Abstract
Around the globe, coral reefs and other marine ecosystems are increasingly overfished. Conventionally, studies of fishing impacts have focused on the population size and dynamics of targeted stocks rather than the broader ecosystem-wide effects of harvesting. Using parrotfishes as an example, we show how coral reef fish populations respond to escalating fishing pressure across the Indian and Pacific Oceans. Based on these fish abundance data, we infer the potential impact on four key functional roles performed by parrotfishes. Rates of bioerosion and coral predation are highly sensitive to human activity, whereas grazing and sediment removal are resilient to fishing. Our results offer new insights into the vulnerability and resilience of coral reefs to the ever-growing human footprint. The depletion of fishes causes differential decline of key ecosystem functions, radically changing the dynamics of coral reefs and setting the stage for future ecological surprises.
Keywords: coral reefs, ecosystem function, fishing, herbivory, grazing, resilience
1. Introduction
Coral reefs and other marine ecosystems are increasingly impacted by overfishing, resulting in distorted food webs and an increased vulnerability to other human impacts [1–4]. Owing to the combined effects of overfishing, pollution and climate change, many reefs have lost their capacity to absorb recurrent natural disturbances such as cyclones, and have undergone long-term phase shifts to degraded ecosystems dominated by fleshy seaweed or other weedy species [5–8].
Overfishing is arguably one of the most pressing human impacts on coral reefs, with a long history of exploitation wherever human populations occur [9,10]. Fishing selectively removes some individuals and species faster than others, with large predatory fishes such as sharks and groupers typically disappearing first [3,9]. Large-bodied species are also among the slowest to recover. However, the broader effects of fishing on ecosystem functions or processes are only just beginning to be understood. In some coral reef systems, there is a clear link between fishing and concomitant changes in benthic community structure. The loss of herbivores, for example, is closely linked to an increase in macroalgae [2,10–13]. In particular, parrotfishes are critically important in maintaining low macroalgal cover [12–16], in removing both live and dead corals [17,18], and in the removal and transport of sediment [17]. Top-down control of macroalgae and removal of dead coral skeletons by excavators are critical processes for promoting the replenishment and recovery of corals. For example, experimental exclusion of large herbivores results in macroalgal blooms that inhibit coral recruitment [2]. In areas of most intense grazing, intact parrotfish populations can scrape the surface of each square metre of reef every 18 days, removing up to 40 kg of sediment from each square metre per year [19]. Each adult of the largest Indo-Pacific parrotfish species, Bolbometopon muricatum, removes over 5000 kg of reef carbonates per year [17]. Consequently, the widespread decline of parrotfishes owing to overfishing is likely to simultaneously impact multiple ecosystem functions.
To examine the effect of fishing on ecosystem function, we first quantified the impact of human activity on parrotfish abundances and population structures. We then calculated the extent to which these changes in fish populations may impact four key ecosystem functions performed by parrotfishes (grazing, sediment removal, bioerosion and coral predation) along a gradient of human population densities. Our results are based on biogeographic, ecological and socio-economic data from 18 reefs, chosen to encompass a range of human population densities, spanning the major biodiversity gradients of the Indian and Pacific Oceans. Specifically, we ask to what extent are rates of grazing, bioerosion, coral predation and sediment removal by parrotfishes influenced by human activity. Significant changes in ecosystem function are likely to have serious, but poorly understood, implications for the future trajectory of coral reefs and their capacity to cope with climate change and other anthropogenic impacts.
2. Material and methods
(a). Reef locations
We sampled 18 reefs that spanned 24° latitude and 93° longitude, from Mauritius (20° S, 57° E) in the western Indian Ocean to Tahiti (17° S, 149° W) in the central Pacific. They were chosen to encompass a broad range of human population densities, from 0 (in remote, highly protected marine park reserves) to over 600 people per square kilometre (electronic supplementary material, table S1 and figure S1). The 18 reefs from west to east were Mauritius and Rodrigues in the western Indian Ocean, Cocos-Keeling and Rowley Shoals off Western Australia, the Togean Islands off Sulawesi, five reefs on the outer, northern Great Barrier Reef (GBR) (Yonge, Carter, Day, Hicks and Hilder Reefs), Binnegem and Kavieng in Papua New Guinea, Pohnpei and Kosrae in Micronesia, Apia and Nu'utele in Western Samoa, and Tahiti and Moorea in French Polynesia. Parrotfishes are heavily targeted by fishers on coral reefs [15,20] and 10 of the reefs have a strong and ongoing local tradition of artisanal fishing. At the opposite end of the spectrum, Hilder and Carter reefs—on the outer edge of the GBR—are afforded the highest level of protection and have both been closed to fishing for 18 years [21]. Rowley Shoals and the three remaining GBR reefs are also partially protected, with very low levels of fishing for parrotfishes.
(b). Quantifying functional roles
This study focuses on parrotfishes because of their dominant or substantial role in a number of ecosystem processes, and because they are a major target for artisanal fisheries throughout the tropics. Compared with any other group of fishes, parrotfishes encompass virtually all the scraping grazers and external bioeroders on coral reefs. There are many species of fish that graze coral reefs but only parrotfishes exhibit the unique scraping grazing mode [19]. However, for clarity, these scraping grazers are referred to herein as grazers. Coral predation, marked by the removal of golf ball-sized chunks of coral skeleton and live tissue, is the primary feeding behaviour of just one Indo-Pacific species, the giant humphead parrotfish B. muricatum. Growing up to 130 cm in length, it is the world's largest parrotfish species. External bioerosion is also a major function undertaken by Bolbometopon and by up to three large-bodied species of Chlorurus (depending on the biogeographic setting).
The contribution of parrotfishes to each functional role was calculated based on their abundance, size and feeding activities. Parrotfish abundances and sizes were recorded at four replicate sites on each of the 18 reefs (islands) across the Indian and Pacific Oceans. At each site, four habitats were censused (the reef slope, crest, flat and back reef). Each of the 288 censuses (16 per reef) consisted of a 5 m wide transect that was surveyed while swimming for 20 min (each census covered approx. 1250 m2). Timed transects allow a large area to be surveyed while minimizing diver avoidance by fishes, especially in areas where fishing may have modified their behaviour [22,23]. All parrotfishes greater than 15 cm total length were counted and categorized into eight 10 cm size classes. Exclusion of the smallest fishes minimized spatial variation in abundances owing to episodic recruitment. Juveniles make a negligible contribution to coral predation and bioerosion, which is almost exclusively restricted to larger individuals, and they play only a minor role in grazing and sediment removal [24,25].
The contributions of parrotfishes to four ecosystem processes were estimated: external bioerosion, coral predation, grazing and sediment removal. External bioerosion and coral predation rates were estimated based on the product of the measured abundances of each species (pooled over the 16 censuses per reef) and the estimated mass of carbonate or coral removed by individual fish per year [17,19]. The per capita mass of carbonate removed was based on published daily bite rates, bite volumes and proportion of bites from particular substrata (corals, algal turfs, etc.) [17,19]. To reduce the impacts of ontogenetic changes in functional category classifications, only adult specimens are included in the analyses (greater than 15 cm total length for Scarus, Hipposcarus and small Chlorurus species; greater than 25 cm for large Chlorurus species, Chlorurus microrhinos, Chlorurus strongylocephalus, Chlorurus frontalis and Cetoscarus bicolor; greater than 50 cm for B. muricatum). These cut-offs provide a conservative estimate of the magnitude of the four ecosystem functions. Grazing and sediment removal were likewise estimated from the product of fish abundances in the censuses and the area of substratum grazed, based on published bite rates and bite scar areas [17,19]. Where values of volumes and areas for individual species were unavailable, those of similar sized congenerics were used.
(c). Measuring human impacts
We anticipated that human population density could explain at least some of the variation in fish abundance and ecosystem function, but also that locations with higher incomes might have better access to alternative livelihoods and could afford more expensive fisheries management options. Therefore, we considered three human impact metrics: human population densities, regional per capita incomes and extent of environmental management. Population densities are expressed as people per square kilometre on the adjacent landmass. The values, therefore, represent conservative estimates of human population pressure. Two of the 18 reefs, Hilder and Carter Reefs on the GBR, have exceptional environmental protection, with an effective ban on all fishing (protected as ‘no entry’ locations for 18 years). For Rowley Shoals (Clerke Reef) in the Indian Ocean and the remaining GBR reefs, some fishing is permitted, although remoteness from human settlements is probably the primary factor in limiting exploitation. Each location was placed into three categories of fishing protection: high (no-entry reserves), medium (some fishing permitted but with gear controls, e.g. no spearing on SCUBA) and low protection (fishing permitted with little or no restrictions).
Because the sites have a wide biogeographic span that encompasses considerable variation in regional biodiversity, preliminary analyses also included regional biodiversity of parrotfishes (data from the present study) and the species richness of 15 reef fish families [5] as covariates. The relationships between these two measures of regional biodiversity, the three metrics of human activity, and each of the ecosystem functions at the 18 reefs were examined, using regression trees [26] to identify the primary structure in the data. All explanatory variables were considered simultaneously in the analyses. These analyses identified human population density as the primary metric accounting for most variance in ecosystem function among locations, explaining over 70 per cent of the variance in erosion and coral predation (electronic supplementary material, figure S2). We therefore present the univariate relationship between human population densities and each of the ecosystem functions, using nonlinear regressions (inverse polynomials).
During the field surveys, a consistent effort was made to interview older local fishers, especially spearfishers, to ascertain if parrotfish stocks were different in the past at seven sites that are now moderately to heavily fished. The main focus was on those people who were actively spearfishing in the 1960s (when relatively widespread access to goggles made it possible to see what was present underwater). In many cases, there was a strong familiarity with, and an oral history of, collecting parrotfishes. In most cases, there was a clear recollection of large parrotfish schools with accurate identifications (e.g. separating Bolbometopon from Chlorurus). Taking fishers' estimates of fish sizes and abundances into account, and using census records of human populations for each location, we broadly estimated the functional capabilities of parrotfish populations in the 1960s for comparison with today.
3. Results
(a). Human impacts on ecosystem functions
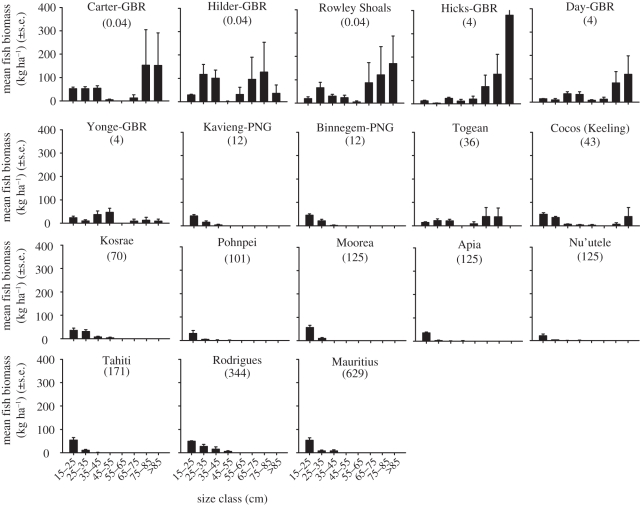
Our analyses show that the most heavily fished reefs have lost virtually all of their large parrotfishes, with individuals larger than 25 cm accounting for just 3–6% of the remaining stocks on the five most heavily fished reefs. In marked contrast, 43–67% of fishes on the five most lightly fished and un-fished reefs were larger than 25 cm. The disparity is even greater in terms of biomass (figure 1), with the lightly fished reefs having more than 50 times the biomass of large (greater than 25 cm) parrotfishes when compared with heavily fished reefs.
Figure 1.
The size structure of parrotfish populations on reefs from highest to lowest human population densities (given in parentheses). Carter and Hilder (Great Barrier Reef, GBR) are no access preservation zones. Rowley Shoals and the remaining three GBR reefs are largely protected. All other reefs are open to fishing.
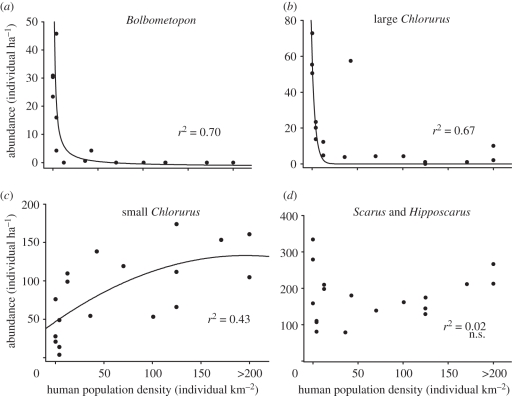
The impacts of humans are even more marked for specific parrotfish taxa (figure 2), with the rapid and almost total loss of Bolbometopon, and large Chlorurus, even at low human population densities. Interestingly, Scarus and Hipposcarus show no clear trend with human population density, whereas small Chlorurus appear to show a marginal increase. However, these changes are even more striking when the functional roles of the various taxa are examined. Our results indicate that the functional roles provided by the larger parrotfish species all but disappear when human population densities reach just 16 people per square kilometre and reefs are open to fishing (figures 3a,b and 4). This pattern of widespread depletion is independent of biogeography and variation in regional biodiversity across the Indo-Pacific, and it has almost certainly arisen in most places within the last 50 years owing to growing human pressures (figure 5). Although many areas have a long tradition of hunting parrotfishes, there were widespread reports of sharp declines in the 1960s and 1970s.
Figure 2.
The relationships between parrotfish groups and human population density at 18 reefs across the Indo-Pacific. Reefs from left to right are Carter, Hilder, Rowley Shoals, Hicks, Day, Yonge, Kavieng, Binnegem, Togean Islands, Cocos-Keeling, Kosrae, Pohnpei, Moorea, Apia, Nu'utele, Tahiti, Rodrigues and Mauritius. The functionally similar Scarus and the rarer Hipposcarus are combined, whereas the functionally different large and small Chlorurus species are separated. The fitted trend lines are inverse polynomials, with the exception of small Cholurus which is quadratic.
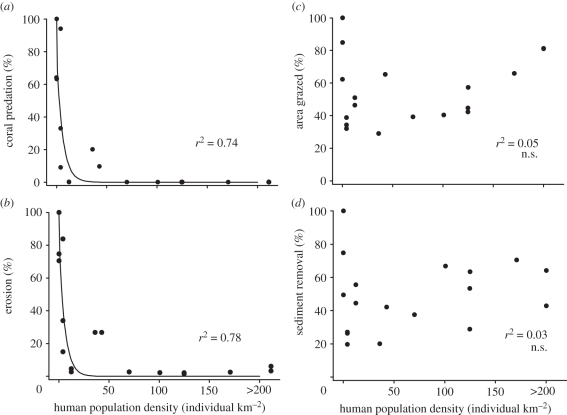
Figure 3.
The relationship between four critical ecosystem processes undertaken by parrotfishes and human population density at 18 reefs across the Indo-Pacific. The four ecosystem processes are (a) coral predation, (b) bioerosion, (c) grazing and (d) sediment removal. Values are expressed as a proportion of the maximum estimated rates recorded in the present study (rates are estimated based on the abundance of parrotfishes on the study reefs). Reefs follow the order listed for figure 2.
Figure 4.
Parrotfishes play critical roles in coral reef ecosystems. (a) The giant humphead parrotfish (Bolbometopon muricatum) is a major external bioeroder and coral predator on Indo-Pacific reefs. Despite its critical functional roles, as a large species it is one of the first to be depleted by fishing. (b) Smaller-bodied parrotfish species such as Scarus schegeli are able to maintain their ecosystem role as grazers in the face of intensive fishing pressure. (c) Fishing activity such as spearing is highly selective, thereby affecting some ecosystem functions more than others. (d) Artisanal fishing of small parrotfishes may be sustainable in the short-term but the reefs that support them are increasingly vulnerable to loss of ecosystem functions (photos: (a,b) J. P. Krajewski; (c) J. Kritzer; (d) D.R.B.).
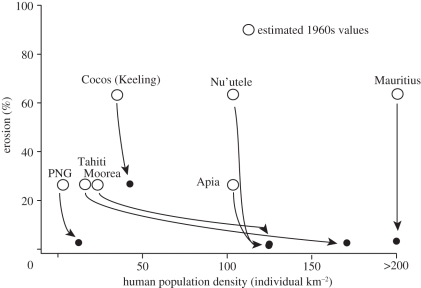
Figure 5.
Changes in estimated rates of bioerosion at seven heavily fished locations from the 1960s to today. Open circles depict estimates of historical bioerosion, based on the oral history of fishing and fish densities at each location. The rapid decline in function reflects the reported loss of large fishes. This decline, even where human population growth has been modest, points to a rapid change in fishing methods and fishing efficiency during the last 50 years. This figure is modified after figure 3; the erosion rates are therefore standardized to the highest values recorded (=100%) among un-fished reefs (these locations are not included in this figure).
The decline in estimated rates of bioerosion and coral predation stands in marked contrast to the relative resilience of rates of grazing and sediment removal by parrotfishes, which show no significant response to variation in human densities (figure 3c,d). Counterintuitively, heavily fished reefs have greater densities of small parrotfish, with roughly twice as many fishes in the smallest (less than 25 cm) size class compared with elsewhere (figure 1). These small-bodied species in the genus Scarus, Hipposcarus or Chlorurus have a unique feeding mode, which removes both turf algae and the sediments trapped within the turf; they are therefore responsible for most parrotfish grazing and sediment removal. In contrast to the patterns for coral predation and bioerosion, grazing and sediment removal varied substantially among reefs with similar human densities (figure 3). This may reflect the greater local variation in the species richness and composition of small-bodied parrotfishes (8–17 species in any location), and the occasional occurrence of large schools of small fishes in our samples. Overall, processes supported by small parrotfish species appear to be relatively resilient and able to withstand significant fishing pressure.
4. Discussion
(a). Humans, reef fish populations and the resilience of ecosystem functions
We found a strong relationship between human population densities and the population structure of reef fishes, with the greatest declines in large individuals and species, a pattern that is consistent with previous studies [3,9]. What was most striking, however, was the extent of variation among the different parrotfish taxa and the probable flow-on effects for ecosystem processes. Bolbometopon and large Chlorurus spp. exhibited a precipitous decline in abundances with increasing human densities while Scarus, Hipposcarus and small Chlorurus spp. showed relatively little response. This variation in the response of groups to human activity had a dramatic influence on our estimates of ecosystem processes. The most striking result was the susceptibility of bioerosion and coral predation by parrotfishes to very low population densities of humans. Equally surprising, was the finding that the two other ecosystem processes, grazing and sediment removal, appear to be relatively resilient to a broad range of human populations. These major differences highlight the complex ecological effects of exploitation.
The decline of larger individuals is likely to have the greatest ecological impact, because of their critical role in maintaining high rates of bioerosion and coral predation. Where human densities exceeded 16 people per square kilometre, large-bodied species and individuals virtually disappeared (figure 1). This is important, because ecosystem functions performed by parrotfishes are strongly dependant on their abundance, body and jaw size, and on the resultant biomechanics of feeding [19]. Foremost among these large-bodied species is the humphead parrotfish B. muricatum, which grows to 130 cm or 46 kg in size. This gregarious species is among the first to be depleted by spearfishing [17,27], because whole schools of fish can be removed overnight when the fish are resting on the reef. Because of this vulnerability, processes dominated by Bolbometopon and other large species were only fully intact in remote locations with low human population densities. Although most declines in parrotfish numbers were reported from the 1960s and 1970s, earlier declines were possible in some areas. The later declines were linked by some fishers to the arrival, and increasingly widespread use, of new hunting tools, including goggles, masks, rubber-powered spearguns, underwater torches and SCUBA. One of the earliest reported gear changes was the widespread use of rubber-powered spearguns following the arrival of rubber (in the form of vehicle inner tubes) in the decades following World War II.
While we found that human population density was the dominant factor in explaining the loss of large fishes and the erosion of ecosystem function, the role of economic status and management practices may also be important [20,28]. Indeed, in our study, relatively intact ecosystem processes were recorded only when all three variables (low population, high income and full protection) are in juxtaposition. Furthermore, although the most intact reefs differ in reef morphology (atolls and barrier reefs) and geographical location (Indian and Pacific Oceans), they all lie in Australian waters. Care is therefore needed in ascribing their status to any specific socio-economic traits, although fishing activity does appear to be a key element.
In marked contrast to bioerosion and coral predation, grazing and sediment removal are relatively resilient to human activity. We hypothesize that the latter two functions are resilient to fishing pressure for four reasons. First, the small species responsible for grazing and sediment removal are targeted less by fishers when compared with larger, more profitable species. Second, small, short-lived species are likely to have faster turnover rates, with earlier reproduction and higher per capita rates of recruitment. Third, there is a strong probability of a trophic cascade occurring on heavily fished reefs where small parrotfish species proliferate following the depletion of large piscivores, such as sharks and groupers [29]. We recorded the highest densities of large coral predators, bioeroders and grazers in the most protected reefs, where large sharks and other piscivores are also abundant [21]. This suggests that predation has a limited effect on the larger species and that it is the small species that are most at risk from predators [29]. The loss of predators is also likely to enhance the capacity of parrotfishes, especially the small species, to increase foraging rates, expand home ranges and exploit new areas [29–31]. If so, our GBR-based feeding rate data may underestimate the extent of feeding by small parrotfishes in heavily fished areas when released from predation pressure. Thus, the ecosystem effects of fishing may be even stronger than those we describe. Fourth, the high species richness of these small grazing parrotfishes, in comparison with bioeroders and coral predators, is likely to afford some functional redundancy, promoting their resilience [11,32].
The patterns observed in parrotfishes are likely to apply to other species in these particular functional groups. Although in the Indo-Pacific, external bioerosion is almost exclusively restricted to parrotfishes [19], grazing is undertaken by a wide range of species in the Acanthuridae and Siganidae [33,34], as well as sea urchins [14,35]. Sediment removal is likewise shared by parrotfishes and surgeonfishes, especially Ctenochaetus spp. [36]. Because Ctenochaetus and the vast majority of non-parrotfish grazers are also relatively small and fast growing [37], it is highly likely that these species will follow the patterns described for the parrotfishes. Coral predation is undertaken by numerous groups [38], but corallivory by parrotfishes is unusual in that large amounts of the coral skeleton are removed. Indeed, our focus on parrotfishes has probably underestimated the effect of humans on ecosystem processes because the sensitive functions, bioerosion and coral predation, have few alternate species (limited redundancy), while the less sensitive functions, grazing and sediment removal, are also performed by numerous alternative species (extensive redundancy).
(b). Implications for ecosystem management
The most positive aspect of our findings is that even in the face of moderately high human population densities (to 600 individual km−2) and intensive fishing, the Indo-Pacific reefs we examined still retain enough grazing activity to prevent the phase shifts to macroalgae that are occurring elsewhere, particularly in the Caribbean [8]. At all the reefs we examined, mean coral cover ranged across sites from 15 to 45 per cent, whereas macroalgae varied from 1 to 5 per cent. Grazing, with the associated removal of algae and sediment, supports coral recruitment onto calcareous substrates and is crucial for maintaining the capacity for reef regeneration [39–41]. In French Polynesia, for example, where fishing intensity is very high, a cyclone and a bleaching event reduced coral cover from 51 to 24 per cent, with turf algae increasing from 16 to 49 per cent over the subsequent 3 years. Over the following decade, grazing intensity was sufficient to slowly reduce the turfs and for coral cover to gradually increase [42]. Grazing parrotfishes, and their counterparts in other taxonomic groups, may therefore help maintain reef resilience, even when moderately exploited. As such, local action to preserve these stocks will potentially buy time for coral reefs, while the long-term challenges presented by climate change and greenhouse gas emissions are addressed [1,2]. Furthermore, the resilience of these small- and medium-sized herbivorous fishes to fishing is critical for supporting the livelihoods of artisanal fishers throughout the tropics [43].
The apparent resilience of grazing on Indo-Pacific reefs may, however, conceal hidden dangers. First, ecosystems can degrade to other undesirable states, not just to assemblages dominated by macroalgae [6,11,44]. Second, ongoing fishing pressure increases the prevalence of small-bodied fast-growing parrotfish species that may be less capable of coping with future change. These relatively recently evolved small parrotfish species have exhibited little trophic diversification [45] and are, for example, incapable of consuming mature stands of macroalgae [4,46]. They may therefore be unable to reverse future phase shifts. Third, the widespread loss of Bolbometopon across its geographical range is likely to be slowly changing the species composition of corals, in favour of its usual diet of fast-growing table corals, such as Acropora hyacynthus [17]. The longer-term consequences of such a shift are poorly understood. However, it may increase the chances of boom-and-bust dynamics, as these table corals are more vulnerable to cyclones and to coral bleaching than most massive or encrusting species [47–49]. Finally, and most importantly, the sustainability of the grazing parrotfishes influences human behaviour. The ability of degraded systems to support ongoing artisanal fishing makes it worthwhile to continue harvesting parrotfishes. This is beneficial for fishers, but it also maintains unsustainable fishing pressure on larger parrotfish species long after they have collapsed, and may eventually lead to local extinction. A similar situation exists in Maine lobsters where a simplified ecosystem provides short-term gains but lays the foundations for an unstable future [50]. Although reefs may appear resilient, the selective loss of large parrotfishes and the erosion of ecosystem function that we have documented increases the chances of future ecological surprises [30,51].
Our focus on functional groups of parrotfishes highlights the importance of implementing a resilience-based approach to sustaining ecosystem functions [11,52]. Many Indo-Pacific coral reefs exhibit considerable resilience to the impacts of fishing, with continued coral recruitment, regeneration of damaged reefs by fast-growing Acropora species, and low macroalgal densities [1,2,53–55]. Nonetheless, many reef systems are operating with compromised or fragmentary ecosystem processes, and are increasingly unable to absorb the impacts of fishing, pollution, climate change and ocean acidification [1,3,8,11,56]. The current conservation focus on iconic species such as sharks, on establishing small highly protected areas, and on biodiversity hotspots is not sufficient to secure the future of the world's reefs. A much broader effort is required, grounded by a clear understanding of reef processes and ecosystem functions at a seascape scale.
Acknowledgements
Research was conducted under permits from the Great Barrier Reef Marine Park Authority and James Cook University Animal Ethics Committee.
We thank J. Tanner, S. Wismer, J. Hodge and M. Sheaves for technical assistance, and N. Graham, M. Pratchett, J. Cinner, S. Foale and two anonymous reviewers for helpful discussions or comments on earlier drafts. This work was supported by the Australian Research Council.
References
- 1.Hughes T. P., et al. 2003. Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933 10.1126/science.1085046 (doi:10.1126/science.1085046) [DOI] [PubMed] [Google Scholar]
- 2.Hughes T. P., et al. 2007. Phase shifts, herbivory and the resilience of coral reefs to climate change. Curr. Biol. 17, 1–6 10.1016/j.cub.2006.12.049 (doi:10.1016/j.cub.2006.12.049) [DOI] [PubMed] [Google Scholar]
- 3.Pandolfi J. M., et al. 2003. Global trajectories of the long-term decline of coral reef ecosystems. Science 301, 955–958 10.1126/science.1085706 (doi:10.1126/science.1085706) [DOI] [PubMed] [Google Scholar]
- 4.Bellwood D. R., Hughes T. P., Hoey A. S. 2006. Sleeping functional group drives coral reef recovery. Curr. Biol 16, 2434–2439 10.1016/j.cub.2006.10.030 (doi:10.1016/j.cub.2006.10.030) [DOI] [PubMed] [Google Scholar]
- 5.Bellwood D. R., Hughes T. P., Connolly S. R., Tanner J. 2005. Environmental and geometric constraints on Indo-Pacific coral reef biodiversity. Ecol. Lett. 8, 643–651 10.1111/j.1461-0248.2005.00763.x (doi:10.1111/j.1461-0248.2005.00763.x) [DOI] [Google Scholar]
- 6.Norström A. V., Nyström M., Lokrantz J., Folke C. 2009. Alternative states on coral reefs: beyond coral-macroalgal phase shifts. Mar. Ecol. Prog. Ser. 376, 295–306 10.3354/meps07815 (doi:10.3354/meps07815) [DOI] [Google Scholar]
- 7.Wilson S. K., Fisher R., Pratchett M. S., Graham N. A. J., Dulvy N. K., Turner R. A., Cakacaka A., Polunin N. V. C., Rushton S. P. 2008. Exploitation and habitat degradation as agents of change within coral reef fish communities. Glob. Change Biol. 14, 2796–2809 10.1111/j.1365-2486.2008.01696.x (doi:10.1111/j.1365-2486.2008.01696.x) [DOI] [Google Scholar]
- 8.Hughes T. P., Graham N. A. J., Jackson J. B. C., Mumby P. J., Steneck R. S. 2010. Rising to the challenge of sustaining coral reef resilience. Trends Ecol. Evol. 25, 633–642 10.1016/j.tree.2010.07.011 (doi:10.1016/j.tree.2010.07.011) [DOI] [PubMed] [Google Scholar]
- 9.Jackson J. B. C., et al. 2001. Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–638 10.1126/science.1059199 (doi:10.1126/science.1059199) [DOI] [PubMed] [Google Scholar]
- 10.Hughes T. P. 1994. Catastrophes, phase-shifts, and large-scale degradation of a Caribbean coral-reef. Science 265, 1547–1551 10.1126/science.265.5178.1547 (doi:10.1126/science.265.5178.1547) [DOI] [PubMed] [Google Scholar]
- 11.Bellwood D. R., Hughes T. P., Folke C., Nyström M. 2004. Confronting the coral reef crisis. Nature 429, 827–833 10.1038/nature02691 (doi:10.1038/nature02691) [DOI] [PubMed] [Google Scholar]
- 12.Mumby P. J., et al. 2006. Fishing, trophic cascades, and the process of grazing on coral reefs. Science 311, 98–101 10.1126/science.1121129 (doi:10.1126/science.1121129) [DOI] [PubMed] [Google Scholar]
- 13.Burkepile D. E., Hay M. E. 2008. Herbivore species richness and feeding complementarity affect community structure and function on a coral reef. Proc. Natl Acad. Sci. USA 105, 16 201–16 206 10.1073/pnas.0801946105 (doi:10.1073/pnas.0801946105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hay M. E. 1984. Patterns of fish and urchin grazing on Caribbean coral reefs: are previous results typical? Ecology 65, 446–454 10.2307/1941407 (doi:10.2307/1941407) [DOI] [Google Scholar]
- 15.Graham N. A. J., Wilson S. K., Jennings S., Polunin N. V. C., Bijoux J. P., Robinson J. 2006. Dynamic fragility of oceanic coral reef ecosystems. Proc. Natl Acad. Sci. USA 103, 8425–8429 10.1073/pnas.0600693103 (doi:10.1073/pnas.0600693103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adam T. C., Schmitt R. J., Holbrook S. J., Brooks A. J., Edmunds P. J., Carpenter R. C., Bernardi G. 2011. Herbivory, connectivity, and ecosystem resilience: response of a coral reef to a large-scale perturbation. PLoS ONE 6, e23717. 10.1371/journal.pone.0023717 (doi:10.1371/journal.pone.0023717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellwood D. R., Hoey A. S., Choat J. H. 2003. Limited functional redundancy in high diversity systems: resilience and ecosystem function on coral reefs. Ecol. Lett. 6, 281–285 10.1046/j.1461-0248.2003.00432.x (doi:10.1046/j.1461-0248.2003.00432.x) [DOI] [Google Scholar]
- 18.Rotjan R. D., Lewis S. M. 2008. Impact of coral predators on tropical reefs. Mar. Ecol. Prog. Ser. 367, 73–91 10.3354/meps07531 (doi:10.3354/meps07531) [DOI] [Google Scholar]
- 19.Hoey A. S., Bellwood D. R. 2008. Cross-shelf variation in the role of parrotfishes on the Great Barrier Reef. Coral Reefs 27, 37–47 10.1007/s00338-007-0287-x (doi:10.1007/s00338-007-0287-x) [DOI] [Google Scholar]
- 20.Aswani S., Hamilton R. J. 2004. Integrating indigenous ecological knowledge and customary sea tenure with marine and social science for conservation of bumphead parrotfish (Bolbometopon muricatum) in the Roviana Lagoon, Solomon Islands. Environ. Conserv. 31, 69–83 10.1017/S037689290400116X (doi:10.1017/S037689290400116X) [DOI] [Google Scholar]
- 21.Robbins W. D., Hisano M., Connolly S. R., Choat J. H. 2006. Ongoing collapse of coral-reef shark populations. Curr. Biol. 16, 2314–2319 10.1016/j.cub.2006.09.044 (doi:10.1016/j.cub.2006.09.044) [DOI] [PubMed] [Google Scholar]
- 22.Feary D. A., Cinner J. E., Graham N. A. J., Hartley F. A. 2010. Effects of customary marine closures on fish behavior, spear-fishing success, and underwater visual surveys. Conserv. Biol. 25, 341–349 [DOI] [PubMed] [Google Scholar]
- 23.Dickens L. C., Goatley C. H. R., Tanner J. K., Bellwood D. R. 2011. Quantifying relative diver effects in underwater visual censuses. PLoS ONE 6, e18965. 10.1371/journal.pone.0018965 (doi:10.1371/journal.pone.0018965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonaldo R. M., Bellwood D. R. 2008. Size-dependent variation in the functional role of the parrotfish Scarus rivulatus on the Great Barrier Reef, Australia. Mar. Ecol. Prog. Ser. 360, 237–244 10.3354/meps07413 (doi:10.3354/meps07413) [DOI] [Google Scholar]
- 25.Lokrantz J., Nyström M., Thyresson M., Johansson C. 2008. The non-linear relationship between body size and function in parrotfishes. Coral Reefs 27, 967–974 10.1007/s00338-008-0394-3 (doi:10.1007/s00338-008-0394-3) [DOI] [Google Scholar]
- 26.De'ath G., Fabricius K. E. 2000. Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology 81, 3178–3192 10.1890/0012-9658(2000)081[3178:CARTAP]2.0.CO;2 (doi:10.1890/0012-9658(2000)081[3178:CARTAP]2.0.CO;2) [DOI] [Google Scholar]
- 27.Dalzell P., Adams T. J. H., Polunin N. V. C. 2006. Coastal fisheries in the Pacific Islands. Oceanogr. Mar. Biol. Annu. Rev. 34, 395–531 [Google Scholar]
- 28.Cinner J. E., McClanahan T. R., Daw T. M., Graham N. A. J., Maina J., Wilson S. K., Hughes T. P. 2009. Linking social and ecological systems to sustain coral reef fisheries. Curr. Biol. 19, 206–212 10.1016/j.cub.2008.11.055 (doi:10.1016/j.cub.2008.11.055) [DOI] [PubMed] [Google Scholar]
- 29.Madin E. M. P., Gaines S. D., Warner R. R. 2010. Field evidence for pervasive indirect effects of fishing on prey foraging behaviour. Ecology 91, 3563–3571 10.1890/09-2174.1 (doi:10.1890/09-2174.1) [DOI] [PubMed] [Google Scholar]
- 30.Madin E. M. P., Gaines S. D., Madin J. S., Warner R. R. 2010. Fishing indirectly structures macroalgal assemblages by altering herbivore behavior. Am. Nat. 176, 785–801 10.1086/657039 (doi:10.1086/657039) [DOI] [PubMed] [Google Scholar]
- 31.Welsh J. Q., Bellwood D. R. In press Spatial ecology of the steephead parrotfish (Chlorurus microrhinos): an evaluation using acoustic telemetry. Coral Reefs. 10.1007/s00338-011-0813-8 (doi:10.1007/s00338-011-0813-8) [DOI] [Google Scholar]
- 32.Folke C., Carpenter S., Walker B., Scheffer M., Elmqvist T., Gunderson L., Holling C. S. 2004. Regime shifts, resilience, and biodiversity in ecosystem management. Annu. Rev. Ecol. Evol. Syst. 35, 557–581 10.1146/annurev.ecolsys.35.021103.105711 (doi:10.1146/annurev.ecolsys.35.021103.105711) [DOI] [Google Scholar]
- 33.Choat J. H., Robbins W. D., Clements K. D. 2004. The trophic status of herbivorous fishes on coral reefs. II. Food processing modes and trophodynamics. Mar. Biol. 145, 445–454 10.1007/s00227-004-1341-7 (doi:10.1007/s00227-004-1341-7) [DOI] [Google Scholar]
- 34.Cheal A. J., MacNeil M. A., Cripps E., Emslie M. J., Jonker M., Schaffelke B. 2010. Coral-macroalgal phase shifts or reef resilience: links with diversity and functional roles of herbivorous fishes on the Great Barrier Reef. Coral Reefs 29, 1005–1015 10.1007/s00338-010-0661-y (doi:10.1007/s00338-010-0661-y) [DOI] [Google Scholar]
- 35.Carpenter R. C. 1986. Partitioning herbivory and its effects on coral reef algal communities. Ecol. Monogr. 56, 345–363 10.2307/1942551 (doi:10.2307/1942551) [DOI] [Google Scholar]
- 36.Goatley C. H. R., Bellwood D. R. 2010. Biologically mediated sediment fluxes on coral reefs: sediment removal and off-reef transportation by the surgeonfish Ctenochaetus striatus. Mar. Ecol. Prog. Ser. 415, 237–245 10.3354/meps08761 (doi:10.3354/meps08761) [DOI] [Google Scholar]
- 37.Choat J. H., Axe L. M. 1996. Growth and longevity in Acanthurid fishes: an analysis of otolith increments. Mar. Ecol. Prog. Ser. 134, 15–26 10.3354/meps134015 (doi:10.3354/meps134015) [DOI] [Google Scholar]
- 38.Cole A. J., Pratchett M. S., Jones G. P. 2008. Diversity and functional importance of coral-feeding fishes on tropical coral reefs. Fish Fish. 9, 286–307 10.1111/j.1467-2979.2008.00290.x (doi:10.1111/j.1467-2979.2008.00290.x) [DOI] [Google Scholar]
- 39.Steneck R. S. 1997. Crustose corallines, other algal functional groups, herbivores and sediments: complex interactions along reef productivity gradients. Proc. 8th Int. Coral Reef Symp. 1, 695–700 [Google Scholar]
- 40.Birrell C. L., McCook L. J., Willis B. L., Diaz-Pulido G. A. 2008. Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. Oceanogr. Mar. Biol. Annu. Rev. 46, 25–63 10.1201/9781420065756.ch2 (doi:10.1201/9781420065756.ch2) [DOI] [Google Scholar]
- 41.Rasher D. B., Hay M. E. 2010. Chemically rich seaweeds poison corals when not controlled by herbivores. Proc. Natl Acad. Sci. USA 107, 9683–9688 10.1073/pnas.0912095107 (doi:10.1073/pnas.0912095107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adjeroud M., et al. 2009. Recurrent disturbances, recovery trajectories, and resilience of coral assemblages on a South Central Pacific reef. Coral Reefs 28, 775–780 10.1007/s00338-009-0515-7 (doi:10.1007/s00338-009-0515-7) [DOI] [Google Scholar]
- 43.McClanahan T. R., Hicks C. C., Darling E. S. 2008. Malthusian overfishing and efforts to overcome it on Kenyan coral reefs. Ecol. Appl. 18, 1516–1529 10.1890/07-0876.1 (doi:10.1890/07-0876.1) [DOI] [PubMed] [Google Scholar]
- 44.McClanahan T. R., Polunin N. V. C., Done T. J. 2002. Ecological states and the resilience of coral reefs. Conserv. Ecol. 6, 18 [Google Scholar]
- 45.Price S. A., Wainwright P. C., Bellwood D. R., Kazancioglu E., Collar D. C., Near T. J. 2010. Functional innovations and morphological diversification in parrotfish. Evolution 64, 3057–3068 10.1111/j.1558-5646.2010.01036.x (doi:10.1111/j.1558-5646.2010.01036.x) [DOI] [PubMed] [Google Scholar]
- 46.Hoey A. S., Bellwood D. R. 2011. Suppression of herbivory by macroalgal density: a critical feedback on coral reefs? Ecol. Lett. 14, 267–273 10.1111/j.1461-0248.2010.01581.x (doi:10.1111/j.1461-0248.2010.01581.x) [DOI] [PubMed] [Google Scholar]
- 47.Hughes T. P., Connell J. H. 1999. Multiple stresses on coral reefs. Limnol. Oceanogr. 44, 932–940 10.4319/lo.1999.44.3_part_2.0932 (doi:10.4319/lo.1999.44.3_part_2.0932) [DOI] [Google Scholar]
- 48.Loya Y., Sakai K., Yamazato K., Nakano Y., Sambali H., Van Woesik R. 2001. Coral bleaching: the winners and losers. Ecol. Lett. 4, 122–131 10.1046/j.1461-0248.2001.00203.x (doi:10.1046/j.1461-0248.2001.00203.x) [DOI] [Google Scholar]
- 49.Madin J. S., Connolly S. R. 2006. Ecological consequences of major hydrodynamic disturbances on coral reefs. Nature 444, 477–480 10.1038/nature05328 (doi:10.1038/nature05328) [DOI] [PubMed] [Google Scholar]
- 50.Steneck R. S., et al. 2011. Creation of a gilded trap by the high economic value of the Maine lobster fishery. Conserv. Biol. 25, 904–912 10.1111/j.1523-1739.2011.01717.x (doi:10.1111/j.1523-1739.2011.01717.x) [DOI] [PubMed] [Google Scholar]
- 51.Scheffer M., Carpenter S., Foley J. A., Folke C., Walker B. 2001. Catastrophic shifts in ecosystems. Nature 413, 519–596 10.1038/35098000 (doi:10.1038/35098000) [DOI] [PubMed] [Google Scholar]
- 52.Hughes T. P., Bellwood D. R., Folke C., Steneck R. S., Wilson J. 2005. New paradigms for supporting resilience of marine ecosystems. Trends Ecol. Evol. 20, 380–386 10.1016/j.tree.2005.03.022 (doi:10.1016/j.tree.2005.03.022) [DOI] [PubMed] [Google Scholar]
- 53.Diaz-Pulido G. A., et al. 2009. Doom and boom on a resilient reef: climate change, algal overgrowth and coral recovery. PLoS ONE 4, e5239. 10.1371/journal.pone.0005239 (doi:10.1371/journal.pone.0005239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stockwell B., Jadloc C. R. L., Abesamis R. A., Alcala A. C., Russ G. R. 2009. Trophic and benthic responses to no-take marine reserve protection in the Philippines. Mar. Ecol. Prog. Ser. 389, 1–15 10.3354/meps08150 (doi:10.3354/meps08150) [DOI] [Google Scholar]
- 55.Wismer S., Hoey A. S., Bellwood D. R. 2009. Cross-shelf benthic community structure on the Great Barrier Reef: relationships between macroalgal cover and herbivore biomass. Mar. Ecol. Prog. Ser. 376, 45–54 10.3354/meps07790 (doi:10.3354/meps07790) [DOI] [Google Scholar]
- 56.Wilson S. K., Graham N. A. J., Pratchett M. S., Jones G. P., Polunin N. V. C. 2006. Multiple disturbances and global degradation of coral reefs: are reef fishes at risk or resilient? Glob. Change Biol. 12, 2220–2234 10.1111/j.1365-2486.2006.01252.x (doi:10.1111/j.1365-2486.2006.01252.x) [DOI] [Google Scholar]