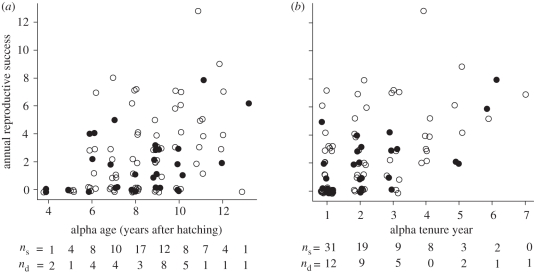
An ontogenetic change in annual success measures may reflect increases attributable to age itself (for example via female preference for older males), or may be attributable to increased experience associated with age. Alphas in their initial year of alpha tenure had relatively poor reproductive success regardless of age: when only first-year alphas were considered, there was no significant correlation between age and reproductive success in that year (rs = 0.13, p = 0.41, n = 43 alphas). For known-aged individuals in their final year of alpha status, ARS was correlated with alpha tenure but not individual age (figure 3: correlation of tenure with siring success in the final year of alpha status, rs = 0.60, p = 0.0005; correlation of age with siring success in the final year of alpha status, rs = 0.30, p = 0.11; n = 30 alphas).
Figure 3.
Tenure but not individual age of lance-tailed manakins was correlated with siring success in the final year of alpha status. Plots show the relationships of (a) individual alpha age (years after hatching) and (b) year of alpha tenure to ARS, measured as number of chicks sired. Data are 102 observations on 43 alpha individuals for which age was known and the start of alpha tenure was directly observed. Filled circles represent males in their final year of alpha status (n = 30 observations), while open circles represent males that survived to the following age or tenure category (n = 72 observations). Data markers are jittered to visualize overlapping values, with sample sizes provided below the x-axis as the number of individuals that survived (ns) and the number that disappeared (nd) in each age or tenure class.
(d). Sources of variation in annual reproductive success
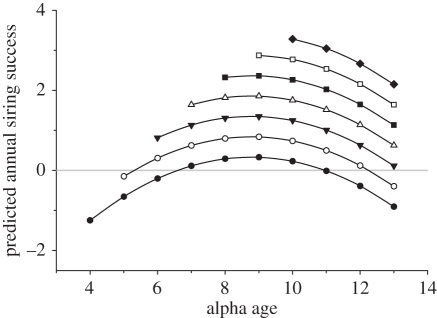
Investigating predicted relationships of each independent variable individually neglects the potential for additive or interactive effects on ARS. I therefore examined the combined effects on ARS of life-history variables representing age, age2, year of alpha tenure, recruitment, survival, and interactions of age with the other variables using a series of GLMMs (see §2 for further details of the theoretical and biological basis of models under consideration). Model selection by AICc revealed strongest support for a model which included year of alpha tenure, individual age, age2 and a binary variable indicating whether it was a male's final year as alpha (table 1; electronic supplementary material, table S1). Approximately equivalent support was detected for a model which excluded the term for final year as alpha. This final year effect was not restricted to any particular age or tenure group (all interactions of age or tenure with final year as alpha were non-significant) and was non-significant in the final model (table 2), but was retained here for completeness because it approached significance. Excluding the final year term from the model did not substantially affect parameter estimates for the remaining terms (electronic supplementary material, table S2). Bootstrapped 95% CIs of parameter estimates confirmed that alpha males increased in siring success with both age and tenure, but also experienced senescence in siring success at advanced ages (table 2). This best-fit model indicated that each year of successive alpha tenure increased alpha siring success by an average of 0.51 chicks, and that this continuing increase in older age classes partially offset the negative effect of senescence (age2). The partial derivative of success with respect to age revealed that males increased in siring success with age until the age of 8.6 years after hatching (bootstrapped 95% CI: 7.59–10.31 years after hatching), then experienced age-related declines in reproductive success with each additional year of life (figure 4). Individual reproductive declines were not restricted to the final year of alpha status; when males in their final year as alpha were excluded, the negative relationship of age2 with annual siring success remained significant (GLMM using LMER with final-year alphas excluded—intercept: −7.44 ± 2.34, z = −3.17, p = 0.0002; year of alpha tenure: 0.42 ± 0.14, z = 3.06, p = 0.002; age: 1.58 ± 0.51, z = 3.10, p = 0.002; age2: −0.08 ± 0.03, z = −2.99, p = 0.003, n = 72 observations on 31 individuals).
Table 1.
ARS of alpha males was best explained by a model incorporating year of alpha tenure, age, a quadratic effect of age and a binary variable indicating whether a male was in his final season as alpha. (GLMMs assessed the effect on ARS of tenure as an alpha male (T), individual age (A), age2 (A2), and two separate binary variables indicating whether a male was in his initial (I) or final (F) year of alpha status. All models considered individual identity as a random effect, and interactions are indicated with an asterisk. Model 6 represents the global model used for overdispersion parameter estimates (see §2). AICc is the Akaike's Information Criterion with small sample size correction, calculated from the negative log-likelihood of the model (−logLik) and k, where k is the number of parameters with one parameter included for random effects. AICc weights are an indicator of the relative support for the model in the set of models considered here. Parameter estimates for the best-fit model are given in table 2. Models shown are those that were within 10% of the AICc weight of the best-supported models, plus the global model (model 6) and models showing singular effects of tenure or age (models 7 and 8). The full array of considered models is reported in the electronic supplementary material, appendix S1.)
| model rank | model | −logLik | k | AICc | ΔAICc | wi |
|---|---|---|---|---|---|---|
| 1 | T + A + A2 + F | −104.89 | 6 | 222.67 | 0 | 0.43 |
| 2 | T + A + A2 | −106.58 | 5 | 223.79 | 1.12 | 0.25 |
| 3 | A * T + F | −105.91 | 6 | 224.71 | 2.05 | 0.15 |
| 4 | A * T | −107.62 | 5 | 225.87 | 3.20 | 0.09 |
| 5 | T + F + I | −108.19 | 5 | 227.00 | 4.34 | 0.05 |
| 6 | A * I + A * F + A2 + T | −104.08 | 9 | 228.12 | 5.45 | 0.03 |
| 7 | T | −113.87 | 3 | 233.98 | 11.32 | 0.002 |
| 8 | A | −119.89 | 3 | 246.02 | 23.36 | <0.001 |
Table 2.
Parameter estimates from the best-fit GLMM, which explained ARS as a function of individual alpha tenure, final year as an alpha, and linear and quadratic effects of individual age. (Individual identity was included as a random effect in this model, and data were 102 observations on 43 individuals. Bootstrapped 95% CIs for the fixed effects were generated by sampling 10 000 times with replacement to create datasets of 43 individuals' reproductive histories as alpha, including all sampled years for each selected individual. The electronic supplementary material, table S2 reports parameter estimates in the model excluding the final year term.)
| model predictors | estimates ± s.e. | z | p | mean estimate from bootstrap | 95% CI from bootstrap |
|
|---|---|---|---|---|---|---|
| lower | upper | |||||
| intercept | −5.51 ± 1.61 | −3.43 | 0. 0006 | −7.84 | −13.83 | −3.30 |
| year of alpha tenure | 0.51 ± 0.11 | 4.69 | <0. 0001 | 0.62 | 0.33 | 1.08 |
| age | 1.22 ± 0.35 | 3.43 | 0.0006 | 1.72 | 0.75 | 3.02 |
| age2 | −0.07 ± 0.02 | −3.48 | 0. 0005 | −0.10 | −0.18 | −0.04 |
| final year | −0.35 ± 0.19 | −1.81 | 0.07 | −0.39 | −0.97 | 0.13 |
Figure 4.
Predicted ARS is shown for lance-tailed manakin alphas of different age and tenure classes. Predicted values are derived from the bootstrapped mean parameter estimates of the best-fit model, which explained ARS as a function of alpha tenure year, individual age, age2, and final year of alpha status (table 2). Declines in average annual siring success at advanced ages indicate senescence in fecundity after males reach 8.6 years after hatching. Note that despite this effect of senescence, individual males are expected to increase in siring success with each successive year of alpha status owing to positive effects of successive tenure classes and the linear term for age. Predicted values that fall below the grey line indicate that a male of the identified age and status category is unlikely to sire any chick. The influence of the final year of alpha status received weak support in the final model and is not shown graphically. Confidence intervals of model predictions for specific tenure categories are presented in the electronic supplementary material, figure S1. Filled circles, tenure year 1; open circles, tenure year 2; inverted filled triangles, tenure year 3; open triangles, tenure year 4; filled squares, tenure year 5; open squares, tenure year 6; filled diamonds, tenure year 7.
4. Discussion
Understanding how variation in individual reproductive success generates patterns at the population level is fundamental to an evolutionary perspective in animal behaviour. This study demonstrated a nonlinear relationship between breeding tenure and LRS, which was not explained by compositional change in the population of breeding males. Instead, this relationship reflected a combination of poor performance early in alpha tenure, ontogenetic increases attributable to both age and experience as an alpha, and a balance of the positive effects of accumulated experience (years of alpha tenure) with negative effects of senescence in fecundity for males beyond their eighth year after hatching. Together, these results indicate that individual ontogeny is the primary factor explaining the population-level relationship between breeding tenure and LRS, and highlight the independent effects of age and breeding experience on reproductive success in this system.
Improvement in individual reproductive performance over time is ubiquitous in long-lived animals [7,11,33–36], and senescence in wild populations is likewise well-established [37]. However, relatively few studies have been able to separate the influences of age and breeding experience on reproductive success, as these variables are highly correlated in most systems. In social species, experience in a social role may include increased skill or improvements in the quality of social alliances that could partially or wholly counteract the negative effects of senescence on individual reproductive output. This predicts an antagonistic relationship between the effects of age and experience on reproductive success at advanced ages. To date, studies which examined both age and experience revealed similar positive effects on reproductive success [11,33], or a negative effect of experience but not age in older age categories [38]. Results from this study instead suggest that increases in reproductive success associated with alpha tenure (experience) may partially offset declines in individual reproductive success associated with age. A balance of positive effects from alpha experience and negative effects from senescence probably influence the benefits and timing of delayed breeding, which is an important component of this mating system. This study quantified fitness as LRS, but incorporating information on individual reproductive rate relative to the population growth rate (e.g. a Fisherian definition of fitness [39]) will provide a more complete picture of selection on the timing of reproduction.
Male age is often correlated with breeding success on leks [21], but most studies of lekking species have poor information on exact ages of lekking males and have instead compared male age categories (e.g. adult versus sub-adult), which precludes detection of age effects that continue past adulthood. The relative success of older males in lekking populations is generally ascribed to age-dependent social structuring among long-lived individuals that return to the same lek in multiple years [19,20]. While age-related success in dominance interactions very probably affects the success of male lance-tailed manakins in attaining alpha status, it seems unlikely to influence the patterns of success reported here among established alphas because alpha males rarely interact with each other and are generally site-faithful throughout their alpha tenures [24].
What is the mechanism by which age and alpha experience influence siring success? Reproductive improvements observed within individuals are most pronounced after the first or second year as a breeder, as reported here for lance-tailed manakins. While poor first-year performance of breeders in many avian species generally results from inexperience in nesting or nestling provisioning [40], the poor performance of lance-tailed manakins in their initial alpha year occurs independent of nesting or chick-rearing behaviours.
Ontogenetic increases in siring success among alphas in this species are most probably achieved through mate choice interactions with females, changes in alliance dynamics among cooperative partners, or developmental changes in individual physiology. The models presented here suggest that the different effects of age and of tenure on reproductive success are additive in nature, and therefore cannot be acting through identical aspects of the events leading to fertilizations (e.g. male display performance cannot simultaneously improve with experience while declining with age). Increased success associated with male age independent of breeding experience could result from intrinsic factors such as physiological development [13] or via female preference for older males [41,42]. Senescence in siring success may result from decreased ability of older males to attract mates, perform vigorous displays or defend against social challenges from younger males. Recent evidence of age-related declines in avian sperm quality suggests that declines in siring success may reflect decreased fertilization success rather than decreased success in attracting females [43,44]. Behavioural differences between alphas of different age categories may provide further insight into the causes of observed fecundity senescence.
By contrast, increased success associated with experience as an alpha, independent of age, suggests that males benefit from social or behavioural changes associated with holding the alpha role. These could include increased stability of social alliances which may improve coordination and success in mate attraction [45], increased skill in the performance of coordinated courtship displays [46], improved responsiveness to female cues of receptivity [47], or increased familiarity with or preference for males with long-established alpha tenures by mate-searching females. Non-independent mate preferences by females could also drive an effect of tenure on reproductive success if older females prefer familiar males and younger females copy their mate choice.
Senescence in annual siring success among old alphas raises the question of why males do not recruit into the alpha role at younger ages. The effects of fecundity senescence in lance-tailed manakins began to influence expected reproductive output at approximately the median age of becoming alpha, and 60 per cent of males held alpha positions beyond this age. Though a long alpha tenure was a critical component of male LRS, early ascent to alpha status did not guarantee long tenure. Furthermore, males did not experience clear trade-offs in timing of attaining alpha status and duration of alpha tenure: males that became alphas early did not differ in length of alpha tenure compared with those that became alpha later in life. Factors that influence the considerable variation in longevity and in the duration of alpha tenure are currently unknown.
Senescence in performance may include components of decline in both survival and fecundity. This study has focused primarily on fecundity senescence, which has received comparatively little attention [37]. While an in-depth analysis of survival across males' lifetimes is necessary to assess the influence of mortality senescence on reproductive decisions, I found no relationship of survival with the following breeding season (i.e. the term indicating final year as alpha) with individual age in alphas, providing preliminary evidence that males senesce in fecundity but not in mortality rate during alpha tenure.
Importantly, these results highlight the need to consider heterogeneity in age and experience when investigating reproductive success in relation to male phenotype [41]. When breeders present in any one year include individuals of widely varied ages and levels of breeding experience, substantial ontogenetic variation in reproductive success among males has the potential to overwhelm variation in individual success owing to other causes. Analyses that seek to investigate the phenotypic correlates of fitness must therefore consider the influence of heterogeneity in individual age and breeding experience on annual measures of success, but such heterogeneity is often neglected.
While this study has considered years of alpha tenure as a measure of ‘experience,’ male lance-tailed manakins may also gain display experience prior to attaining an alpha position. Subordinate beta males frequently form alliances with alpha individuals and may participate in paired courtship displays for more than four years before attaining a breeding alpha position [24]. The influence of experience gained as a beta on reproductive performance as an alpha remains to be determined. Long beta tenures may compensate for lack of alpha display experience, increasing rates of reproductive success once betas attain an alpha position. The role of individual variation in pathways to alpha status is a promising area for future research.
Acknowledgements
All protocols were approved by the Florida State University Animal Care and Use Committee (protocol no. 0718).
This work was made possible by the assistance of 10 volunteer field crews. D. Houle, K. Hughes, B. Inouye and A. Winn provided useful comments on analyses and manuscript preparation. M. Festa-Bianchet, D. Promislow and D. Westneat contributed constructive reviews which improved the final paper. Funding was provided by the NSF IOS-Animal Behaviour 0843334 and by Florida State University. Previous support for this long-term research came from the Max Planck Institute for Ornithology and the University of California, Berkeley. Jorge Garcia and the staff of ANAM, Panamá, and the MVUP assisted with research permits; F. Köhler and E. Y. Pinzon kindly allowed field site access.
References
- 1.Clutton-Brock T. H. 1988. Reproductive success. Chicago, IL: University of Chicago Press [Google Scholar]
- 2.Grant P. R., Grant B. R. 2011. Causes of lifetime fitness of Darwin's finches in a fluctuating environment. Proc. Natl Acad. Sci. USA 108, 674–679 10.1073/pnas.1018080108 (doi:10.1073/pnas.1018080108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez-Vaamonde C., Raine N. E., Koning J. W., Brown R. M., Pereboom J. J. M., Ings T. C., Ramos-Rodriguez O., Jordan W. C., Bourke A. F. G. 2009. Lifetime reproductive success and longevity of queens in an annual social insect. J. Evol. Biol. 22, 983–996 10.1111/j.1420-9101.2009.01706.x (doi:10.1111/j.1420-9101.2009.01706.x) [DOI] [PubMed] [Google Scholar]
- 4.Spong G. F., Hodge S. J., Young A. J., Clutton-Brock T. 2008. Factors affecting the reproductive success of dominant male meerkats. Mol. Ecol. 17, 2287–2299 10.1111/j.1365-294X.2008.03734.x (doi:10.1111/j.1365-294X.2008.03734.x) [DOI] [PubMed] [Google Scholar]
- 5.Vaupel J. W., Yashin A. I. 1985. Heterogeneity's ruses: some surprising effects of selection on population dynamics. Am. Stat. 39, 176–185 10.2307/2683925 (doi:10.2307/2683925) [DOI] [PubMed] [Google Scholar]
- 6.Forslund P., Pärt T. 1995. Age and reproduction in birds: hypotheses and tests. Trends Ecol. Evol. 10, 374–378 10.1016/S0169-5347(00)89141-7 (doi:10.1016/S0169-5347(00)89141-7) [DOI] [PubMed] [Google Scholar]
- 7.Rebke M., Coulson T., Becker P. H., Vaupel J. W. 2010. Reproductive improvement and senescence in a long-lived bird. Proc. Natl Acad. Sci. USA 107, 7841–7846 10.1073/pnas.1002645107 (doi:10.1073/pnas.1002645107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richard M., Lecomte J., de Fraipont M., Clobert J. 2005. Age-specific mating strategies and reproductive senescence. Mol. Ecol. 14, 3147–3155 10.1111/j.1365-294X.2005.02662.x (doi:10.1111/j.1365-294X.2005.02662.x) [DOI] [PubMed] [Google Scholar]
- 9.Seamons T. R., Quinn T. P. 2010. Sex-specific patterns of lifetime reproductive success in single and repeat breeding steelhead trout (Oncorhynchus mykiss). Behav. Ecol. Sociobiol. 64, 505–513 10.1007/s00265-009-0866-7 (doi:10.1007/s00265-009-0866-7) [DOI] [Google Scholar]
- 10.McDonald D. B. 1993. Delayed plumage maturation and orderly queues for status: a manakin mannequin experiment. Ethology 94, 31–45 10.1111/j.1439-0310.1993.tb00545.x (doi:10.1111/j.1439-0310.1993.tb00545.x) [DOI] [Google Scholar]
- 11.Weimerskirch H. 1992. Reproductive effort in long-lived birds: age-specific patterns of condition, reproduction, and survival in the wandering albatross. Oikos 64, 464–473 10.2307/3545162 (doi:10.2307/3545162) [DOI] [Google Scholar]
- 12.Curio E. 1983. Why do young birds produce less well? Ibis 125, 400–404 10.1111/j.1474-919X.1983.tb03130.x (doi:10.1111/j.1474-919X.1983.tb03130.x) [DOI] [Google Scholar]
- 13.Angelier F., Weimerskirch H., Dano S., Chastel O. 2007. Age, experience and reproductive performance in a long-lived bird: a hormonal perspective. Behav. Ecol. Sociobiol. 61, 611–621 10.1007/s00265-006-0290-1 (doi:10.1007/s00265-006-0290-1) [DOI] [Google Scholar]
- 14.Williams G. C. 1966. Adaptation and natural selection. Princeton, NJ: Princeton University Press [Google Scholar]
- 15.Williams G. C. 1957. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411 10.2307/2406060 (doi:10.2307/2406060) [DOI] [Google Scholar]
- 16.Saino N., Ambrosini R., Martinelli R., Moller A. P. 2002. Mate fidelity, senescence in breeding performance and reproductive trade-offs in the barn swallow. J. Anim. Ecol. 71, 309–319 10.1046/j.1365-2656.2002.00600.x (doi:10.1046/j.1365-2656.2002.00600.x) [DOI] [Google Scholar]
- 17.DuVal E. H., Kempenaers B. 2008. Sexual selection in a lekking bird: the relative opportunity for selection by female choice and male competition. Proc. R. Soc. B 275, 1995–2003 10.1098/rspb.2008.0151 (doi:10.1098/rspb.2008.0151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DuVal E. H. 2005. Age-based plumage changes in the lance-tailed manakin: a two-year delay in plumage maturation. Condor 107, 917–922 10.1650/7793.1 (doi:10.1650/7793.1) [DOI] [Google Scholar]
- 19.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- 20.Alonso J. C., Magana M., Palacin C., Martin C. A. 2010. Correlates of male mating success in great bustard leks: the effects of age, weight, and display effort. Behav. Ecol. Sociobiol. 64, 1589–1600 10.1007/s00265-010-0972-6 (doi:10.1007/s00265-010-0972-6) [DOI] [Google Scholar]
- 21.Fiske P., Rintamaki P. T., Karvonen E. 1998. Mating success in lekking males: a meta-analysis. Behav. Ecol. 9, 328–338 10.1093/beheco/9.4.328 (doi:10.1093/beheco/9.4.328) [DOI] [Google Scholar]
- 22.Van de Pol M., Verhulst S. 2006. Age-dependent traits: a new statistical model to separate within- and between-individual effects. Am. Nat. 167, 766–773 10.1086/503331 (doi:10.1086/503331) [DOI] [PubMed] [Google Scholar]
- 23.DuVal E. H. 2007. Cooperative display and lekking behavior of the lance-tailed manakin (Chiroxiphia lanceolata). Auk 124, 1168–1185 10.1642/0004-8038(2007)124[1168:CDALBO]2.0.CO;2 (doi:10.1642/0004-8038(2007)124[1168:CDALBO]2.0.CO;2) [DOI] [Google Scholar]
- 24.DuVal E. H. 2007. Social organization and variation in cooperative alliances among male lance-tailed manakins. Anim. Behav. 73, 391–401 10.1016/j.anbehav.2006.05.017 (doi:10.1016/j.anbehav.2006.05.017) [DOI] [Google Scholar]
- 25.R Development Core Team 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org [Google Scholar]
- 26.Wood S. N. 2006. Generalized additive models: an introduction with R. New York, NY: Chapman and Hall/CRC Press [Google Scholar]
- 27.Bolker B., Brooks M. E., Clark C. J., Geange S. W., Poulsen J. R., Stevens M. H. H., White J. S. 2008. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135 10.1016/j.tree.2008.10.008 (doi:10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 28.Mauck R. A., Huntington C. E., Grubb T. C. 2004. Age-specific reproductive success: evidence for the selection hypothesis. Evolution 58, 880–885 [DOI] [PubMed] [Google Scholar]
- 29.Pinheiro J. C., Bates D. M. 2000. Mixed-effects models in S and S-plus. New York, NY: Springer [Google Scholar]
- 30.Burnham K. P., Anderson D. J. 2002. Model selection and multimodel inference. New York, NY: Springer [Google Scholar]
- 31.Vaida F., Blanchard S. 2005. Conditional Akaike information for mixed-effects models. Biometrika 92, 351–370 10.1093/biomet/92.2.351 (doi:10.1093/biomet/92.2.351) [DOI] [Google Scholar]
- 32.Hox J. 2002. Multilevel analysis: techniques and applications. Mahwah, NJ: Lawrence Erlbaum Associated, Inc [Google Scholar]
- 33.Limmer B., Becker P. H. 2010. Improvement of reproductive performance with age and breeding experience depends on recruitment age in a long-lived seabird. Oikos 119, 500–507 10.1111/j.1600-0706.2009.16673.x (doi:10.1111/j.1600-0706.2009.16673.x) [DOI] [Google Scholar]
- 34.Cam E., Monnat J. Y. 2000. Stratification based on reproductive state reveals contrasting patterns of age-related variation in demographic parameters in the kittiwake. Oikos 90, 560–574 10.1034/j.1600-0706.2000.900314.x (doi:10.1034/j.1600-0706.2000.900314.x) [DOI] [Google Scholar]
- 35.Reid J. M., Bignal E. M., Bignal S., McCracken D. I., Monaghan P. 2003. Age-specific reproductive performance in red-billed choughs Pyrrhocorax pyrrhocorax: patterns and processes in a natural population. J. Anim. Ecol. 72, 765–776 10.1046/j.1365-2656.2003.00750.x (doi:10.1046/j.1365-2656.2003.00750.x) [DOI] [Google Scholar]
- 36.Weladji R. B., Gaillard J. M., Yoccoz N. G., Holand O., Mysterud A., Loison A., Nieminen M., Stenseth N. C. 2006. Good reindeer mothers live longer and become better in raising offspring. Proc. R. Soc. B 273, 1239–1244 10.1098/rspb.2005.3393 (doi:10.1098/rspb.2005.3393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monaghan P., Charmantier A., Nussey D. H., Ricklefs R. E. 2008. The evolutionary ecology of senescence. Funct. Ecol. 22, 371–378 10.1111/j.1365-2435.2008.01418.x (doi:10.1111/j.1365-2435.2008.01418.x) [DOI] [Google Scholar]
- 38.Pyle P., Snydeman W. J., Hester M. 2001. Effects of age, breeding experience, mate fidelity and site fidelity on breeding performance in a declining population of Cassin's auklets. J. Anim. Ecol. 70, 1088–1097 [Google Scholar]
- 39.Fisher R. A. 1930. The genetical theory of natural selection, 3rd ed. New York, NY: Oxford University Press [Google Scholar]
- 40.Förschler M. L., Kalko E. K. V. 2006. Age-specific reproductive performance in citril finches Carduelis [citrinella]. Ardea 94, 275–279 [Google Scholar]
- 41.Manning J. T. 1985. Choosy females and correlates of male age. J. Theor. Biol. 116, 349–354 10.1016/s0022-5193(85)80273-3 (doi:10.1016/s0022-5193(85)80273-3) [DOI] [Google Scholar]
- 42.Kirkpatrick M. 1987. Sexual selection by female choice in polygynous animals. Annu. Rev. Ecol. Syst. 18, 43–70 10.1146/annurev.es.18.110187.000355 (doi:10.1146/annurev.es.18.110187.000355) [DOI] [Google Scholar]
- 43.Velando A., Noguera J. C., Drummond H., Torres R. 2011. Senescent males carry premutagenic lesions in sperm. J. Evol. Biol. 24, 693–697 10.1111/j.1420-9101.2010.02201.x (doi:10.1111/j.1420-9101.2010.02201.x) [DOI] [PubMed] [Google Scholar]
- 44.Preston B. T., Saint Jalme M., Hingrat Y., Lacroix F., Sorci G. 2011. Sexually extravagant males age more rapidly. Ecol. Lett. 14, 1017–1024 10.1111/j.1461-0248.2011.01668.x (doi:10.1111/j.1461-0248.2011.01668.x) [DOI] [PubMed] [Google Scholar]
- 45.Trainer J. M., McDonald D. B. 1995. Singing performance, frequency matching and courtship success of long-tailed manakins (Chiroxiphia linearis). Behav. Ecol. Sociobiol. 37, 249–254 10.1007/BF00177404 (doi:10.1007/BF00177404) [DOI] [Google Scholar]
- 46.Byers J., Hebets E., Podos J. 2010. Female mate choice based upon male motor performance. Anim. Behav. 79, 771–778 10.1016/j.anbehav.2010.01.009 (doi:10.1016/j.anbehav.2010.01.009) [DOI] [Google Scholar]
- 47.Patricelli G. L., Uy J. A. C., Walsh G., Borgia G. 2002. Sexual selection: male displays adjusted to female's response. Nature 415, 279–280 10.1038/415279a (doi:10.1038/415279a) [DOI] [PubMed] [Google Scholar]