Abstract
Reported declines of pollinator populations around the world have led to increasing concerns about the consequences for pollination as a critical ecosystem function and service. Pollination could be maintained through compensation if remaining pollinators increase their contribution or if novel species are recruited as pollinators, but empirical evidence of this compensation is so far lacking. Using a natural experiment in New Zealand where endemic vertebrate pollinators still occur on one offshore island reserve despite their local extinction on the adjacent North Island, we investigated whether compensation could maintain pollination in the face of pollinator extinctions. We show that two recently arrived species in New Zealand, the invasive ship rat (Rattus rattus) and the recent colonist silvereye (Zosterops lateralis; a passerine bird), at least partly maintain pollination for three forest plant species in northern New Zealand, and without this compensation, these plants would be significantly more pollen-limited. This study provides empirical evidence that widespread non-native species can play an important role in maintaining ecosystem functions, a role that needs to be assessed when planning invasive species control or eradication programmes.
Keywords: extinction, ecosystem function, compensation, pollination, New Zealand, vertebrate pollinators
1. Introduction
Considerable attention over the last few decades has been focused on understanding how the loss of species affects ecosystem functions such as pollination [1–5]. Pollination by animals is required for reproduction by 87.5 per cent of flowering plant species [6], and is a critically important ecosystem service for crop production [7]. Many authors have expressed concern about the stability of pollination systems in the face of global declines of pollinators [5,7–9], and the majority of studies address the scenario where pollination decreases as a result of species loss.
However, in the case of mutualisms that are driven through the attraction of mutualists to resource rewards (pollen and nectar in the case of pollination), the loss of a species from a system should lead to greater availability of these resources for other species to exploit. Pollination as an ecosystem function could be maintained if other species are able to effectively pollinate the flowers while using these newly available floral rewards, thus compensating for the loss of the original pollinator. Compensation has been suggested as one mechanism that may help to maintain crop pollination despite inter-annual variability in the density of insect pollinator populations [2,4,8,10,11], but evidence of compensation among crop pollinators is lacking to date [8].
In this study, we ask whether compensation can maintain pollination function in the extreme scenario of species extinction. Compensation could occur through either an increase in the interaction rate or efficiency of the remaining pollinator species [1,8], or through the recruitment of novel species [12]. We define full compensation as that which maintains pollination at a level equal to or greater than that which occurred in the original condition (prior to species loss), while partial compensation refers to the case when pollination is significantly reduced yet there is a significant increase in contributions by the remaining native species or significant contributions by novel species.
The contribution of novel species to the maintenance of ecosystem function is a core component in the concept of ‘novel ecosystems’ (ecosystems comprised of a mixture of native and exotic species) [13,14], and has been discussed in other studies in terms of the replacement or displacement of native species (e.g. [15]), and mostly with regard to the contribution to ecosystem function by invasive plants [16,17]. The concept of compensation has not yet been incorporated into models of pollination network dynamics (e.g. [9,18]), but observations of flower visitation by introduced species have led some authors to suggest that the introduced species may play a limited role in replacing extirpated native species [19–21]. While one study has documented a non-native bird species maintaining pollination in the absence of native vertebrate pollinators [12], the invasion of native pollination networks by non-native insect species such as the honeybee (Apis melifera) and bumble-bees (Bombus spp.) is generally reported to have led to negative consequences for native pollination systems [22,23].
If compensation fully or partially maintains ecosystem function, concerns about potential declines in pollination may be somewhat allayed (cf. [5,7–9,13]), and investigations into the factors that limit compensation should become a research priority. Specialized floral structures that restrict access to floral rewards would limit the pool of potential pollinators that could compensate. Remaining native pollinators may be limited in their ability to compensate if other factors such as nest site availability or predation restrict their potential to respond to the increased availability of floral rewards; novel species may be limited by mismatches in morphology or behaviour that restrict their ability to exploit new floral resources or that prevent effective pollination.
New Zealand affords a unique opportunity to investigate whether compensation can maintain pollination function following the loss of pollinator species. Nine North Island bird species, one bat species and five gecko species—all endemic to New Zealand—have been recorded as flower visitors [24,25], but the introduction of invasive mammalian predators (such as ship rats Rattus rattus, stoats Mustela ermina and cats Felis catus) has led to the local or functional extinction of most of these species from much of the upper North Island. Offshore island sanctuaries lacking invasive predators act as refuges for many of these endemic vertebrates, thus providing a natural experiment that allows us to compare pollination at sites with and without the endemic vertebrate flower visitors [26–28]. A recent study showed that the loss of native birds has led to a decline in reproductive output of a plant with tubular flowers that is only effectively pollinated by native birds [26], but little is known about the consequences of losing the endemic vertebrate pollinators for plants with more openly accessible flowers.
The aim of this study was to investigate whether the loss of endemic vertebrate pollinators in the North Island of New Zealand has led to a decline in pollination for three New Zealand plant species that have readily accessible brush-inflorescence flowers, or whether remaining and novel species compensate for the loss of the native pollinators. Our study involved a comparison of pollination ecology on the 2800 ha Little Barrier Island (LBI) nature reserve, which is the only site that retains self-sustaining populations of all known North Island vertebrate flower visitors, with sites on the adjacent North Island, where only one endemic vertebrate flower-visitor remains common (the tui Prosthemadera novaezelandiae, an endemic honeyeater). These North Island forests now host populations both of alien vertebrate and invertebrate species, and of species that have colonized New Zealand since European settlement, around 1840 (henceforth, we term all alien species and species that have colonized New Zealand since European settlement ‘novel species’ to reflect their lack of a long evolutionary relationship with the native flora). We assessed the importance of vertebrate pollinators on LBI and on the North Island using exclusion experiments and by recording flower-visitation rates, and we compared overall pollination between sites to test whether the loss of native pollinator fauna is associated with a decline in pollination.
2. Material and methods
We studied three endemic New Zealand forest plants with brush-like inflorescences that are visited by diverse vertebrate and invertebrate species, and produce nectar in quantities likely to attract vertebrate flower visitors [25].
Metrosideros excelsa (Myrtaceae) is a large, endemic, canopy species that is dominant in coastal forest in the northern half of the North Island (figure 1a), and flowers in December each year when scarlet blossoms cover the canopy. Significant overlap in the timing of male and female developmental stages means that self-pollination is possible, and populations comprise a mix of self-compatible and self-incompatible individuals [29]. While most seeds produced at North Island sites are self-pollinated, late-acting inbreeding depression reduces the vigour of self-pollinated seedlings [30]. One study identified native bird species as important pollinators, but concluded that the loss of these bird species was unlikely to have a detrimental impact on the survival of the M. excelsa owing to the large number of seeds produced by an individual each year [30]. These previous studies did not consider the role of nocturnal pollinators, and did not assess the degree to which compensation might be maintaining pollination for this species at North Island sites.
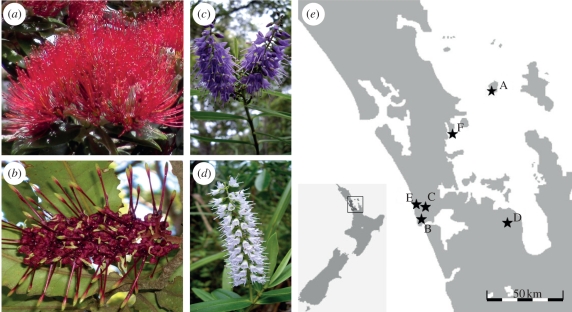
Figure 1.
Study species and study sites. (a) Metrosideros excelsa. (b) Knightia excelsa. (c) Veronica macrocarpa var. latisepala. (d) Veronica macrocarpa var. macrocarpa. (e) This study was conducted in the Auckland region of New Zealand on Little Barrier Island (A, ‘intact’) and five North Island sites (‘invaded’): Metrosideros excelsa at sites E and F; Knightia excelsa at sites C and D; and Veronica macrocarpa at sites B and C. A, Little Barrier Island Nature Reserve; B, Pararaha, Waitakere Ranges Regional Park; C, Quarry Track, Waitakere Ranges Regional Park; D, Hunua Ranges Regional Park; E, White's Beach, Waitakere Ranges Regional Park; F, Mahurangi Regional Park. Map credit—Wikicommons.
Knightia excelsa (Proteaceae) is a tall, emergent forest tree species common throughout the North Island (figure 1b), and no study has been published to date on its reproductive biology. Birds and non-flying mammals have been identified as important pollinators for other Proteaceae species [31], and in New Zealand the floral structure and frequency of bird visitation has led other authors to classify this species as bird-pollinated [25,32]. Species in the Proteaceae show a mix of self-compatibility and self-incompatibility, and low seed-set is reported for most species [31].
Veronica (formerly Hebe) macrocarpa (Plantaginaceae) is an understorey shrub that is found in forests in the north of the North Island and occurs in two variants: var. latisepala generally has purple flowers and is found only on LBI and the adjacent Great Barrier Island (figure 1c), while var. macrocarpa generally has white flowers and is common in forests on the North Island in the Auckland region (figure 1d). There is considerable overlap in the morphological characteristics of these two V. macrocarpa variants, and for the purpose of this study they are treated as a single species. While most Veronica species are thought to be insect-pollinated [25], there have been reports of infrequent bird visitation to flowers [25,32].
Each species was studied in forest on the southwestern tip of LBI (‘intact’), and at two sites on the adjacent North Island (‘invaded’; five North Island sites in total to cover all three species; figure 1e) in the Auckland region. Invaded study sites were located in native forest in the Waitakere Ranges Regional Park, Hunua Ranges Regional Park and Mahurangi Regional Park (figure 1e) at locations where there was no ongoing programme of exotic animal pest control apart from periodic control of brush-tailed possum (Trichosurus vulpecula). Study sites of M. excelsa were chosen on the basis of the presence of mature M. excelsa canopy, and all sites were within 200 m of MHWS in coastal forest dominated by M. excelsa with an understorey that included Coprosma spp., Macropiper excelsum and Rhopalostylis sapida. Study sites of K. excelsa and V. macrocarpa were located in forests on ancient weathered volcanic ridges between 100 and 400 m in elevation, and sites were chosen based on adequate numbers of accessible plants. Forests at these sites were characterized by a sparse canopy of ageing Kunzea ericodes, with a developing secondary canopy comprising species such as Pseudopanax arboreus, Coprosma arborea and Pittosporum spp. interspersed with young Podocarps and Agathis australis. These site-selection criteria resulted in comparable densities of each study species and similar forest structure and composition at the intact and both invaded sites for each species.
Individual plants at a site were selected on the basis of accessibility of adequate numbers of inflorescences (up to 4 m above ground level), and appropriate distance from walking trails to minimize disturbance of experimental treatments by members of the public. At least three standard pollination treatments were conducted on each individual plant at each site: supplementary cross pollen from at least four donors on newly open inflorescences (‘cross’, maximum pollination control), inflorescences left open to be pollinated naturally (‘open’) and inflorescences bagged to prevent access by all pollinators (‘autonomous’, self-pollination control). In addition, on each plant we conducted a ‘cage’ treatment where flowers were enclosed before opening in 19 mm wire mesh cages to prevent access by birds and mammals. Each treatment was conducted on a minimum of one inflorescence per plant (on all florets of an inflorescence), or more if enough inflorescences were open on a given plant. To minimize the effects of resource allocation on fruit- and seed-set, cross-pollination treatments were conducted on a separate stem or branch from other treatments [33,34].
After flowering finished, all bags and cages were removed from inflorescences, and treatments were monitored every month. Seed capsules of M. excelsa and V. macrocarpa were harvested when they were close to opening (determined by colour change and presence of capsules starting to open within the wider population). Capsules were dried and then opened to count developed seeds. Developed seeds were defined as those that had a clearly visible endosperm; such seeds were easy to distinguish from aborted seeds for both species. Aborted V. macrocarpa seeds were too small to reliably count, so developed seeds from at least 20 capsules per treatment were counted (or all capsules if less than 20 in total), and then the mean number of developed seeds per capsule was multiplied by the total number of capsules in that treatment. Because M. excelsa capsules varied greatly in size and seed number, the seeds from all capsules from a given treatment on each tree were combined and then five samples of 100 seeds were taken out randomly. For each sample, we separated the developed seeds from the aborted seeds (using a dissecting scope) and calculated the proportion of the total weight of the 100 seeds that was attributable to the developed seeds (using a microbalance). To calculate the total number of developed seeds per floret in each treatment, we multiplied the total weight of developed and aborted seeds from each treatment by the mean proportion by weight of developed seeds from the five samples, divided by the average weight of developed seeds, and finally divided by the number of florets in the treatment. The proportion of florets of K. excelsa that had become developing seed capsules were counted one month after flowering, as high capsule-loss rates are a methodological problem for Proteaceae [31,35].
To compare pollination between sites and treatments, we calculated the pollen limitation index (PLI) for individual plants at all sites [36], based on seed-set per floret for M. excelsa and V. macrocarpa, and capsule-set per floret for K. excelsa. PLI measures the degree to which a plant's fruit or seed production is limited by the amount of pollen it receives and controls for individual or site differences in fecundity [36]. PLI equals one minus the ratio of open-pollination rates to cross-pollination rates, so values range from 0 (more pollen received than necessary for maximum fruit- or seed-set) to 1 (not enough pollen to set any seeds). Values greater than 0.75 are generally considered to represent a high degree of pollen limitation [25].
The ability of each individual plant to set seed from its own pollen was estimated using the ratio of the seed-set from the autonomous treatment to the cross treatment (analogous to the self-compatibility index or ‘SCI’ [25]). To determine whether the degree of self-compatibility of plants needed to be considered in our analysis of pollen limitation, we plotted PLI versus SCI values of individual plants for each species at each three sites, and tested for significant correlations.
At each site, for each plant, we calculated PLI values for open inflorescences (able to be pollinated by all available pollinators) and caged inflorescences (birds and mammals unable to access inflorescences). Significantly higher PLI values from caged inflorescences compared with open inflorescences at a site indicate that birds and mammals are important pollinators. Significantly higher PLI values from open inflorescences at invaded sites compared with open inflorescences at the intact site indicate that pollination is reduced in the absence of endemic vertebrate pollinators. As the inclusion of highly self-compatible individuals may underestimate the importance of cross-pollination (which is important for the long-term persistence of plant populations) [37], the analyses were repeated after excluding the individual plants that had SCI values greater than 0.5.
Access to LBI was highly restricted during peak flowering of K. excelsa for reasons wholly unrelated to this study. Consequently, the sample sizes for this species on LBI were necessarily limited because of this access problem. While treatments in 2009 were conducted on nine trees in total, very low flower numbers and high flower-loss rates meant that only four trees could provide the minimum three treatments (cross, open and cage) necessary for an analysis of PLI. An additional analysis was therefore conducted that compared the capsule-set rates of open and caged treatments, which increased the sample size to eight trees, as doing so permitted us to include data from four additional trees that did not have enough accessible flowers to conduct a cross treatment as well as the open and cage treatments.
Vertebrate visitation rates were calculated for each individual plant separately, and then averaged across sites. A visit was defined as an occurrence of an animal at a single inflorescence. To identify flower visitors and to estimate visitation rates, we used a system of five video cameras that recorded continuous footage 24 h d−1 and standardized visitation rates to visits per inflorescence per hour. Video footage was reviewed at 20–30 times normal speed to search for visits by birds and mammals; even very brief visits were detected using this method. Compensation in visitation rate can be concluded from the presence of novel species visiting flowers at invaded sites or from an increase in visitation rates of the remaining endemic species.
While the overall design of this study means that the effect of the presence of an intact or invaded pollinator community is potentially confounded with individual site effects, the existence of only one site with an intact native vertebrate pollinator community is a limitation beyond our control. However, the difference in vertebrate pollinator communities is so pronounced that individual site effects are highly unlikely to be as important as the effects being measured (i.e. the effect of caging inflorescences, and the difference in PLI and visitation rates between intact and invaded sites).
Data from both invaded sites were analysed as one dataset for each species as there were no significant differences in the means or variance of the PLI or SCI data between the two invaded sites, and this combination of invaded datasets allowed us to focus on the key comparison between plants at invaded sites and plants at the intact site. The sample size for all analyses is the number of individual plants. Significant differences were determined using one-tailed t-tests when datasets were normal, or one-tailed Mann–Whitney U (unpaired data) and Wilcoxon (paired data) tests when one or both of the datasets being compared were non-normal. Paired tests were used for comparisons of PLI between open and cage treatments at each site, and unpaired tests were used for comparisons of open PLI between sites. Datasets with n = 4 could not be tested by non-parametric methods, so t-tests were used with caveats on the interpretation of results owing to statistical power. An alpha level of 0.05 was used for all tests. Confidence intervals were derived using the bootstrap BCa estimation (default ‘BOOTCI’ function in Matlab v. 7.7.0.471, NBOOT = 1000) as many datasets were significantly non-normal and sample sizes were small [38]. Normality of datasets was tested using the ‘LILLIETEST’ function in Matlab (a = 0.02).
3. Results
Data on self-compatibility and pollen limitation were obtained for: 15 M. excelsa individuals at the intact site and 15 at the two invaded sites (8 and 7 at each invaded site, respectively); 4 K. excelsa individuals at the intact sites and 13 at the two invaded sites (7 and 6); and 10 V. macrocarpa individuals at the intact site and 19 at the invaded sites (12 and 7).
Self-compatibility was generally low for all species at all sites, and was not significantly different between intact and invaded sites. Mean SCI was 0.23 (s.d. = 0.289) for M. excelsa at the intact site and 0.26 (s.d. = 0.264) at the invaded sites (p = 0.7142; unpaired two-tailed t-test, t = 0.37, d.f. = 28). Mean SCI for K. excelsa was 0.1 (s.d. = 0.224) at the intact site and 0.19 at the invaded sites (p = 0.5124; unpaired two-tailed t-test, t = 0.67, d.f. = 16). Mean SCI was 0.06 (s.d. = 0.101) for V. macrocarpa at the intact site and 0.04 (s.d. = 0.086) at the invaded sites (p = 0.5549; unpaired two-tailed t-test, t = 0.6, d.f. = 21). Just five M. excelsa (spread between all three sites) and one K. excelsa (at one invaded site) had SCI values greater than 0.5. There was no significant correlation between PLI of open flowers and SCI values for any of the three species (p > 0.05; t-test for significance of correlation coefficient), showing that the degree of pollen limitation is not dependent on the degree of self-compatibility of the individual plants (electronic supplementary material, figure S1).
For all three species at the intact site, caged inflorescences showed reduced reproductive output compared with open inflorescences. Caged M. excelsa inflorescences (mean PLI = 0.819) were on average 2.5 times as pollen-limited as open inflorescences (mean PLI = 0.325; p < 0.0001; paired-sample t-test, t = 5.61, d.f. = 14; figure 2a). Caged V. macrocarpa inflorescences (mean PLI = 0.804) were 1.6 times as pollen-limited as open inflorescences (mean PLI = 0.491; p = 0.0408; paired-sample t-test, t = 1.96, d.f. = 9; figure 2c). While the small sample size for K. excelsa on LBI resulted in no significant difference between the pollen limitation of open (mean PLI = 0.402) and caged inflorescences (mean PLI = 0.607; p = 0.0603; paired-sample t-test, t = 2.15, d.f. = 3; figure 2b), the mean capsule set rate of open K. excelsa inflorescences was 0.26, compared with 0.16 from caged inflorescences (p = 0.0143; paired-sample t-test, t = 2.75, d.f. = 7).
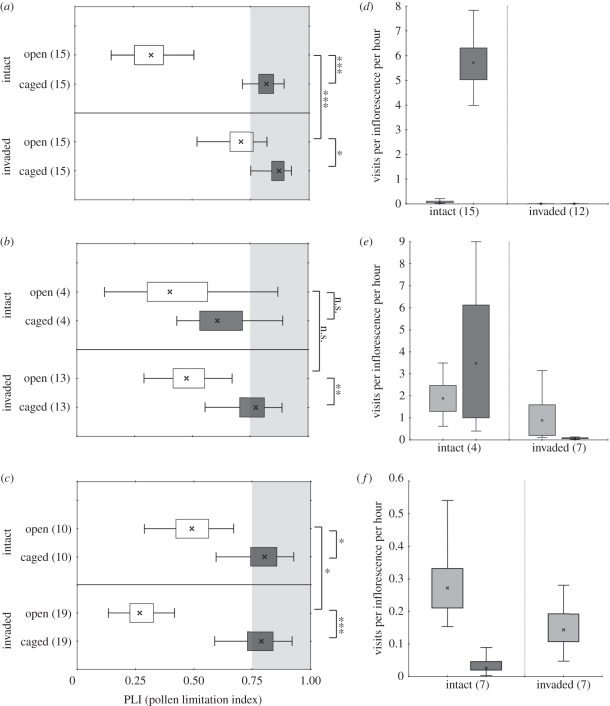
Figure 2.
Novel vertebrate visitors partially maintain pollination levels in three northern New Zealand plant species. (a–c) 95% (error bars) and 50% (boxes) bootstrap confidence intervals of mean PLI values (crosses) for open (clear) and caged (dark grey) flowers at intact (Little Barrier Island) and invaded (North Island) sites. The light grey shading delimits high pollen limitation (PLI > 0.75). Asterisk, double asterisk and triple asterisk signify significance levels of p < 0.05, p < 0.01 and p < 0.001, respectively; n.s. signifies p > 0.05. (d–f) Mean (crosses) bird (light grey) and mammal (dark grey) visitation rates at intact and invaded sites (boxes are 50% bootstrap confidence intervals and error bars are 95% bootstrap confidence intervals). (a,d) present results for M. excelsa, (b,e) for K. excelsa and (c,f) for V. macrocarpa. Numbers in parentheses are sample sizes.
Despite the absence of endemic vertebrate pollinators at the invaded sites, all species at these invaded sites showed significantly higher PLI values for caged inflorescences than open inflorescences, indicating that vertebrates were still important pollinators for all three species. Although caged M. excelsa inflorescences at invaded sites (mean PLI = 0.872) were only 1.2 times as pollen-limited as open inflorescences (mean PLI = 0.709), this difference was still significant (p = 0.0262; paired-sample t-test, t = 2.12, d.f. = 14; figure 2a). Caged K. excelsa inflorescences (mean PLI = 0.775) were 1.6 times as pollen-limited as open inflorescences (mean PLI = 0.474; p = 0.0052; paired-sample t-test, t = 3.03, d.f. = 12; figure 2b). Caged V. macrocarpa inflorescences (mean PLI = 0.790) were 2.9 times as pollen-limited as open inflorescences (mean PLI = 0.268; p = 0.0002; Wilcoxon signed-rank test, w = −163, z = −3.54, d.f. = 18; figure 2c).
The PLI values for caged flowers of all species at invaded sites, and for M. excelsa and V. macrocarpa at the intact site, were greater than 0.75; indicating high pollen limitation. The significant reduction in pollination owing to caging for all species at all sites is strong evidence that birds and mammals are required for adequate pollination at both the invaded North Island sites and at the intact LBI site.
Open M. excelsa inflorescences at invaded sites were 2.2 times more pollen-limited than open inflorescences at the intact site (p = 0.001; unpaired t-test, t = 3.17, d.f. = 28; figure 2a), indicating that pollination function is not being fully maintained at invaded sites. For K. excelsa, there was no significant increase in PLI of open inflorescences at invaded sites compared with intact sites (p = 0.3767; unpaired t-test, t = 0.32, d.f. = 15; figure 2b), but analysis is limited by the small sample size from the intact site. However, the distribution of PLI values of K. excelsa from invaded sites confirms that these populations are not experiencing high pollen limitation (figure 2b). Open V. macrocarpa inflorescences showed significantly lower PLI values at invaded sites than at the intact site (p = 0.0475; unpaired t-test, t = 1.73, d.f. = 27; figure 2c), indicating that V. macrocarpa plants at the invaded sites were, in fact, better pollinated than those at the intact site.
When the six individual plants with an SCI greater than 0.5 were excluded, the analyses showed the same pattern; caged inflorescences were significantly more pollen-limited than open inflorescences at all sites (and on LBI, open K. excelsa inflorescences set more capsules than caged inflorescences), and while open M. excelsa inflorescences at invaded sites were significantly more pollen-limited than at the intact site, K. excelsa and V. macrocarpa inflorescences at invaded sites were not significantly more pollen-limited than at the intact site (electronic supplementary material, tables S1 and S2). This confirms that vertebrates are important pollinators for these species not just in terms of total seed production but also in terms of the production of cross-pollinated seed.
A total of 1239 h of video footage were reviewed for this study. Video footage confirmed that caged flowers of all three species were visited by large pollinating insects (including moths and introduced bumble-bees), but the cages successfully prevented access by birds and mammals. On LBI, Pacific geckos (Hoplodactylus pacificus) were recorded visiting M. excelsa (mean of 0.06 visits per inflorescence per hour) and K. excelsa (mean of 0.25 visits per inflorescence per hour), but because they were able to get through the exclusion cages their contribution could not be distinguished from that of insect pollinators. Data on pollinator visitation rates were obtained for: 15 M. excelsa individuals at the intact site and 12 at the two invaded sites (5 and 7 at each invaded site, respectively); 4 K. excelsa individuals at the intact site and 7 individuals at the invaded sites (4 and 3); and 7 individuals of V. macrocarpa at both the intact and the invaded sites (5 and 2).
All vertebrate visitors recorded at the intact site were endemic species (see electronic supplementary material, video S1 for sample footage from this site). The threatened, endemic short-tailed bat (Mystacina tuberculata) was the most frequent visitor to M. excelsa (97.4% of 5424 recorded visits; figure 2d) and K. excelsa (61.1% of 265 recorded visits; figure 2e); it also regularly visited V. macrocarpa (figure 2f). Bellbirds (Anthornis melanura) were the most common bird visitors to the three species at the intact site, and were the most common visitors overall to V. macrocarpa (85.4% of 466 recorded vertebrate visits; figure 2f). Tui, saddlebacks (Philesturnus carunculatus) and red-crowned parakeets (Cyanorhamphus novaezelandiae) were also recorded visiting M. excelsa at the intact site, and tui and stitchbirds (Notiomystis cincta) were recorded visiting K. excelsa. Stitchbirds were also recorded visiting V. macrocarpa.
Vertebrate visitation rates at invaded sites were significantly lower than at the intact site for M. excelsa (p = 0.0001; one-tailed Mann–Whitney U-test, U = 0, z = 4.31, na = 15, nb = 12; figure 2d), but not for K. excelsa (p = 0.13847; unpaired unequal variance t-test, t = 2, d.f. = 3.66; figure 2e) or V. macrocarpa (p = 0.1539; one-tailed Mann–Whitney U-test, U = 16, z = 1.02, na = 7, nb = 7; figure 2f; see electronic supplementary material, video S2 for sample footage from these sites). The most common vertebrate species recorded visiting these three plant species at invaded sites were the recently arrived silvereye (90.6% of 202 total vertebrate visits) and the invasive ship rat (5.9% of 202 vertebrate visits). Only 15 vertebrate visits to M. excelsa were recorded at mainland sites; 8 (53.3%) were by silvereyes and 4 (26.7%) were by ship rats. Of the 133 visits recorded to K. excelsa, 121 (91%) were by silvereyes to three individual trees at one site, and 8 (6%) were by ship rats to four different trees at two sites. Silvereyes were the only recorded vertebrate visitors to V. macrocarpa at invaded sites, and were recorded making 54 visits to six plants at two sites. The only endemic bird visitor recorded on camera at invaded sites was the grey gerygone (Gerygone igata), which visited one K. excelsa inflorescence four times in one day (3% of 133 recorded visits to K. excelsa at invaded sites). Metrosideros excelsa was also visited by the introduced blackbird (Turdus merula).
Observations of visitation behaviour show that birds tend to peck at flowers from adjacent perches, while bats and rats tend to crawl over the inflorescences, suggesting that stigma contact rates might differ significantly between these groups (electronic supplementary material, videos S1 and S2). Ship rats were not observed eating or damaging any floral parts while feeding on nectar (electronic supplementary material, video S2).
4. Discussion
Our invaded North Island sites had lost almost all of their endemic vertebrate pollinators (five birds, one bat and one gecko species were recorded as regular visitors to the three plant species at the intact LBI site), and the one remaining common endemic bird (tui) did not visit any of the flowers videoed at these sites. Yet the exclusion experiments showed that vertebrate pollinators were still important at these invaded sites, and the video footage revealed the identity of these pollinators as recent colonist silvereyes and invasive ship rats, both of which have only established in New Zealand following European colonization in the mid-1800s [26,27]. Our results show that these novel vertebrate species are maintaining pollination to some degree for each of the three plant species at these invaded sites, and without this compensation, the plants would probably be experiencing high pollen limitation.
A recent study linked the local extinction of native pollinating bird species in New Zealand to serious reproduction failure in a bird-specialized plant species. We show that these three more generalized flowers would probably be in a similar position if silvereyes and ship rats were not now contributing to pollination, as insects do not appear to maintain adequate cross-pollination for these species. Nectar draws these omnivorous novel species to the flowers, and their role as pollinators for the wider flora of New Zealand will therefore depend on the availability and attractiveness of nectar compared with other food sources, and the matching of floral morphology to feeding behaviour. Our results suggest that other New Zealand plant species that are adapted for pollination by endemic birds and bats (such as those with inflorescences that produce significant quantities of accessible nectar) might experience reduced reproductive output were it not for compensation by novel birds and rats.
While it is common knowledge that many endemic animal species have been lost from the North and South Islands, and that a broad suite of introduced animal species now occupies these forests, little work has been done to date to assess whether these novel species are now important for the maintenance of ecosystem function. An earlier review of the role of non-native species in pollination concluded on the basis of incomplete visitation data that non-natives were unimportant [21]. Although silvereyes have been recorded visiting a wide range of flowers in New Zealand, they act as nectar robbers of bird-specialized tubular flowers [24,26], and until now their importance as pollinators of more generalized flowers has not been assessed. A related species, Zosterops japonica, was shown to pollinate a Hawaiian member of the Pandanaceae family following the local extinction of endemic birds [12].
While the subset Hebe in the genus Veronica are generally considered to be insect-pollinated species, we have shown that native birds and bats would have been important pollinators for V. macrocarpa historically, and that the silvereye is now an important pollinator at North Island sites. Previous studies have suggested that M. excelsa and K. excelsa are specialized for bird pollination on the basis of copious nectar production, the size of floral parts [25] and, in the case of M. excelsa, exclusion experiments [39]. However, the continued presence of these species at North Island sites where most native bird pollinators are absent has been suggested as evidence that invertebrates may be adequate pollinators [25]. In this study, we have confirmed that birds are important pollinators for these species, but that mammals are also important. Schmidt-Adam et al. [30] suggested that the loss of native bird pollinators was probably not a major concern because of the total quantity of seeds produced in a year by a single plant, but here we show that a significant proportion of seed-set in North Island M. excelsa populations is now dependent on novel bird and rat pollinators [30].
These results show that despite the open-access structure of these flowers, which allows visitation by numerous insect species, cross-pollination by large vertebrates is still required. While pollination by birds and mammals is generally considered to be uncommon globally [6,25,35], pollination studies are usually conducted during periods of high bee activity and measurement of visitation rates is usually conducted by observers close to the flowers, so this is likely to have led to an underestimation of the importance of vertebrates as pollinators. However, there is increasing recognition of the importance of pollination by birds, mammals and geckos, especially in tropical and island systems [24,35,40–42]. While the results of this study are likely to be most relevant to pollination systems where vertebrates are known to play an important role, our demonstration of beneficial contribution by invasive species to ecosystem function has broad relevance to the maintenance of ecosystem function in diverse ecosystems globally. Other authors have suggested that non-native species may be able to functionally replace native species [12–17,43], but clear empirical evidence for this in the case of animals has been lacking.
Relatively rare visits by an effective pollinator may be more important than frequent visits by an ineffective pollinator. For this reason, we do not believe it is appropriate to draw conclusions about the relative importance of silvereyes and ship rats on the basis of visitation frequency alone. The relative effectiveness of pollinators may vary widely across taxonomic groups, and in this case the difference in behaviour between birds and mammals may result in very different rates of stigma contact. Stigma contact rates are likely to be a better proxy of pollinator effectiveness than visitation rates to inflorescences [44].
The importance of mammal-mediated pollination in New Zealand may have been overlooked because most previous pollination studies either ignored the short-tailed bat or regarded it as an incidental flower visitor [24,25,39], despite reports of nectar-feeding behaviour and pollen in guano [45–48]. As a consequence, the potential role of introduced mammals in maintaining pollination function for native plant species has not been seriously considered previously [45,46]. Ironically, ship rats have been implicated in the decline and local extinction of many native vertebrate species in New Zealand and around the world, including some of the endemic pollinators whose role they now appear to be filling on the North Island [49,50].
This study highlights the difficulty of assigning categories such as ‘problematic’ and ‘non-problematic’ to alien species. While the ship rat is known to have severely negative impacts on biodiversity, we provide an example here of this species acting to maintain crucial ecosystem function. While we do not advocate changing current rat-control strategies in New Zealand or elsewhere, we do believe that the consequences of rat control for functions such as pollination must be considered.
Likewise, silvereyes are largely seen by the public as a benign species. Some ecologists, however, have taken a dimmer view of them, pointing to their record as nectar robbers of plants such as Rhabdothamnus solandri, which is morphologically adapted for native bird pollinators that have longer beaks and tongues [26]. However, here we show evidence of their importance as pollinators of brush-inflorescence flowers on the North Island.
This study is an initial step in understanding the role of novel species in maintaining pollination function, as we have shown that reproductive output is maintained, at least in part, by ship rats and silvereyes. While pollen limitation studies usually only measure the effect of pollen quantity [37], by repeating the analysis after excluding highly self-compatible individuals, we have shown that these vertebrates are important for the transfer of high-quality (out-crossed) pollen. Although seed-set does provide a quantifiable measure of the relative contribution of different pollinators, the complex relationship between seed production and population dynamics is poorly understood [33], and long-term studies of these three plant species are necessary to determine whether this compensation in pollination is sufficient to maintain their populations over time. Gene flow and the genetic structure of populations could change with a shift to novel pollinators if these new species have different movement patterns or differently sized home ranges. Spatially explicit predictions of genetic structure could be developed by using data on movement patterns of different species, and this information could be used to experimentally investigate consequences of pollinator shifts in the field. The unique contribution of this study is a clear demonstration that vertebrates contribute significantly to seed-set of these three generalist plant species, and that the vertebrate visitors at North Island sites are novel species.
While the detrimental impact of invasive rats on native plants and animals is incontrovertible, our study suggests that the functional roles of novel species need to be better understood to ensure that management programmes that specifically target invasive species do not reduce critical ecosystem functions such as pollination [43]. However, while invasive rodents may help maintain pollination for some plants (thus disguising the negative effects of endemic pollinator loss), they cannot completely replace endemic vertebrate pollinators, and their presence is a contributing factor in the loss of endemic pollinators. Therefore, the restoration of endemic pollinator populations should be a priority following invasive mammal eradication programmes, especially the short-tailed bat, which we have shown to be a much more important pollinator than previously considered.
Our results provide evidence that these two widespread species that have broad, adaptable niches can be important in maintaining ecosystem functions in degraded ecosystems, a phenomenon likely to be observed elsewhere. Our study shows that it is critically important to understand this potential compensatory role of novel species, and we suggest that the factors limiting compensation should be a priority for researchers seeking to understand the stability of ecosystem functions in the face of ongoing species extinctions.
Acknowledgements
We thank two anonymous reviewers, J. Adelman, C. Nunez and especially R. Winfree for comments on this manuscript, and A. Barrell, S. Johnson, H. Reynolds, J. Smith and J. Waite for assistance with fieldwork and seed counting. Research funding was provided by High Meadows Foundation, Todd Foundation for Excellence, Princeton University and Auckland Regional Council. This research was conducted under permits from the Auckland Regional Council and the Department of Conservation (AK-23484-FAU).
References
- 1.Naeem S., Li S. 1997. Biodiversity enhances ecosystem reliability. Nature 390, 507–509 10.1038/37348 (doi:10.1038/37348) [DOI] [Google Scholar]
- 2.Tilman D. 1999. The ecological consequences of changes in biodiversity: a search for general principles. Ecology 80, 1455 [Google Scholar]
- 3.Loreau M., et al. 2001. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294, 804–808 10.1126/science.1064088 (doi:10.1126/science.1064088) [DOI] [PubMed] [Google Scholar]
- 4.Hooper D. U., et al. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35 10.1890/04-0922 (doi:10.1890/04-0922) [DOI] [Google Scholar]
- 5.Kremen C. 2005. Managing ecosystem services: what do we need to know about their ecology? Ecol. Lett. 8, 468–479 10.1111/j.1461-0248.2005.00751.x (doi:10.1111/j.1461-0248.2005.00751.x) [DOI] [PubMed] [Google Scholar]
- 6.Ollerton J., Winfree R., Tarrant S. 2011. How many flowering plants are pollinated by animals? Oikos 120, 321–326 10.1111/j.1600-0706.2010.18644.x (doi:10.1111/j.1600-0706.2010.18644.x) [DOI] [Google Scholar]
- 7.Klein A., Vaissière B. E., Cane J. H., Steffan-Dewenter I., Cunningham S. A., Kremen C., Tcharntke T. 2007. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 274, 303–313 10.1098/rspb.2006.3721 (doi:10.1098/rspb.2006.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winfree R., Kremen C. 2009. Are ecosystem services stabilized by differences among species? A test using crop pollination. Proc. R. Soc. B 276, 229–237 10.1098/rspb.2008.0709 (doi:10.1098/rspb.2008.0709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Memmott J., Waser N. M., Price M. V. 2004. Tolerance of pollination networks to species extinctions. Proc. R. Soc. Lond. B 271, 2605–2611 10.1098/rspb.2004.2909 (doi:10.1098/rspb.2004.2909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGrady-Steed J., Morin P. J. 2000. Biodiversity, density compensation, and the dynamics of populations and functional groups. Ecology 81, 361–373 10.1890/0012-9658(2000)081[0361:BDCATD]2.0.CO;2 (doi:10.1890/0012-9658(2000)081[0361:BDCATD]2.0.CO;2) [DOI] [Google Scholar]
- 11.Cottingham K., Brown B., Lennon J. 2001. Biodiversity may regulate the temporal variability of ecological systems. Ecol. Lett. 4, 72–85 10.1046/j.1461-0248.2001.00189.x (doi:10.1046/j.1461-0248.2001.00189.x) [DOI] [Google Scholar]
- 12.Cox P. A. 1983. Extinction of the Hawaiian avifauna resulted in a change of pollinators for the ieie, Freycinetia arborea. Oikos 41, 195–199 [Google Scholar]
- 13.Hobbs R. J., et al. 2006. Novel ecosystems: theoretical and management aspects of the new ecological world order. Glob. Ecol. Biogeogr. 15, 1–7 10.1111/j.1466-822X.2006.00212.x (doi:10.1111/j.1466-822X.2006.00212.x) [DOI] [Google Scholar]
- 14.Hobbs R. J., Higgs E., Harris J. A. 2009. Novel ecosystems: implications for conservation and restoration. Trends Ecol. Evol. 24, 599–605 10.1016/j.tree.2009.05.012 (doi:10.1016/j.tree.2009.05.012) [DOI] [PubMed] [Google Scholar]
- 15.Kiers T. E., Palmer T. M., Ives A. R., Bruno J. F., Bronstein J. L. 2010. Mutualisms in a changing world: an evolutionary perspective. Ecol. Lett. 13, 1459–1474 [DOI] [PubMed] [Google Scholar]
- 16.Westman W. E. 1990. Park management of exotic plant species: problems and issues. Conserv. Biol. 4, 251–260 10.1111/j.1523-1739.1990.tb.00286.x (doi:10.1111/j.1523-1739.1990.tb.00286.x) [DOI] [Google Scholar]
- 17.Ewel J. J., Putz F. E. 2004. A place for alien species in ecosystem restoration. Front. Ecol. Environ. 2, 354–360 10.1890/1540-9295(2004 (doi:10.1890/1540-9295(2004) [DOI] [Google Scholar]
- 18.Bascompte J., Jordano P. 2007. Plant–animal mutualistic networks: the architecture of biodiversity. Annu. Rev. Ecol. Evol. Syst. 38, 567–593 10.1146/annurev.ecolsys.38.091206.095818 (doi:10.1146/annurev.ecolsys.38.091206.095818) [DOI] [Google Scholar]
- 19.Olesen J. M., Rønsted N., Tolderlund U., Cornett C., Mølgaard P., Madsen J., Jones C. G., Olsen C. E. 1998. Mauritian red nectar remains a mystery. Nature 393, 529. 10.1038/31128 (doi:10.1038/31128) [DOI] [Google Scholar]
- 20.Olesen J. M., Eskildsen L. I., Venkatasamy S. 2002. Invasion of pollination networks on oceanic islands: importance of invader complexes and endemic super generalists. Divers. Distrib. 8, 181–192 10.1046/j.1472-4642.2002.00148.x (doi:10.1046/j.1472-4642.2002.00148.x) [DOI] [Google Scholar]
- 21.Kelly D., Robertson A., Ladley J., Anderson S., McKenzie R. 2010. Relative (un)importance of introduced animals as pollinators and dispersers of native plants. In Biological invasions in New Zealand (eds Allen R. B., Lee W. G.), pp. 227–245 Berlin, Germany: Springer; 10.1007/3-540-30023-6_15 (doi:10.1007/3-540-30023-6_15) [DOI] [Google Scholar]
- 22.Traveset A., Richardson D. M. 2006. Biological invasions as disruptors of plant reproductive mutualisms. Trends Ecol. Evol. 21, 208–216 10.1016/j.tree.2006.01.006 (doi:10.1016/j.tree.2006.01.006) [DOI] [PubMed] [Google Scholar]
- 23.Goulson D. 2003. Effects of introduced bees on native ecosystems. Annu. Rev. Ecol. Evol. Syst. 34, 1–26 10.1146/annurev.ecolsys.34.011802.132355 (doi:10.1146/annurev.ecolsys.34.011802.132355) [DOI] [Google Scholar]
- 24.Kelly D., Ladley J., Robertson A., Anderson S., Wotton D., Wiser S. 2010. Mutualisms with the wreckage of an avifauna: the status of bird pollination and fruit-dispersal in New Zealand. NZ J. Ecol. 34, 66–85 [Google Scholar]
- 25.Newstrom L., Robertson A. 2005. Progress in understanding pollination systems in New Zealand. NZ J. Bot. 43, 1–59 [Google Scholar]
- 26.Anderson S. H., Kelly D., Ladley J. J., Molloy S., Terry J. 2011. Cascading effects of bird functional extinction reduce pollination and plant density. Science 331, 1068–1071 10.1126/science.1199092 (doi:10.1126/science.1199092) [DOI] [PubMed] [Google Scholar]
- 27.Atkinson I. A. E., Cameron E. K. 1993. Human influence on the terrestrial biota and biotic communities of New Zealand. Trends Ecol. Evol. 8, 447–451 10.1016/0169-5347(93)90008-D (doi:10.1016/0169-5347(93)90008-D) [DOI] [PubMed] [Google Scholar]
- 28.Towns D. R., Ballantine W. J. 1993. Conservation and restoration of New Zealand island ecosystems. Trends Ecol. Evol. 8, 452–457 10.1016/0169-5347(93)90009-E (doi:10.1016/0169-5347(93)90009-E) [DOI] [PubMed] [Google Scholar]
- 29.Schmidt-Adam G., Gould K. S., Murray B. G. 1999. Floral biology and breeding system of Pohutukawa (Metrosideros excelsa, Myrtaceae). NZ J. Bot. 37, 687–702 [Google Scholar]
- 30.Schmidt-Adam G., Young A. G., Murray B. G. 2000. Low outcrossing rates and shift in pollinators in New Zealand Pohutukawa (Metrosideros excelsa; Myrtaceae). Am. J. Bot. 87, 1265–1271 [PubMed] [Google Scholar]
- 31.Collins B. G., Rebelo T. 1987. Pollination biology of the Proteaceae in Australia and southern Africa. Aust. J. Ecol. 12, 387–421 10.1111/j.1442-9993.1987.tb00958.x (doi:10.1111/j.1442-9993.1987.tb00958.x) [DOI] [Google Scholar]
- 32.Castro I., Robertson A. W. 1997. Honeyeaters and the New Zealand forest flora: the utilisation and profitability of small flowers. NZ J. Ecol. 21, 169–179 [Google Scholar]
- 33.Ashman T., et al. 2004. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85, 2408–2242.1 10.1890/03-8024 (doi:10.1890/03-8024) [DOI] [Google Scholar]
- 34.Knight T. M., Steets J. A., Ashman T. 2006. A quantitative synthesis of pollen supplementation experiments highlights the contribution of resources reallocation to estimates of pollen limitation. Am. J. Bot. 93, 271–277 10.1890/03-8024 (doi:10.1890/03-8024) [DOI] [PubMed] [Google Scholar]
- 35.Carthew S. M., Goldingay R. L. 1997. Non-flying mammals as pollinators. Trends Ecol. Evol. 12, 104–108 10.1016/S0169-5347(96)10067-7 (doi:10.1016/S0169-5347(96)10067-7) [DOI] [PubMed] [Google Scholar]
- 36.Larson B. M. H., Barrett S. C. H. 2000. A comparative analysis of pollen limitation in flowering plants. Biol. J. Linn. Soc. 69, 503–520 10.1111/j.1095-8312.2000.tb01221.x (doi:10.1111/j.1095-8312.2000.tb01221.x) [DOI] [Google Scholar]
- 37.Aizen M. A., Harder L. D. 2007. Expanding the limits of the pollen-limitation concept: effects of pollen quantity and quality. Ecology 88, 271–281 10.1890/06-1017 (doi:10.1890/06-1017) [DOI] [PubMed] [Google Scholar]
- 38.Wang F. K. 2001. Confidence interval for the mean of non-normal data. Qual. Reliab. Eng. Int. 17, 257–267 [Google Scholar]
- 39.Schmidt-Adam G., Murray B., Young A. 2009. The relative importance of birds and bees in the pollination of Metrosideros excelsa (Myrtaceae). Austral. Ecol. 34, 490–498 10.1111/j.1442-9993.2009.01949.x (doi:10.1111/j.1442-9993.2009.01949.x) [DOI] [Google Scholar]
- 40.Fleming T. H., Geiselman C., Kress W. J. 2009. The evolution of bat pollination: a phylogenetic perspective. Ann. Bot. 104, 1017–1043 10.1093/aob/mcp197 (doi:10.1093/aob/mcp197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sekercioglu C. H. 2006. Increasing awareness of avian ecological function. Trends Ecol. Evol. 21, 464–471 10.1016/j.tree.2006.05.007 (doi:10.1016/j.tree.2006.05.007) [DOI] [PubMed] [Google Scholar]
- 42.Olesen J. M., Valido A. 2003. Lizards as pollinators and seed dispersers: an island phenomenon. Trends. Ecol. Evol. 18, 177–181 10.1016/S0169-5347(03)00004-1 (doi:10.1016/S0169-5347(03)00004-1) [DOI] [Google Scholar]
- 43.Zavaleta E. S., Hobbs R. J., Mooney H. A. 2001. Viewing invasive species removal in a whole-ecosystem context. Trends Ecol. Evol. 16, 454–459 10.1016/S0169-5347(01)02194-2 (doi:10.1016/S0169-5347(01)02194-2) [DOI] [Google Scholar]
- 44.Vázquez D. P., Morris W. F., Jordano P. 2005. Interaction frequency as a surrogate for the total effect of animal mutualists on plants. Ecol. Lett. 8, 1088–1094 10.1111/j.1461-0248.2005.00810.x (doi:10.1111/j.1461-0248.2005.00810.x) [DOI] [Google Scholar]
- 45.Lord J. 1991. Pollination and seed dispersal in Freycinetia baueriana a dioecious liane that has lost its bat pollinator. NZ J. Bot. 29, 83–86 [Google Scholar]
- 46.Ecroyd C. 1996. The ecology of Dactylanthus taylorii and threats to its survival. NZ J. Zool. 20, 81–100 [Google Scholar]
- 47.Arkins A. M., Winnington A. P., Anderson S., Clout M. N. 1999. Diet and nectarivorous foraging behaviour of the short-tailed bat (Mystacina tuberculata). J. Zool. 247, 183–187 [Google Scholar]
- 48.Peterson P. G., Robertson A. W., Lloyd B., McQueen S. 2006. Non-native pollen found in short-tailed bat (Mystacina tuberculata) guano from the central North Island. New Zeal. J. Ecol. 30, 267–272 [Google Scholar]
- 49.Holdaway R. N. 1989. New Zealand's pre-human avifauna and its vulnerability. NZ J. Ecol. 12, 11–25 [Google Scholar]
- 50.Towns D. R., Atkinson I. A. E., Daugherty C. H. 2006. Have the harmful effects of introduced rats on islands been exaggerated? Biol. Invasions 8, 863–891 10.1007/s10530-005-0421-z (doi:10.1007/s10530-005-0421-z) [DOI] [Google Scholar]