Abstract
Successful invasive species often are established for a long time period before increasing exponentially in abundance. This lag phase is one of the least understood phenomena of biological invasions. Plant invasions depend on three factors: a seed source, suitable habitat and a seed disperser. The non-native walnut, Juglans regia, has been planted for centuries in Central Europe but, until recently, has not spread beyond planted areas. However, in the past 20 years, we have observed a rapid increase in walnut abundance, specifically in abandoned agricultural fields. The dominant walnut disperser is the rook, Corvus frugilegus. During the past 50 years, rooks have increased in abundance and now commonly inhabit human settlements, where walnut trees are planted. Central Europe has, in the past few decades, experienced large-scale land abandonment. Walnut seeds dispersed into ploughed fields do not survive, but when cached into ploughed and then abandoned fields, they successfully establish. Rooks preferentially cache seeds in ploughed fields. Thus, land-use change combined with disperser changes can cause rapid increase of a non-native species, allowing it to become invasive. This may have cascading effects on the entire ecosystem. Thus, species that are non-native and not invasive can become invasive as habitats and dispersers change.
Keywords: agriculture, behaviour, caching, lag-phase, land management, policy
1. Introduction
Invasive alien species can change the entire ecosystem and can cause declines in biodiversity [1,2]; therefore, biological invasions are a major research topic in ecology and conservation biology [3–5]. Factors such as fast growth, allelopathy and shading can provide competitive advantages for non-native species that allow them to successfully colonize new habitats [6–9]. However, for many invasions, a lag-phase is observed [4,10], in which alien species are present at a low population size before a demographic explosion in abundance occurs [4,10,11]. This lag phase can be explained by changes in biotic (e.g. availability of pollinators or dispersers), abiotic (e.g. climate, habitat fragmentation) and/or human-related factors (e.g. land-use changes), but documented examples are rare [10,11].
One such alien, long-established, but non-invasive plant species in Europe is the Persian walnut (Juglans regia, hereafter walnut; see electronic supplementary material, S1). Walnut trees produce heavy, fat seeds and are valued for their wood. Extensively cultivated in residential areas, walnut trees were first introduced to monasteries in Europe in the Middle Ages [12]. However, recently, we have observed a rapid increase in their abundance in semi-natural habitats, which raises the question of what has changed in the ecology of this species that has caused this increase.
Invasive alien species often have small, wind-dispersed seeds, but for many species an effective animal disperser is crucial for successful spread [13]. Alien plant dispersal by animals can be either by a generalized disperser, such as canids and bears [14,15] or by a more specialized, mutualistic disperser, such as ants [16] or some birds consuming fleshy fruits [13,17]. Plants that produce large seeds are rarely invasive [13,18]. However, large seeds can be an attractive food source, and dispersers that transport and cache seeds may strongly facilitate an invasion [19–21]. Large tree seeds like walnuts are frequently eaten and cached by corvids [20,22], such as the rook Corvus frugilegus [21,23,24]. Rooks traditionally avoided humans and restricted their foraging to open areas. However, in recent decades, because of restricted hunting, rooks have changed their behaviour, do not avoid humans, and commonly occur in residential areas [25,26]. This change in behaviour has led rooks to frequently harvest and cache walnut seeds.
Agricultural land abandonment induces huge ecosystem changes in soils, biodiversity and ecosystem services [27,28], but detailed records of these changes are rare. Recent studies show that land abandonment can induce both positive and negative changes in native fauna and flora [29,30]. However, land-use change, and especially land abandonment, can also lead to drastic disturbance changes [28] and thereby provide new opportunities for alien species to become invasive. In many countries, land abandonment is often related to major political changes. For example, in Central Europe, the collapse of socialism in the 1990s led to dramatic changes in agricultural landscapes [31–33]. However, not much is known about the biodiversity or ecosystem consequences of this change [34,35]. In Poland, abandoned agricultural fields currently amount to 2 million ha (12% of all agricultural land) [32].
In this study, we examined the different aspects of the walnut invasion in Poland. Specifically, we examined: (i) where the walnut seed sources are located, (ii) who disperses the seeds, (iii) into which habitats seeds are dispersed, (iv) which habitats are invaded; (v) then we examined how land-use change and disperser changes have induced the recent rapid invasion of walnut in Central Europe.
2. Material and methods
(a). Walnut distribution at the landscape and regional scales
We randomly selected ten 1.5 × 1.5 km landscape plots in Southern Poland located in agricultural landscapes. Within these plots, we mapped the areas of abandoned fields (former ploughed fields, n = 479 in total), ploughed fields, grasslands, woodlands and human settlements. The mean (±s.e.) area covered by abandoned fields in the 10 landscape plots was 12.5 ± 2.2%; ploughed fields covered 60.2 ± 4.3%, grasslands 10.5 ± 2.7%, woodlands 7.2 ± 1.5% and human settlements 9.2 ± 1.4%. We counted all walnut trees growing within these landscape plots in the summer and autumn of 2007 (three plots) or 2008 (seven plots). We noted the habitat where wild walnuts grew, the diameter and height of the trees, and the number of seed-bearing trees.
The age of wild walnut trees was calculated on the basis of their diameters measured at 30 cm above ground level, or at ground level in the case of saplings less than 3 years old [36] (for details see electronic supplementary material, S1). Because determining the exact time of field abandonment was very difficult, we have abandonment times for only 215 out of 479 fields.
To estimate the factors affecting the presence and abundance of walnut trees at the regional scale, we randomly selected 130 plots (1 × 1 km each) spread throughout agricultural landscapes in Poland (figure 1). We counted walnut trees and corvids in these plots between June and September annually from 2007 to 2010 (electronic supplementary material, S1).
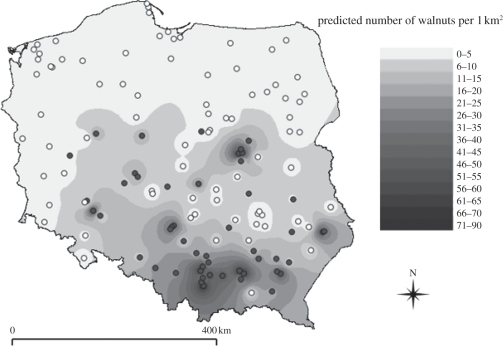
Figure 1.
The absence/presence in 1 × 1 km plots and interpolated density of wild walnut trees and seedlings in agricultural landscapes of Poland. Dark circles indicate plots where at least one wild walnut was found, white circles are plots where walnuts were absent. The interpolation was performed with an inverse distance method in Quantum GIS software.
(b). Corvid–walnut interactions
We examined corvid abundance and behaviour in three plots randomly selected from the 10 used for the walnut tree surveys. Counts were made from the middle of August to the end of December 2008 at roughly 7-day intervals. Each visit lasted 2 h. We noted the number of birds and their foraging habitat as well as all cases of caching walnut seeds and the habitat where the seeds were cached. We also determined how many walnut seeds were available for dispersers during the season, distances covered by corvids carrying walnuts and the number of cached seeds (see electronic supplementary material, S1). Moreover, we estimated the exact rate of seed caching, excavating and crushing in selected ploughed fields in the three landscape plots (electronic supplementary material, S1). Because rooks are the dominant disperser of walnut seeds, we only describe the rook behaviour.
(c). Analysis
We checked for spatial autocorrelation in the data by means of Moran's I statistics [37]. If significant autocorrelation was found (the presence and abundance of walnut trees at a regional scale—see below), we used spatial statistics: autologistic or simultaneous autoregressive models [38]. Otherwise, traditional general linear mixed models were used. In regression models, data were standardized before calculations to allow direct comparison of beta values. For more details on statistical methods, see electronic supplementary material, S1.
Estimates of statistical parameters are quoted with standard errors (s.e.) throughout the text. All analyses were performed in SAM v. 4.0 [39] and SAS v. 9.1.
3. Results
(a). Walnut seed source
We found a total of 725 mature (older than 30 years) walnut trees in farmyards and gardens within human settlements in each of the 10 study landscape plots. We did not find any mature walnut trees in other habitats within the landscape plots. Thus, planted trees are the current seed source.
(b). Rook foraging behaviour
Among all corvids observed during weekly surveys (n = 3927 individuals), 88% were rooks, 10% jackdaws (Corvus monedula) and 2% carrion crows (Corvus corone cornix). We observed 325 caching events by rooks and 23 by carrion crows, but none by jackdaws. The first two species carried walnut seeds collected on or below trees from villages to agricultural habitats.
At the regional scale, we found a positive correlation (corrected for spatial autocorrelation) between the number of foraging rooks and the number of walnut trees in plots (r = 0.604, p < 0.001, n = 130 plots; figure S1 in electronic supplementary material, 2). This relationship was also significant (r = 0.567, p < 0.001, n = 130) after excluding the effects of the proximity of towns.
The abundance changes of rooks in three selected landscape plots significantly correlated with the availability of walnut seeds (figure S2 in electronic supplementary material, 2). The number of birds crushing seeds followed the dynamics of birds caching seeds with about a two-week lag (correlation coefficient between number of birds caching and number crushing seeds two weeks later: r2 = 0.650, p = 0.005, n = 17; correlation coefficient between the number of birds caching and the number crushing seeds at the same time: r2 = 0.352, p = 0.193, n = 19; figure S2 in electronic supplementary material, 2). Over the entire season, we counted 325 rooks caching seeds and 129 rooks crushing seeds in fields, which indicated that 2.5 times more seeds were being hidden than eaten during the study period.
In total, from the beginning of September until the end of November 2008, we observed 968 incidences of rooks caching walnut seeds and 348 incidences of these birds excavating and crushing nuts (ratio of seeds dug up and crushed to cache was 0.359) in selected ploughed fields. Rooks cached 3.78 ± 0.37 walnut seeds h−1 ha−1 (n = 378 1 h observation samples), and during the same observation periods crushed 1.25 ± 0.14 seeds h−1 ha−1 (n = 378). The rate of seed excavating and crushing was highest at the end of October and beginning of November and was not influenced by the time of day (figure S3 in electronic supplementary material, 2).
(c). Rook walnut seed dispersal
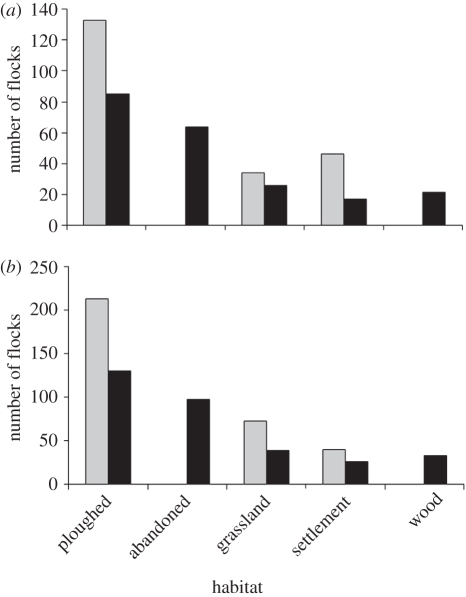
Rooks preferred ploughed fields and grasslands when foraging on invertebrates and weeds (figure 2; electronic supplementary material, 3). Rooks hid walnut seeds in ploughed fields but never cached seeds in abandoned fields (figure 2). We also observed jays (Garrulus glandarius) and red squirrels (Sciurus vulgaris) carrying seeds but these species always cached them in forests; because we observed only few seedlings in forest habitats we do not consider them further here. Rooks carried walnuts from villages to ploughed fields for distances that were usually below 500 m, although sometimes up to or over 1 km (figure S4 in electronic supplementary material, 2).
Figure 2.
Rook preferences for (a) foraging habitats (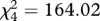 , p < 0.001, n = 213 flocks) and (b) habitats for seed caching (
, p < 0.001, n = 213 flocks) and (b) habitats for seed caching (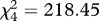 , p < 0.001, n = 325 rooks). Grey bars indicate observed values; black bars indicate expected values calculated on the assumption that there were no preferences (number of flocks and birds should be proportional to the share of the habitats in the landscape). Observed values are pooled numbers of flocks/birds noted in three 1.5 × 1.5 km landscape plots where detailed behavioural observations were made (ploughed, managed ploughed fields; abandoned, abandoned fields (former ploughed fields); grassland, grasslands (meadows, pastures); settlement, human settlements; wood, glades in woods).
, p < 0.001, n = 325 rooks). Grey bars indicate observed values; black bars indicate expected values calculated on the assumption that there were no preferences (number of flocks and birds should be proportional to the share of the habitats in the landscape). Observed values are pooled numbers of flocks/birds noted in three 1.5 × 1.5 km landscape plots where detailed behavioural observations were made (ploughed, managed ploughed fields; abandoned, abandoned fields (former ploughed fields); grassland, grasslands (meadows, pastures); settlement, human settlements; wood, glades in woods).
In 2008, six ploughed fields were abandoned in the study area. Seventy-nine foraging rooks, including those caching walnuts (n = 13), were observed in these fields in 2007 but not in 2008, 2009 or 2010. In the autumn of 2008, we found 42, 15, 20, 5, 11 and 20 walnut seedlings, respectively, in those fields. One field was ploughed again in 2009 (the one with 20 walnut seedlings). The numbers of seedlings on the remaining fields 2 years after abandonment were 50, 13, 29, 19, 14, respectively, while in 2010 there were 51, 10, 31, 24, 13 (table S1 in electronic supplementary material, 2).
We found in 2008 that 21 of the 30 examined ploughed fields contained walnut first-year seedlings, with a mean density of 19.6 ± 2.8 seedlings ha−1 (electronic supplementary material, S3). In 2009, in another set of 30 ploughed fields, walnut seedlings were found in 14 fields, with a mean density of 15.4 ± 3.8 seedlings ha−1. All these seedlings were short enough not to be cut by the combine grain harvester. In both years, all seedlings were later destroyed during ploughing.
(d). Walnut-invaded habitat
In the 10 surveyed 1.5 × 1.5 km landscape plots, we found a total of 12 529 walnut seedlings, of which 96 per cent grew in abandoned fields and 4 per cent in ploughed field margins (e.g. roadsides, drainage ditches; electronic supplementary material, S3). Among all walnut seedlings, 10 per cent were producing seeds (electronic supplementary material, S3). We did not find any walnut seedlings in grasslands. Walnut occupied 48.4 ± 5.1% of the abandoned fields in the 10 landscape plots. Where walnuts occurred, the average density within abandoned fields was 125.2 ± 9.0 seedlings ha−1, or with abandoned fields comprising 12.5 per cent the mosaic of habitats within this agricultural landscape, 557 individuals per 1 km2.
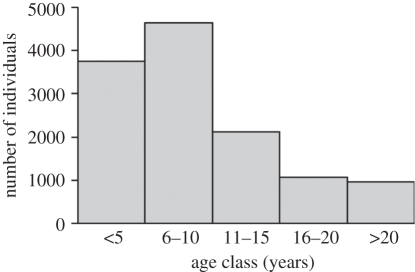
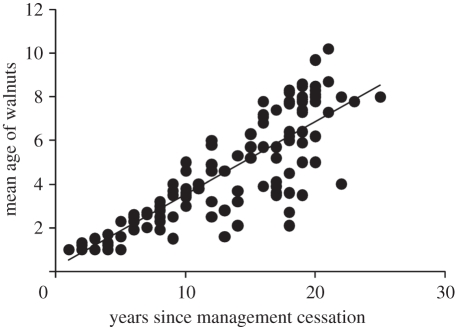
The age distribution of the walnut seedlings was right-skewed, with the oldest individuals slightly over 20 years old (figure 3 and electronic supplementary material, 3). We found a positive relationship between years since management cessation and the mean age of walnuts (GLMM: F1,5.9 = 107.21, p < 0.001, r2 = 0.724; figure 4).
Figure 3.
Age distribution of wild walnuts growing in abandoned fields in Poland. Data comes from 10 1.5 × 1.5 km landscape plots in Southern Poland.
Figure 4.
Relationship between years since field abandonment and mean age of walnuts. Dots represent mean age of walnuts calculated per abandoned field.
The occurrence and density of walnut seedlings in the abandoned fields in 10 landscape plots were positively correlated with distance to the nearest human settlement (tables S2, S3 and figures S5, S6 in electronic supplementary material, 2) and significantly increased with years since cessation of field management (electronic supplementary material, S2).
We found walnut seedlings in 50 out of 130 examined 1 × 1 km plots. At the regional scale, the probability of walnut seedling occurrence increased with the cover of abandoned fields within the plot and the proximity of towns (table S4 and figure S7 in electronic supplementary material, 2). The probability of walnut seedling occurrence was negatively related to geographical latitude (β = −1.001 ± 0.241, r2 = 0.25, p < 0.001); i.e. in Southern Poland, walnuts occurred more frequently (figure 1). We did not find a significant effect of altitude on walnut seedling occurrence (β = 0.133 ± 0.116, p = 0.254). We also found a statistically significant positive spatial autocorrelation at distances of up to 100 and between 200 and 300 km and a negative spatial autocorrelation at distances above 300 km (figure S8 in electronic supplementary material, 2).
The number of walnut seedlings in the plots where this species was found varied from 1 to 86 individuals (mean 23.8 ± 3.8) and was positively dependent on the cover of abandoned fields in the plots and distance to the nearest town (table S5 and figure S9 in electronic supplementary material, 2). A statistically significant negative spatial autocorrelation was also found for the number of individuals at a distance of 200 km (figure S8 in electronic supplementary material, 2).
4. Discussion
Many more species are introduced than actually spread and become successful invaders. This is especially the case for plant species where, because of agricultural or ornamental values, numerous species have been introduced that have not spread beyond planted individuals. However, ultimately some species do spread, often following an extended period after first planting. This observed lag-phase in abundance leads to the question of why and what changes after introduction induce a species to become invasive. To answer this question, we need to consider that invasion is a multi-step process that depends on three key factors: seed availability, availability of suitable habitat and dispersal from seed source to the suitable habitat.
The walnut tree in Central Europe provides an example of a lag-phase in a plant invasion. Monks in the middle Ages introduced walnut trees to this area because of the trees' edible seeds, wood, and medical properties [12]. Walnut trees are widely planted in Poland in gardens within villages and towns, providing a widespread seed source, but walnuts have not spread and become invasive until the past two decades. This recent spread can be linked to changes in land use that provide a suitable habitat and changes in the behaviour of the dominant disperser. We found that mature walnut trees occurred only within human settlements. However, we found walnut seedlings in many regions of Poland, indicating that the dispersal of this species from gardens to semi-natural habitats has occurred across the entire country. This recent increase in growth of walnut trees can be linked with the abundance and behaviour of the rook, the dominant disperser. Since the cessation of hunting 50 years ago, rooks have increased in abundance and changed their behaviour by increasingly using human settlements [25,26]. In human settlements, rooks are now collecting and caching walnut seeds, and thereby have become an efficient seed-disperser for walnuts. Rooks cached seeds only in ploughed fields, and all walnut saplings that we found are in recently abandoned ploughed fields. Widespread agricultural land abandonment started in Poland after the political changes of 1989, and the walnut age distribution in abandoned fields correlates with the time of agricultural abandonment. Thus, all three key factors—seed source, disperser and habitat—fit together to allow the walnut to become a successful invasive species.
The current main disperser of walnut seeds is the rook and, to a lesser extent, the carrion crow—both native birds in Europe [25]. According to historical ornithological data, rooks were much less numerous in Europe at the beginning of the twentieth century and, more importantly, rookeries and roosts occurred mostly in small forests in agricultural areas [26]. Thus, chances for rooks to interact with planted walnut trees were very rare. A substantial change in the rook population started about 50 years ago when the species acquired legal protection in many countries [26,40]. The population of rooks grew rapidly and birds started to use human settlements where easily available human-related food resources could be found [25]. There also was a well-documented shift from breeding colonies located in small forests in agricultural areas to colonies in parks within towns and villages [26,40]. Our study also demonstrates that the phenology of walnut seed maturation and the abundance of rooks coincide with each other. Most rooks remain in the urban environment after the breeding season, often roost in large flocks and forage in surrounding farmlands, with an increase in abundance during September and October caused by the appearance of migrating birds in this period [26]. This explains the positive correlation between human settlements and the occurrence and abundance of walnut trees at the landscape and regional scales in our study. The synanthropization and following synurbanization of the rook inevitably has led to the increased number of interactions with planted walnut trees. The other corvid caching walnut seed, the carrion crow, has also been colonizing cities and villages, but its population size is still much smaller than the rook [25].
During foraging and caching of walnut seeds, rooks preferentially choose ploughed fields and avoid older abandoned fields. In comparison with ploughed fields, abandoned fields are usually covered by tall grasses, dense herbaceous plants and shrubs (electronic supplementary material, S3; [28]). Insects, spiders and snails are unable to hide on bare ground. Birds can find large amounts of easy-to-catch and injured invertebrates on freshly ploughed fields [41]. Short or absent vegetation also guarantee good visibility and early detection of predators or competitors [42]. Rooks are not an exception; many birds, including shrikes (Lanidae), birds of prey and gulls (Laridae) prefer to forage among short vegetation on ploughed fields and grasslands [43,44]. The habitat where rooks cached walnut seeds in our study area corresponds to results of earlier studies indicating that corvids preferred ploughed fields in agricultural landscapes [20,22]. Caching food is common among different groups of animals, especially rodents and corvids [23,45,46]. Their brains are developed well enough to provide good cognitive skills and spatial memory [21,47,48]. Their memory is not perfect, however, and they forget many stockpiles [21,45].
Rooks flew long distances, often more than 500 m, with walnut seeds providing a long-distance dispersal vector for the walnut that facilitates a rapid spread in agricultural landscapes. Rooks' stockpiling habits may help explain these longer flights. These birds usually keep an eye on other individuals known to covet and rob others' stockpiles [49]. Therefore, with seeds predestined to be hidden, it may be profitable for a bird to fly alone and further, to hide the seeds in a safer, secluded place where there are no other corvids present (but see [20]). We observed many walnut seeds being buried in ploughed fields over short time periods. This indicates that the seed bank of this plant in ploughed fields is probably large. Seeds hidden by corvids in soil are buried; birds act in the same way as walnut growers who bury them in a few centimetres of soil. Seed burial is also a critical step in the growth process because it reduces the chances of seed predation by other animals [50], and enhances seed stratification.
There are several other studies that have also found that native dispersers and land abandonment can facilitate plant invasions. For instance, buckthorn Rhamnus cathartica is rapidly dispersed by frugivorous birds following agricultural abandonment in the USA [51]. The importance of dispersers and seed sources was also pointed out by Gosper et al. [13], who showed that native birds caused the invasion of many fleshy fruited plants in Australia. These studies show that land-use changes may lead to the creation of suitable habitat for invasive species and that co-occurrence of appropriate native dispersers can enable a spread.
We found that 10 per cent of all wild walnut seedlings in the abandoned fields already bear seeds. This seed source's location, far from human settlements, may lead to further expansion as these seeds are now available to other dispersers like red squirrels and jays that do not forage in human settlements. In addition, as these dispersers do not preferentially cache seeds in ploughed fields, they may move seeds to other landscape elements, leading to the spread of walnuts beyond the recently abandoned ploughed fields.
Walnut trees' dominance may also lead to cascading, large ecosystem and diversity changes, because walnut trees release juglone, an allelopathic chemical, which can have strong impacts on the growth of other plants ([4,5], M. Lenda, P. Skórka, D. Moroń & M. Woyciechowski 2007–2011, unpublished data). This allelopathic chemical can have a widespread impact; the North American-native black walnut Juglans nigra, for instance, has a toxic zone that extends up to 25 m from the trunk [52].
The walnut invasion that we observed is not restricted to Poland. Casual observations in the Czech Republic, Slovakia, Hungary and Ukraine also revealed that wild walnuts grow in many agricultural areas (M. Lenda, P. Skórka, D. Moroń & M. Woyciechowski 2007–2011, unpublished data), suggesting a spread in other Central and Eastern European countries. Interestingly, floristic monographs on walnut either do not mention that it may spread in large numbers to natural or semi-natural habitats or that it was believed that wild walnuts were rarely found [53,54].
5. Conclusion
Plant invasions are a multistep process requiring a seed source, seed dispersal and availability of suitable habitat that can be colonized. Here, we show that changes in the native rook's population size and caching behaviour combined with vast land abandonment led to the rapid invasions of the long-established, but not invasive, walnut. If habitats and dispersers change owing to changes in climate or human socioeconomics, other previously non-invasive alien species may become invasive. Poland has a native flora of 2500 species and 460 introduced alien plant species [55,56], and even more exotic species are established in other countries [57]. We cannot predict which species will become invasive in the future. However, the large number of established non-native species makes it certain that other previously non-invasive species will become invasive. Thus, as habitat destruction can lead to an extinction debt [58], introduced alien species can provide an invasion debt—a future ecological cost when habitats or dispersers change.
Acknowledgements
We would like to thank all field assistants for their help with this study. We are grateful to Lars Chittka and two anonymous referees for their critical comments. We thank Professor Tim H. Sparks and Tamara Kaup for valuable comments and linguistic corrections. This study was financed by project NN 304074635 from the Polish Ministry of Science and Higher Education and the Jagiellonian University, DS/BiNoZ/INoŚ/761/10-11.
Footnotes
Electronic supplementary material is available at http://dx.doi.org/10.1098/rspb.2011.2153 or via http://rspb.royalsocietypublishing.org.
References
- 1.Pyšek P., Prach K., Mandák B. 1998. Invasions of alien plants into habitats of Central European landscape: an historical pattern. In Plant invasions: ecological mechanisms and human responses (eds Starfinger U., Edwards K., Kowarik I., Williamson M.), pp. 23–32 Leiden, The Netherlands: Backhuys Publishers [Google Scholar]
- 2.Drake J. A. (ed.) 2009. Handbook of alien species in Europe. Invading Nature Springer Series in Invasion Ecology, vol. 3 Knoxville, TN: University of Tennessee [Google Scholar]
- 3.Mack R. N. 1996. Predicting the identity and fate of plant invaders: emergent and emerging approaches. Biol. Conserv. 78, 107–121 (doi:10.1016/0006-3207(96)00021-3) [Google Scholar]
- 4.Mack R. N. 2003. Plant naturalizations and invasions in the eastern United States: 1634–1860. Ann. Missouri Bot. Garden 90, 77–90 (doi:10.2307/3298528) [Google Scholar]
- 5.Pyšek P., Hulme P. E. 2005. Spatio-temporal dynamics of plant invasions: linking pattern to process. Ecoscience 12, 302–315 (doi:10.2980/i1195-6860-12-3-302.1) [Google Scholar]
- 6.Brown B. J., Mitchell R. J. 2001. Competition for pollination: effects of pollen of an invasive plant on seed set of a native congener. Oecologia 129, 43–49 (doi:10.1007/s004420100700) [DOI] [PubMed] [Google Scholar]
- 7.Chittka L., Schürkens S. 2001. Successful invasion of a floral market. Nature 411, 653 (doi:10.1038/35079676) [DOI] [PubMed] [Google Scholar]
- 8.Weston L. A., Duke S. O. 2003. Weed and crop allelopathy. Cr. Rev. Plant Sci. 22, 367–389 (doi:10.1002/ps.1320) [Google Scholar]
- 9.Totland Ø., Nielsen A., Bjerknes A. L., Ohlson M. 2006. Effects of an exotic plant and habitat disturbance on pollinator visitation and reproduction in a boreal forest herb. Am. J. Bot. 93, 868–873 (doi:10.3732/ajb.93.6.868) [DOI] [PubMed] [Google Scholar]
- 10.Aikio S., Duncan R. P., Hulme P. E. 2010. Lag-phases in alien plant invasions: separating the facts from the artefacts. Oikos 119, 370–378 (doi:10.1111/j.1600-0706.2009.17963.x) [Google Scholar]
- 11.Binggeli P. 2000. Time-lags between introduction, establishment and rapid spread of introduced environmental weeds. Proc. Int. Weed Sci. Congr. 3, 2–14 [Google Scholar]
- 12.Zdyb H. 2010. Walnut. Warszawa, Poland: PWRiL [Google Scholar]
- 13.Gosper C. R., Stansbury C. D., Vivian-Smith G. 2005. Seed dispersal of fleshy-fruited invasive plants by birds: contributing factors and management options. Divers. Distrib. 11, 549–558 (doi:10.1111/j.1366-9516.2005.00195.x) [Google Scholar]
- 14.Stiles E. W. 1989. Fruits, seeds and dispersal agents. In Plant–animal interactions (ed. Abrahamson W. G.), pp. 87–122 New York, NY: McGraw-Hill [Google Scholar]
- 15.Richardson D. M., Allsopp N., D'Antonio C. M., Milton S. J., Rejmánek M. 2000. Plant invasions: the role of mutualisms. Biol. Rev. 75, 65–93 (doi:10.1111/j.1469-185X.1999.tb00041.x) [DOI] [PubMed] [Google Scholar]
- 16.Pemberton R. W. 1988. Myrmecochory in the introduced range weed, leafy spurge (Euphorbia esula L.). Am. Midl. Nat. 119, 431–435 (doi:10.2307/2425826) [Google Scholar]
- 17.Bartuszevige A. M., Gorchov D. L. 2006. Avian seed dispersal of an invasive shrub. Biol. Invasions 8, 1013–1022 (doi:10.1007/s11252-011-0193-4) [Google Scholar]
- 18.Gosper C. R., Vivian-Smith G. 2010. Fruit traits of vertebrate-dispersed alien plants: smaller seeds and more pulp sugar than indigenous species. Biol. Invasions 12, 2153–2163 (doi:10.1007/s10530-009-9617-y) [Google Scholar]
- 19.Emery N. J., Clayton N. S. 2001. Effects of experience and social context on prospective caching strategies by scrub jays. Nature 414, 443–446 (doi:10.1038/35106560) [DOI] [PubMed] [Google Scholar]
- 20.Cristol D. A. 2005. Walnut caching behavior of American Crows. J. Field. Ornithol. 76, 27–32 (doi:10.1648/0273-8570(2005)076[0027:WBOAC]2.0.CO;2) [Google Scholar]
- 21.Clayton N. S., Dally J. M., Emery N. J. 2007. Social cognition by food-caching corvids. The western scrub-jay as a natural psychologist. Phil. Trans. R. Soc. B 362, 507–522 (doi:10.1098/rstb.2006.1992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Källander H. 1978. Hoarding in the rook. Corvus frugilegus. Anser 3(suppl.), 124–128 [Google Scholar]
- 23.Kort S. R., Clayton N. S. 2006. An evolutionary perspective on caching by corvids. Proc. R. Soc. B 273, 417–423 (doi:10.1098/rspb.2005.3350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Czarnecka J., Kitowski I. 2010. Seed dispersal by the Rook (Corvus frugilegus L.) in agricultural landscape. Pol. J. Ecol. 58, 511–523 [Google Scholar]
- 25.Tomiałojć L., Stawarczyk T. 2003. Avifauna of Poland. Distribution, numbers and trends. Wrocław: PTPP ‘pro Natura’ [Google Scholar]
- 26.Hordowski J. 2009. The rook in the Carpathian Foothills: species monograph and economic importance. Poland: Bolestraszyce (Przemyśl) [Google Scholar]
- 27.Moreira F., Russo D. 2007. Modelling the impact of agricultural abandonment and wildfires on vertebrate diversity in Mediterranean Europe. Land. Ecol. 22, 1461–1476 (doi:10.1007/s10980-007-9125-3) [Google Scholar]
- 28.Moroń D., Lenda M., Skórka P., Szentgyorgyi H., Settele J., Woyciechowski M. 2009. Wild pollinator communities are negatively affected by invasion of alien goldenrods in grassland landscape. Biol. Conserv. 142, 1322–1332 (doi:10.1016/j.biocon.2008.12.036) [Google Scholar]
- 29.MacDonald D., Crabtree J. R., Weisinger G., Dax Y., Stamou N., Fleury P., Guttierez-Lazpita J., Gibon A. 2000. Agricultural abandonment in mountain areas of Europe: environmental consequences and policy response. J. Environ. Manage. 59, 47–69 (doi:10.1006/jema.1999.0335) [Google Scholar]
- 30.Fonderflick J., Lepart J., Caplat P., Debusshe M., Marty P. 2010. Biodiversity and landscape dynamics under different scenarios of agricultural changes: a case study in a Mediterranean upland. Biol. Conserv. 143, 737–746 (doi:10.1016/j.biocon.2009.12.014) [Google Scholar]
- 31.Stoate C., Báldi A., Beja P., Boatman N. D., Herzon I., van Doorn A., de Snoo G. R., Rakosy L., Ramwell C. 2009. Ecological impacts of early 21st century agricultural change in Europe. J. Environ. Manage. 91, 22–46 (doi:10.1016/j.jenvman.2009.07.005) [DOI] [PubMed] [Google Scholar]
- 32.Orłowski G. 2005. Endangered and declining bird species of abandoned farmland in south-western Poland. Agr. Ecosyst. Environ. 111, 231–236 (doi:10.1016/j.agee.2005.06.012) [Google Scholar]
- 33.Bański J. 2007. Geography of agriculture in Poland. Warszawa, Poland: Polskie Wydawnictwo Ekonomiczne [Google Scholar]
- 34.Cramer V. A., Hobbs R. J., Standish R. J. 2008. What's new about old fields? Land abandonment and ecosystem assembly. Trends Ecol. Evol. 23, 104–122 (doi:10.1016/j.tree.2007.10.005) [DOI] [PubMed] [Google Scholar]
- 35.Pullin A. S., et al. 2009. Conservation focus on Europe: major conservation policy issues that need to be informed by conservation science. Conserv. Biol. 23, 818–824 (doi:10.1111/j.1523-1739.2009.01283.x) [DOI] [PubMed] [Google Scholar]
- 36.Loacker K., Kofler W., Pagitz K., Oberhuber W. 2007. Spread of walnut (Juglans regia L.) in an Alpine valley is correlated with climate warming. Flora 202, 70–78 (doi:10.1016/j.flora.2006.03.003) [Google Scholar]
- 37.Legendre P., Legendre L. 1998. Numerical ecology: developments in environmental modeling 20. Amsterdam, The Netherlands: Elsevier Science B.V [Google Scholar]
- 38.Dormann C. F., et al. 2007. Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30, 609–628 (doi:10.1111/j.2007.0906-7590.05171.x) [Google Scholar]
- 39.Rangel T. F. L. V. B., Diniz-Filho J. A. F., Bini L. M. 2010. SAM: a comprehensive application for spatial analysis in macroecology. Ecography 33, 46–50 (doi:10.1111/j.1600-0587.2009.06299.x) [Google Scholar]
- 40.Little D. I., Little A. E., Levell D. 2001. A review of rook status, with new south Pembrokeshire data, 1986–1996. Field Studies 10, 37–56 [Google Scholar]
- 41.Morris L. P., Thompson F. R., III 1998. Effect of habitat and invertebrate density on abundance and foraging behavior of Brown-headed Cowbirds. Auk 115, 376–385 [Google Scholar]
- 42.Butler S. J., Bradbury R. B., Whittingham M. J. 2005. Stubble height affects the use of stubble fields by farmland birds. J. Appl. Ecol. 42, 469–476 (doi:10.1111/j.1365-2664.2005.01027.x) [Google Scholar]
- 43.Schwemmer P., Garthe S., Mundry R. 2008. Area utilization of gulls in a coastal farmland landscape: habitat mosaic supports niche segregation of opportunistic species. Land. Ecol. 23, 355–367 (doi:10.1007/s10980-008-9194-y) [Google Scholar]
- 44.Zub K., Pugacewicz E., Jędrzejewska B., Jędrzejewski W. 2010. Factors affecting habitat selection by breeding lesser spotted eagles Aquila pomarina in northeastern Poland. Acta Ornithol. 45, 105–114 (doi:10.3161/000164510X516155) [Google Scholar]
- 45.Vander Wall S. B. 2001. The evolutionary ecology of nut dispersal. Bot. Rev. 67, 74–117 (doi:10.1007/BF02857850) [Google Scholar]
- 46.Cristol D. A., Switzer P. V. 1999. Avian prey-dropping behavior. II. American crows and Walnuts. Behav. Ecol. 10, 220–226 (doi:10.1093/beheco/10.3.220) [Google Scholar]
- 47.Kamil A. C., Balda R. P. 1990. Spatial memory in seed-caching Corvids. Psych. Learn. Motiv. 26, 1–25 (doi:10.1016/S0079-7421(08)60050-X) [Google Scholar]
- 48.Gibson B. M., Kamil A. C. 2005. The fine-grained spatial abilities of three seed-caching corvids. Learn. Behav. 33, 59–66 (doi:10.1007/BF02857850) [DOI] [PubMed] [Google Scholar]
- 49.Baglione V., Canestrari D. 2009. Kleptoparasitism and temporal segregation of sympatric corvids foraging in a refuse dump. Auk 126, 566–578 (doi:10.1525/auk.2009.08146) [Google Scholar]
- 50.Barnett R. J. 1977. The effect of burial by squirrels on germination and survival of oak and hickory nuts. Am. Midland Nat. 98, 319–330 (doi:10.2307/2424983) [Google Scholar]
- 51.McCay T. S., McCay D. H. 2009. Processes regulating the invasion of European buckthorn (Rhamnus cathartica) in three habitats of the northeastern United States. Biol. Invasions 11, 1835–1844 (doi:10.1007/s10530-008-9362-7) [Google Scholar]
- 52.Ponder F., Tadros S. H. 1985. Juglone concentration in soil beneath black walnut interplanted with nitrogen-fixing species. J. Chem. Ecol. 11, 937–942 (doi:10.1007/BF01012079) [DOI] [PubMed] [Google Scholar]
- 53.Fiek E. 1881. Flora von Schlesien, preussischen und österreichischen Anteils. Breslau, Germany: Verl. J. U. Kern [Google Scholar]
- 54.Zając A., Zając M., Tokarska-Guzik B. 1998. Kenophtyes in the flora of Poland: list, status and origin. Phytocoenosis 9, 107–116 [Google Scholar]
- 55.Zając A., Zając M. (eds) 2001. Distribution atlas of vascular plants in Poland, pp. 714 Kraków, Poland: Laboratory of Computer Chorology. Institute of Botany, Jagiellonian University [Google Scholar]
- 56.Tokarska-Guzik B. 2005. The establishment and spread of alien plant species (kenophytes) in the flora of Poland. Prace Naukowe Uniwersytetu Sląskiego w Katowicach 2372, 1–192 [Google Scholar]
- 57.Chytrý M., Pyšek P., Wild J., Pino J., Maskell L. C., Vila M. 2009. European map of alien plant invasions based on the quantitative assessment across habitats. Divers. Distrib. 15, 98–107 (doi:10.1111/j.1472-4642.2008.00515.x) [Google Scholar]
- 58.Tilman D., May R. M., Lehman C. L., Nowak M. A. 1994. Habitat destruction and the extinction debt. Nature 371, 65–66 (doi:10.1038/371065a0) [Google Scholar]