Abstract
Competition between reef-building corals and benthic algae is of key importance for reef dynamics. These interactions occur on many spatial scales, ranging from chemical to regional. Using microprobes, 16S rDNA pyrosequencing and underwater surveys, we examined the interactions between the reef-building coral Montastraea annularis and four types of benthic algae. The macroalgae Dictyota bartayresiana and Halimeda opuntia, as well as a mixed consortium of turf algae, caused hypoxia on the adjacent coral tissue. Turf algae were also associated with major shifts in the bacterial communities at the interaction zones, including more pathogens and virulence genes. In contrast to turf algae, interactions with crustose coralline algae (CCA) and M. annularis did not appear to be antagonistic at any scale. These zones were not hypoxic, the microbes were not pathogen-like and the abundance of coral–CCA interactions was positively correlated with per cent coral cover. We propose a model in which fleshy algae (i.e. some species of turf and fleshy macroalgae) alter benthic competition dynamics by stimulating bacterial respiration and promoting invasion of virulent bacteria on corals. This gives fleshy algae a competitive advantage over corals when human activities, such as overfishing and eutrophication, remove controls on algal abundance. Together, these results demonstrate the intricate connections and mechanisms that structure coral reefs.
Keywords: turf algae, crustose coralline algae, bacteria, competition, ecology
1. Introduction
Coral reefs, the most biologically diverse marine ecosystems, are supported by the structural complexity provided by hermatypic corals [1]. However, coral reefs around the world are becoming increasingly dominated by benthic algae, resulting in a loss of habitat and biodiversity [2–5]. This trend is driven by algal overgrowth of live and recently dead corals, a trend facilitated by modern environmental changes such as decreased herbivory [6–9], eutrophication [8,10], increased coral bleaching associated with climate change [11–13] and coral disease [14–16]. While this phenomenon has been well documented, the mechanisms by which algae overtake corals are not well understood. Competition between corals and benthic algae is common on coral reefs worldwide [17–20], and these interactions are frequently harmful to the coral, causing tissue damage and necrosis [3,20–23], reduced zooxanthellar function [23–26] and reduced coral fecundity [3,20,27]. On the other hand, some algae have little effect on corals [28,29], such as certain species of CCA that promote coral settlement [30,31] and inhibit recruitment of macroalgae that would otherwise compete with corals [32,33].
Benthic algae also influence the microbes associated with corals, disrupting the complex community of the healthy coral holobiont—the symbiotic consortium including the coral animal, zooxanthellae, Bacteria, Archaea, fungi and viruses [34]. For example, algae can transmit pathogens to adjacent corals, causing disease [35]. Allelochemicals released by the algae may also stress the corals and disrupt the holobiont, causing loss of normal functions and coral mortality [36,37]. Several lines of evidence suggest that one mechanism for the deleterious effects of algae on corals is the release of organic carbon by the algae that fuels increased local activity of microbes. Coral–algal interactions in aquaria, for example, result in coral necrosis and hypoxia as a result of bacterial activity [38], and hypoxia has been observed in situ when turf and macroalgae border coral [17]. Consistent with this hypothesis are findings that experimental addition of dissolved organic carbon (DOC) alters the coral holobiont by increasing potential pathogens [39], leads to coral mortality and disease symptoms [40,41] and is deadly to corals, whereas addition of inorganic nutrients is not [25,40,41].
We hypothesized that stressful coral–algal interactions compromise the normal function of the coral holobiont, allowing potentially pathogenic microbes to invade and the algae to overgrow the coral. In order to better understand these micro-scale dynamics and how they affect coral reef composition, we investigated the physiological and bacterial responses of the coral holobiont to interactions with different functional groups of benthic algae and quantified the prevalence of coral–algal interactions at reefs with different levels of human influence (electronic supplementary material, figure S1; [42]). In situ interactions between the dominant reef-building Caribbean coral Montastraea annularis and four types of benthic algae were studied: encrusting calcified red algae (CCA); fleshy brown macroalgae (Dictyota bartayresiana); upright calcareous green algae (Halimeda opuntia) and a mixed assemblage of turf algae (figure 1). Physiological changes across these four types of coral–algal interfaces were compared by measuring the dissolved oxygen (DO) levels at the interaction zones with and without algae removal. Algal-induced changes to the bacterial constituents of the holobiont were assessed by identifying the taxonomic composition of coral-associated bacteria across the same four types of interactions by pyrosequencing of the 16S rRNA gene. Our results demonstrate that each alga exerts its own characteristic suite of effects on the coral holobiont, and that these micro-scale dynamics have the potential to drive changes in reef community composition.
Figure 1.
Typical interaction zones between the coral Montastraea annularis and the four types of algae examined. (a) Intact interactions and (b) interactions after algal removal.
2. Material and methods
(a). Physiology of the coral holobiont at algal interaction zones
This study was conducted on the island of Curacao, former Netherlands Antilles, under the auspices of Caribbean Research and Management of Biodiversity (CARMABI). Interactions between the dominant reef-building coral M. annularis bordering one of four groups of algae: CCA, D. bartayresiana, H. opuntia and turf algae were studied (figure 1). Ten colonies of each interaction type (40 total) were identified on the reef (8–10 m deep, Water Factory; electronic supplementary material, figure S1). The algae were removed from five of the 10 coral–algal interactions of each type, taking care not to damage the adjacent coral tissue (figure 1). All colonies were removed from the reef 10–12 days later by breaking off columns below the live coral to avoid tissue damage. The concentration of DO was measured within 1 mm of the surface of each interaction using an oxygen microprobe (Unisense, Denmark) as previously described (electronic supplementary material; [17]). Four replicate readings were taken within each of the three zones of interaction: (i) coral tissue from the centre of the colony, (ii) coral tissue less than 0.5 cm from the algae and (iii) algal tissue.
(b). Microbial sampling
Tissue samples were collected from each of the four types of coral–algal interactions using a hollow punch (diameter = 0.64 cm) and hammer (8–10 m deep, Water Factory; electronic supplementary material, figure S1). Tissue punches were collected from five different zones: (i) coral tissue from the centre of the colony (less than 10 cm away from algae), (ii) coral tissue adjacent to the algae, (iii) the interaction zone, (iv) algal tissue adjacent to the coral and (v) algal tissue less than 10 cm away from the interface. Five different interactions of each type were sampled, for a total of five replicate tissue samples per zone per coral–algal interaction type. DNA was extracted from each sample and the bacterial 16S rRNA genes were amplified and pyrosequenced (see electronic supplementary material, table S2; [43]). Sequences were screened for quality, sorted by barcode, grouped into operational taxonomic unit (OTU, 97% similarity) and classified as previously described (electronic supplementary material; [43]). A resampling-based rank comparison was employed to identify the taxa that were over- or under-represented in the five libraries from across each type of interaction (electronic supplementary material).
(c). Metabolic reconstruction
The metabolic profiles of the bacterial communities present in coral tissue away from algal interactions and those over-represented in coral tissue near or at each algal interface were estimated. For each taxon, the closest relative with a sequenced genome was selected and the metabolic profile from that genome (determined by the SEED database; www.theseed.org) was included and weighted by the taxon's relative abundance. The metabolic profile for each community was then calculated as the linear combination of the metabolic profiles of each included taxon, weighted by its relative abundance, and XIPE was used to determine which metabolic subsystems were statistically different at the interfaces (90% confidence level, 5000 iterations; [44]). Statistical ranking was again performed to determine which metabolic subsystems were over-represented at the different coral–algal interaction zones relative to each other. The metabolic shifts observed at algal interfaces were then compared with those previously observed in corals subjected to abiotic stress [39] by principal component analysis (PCA).
(d). Surveys of coral–algal interactions
Survey sites spanned the leeward side of Curacao and included different levels of human impact (e.g. adjacent population and sewage signature [42]) that declined with increasing distance from the capital, Willemstad (electronic supplementary material, figure S1). Surveys to quantify the types and abundances of interactions between corals and algae were conducted at 10 m depth as previously described (electronic supplementary material; [17]). Per cent cover of benthic organisms was determined from photoquadrats at 10 m depth (electronic supplementary material).
3. Results
(a). Physiological changes of the coral holobiont owing to algal interactions
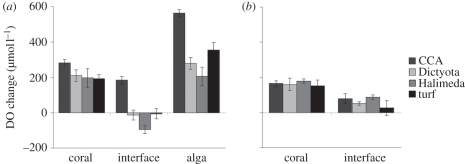
DO concentrations in the boundary layer above M. annularis tissue distant from the site of algal interaction were hyperoxic relative to ambient sea water (192–282 µmol l−1 above ambient; ambient = 212 µmol l−1; figure 2a). Likewise, the algal boundary layer was hyperoxic for all four types of algae examined, ranging from 418 to 775 µmol l−1 above ambient (figure 2a). However, when M. annularis was interacting with any of the four types of algae, the DO concentration at the interaction zone was decreased (paired t-test: p < 0.02 for each interaction type; figure 2a). These decreases resulted in DO levels below ambient for corals bordering H. opuntia, D. bartayresiana or turf algae (95, 12 and 5.2 µmol l−1 below ambient, respectively) while corals adjacent to CCA maintained hyperoxia (184 µmol l−1 above ambient). Algal removal resulted in significant DO increase for H. opuntia (70%; t-test, p = 0.003) and D. bartayresiana (52%; p = 0.03), restoring hyperoxia at these interfaces (figure 2b). Removal of turf algae restored hyperoxia but recovery was not statistically significant (36%, p = 0.21); coral–CCA interfaces remained hyperoxic after algal removal (figure 2b).
Figure 2.
Dissolved oxygen (DO) concentration changes at the zone of interaction between the coral Montastraea annularis and benthic algae. (a) Unaltered coral–algal interactions. (b) Coral–algal interactions 10 days after removal of the algae. DO concentrations are shown relative to atmospheric saturation of sea water (212 µmol l−1). CCA, crustose coralline algae; Dictyota, Dictyota bartayresiana; Halimeda, Halimeda opuntia. n = 5 for all treatments; ±s.e.m.
(b). Changes to coral-associated bacteria owing to algal interactions
The number of observed and predicted (Chao1) bacterial OTUs increased in coral tissue near all types of algae except H. opuntia relative to coral tissue distant from algae (electronic supplementary material, table S1). In addition, the Shannon–Weiner diversity (H′) of the coral-associated bacterial communities increased in tissues near CCA (from 3.26 to 4.72) and D. bartayresiana (from 2.84 to 3.28), but decreased for coral tissue adjacent to H. opuntia or turf algae (electronic supplementary material, table S1). Three of the four coral–algal interfaces showed high diversity (5.70–7.64) comparable with that observed for the corresponding algal tissues (6.22–7.82), the exception being the H. opuntia interface (4.58; electronic supplementary material, table S1). When the phylogenetic distance between the 16S rDNA libraries was analysed by PCA, the coral-associated bacteria distant from algae clustered together along with those from coral tissue adjacent to H. opuntia, while those adjacent to CCA, D. bartayresiana, and turf algae were distant from the coral-associated communities and also from each other (electronic supplementary material, figure S2). Some taxa were over-represented at or near the algal interfaces and the number varied depending on the type of algae involved: near CCA, 20 taxa or 38 per cent relative abundance; near D. bartayresiana, 19 taxa or 21 per cent relative abundance; near turf algae, 14 taxa or 13 per cent relative abundance; or near H. opuntia, 12 taxa or 11 per cent relative abundance (figure 3). A majority (30/45) of over-represented taxa were enriched at only one type of coral–algal interface. Of the remaining 15 taxa, 11 were over-represented at two interfaces, three at three interfaces (all members of the Planctomycetaceae) and one (Actinomycetales) at all four interfaces.
Figure 3.
Heat map of relative abundance of Bacteria associated with coral–algal interfaces. (a–d) Relative abundances across all five zones of interaction with one type of algae. Bacterial taxa are listed at the highest classifiable level; taxa listed above genus (e.g. family or order) include only members that could not be classified at a lower level. Operational taxonomic units at the top of each list are those most abundant in coral tissue; those at the bottom are the most abundant in the algal tissue. Scale bar represents relative abundance (%) of each taxon within each library. Asterisks indicate taxa over-represented in coral tissue at or near the coral–algal interface.
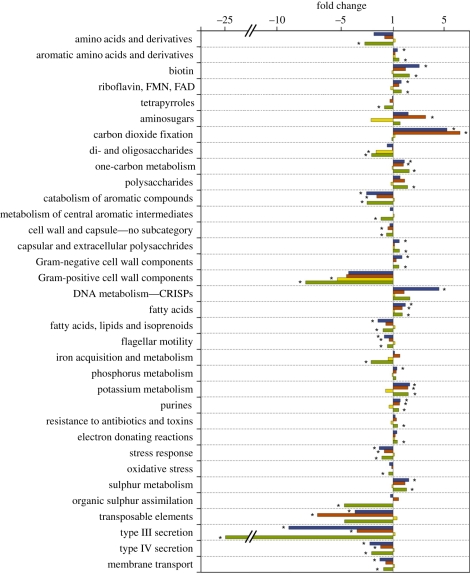
The metabolic capabilities of the coral-associated bacterial communities were also altered by proximity to algal interfaces. For example, coral-associated 16S rDNA libraries were dominated by sequences related to facultative anaerobes [43]. In contrast, we found sequences related to strict anaerobes present in coral tissue near or at interfaces with three of the four groups of algae: 8.5 per cent relative abundance at CCA interfaces; 2.2 per cent relative abundance near D. bartayresiana interfaces; 2 per cent relative abundance near H. opuntia interfaces; but absent near and at interfaces with turf algae. The number of metabolic pathways (from the SEED database) that were over- or under-represented within these over-represented taxa also varied depending on the alga present (turf algae, 29; CCA, 22; D. bartayresiana, 13 and H. opuntia, 2). Interfaces with three of the types of algae (turf algae, CCA and D. bartayresiana) shared several metabolic trends. Specifically, several pathways were under-represented at all three interfaces: membrane transport (including type III and type IV secretion systems), stress response, aromatic catabolism and flagellar motility (figure 4). Likewise, all three showed an increased abundance of pathways for metabolism of single-carbon compounds, fatty acids, potassium and purines. Coral–turf interfaces uniquely showed a reduction in organic sulphur assimilation as well as iron acquisition and metabolism. The two significant changes at H. opuntia interactions were decreased abundance of genes for Gram-positive cell wall components and di- and oligosaccharide metabolism (figure 4).
Figure 4.
Altered metabolic subsystem abundances in coral-associated Bacteria at coral–algal interfaces. Shown are subsystems that were significantly increased or decreased in the Bacteria over-represented in coral tissue at or near at least one type of algal interface. The fold change is relative to corals distant from the interface. Asterisks indicate significant differences (90% confidence levels with 5000 iterations). Blue, CCA; red, Dictyota; yellow, Halimeda; green, turf.
Comparison of the metabolic subsystems in the coral-associated bacterial communities near or at the interfaces with each other showed more virulence and potassium metabolism genes at interactions with turf algae and more carbohydrate metabolism genes at D. bartayresiana interactions (table 1). The communities near or at CCA interactions had more metabolic genes related to cell maintenance than the other interfaces, while those near or at H. opuntia interfaces were similar to coral-associated communities. PCA of the reconstructed metabolic subsystems showed that coral-associated communities clustered closely with the H. opuntia interface community, whereas CCA, D. bartayresiana and turf algae interface communities were distant from the corals and from each other (electronic supplementary material, figure S3), mirroring the taxonomic clustering (electronic supplementary material, figure S2). A PCA was also performed to compare these metabolic changes at coral–algal interactions with previously collected data on the coral holobiont's response to abiotic stress treatments (nutrient addition, temperature increase, decreased pH and DOC addition; [39]). The metabolic changes associated with all four types of algal interactions clustered together with the DOC treatment (electronic supplementary material, figure S4).
Table 1.
Influence of different algal interactions on corals across multiple spatial scales.
| measured attribute | CCA (encrusting) | Halimeda spp. (upright calcareous) | turf algae | fleshy macroalgae | references | |
|---|---|---|---|---|---|---|
| reef scale | interactions on healthy reefa | ↑↑ | ↓↓ | ↓↓ | ↓↓ | this study |
| coral recruitmentb | ↑↑ | ↓↓ | 0/↓↓ | ↓↓ | [45–49] | |
| coral fecundityb | no data | no data | ↓↓ | ↓↓ | [3,20,27] | |
| colony scaleg | shading and abrasionc | 0 | +++ | 0 | +++ | [50] |
| tissue damagec | 0/+++ | 0/+++ | +++ | +++ | [17,18,36,38] | |
| bleachingc | 0 | +++ | +++ | +++ | [17,25,36] and this study | |
| photosynthesis inhibition (expt)d | no data | med | no data | low–high | [36,38] | |
| photosynthesis inhibition (natural)d | none | no data | low | no data | [26] | |
| microbial scale | number of over-represented bacterial taxa at interface | 20 | 12 | 14 | 19 | this study |
| predicted bacterial metabolic subsystems enriched at interface | cell wall, cofactors, nucleotides, photosynthesis, respiration | membrane transport, aromatics, motility, stress response | virulence, potassium | carbohydrates | this study | |
| molecular scale | allelochemical impact on corald | no data | high | no data | high | [36] |
| DOC releasee | med | none–low | high | med–high | [51] (A. Haas 2010, unpublished data) | |
| oxygen change at interfacef | ↑↑ | ↓↓ | ↓↓ | ↓↓ | [17,38] and this study |
aCoral–algal interactions: ↓↓, decrease; ↑↑, increase.
bAlgal impacts on coral reproduction: ↑↑, promotes; 0, none; ↓↓, inhibits.
cPhysical impacts of algae on corals: +++, present; 0, absent.
dRange of algal impacts on holobiont photosynthesis (quantum yield inhibition; [Fv/Fm]): 0.67, none; 0.5–0.65, low; 0.25–0.5, med; 0–0.25, high; expt, experiments.
eDissolved organic carbon release by algae (DOC, µM m−2 h−1): 0–150, low; 151–300, med; >300, high.
fBoundary layer oxygen conditions at interface: ↑↑, hyperoxic; ↓↓, below ambient.
gFor a comprehensive review of physical interaction mechanisms, see McCook et al. [50].
(c). Reef-scale changes in coral–algal interactions
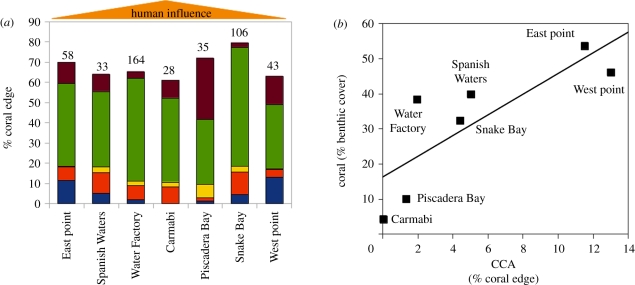
Every coral colony observed was interacting with at least one type of alga, with an average of 61–80% of the coral perimeter involved in any type of algal interaction. Interactions with turf algae were the most abundant, accounting for 32–58% of the coral edge (figure 5a). The percentage of the coral edge bordered by CCA showed the most obvious trend, averaging 12–13% at the eastern and western ends of the island and declining to approximately 0 per cent at sites near the centre of the island where human influence is greatest (figure 5a; [42]). Herbivore biomass was also lowest at sites nearest to the centre of the island (13.5–22.6 g m−2) versus the eastern and western points (27.1–47.3 g m−2; M.J.V. 2011, unpublished data). The number of coral–algal interactions did not correlate with changing per cent cover for either CCA or turf algae (electronic supplementary material, figure S5a,b). Coral cover, however, was higher at sites where a larger percentage of the coral edge interacted with CCA (p = 0.026; figure 5b), but was not correlated with the percentage of coral edges interacting with turf algae (electronic supplementary material, figure S5c).
Figure 5.
Abundance of coral–algal interactions across a range of human impact and coral cover. (a) The average per cent of coral colony edge interacting with the indicated type of algae at the seven surveyed sites east to west across Curacao; the remainder of the perimeter not interacting with algae included interactions with sand, sponges and other corals. Numbers above bars indicate the number of coral colonies observed at each site. (b) Relationship between benthic coral coverage and the average per cent of each colony interacting with crustose coralline algae (CCA) at each site (r2 = 0.66; p = 0.026). (a) Brown, Cyanobacteria; green, turf; yellow, Halimeda; red, Dictyota; blue, CCA.
4. Discussion
(a). Reef to colony-scale responses to algal interactions
Every coral colony observed in this study was interacting with at least one alga, and the frequency of interactions was unrelated to the local percentage of benthic coral or algal cover. The most common coral–algal interactions observed were between corals and turf algae. These interactions were found to negatively affect the physiology of the coral holobiont by eliminating net oxygen production along the interface (figure 2; [17]). While algal removal and coral recovery occurred in situ, DO measurements were taken in an aquarium. This eliminated the effects of local hydrodynamics, permitting measurement of the net flux of oxygen at the interaction zone. The two species of macroalgae examined here also caused DO levels to decrease below ambient, but the magnitudes of their effects differed. Turf algae and many macroalgae have been shown to limit coral growth and negatively impact the bordering coral tissue [17,18,23,24,26], lower coral fecundity [27] and inhibit larval settlement [45–47], thereby impacting corals on multiple scales in time and space (table 1). Given the greater abundance of coral interactions with turf algae relative to other functional groups of algae around the world (figure 5; [17,18]), coral–turf interactions are likely important in influencing the structure of benthic coral reef communities.
In contrast to the algae discussed earlier, interactions with CCA did not exhibit hypoxia (figure 2; [17]). Because CCA appear to cause little stress to coral adults and can also benefit corals by preventing colonization of the coral border by other algae [33], we hypothesize that corals interacting with CCA are more successful on the reef. While some species of CCA can harm corals [52], our hypothesis is supported by the observation that the proportion of an individual coral colony edge interacting with CCA at a given site, regardless of CCA species, correlated positively with benthic coral cover (figure 5). Previous studies have also demonstrated that CCA are generally less detrimental to the health, growth and photosynthetic efficiency of adjacent coral tissue than turf algae [17,26]. Because some species of CCA also promote coral settlement [30,31], their influence on corals is counter to that of turf algae examined here across multiple spatial scales.
(b). Micro-scale interactions between corals and algae
The coral holobiont is a selective environment for bacteria, as evidenced by the variety of stressors that the residents must counteract: host antibiotics [53,54], bacteria–bacteria antagonism [53,55], and dimethylsulphide (DMS), dimethylsulphoniopropionate (DMSP) [56,57] and free radicals [58] released by the zooxanthellae. We hypothesize that the holobiont becomes compromised when stressed by competition with certain algae, allowing microbes to invade that do not possess the suite of metabolisms necessary to survive the normal holobiont landscape and that disproportionately capitalize on DOC released by the algae. This study is the first to identify the types of bacteria present along coral–algal interactions, and we find that bacterial stress response pathways were reduced at coral interfaces with CCA, D. bartayresiana and turf algae (figure 4). Type III and IV secretion pathways, hallmarks of pathogenesis but important for some symbiotic interactions [59,60], were also lower at these three interface types, potentially indicating a breakdown of symbiosis. Carbohydrate metabolisms were enriched along these same three interfaces (figure 4 and table 1) and bacterial communities at all coral–algal interfaces showed changes similar to DOC stressed corals (electronic supplementary material, figure S4), together suggesting that bacteria present at some coral–algal interfaces may be consuming carbohydrates released from the neighbouring algae [51].
Despite the earlier-mentioned similarities, the different types of algae examined here have characteristic impacts on the bacterial component of the neighbouring coral holobiont. CCA presence did not affect holobiont physiology, but did alter the holobiont composition, while H. opuntia had little effect on holobiont composition despite its impact on physiology (DO). Turf algae, on the other hand, affected holobiont physiology and had the most distinct influence on its bacterial community. The coral–turf interface was the only one to show increased bacterial virulence pathways (table 1), suggesting that coral–bacterial symbiosis may be breaking down further here and shifting towards a more pathogenic state compared with the other coral–algal interfaces. Additional support for this is evident in the decrease in organic sulphur assimilation at the coral–turf interface. Organic sulphur compounds, particularly DMS and DMSP, are important for structuring coral-associated bacterial communities [56,57], and loss of this bacterial metabolism at coral–turf interfaces suggests that turf algae may have facilitated invasion of the holobiont by bacteria lacking these pathways. Further investigations are needed to determine the direct effects of these interface-associated microbial communities on coral health. Recent studies have shown that different species of algae alter the growth of coral bacteria [61], supporting the hypothesis that algae may directly alter the structure of the coral holobiont.
(c). Coral–algal interaction mechanisms
This is the first time that algae have been shown to cause lower oxygen levels on corals in naturally occurring interactions; however, the mechanism remains in question. Loss of zooxanthellae owing to shading and possibly allelopathy is the main cause of hypoxia at coral–H. opuntia interaction zones, because coral tissue was bleached but showed little change in the bacterial community. Alternative mechanisms are likely causing hypoxia at coral–D. bartayresiana and coral–turf interaction zones because these algae cause little to no shading. Possibilities include algal photosynthates (i.e. DOC) that stimulate microbial respiration and pathogen invasion [38,40,41], algal allelochemicals that inhibit photosynthesis by the zooxanthellae and cause bleaching at the site of contact [36], direct physical damage or some combination of these (table 1). Physical effects such as abrasion are often minimal compared with the effects of live algae [36,62], and while lipid-soluble extracts (i.e. allelochemicals) from some algae have been shown to damage corals, these compounds are highly specific to the algal species and require direct contact for effect [36]. In contrast, DOC is a water-soluble product of photosynthesis that is potentially released by many algae [51,63] and does not require contact to affect the coral holobiont. Various forms of DOC released by algae have been shown to kill corals and increase microbial growth rates [40,41], while some algae cause coral death and hypoxia that is mediated by microbes [38]. Coral exposure to DOC also induces coral-associated viruses [64] and increases the proportion of pathogens on corals [39], and algae that release more DOC likely show a stronger effect [39]. Because this study demonstrates similar patterns in oxygen levels and microbial composition on corals at some in situ coral–algal interaction zones, DOC is a likely candidate stimulating these changes. The significance of DOC in these interactions does not preclude the action of other mechanisms (e.g. allelochemistry). Indirect interactions within this complex system may also play an as yet unknown but important role [65], such as microinvertebrates associated with the algae that can draw down local oxygen levels or herbivory that may affect algal morphology [66].
(d). Ecological implications
Micro-scale interactions between benthic algae and the coral holobiont have far-reaching implications for the composition of the reef. We propose a model whereby some fleshy algae (e.g. turf algae and fleshy macroalgae) act at the micro-scale to stress corals, leading to macro-scale changes in the ecology of the reef (figure 5 and table 1). On reefs approaching a phase-shift from the coral-dominated to the algae-dominated state, the impacts of fleshy algae on the coral holobiont are worsened by increased fleshy algal cover and more abundant interactions with corals [67]. These negative impacts span the range from micro-scale changes in microbial communities and oxygen drawdown to coral colony-scale effects such as damage to adjacent polyps and lowered fecundity of the adjacent coral colony, likely leading to reef-scale effects on coral abundance and distribution (table 1). Conversely, on healthy coral reefs where CCA and calcified macroalgae (Halimeda spp.) are more abundant, coral–algal interactions have less impact on the holobiont composition and physiology. CCA, in particular, promote coral proliferation through interactions at micro, colony and reef scales (figure 5 and table 1).
Various disturbances on the reef (herbivore removal via overfishing, eutrophication, elevated sea surface temperature, etc.) undoubtedly influence these micro-scale interactions, affecting benthic composition at the reef scale. One prominent factor likely affecting the distribution of the different types of coral–algal interactions is herbivory. Many herbivores preferentially feed on turf algae, lowering algal biomass [26,68,69]. If highly grazed (i.e. short, low density) patches of turf algae are less detrimental to corals than less grazed (i.e. tall, dense) stands, then it is possible that herbivores attenuate the micro- and macro-scale effects of turf algae on corals. High herbivory also preferentially removes algae that compete with CCA [68], thus increasing the proportion of the benthos occupied by CCA, which can in turn lower recruitment of macroalgae to the reef [33]. Environmental disturbances, by affecting the micro- and colony-scale interactions occurring between certain types of algae and the coral holobiont, should manifest at the reef scale by influencing the distribution and outcomes of these interactions and ultimately the composition of the reef benthos.
Acknowledgements
We thank CARMABI in Curacao for use of their facilities, and Dana Willner, Matthew Haynes, Alejandro Reyes and Bahador Nosrat for technical and bioinformatics support. We also thank Andreas Haas for sharing data on algal DOC release. Funding for this work was provided by the National Science Foundation Grant OCE-0927415 to F.L.R. K.L.B. was supported by a National Science Foundation Graduate Research Fellowship.
References
- 1.Knowlton N. 2001. Coral reef biodiversity–habitat size matters. Science 292, 1493–1495 10.1126/science.1061690 (doi:10.1126/science.1061690) [DOI] [PubMed] [Google Scholar]
- 2.Hughes T. P. 1994. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265, 1547–1551 10.1126/science.265.5178.1547 (doi:10.1126/science.265.5178.1547) [DOI] [PubMed] [Google Scholar]
- 3.Hughes T. P., et al. 2007. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr. Biol. 17, 360–365 10.1016/j.cub.2006.12.049 (doi:10.1016/j.cub.2006.12.049) [DOI] [PubMed] [Google Scholar]
- 4.Knowlton N., Jackson J. B. C. 2008. Shifting baselines, local impacts, and global change on coral reefs. PLoS Biol. 6, e54. 10.1371/journal.pbio.0060054 (doi:10.1371/journal.pbio.0060054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkinson C. 2004. Status of coral reefs of the world: 2004. Townsville, Queensland: Australian Institute of Marine Science [Google Scholar]
- 6.Carpenter R. C. 1988. Mass mortality of a Caribbean sea urchin: immediate effects on community metabolism and other herbivores. Proc. Natl Acad. Sci. USA 85, 511–514 10.1073/pnas.85.2.511 (doi:10.1073/pnas.85.2.511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ledlie M. H., Graham N. A. J., Bythell J. C., Wilson S. K., Jennings S., Polunin N. V. C., Hardcastle J. 2007. Phase shifts and the role of herbivory in the resilience of coral reefs. Coral Reefs 26, 641–653 10.1007/s00338-007-0230-1 (doi:10.1007/s00338-007-0230-1) [DOI] [Google Scholar]
- 8.Littler M. M., Littler D. S., Brooks B. L. 2006. Harmful algae on tropical coral reefs: bottom-up eutrophication and top-down herbivory. Harmful Algae 5, 565–585 10.1016/j.hal.2005.11.003 (doi:10.1016/j.hal.2005.11.003) [DOI] [Google Scholar]
- 9.Sandin S. A., et al. 2008. Baselines and degradation of coral reefs in the northern Line Islands. PLoS ONE 3, e1548. 10.1371/journal.pone.0001548 (doi:10.1371/journal.pone.0001548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCook L. J. 1999. Macroalgae, nutrients and phase shifts on coral reefs: scientific issues and management consequences for the Great Barrier Reef. Coral Reefs 18, 357–367 10.1007/s003380050213 (doi:10.1007/s003380050213) [DOI] [Google Scholar]
- 11.Hughes T. P., et al. 2003. Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933 10.1126/science.1085046 (doi:10.1126/science.1085046) [DOI] [PubMed] [Google Scholar]
- 12.Hoegh-Guldberg O., Australia G. 1999. Climate change, coral bleaching and the future of the world's coral reefs. Mar. Freshwater Res. 50, 839–866 10.1071/MF99078 (doi:10.1071/MF99078) [DOI] [Google Scholar]
- 13.Hoegh-Guldberg O., et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742 10.1126/science.1152509 (doi:10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- 14.Sutherland K. P., Porter J. W., Torres C. 2004. Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar. Ecol. Prog. Ser. 266, 265–272 10.3354/meps266273 (doi:10.3354/meps266273) [DOI] [Google Scholar]
- 15.Harvell C. D., et al. 1999. Emerging marine diseases: climate links and anthropogenic factors. Science 285, 1505–1510 10.1126/science.285.5433.1505 (doi:10.1126/science.285.5433.1505) [DOI] [PubMed] [Google Scholar]
- 16.Bourne D. G., Garren M., Work T. M., Rosenberg E., Smith G. W., Harvell C. D. 2009. Microbial disease and the coral holobiont. Trends Microbiol. 17, 554–562 10.1016/j.tim.2009.09.004 (doi:10.1016/j.tim.2009.09.004) [DOI] [PubMed] [Google Scholar]
- 17.Barott K., Smith J., Dinsdale E. A., Hatay M., Sandin S., Rohwer F. 2009. Hyperspectral and physiological analyses of coral–algal interactions. PLoS ONE 4, e8043. 10.1371/journal.pone.0008043 (doi:10.1371/journal.pone.0008043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haas A., el-Zibdah M., Wild C. 2010. Seasonal monitoring of coral–algae interactions in fringing reefs of the Gulf of Aqaba, Northern Red Sea. Coral Reefs 29, 93–103 10.1007/s00338-009-0556-y (doi:10.1007/s00338-009-0556-y) [DOI] [Google Scholar]
- 19.Lirman D. 2001. Competition between macroalgae and corals: effects of herbivore exclusion and increased algal biomass on coral survivorship and growth. Coral Reefs 19, 392–399 10.1007/s003380000125 (doi:10.1007/s003380000125) [DOI] [Google Scholar]
- 20.Tanner J. E. 1995. Competition between scleractinian corals and macroalgae: an experimental investigation of coral growth, survival and reproduction. J. Exp. Mar. Biol. Ecol. 190, 151–168 10.1016/0022-0981(95)00027-O (doi:10.1016/0022-0981(95)00027-O) [DOI] [Google Scholar]
- 21.Miller M. W. 1998. Coral/seaweed competition and the control of reef community structure within and between latitudes. Oceanogr. Mar. Biol. 36, 65–96 [Google Scholar]
- 22.River G. F., Edmunds P. J. 2001. Mechanisms of interaction between macroalgae and scleractinians on a coral reef in Jamaica. J. Exp. Mar. Biol. Ecol. 261, 159–172 10.1016/S0022-0981(01)00266-0 (doi:10.1016/S0022-0981(01)00266-0) [DOI] [PubMed] [Google Scholar]
- 23.Titlyanov E. A., Yakovleva I. M., Titlyanova T. V. 2007. Interaction between benthic algae (Lyngbya bouillonii, Dictyota dichotoma) and scleractinian coral Porites lutea in direct contact. J. Exp. Mar. Biol. Ecol. 342, 282–291 10.1016/j.jembe.2006.11.007 (doi:10.1016/j.jembe.2006.11.007) [DOI] [Google Scholar]
- 24.Quan-Young L. I., Espinoza-Avalos J. 2006. Reduction of zooxanthellae density, chlorophyll a concentration, and tissue thickness of the coral Montastraea faveolata (Scleractinia) when competing with mixed turf algae. Limnol. Oceanogr. 51, 1159–1166 10.4319/lo.2006.51.2.1159 (doi:10.4319/lo.2006.51.2.1159) [DOI] [Google Scholar]
- 25.Haas A., Al-Zibdah M., Wild C. 2009. Effect of inorganic and organic nutrient addition on coral–algae assemblages from the Northern Red Sea. J. Exp. Mar. Biol. Ecol. 380, 99–105 10.1016/j.jembe.2009.09.005 (doi:10.1016/j.jembe.2009.09.005) [DOI] [Google Scholar]
- 26.Vermeij M. J. A., van Moorselaar I., Engelhard S., Hörnlein C., Vonk S. M., Visser P. M. 2010. The effects of nutrient enrichment and herbivore abundance on the ability of turf algae to overgrow coral in the Caribbean. PLoS ONE 5, e14312. 10.1371/journal.pone.0014312 (doi:10.1371/journal.pone.0014312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foster N., Box S., Mumby P. 2008. Competitive effects of macroalgae on the fecundity of the reef-building coral Montastraea annularis. Mar. Ecol. Prog. Ser. 367, 143–152 10.3354/meps07594 (doi:10.3354/meps07594) [DOI] [Google Scholar]
- 28.Jompa J., McCook L. J. 2003. Contrasting effects of turf algae on corals: massive Porites spp. are unaffected by mixed-species turfs, but killed by the red alga Anotrichium tenue. Mar. Ecol. Prog. Ser. 258, 79–86 10.3354/meps258079 (doi:10.3354/meps258079) [DOI] [Google Scholar]
- 29.McCook L. 2001. Competition between corals and algal turfs along a gradient of terrestrial influence in the nearshore central Great Barrier Reef. Coral Reefs 19, 419–425 [Google Scholar]
- 30.Price N. 2010. Habitat selection, facilitation, and biotic settlement cues affect distribution and performance of coral recruits in French Polynesia. Oecologia 163, 747–758 10.1007/s00442-010-1578-4 (doi:10.1007/s00442-010-1578-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morse D. E., Hooker N., Morse A. N. C., Jensen R. A. 1988. Control of larval metamorphosis and recruitment in sympatric agariciid corals. J. Exp. Mar. Biol. Ecol. 116, 193–217 10.1016/0022-0981(88)90027-5 (doi:10.1016/0022-0981(88)90027-5) [DOI] [Google Scholar]
- 32.Denboh T., Suzuki M., Mizuno Y., Ichimura T. 1997. Suppression of Laminaria sporelings by allelochemicals from coralline red algae. Bot. Mar. 40, 249–256 10.1515/botm.1997.40.1-6.249 (doi:10.1515/botm.1997.40.1-6.249) [DOI] [Google Scholar]
- 33.Vermeij M. J. A., Dailer M. L., Smith C. M. 2011. Crustose coralline algae can suppress macroalgal growth and recruitment on Hawaiian coral reefs. Mar. Ecol. Prog. Ser. 422, 1–7 10.3354/meps08964 (doi:10.3354/meps08964) [DOI] [Google Scholar]
- 34.Knowlton N., Rohwer F. 2003. Multispecies microbial mutualisms on coral reefs: the host as a habitat. Am. Nat. 162, S51–S62 10.1086/378684 (doi:10.1086/378684) [DOI] [PubMed] [Google Scholar]
- 35.Nugues M. M., Smith G. W., Hooidonk R. J., Seabra M. I., Bak R. P. M. 2004. Algal contact as a trigger for coral disease. Ecol. Lett. 7, 919–923 10.1111/j.1461-0248.2004.00651.x (doi:10.1111/j.1461-0248.2004.00651.x) [DOI] [Google Scholar]
- 36.Rasher D. B., Hay M. E. 2010. Chemically rich seaweeds poison corals when not controlled by herbivores. Proc. Natl Acad. Sci. USA 107, 9683–9688 10.1073/pnas.0912095107 (doi:10.1073/pnas.0912095107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gross E. M. 2003. Allelopathy of aquatic autotrophs. Crit. Rev. Plant Sci. 22, 313–339 10.1080/713610859 (doi:10.1080/713610859) [DOI] [Google Scholar]
- 38.Smith J. E., et al. 2006. Indirect effects of algae on coral: algae-mediated, microbe-induced coral mortality. Ecol. Lett. 9, 835–845 10.1111/j.1461-0248.2006.00937.x (doi:10.1111/j.1461-0248.2006.00937.x) [DOI] [PubMed] [Google Scholar]
- 39.Thurber R. V., Willner-Hall D., Rodriguez-Mueller B., Desnues C., Edwards R. A., Angly F., Dinsdale E., Kelly L., Rohwer F. 2009. Metagenomic analysis of stressed coral holobionts. Environ. Microbiol. 11, 2148–2163 10.1111/j.1462-2920.2009.01935.x (doi:10.1111/j.1462-2920.2009.01935.x) [DOI] [PubMed] [Google Scholar]
- 40.Kline D. I., Kuntz N. M., Breitbart M., Knowlton N., Rohwer F. 2006. Role of elevated organic carbon levels and microbial activity in coral mortality. Mar. Ecol. Prog. Ser. 314, 119–125 10.3354/meps314119 (doi:10.3354/meps314119) [DOI] [Google Scholar]
- 41.Kuntz N. M., Kline D. I., Sandin S. A., Rohwer F. 2005. Pathologies and mortality rates caused by organic carbon and nutrient stressors in three Caribbean coral species. Mar. Ecol. Prog. Ser. 294, 173–180 10.3354/meps294173 (doi:10.3354/meps294173) [DOI] [Google Scholar]
- 42.Klaus J. S., Janse I., Heikoop J. M., Sanford R. A., Fouke B. W. 2007. Coral microbial communities, zooxanthellae and mucus along gradients of seawater depth and coastal pollution. Environ. Microbiol. 9, 1291–1305 10.1111/j.1462-2920.2007.01249.x (doi:10.1111/j.1462-2920.2007.01249.x) [DOI] [PubMed] [Google Scholar]
- 43.Barott K. L., Rodriguez-Brito B., Janouškovec J., Marhaver K. L., Smith J. E., Keeling P., Rohwer F. L. 2011. Microbial diversity associated with four functional groups of benthic reef algae and the reef–building coral Montastraea annularis. Environ. Microbiol. 13, 1192–1204 10.1111/j.1462-2920.2010.02419.x (doi:10.1111/j.1462-2920.2010.02419.x) [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Brito B., Rohwer F., Edwards R. 2006. An application of statistics to comparative metagenomics. BMC Bioinf. 7, 162. 10.1186/1471-2105-7-162 (doi:10.1186/1471-2105-7-162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birrell C. L., McCook L. J., Willis B. L. 2005. Effects of algal turfs and sediment on coral settlement. Mar. Pollut. Bull. 51, 408–414 10.1016/j.marpolbul.2004.10.022 (doi:10.1016/j.marpolbul.2004.10.022) [DOI] [PubMed] [Google Scholar]
- 46.Kuffner I. B., Paul V. J. 2004. Effects of the benthic cyanobacterium Lyngbya majuscula on larval recruitment of the reef corals Acropora surculosa and Pocillopora damicornis. Coral Reefs 23, 455–458 10.1007/s00338-004-0416-8 (doi:10.1007/s00338-004-0416-8) [DOI] [Google Scholar]
- 47.Kuffner I. B., Walters L. J., Becerro M. A., Paul V. J., Ritson-Williams R., Beach K. S. 2006. Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar. Ecol. Prog. Ser. 323, 107–117 10.3354/meps323107 (doi:10.3354/meps323107) [DOI] [Google Scholar]
- 48.Birrell C. L., McCook L. J., Willis B. L., Diaz-Pulido G. A. 2008. Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. Oceanogr. Mar. Biol. Annu. Rev. 46, 25–64 10.1201/9781420065756.ch2 (doi:10.1201/9781420065756.ch2) [DOI] [Google Scholar]
- 49.Nugues M. M., Szmant A. M. 2006. Coral settlement onto Halimeda opuntia: a fatal attraction to an ephemeral substrate? Coral Reefs 25, 585–591 10.1007/s00338-006-0147-0 (doi:10.1007/s00338-006-0147-0) [DOI] [Google Scholar]
- 50.McCook L., Jompa J., Diaz-Pulido G. 2001. Competition between corals and algae on coral reefs: a review of evidence and mechanisms. Coral Reefs 19, 400–417 10.1007/s003380000129 (doi:10.1007/s003380000129) [DOI] [Google Scholar]
- 51.Haas A. F., Jantzen C., Naumann M. S., IglesiasPrieto R., Wild C. 2010. Organic matter release by the dominant primary producers in a Caribbean reef lagoon: implication for in situ oxygen availability. Mar. Ecol. Prog. Ser. 409, 27–39 10.3354/meps08631 (doi:10.3354/meps08631) [DOI] [Google Scholar]
- 52.Antonius A. 2001. Pneophyllum conicum, a coralline red alga causing coral reef-death in Mauritius. Coral Reefs 1, 418. 10.1007/s003380000126 (doi:10.1007/s003380000126) [DOI] [Google Scholar]
- 53.Ritchie K. B. 2006. Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Mar. Ecol. Prog. Ser. 322, 1–14 10.3354/meps322001 (doi:10.3354/meps322001) [DOI] [Google Scholar]
- 54.Koenig J. E., Bourne D. G., Curtis B., Dlutek M., Stokes H. W., Doolittle W. F., Boucher Y. 2011. Coral-mucus-associated Vibrio integrons in the Great Barrier Reef: genomic hotspots for environmental adaptation. ISME J. 5, 962–972 10.1038/ismej.2010.193 (doi:10.1038/ismej.2010.193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rypien K. L., Ward J. R., Azam F. 2010. Antagonistic interactions among coral-associated bacteria. Environ. Microbiol. 12, 28–39 10.1111/j.1462-2920.2009.02027.x (doi:10.1111/j.1462-2920.2009.02027.x) [DOI] [PubMed] [Google Scholar]
- 56.Raina J. B., Tapiolas D., Willis B. L., Bourne D. G. 2009. Coral-associated bacteria and their role in the biogeochemical cycling of sulfur. Appl. Environ. Microbiol. 75, 3492. 10.1128/AEM.02567-08 (doi:10.1128/AEM.02567-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raina J.-B., Dinsdale E. A., Willis B. L., Bourne D. G. 2010. Do the organic sulfur compounds DMSP and DMS drive coral microbial associations? Trends Microbiol. 18, 101–108 10.1016/j.tim.2009.12.002 (doi:10.1016/j.tim.2009.12.002) [DOI] [PubMed] [Google Scholar]
- 58.Lesser M. P. 2006. Oxidative stress in marine environments: biochemistry and physiological ecology. Annu. Rev. Physiol. 68, 253–278 10.1146/annurev.physiol.68.040104.110001 (doi:10.1146/annurev.physiol.68.040104.110001) [DOI] [PubMed] [Google Scholar]
- 59.Viprey V., Del Greco A., Golinowski W., Broughton W. J., Perret X. 1998. Symbiotic implications of type III protein secretion machinery in Rhizobium. Mol. Microbiol. 28, 1381–1389 10.1046/j.1365-2958.1998.00920.x (doi:10.1046/j.1365-2958.1998.00920.x) [DOI] [PubMed] [Google Scholar]
- 60.Galan J. E., Wolf-Watz H. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444, 567–573 10.1038/nature05272 (doi:10.1038/nature05272) [DOI] [PubMed] [Google Scholar]
- 61.Morrow K. M., Paul V. J., Liles M. R., Chadwick N. E. 2011. Allelochemicals produced by Caribbean macroalgae and cyanobacteria have species-specific effects on reef coral microorganisms. Coral Reefs 3, 309–320 10.1007/s00338-011-0747-1 (doi:10.1007/s00338-011-0747-1) [DOI] [Google Scholar]
- 62.Diaz-Pulido G., Gouezo M., Tilbrook B., Dove S., Anthony K. R. N. 2011. High CO2 enhances the competitive strength of seaweeds over corals. Ecol. Lett. 14, 156–162 10.1111/j.1461-0248.2010.01565.x (doi:10.1111/j.1461-0248.2010.01565.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wild C., Niggl W., Naumann M. S., Haas A. F. 2010. Organic matter release by Red Sea coral reef organisms—potential effects on microbial activity and in situ O2 availability. Mar. Ecol. Prog. Ser. 411, 61–71 10.3354/meps08653 (doi:10.3354/meps08653) [DOI] [Google Scholar]
- 64.Thurber V., et al. 2008. Metagenomic analysis indicates that stressors induce production of herpes-like viruses in the coral Porites compressa. Proc. Natl Acad. Sci. USA 105, 18 413–18 418 10.1073/pnas.0808985105 (doi:10.1073/pnas.0808985105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wootton J. T. 2002. Indirect effects in complex ecosystems: recent progress and future challenges. J. Sea Res. 48, 157–172 10.1016/S1385-1101(02)00149-1 (doi:10.1016/S1385-1101(02)00149-1) [DOI] [Google Scholar]
- 66.Lewis S. M., Norris J. N., Searles R. B. 1987. The regulation of morphological plasticity in tropical reef algae by herbivory. Ecology 68, 636–641 10.2307/1938468 (doi:10.2307/1938468) [DOI] [Google Scholar]
- 67.Pandolfi J. M., et al. 2005. Are US coral reefs on the slippery slope to slime? Science 307, 1725–1726 10.1126/science.1104258 (doi:10.1126/science.1104258) [DOI] [PubMed] [Google Scholar]
- 68.Burkepile D., Hay M. 2009. Nutrient versus herbivore control of macroalgal community development and coral growth on a Caribbean reef. Mar. Ecol. Prog. Ser. 389, 71–84 10.3354/meps08142 (doi:10.3354/meps08142) [DOI] [Google Scholar]
- 69.Carpenter R. C. 1986. Partitioning herbivory and its effects on coral reef algal communities. Ecol. Monogr. 56, 345–363 10.2307/1942551 (doi:10.2307/1942551) [DOI] [Google Scholar]