Abstract
Behavioural consistency or predictability through time and/or different contexts (‘syndromes’ or ‘personality types’) is likely to have substantial influence on animal life histories and fitness. Consequently, there is much interest in the forces driving and maintaining various syndromes. Individual host behaviours have been associated with susceptibility to parasitism, yet the role of pre-existing personality types in acquiring infections has not been investigated experimentally. Using a larval amphibian–trematode parasite model system, we report that tadpoles generally showed consistency in their activity level in response to both novel food and parasite exposure. Not only were individual activity level and exploration in the novel food context correlated with each other and with anti-parasite behaviour, all three were significant predictors of host parasite load. This is the first empirical demonstration that host behaviours in other contexts are related to behaviours mitigating infection risk and, ultimately, host parasite load. We suggest that this system illustrates how reliably high levels of activity and exploratory behaviour in different contexts might maximize both energy acquisition and resistance to trematode parasites. Such benefits could drive selection for the behavioural syndrome seen here owing to the life histories and ecological circumstances typical of wood frog (Lithobates sylvaticus) larvae.
Keywords: personality, disease, behaviour, syndrome, parasite, amphibian
1. Introduction
Many animal behaviours represent adaptations to maximize fitness and have long been considered highly plastic such that individuals can optimize their behaviour in particular conditions [1]. However, there is increasing evidence that, while individual behaviour can be quite flexible, it is also often consistent and predictable across time and/or different situations, suggesting the existence of underlying behavioural types or personalities [2]. Consistency can be most easily thought of in two ways: predictability of a specific behaviour in different functional contexts [3], or a predictable relationship between two seemingly unrelated behaviours, such as intrasexual aggression and activity in novel environments [4]. Such correlations within/among behaviours have been termed ‘behavioural syndromes’ [1] and have been reported for various species [5]. It has been suggested that many behavioural syndromes represent selection for behavioural consistency because this may be advantageous across different situations [1]. Conversely, lack of such behavioural plasticity can have important ecological and evolutionary implications if correlations across contexts prevent an individual from optimizing its behaviour in each separate situation [1]; thus many studies seek to explain why particular syndromes exist or how they are maintained [6,7].
Behaviour and infection are intimately linked because parasites can affect behaviour, perhaps even influencing human personalities and contributing to cultural differences [8], and many individual host behaviours have been separately shown to impact chances of infection [9,10]. However, very few studies of behavioural syndromes have examined whether animal ‘personalities’ predict susceptibility to parasitism [11,12], and none have done so experimentally. In particular, animals have evolved a wide range of anti-parasite behavioural strategies, such as avoidance and removal of parasite infectious stages [13,14]. Since variation in individual behaviour generates the potential for differential parasite exposure, and parasites can be associated with costs comparable with predation [15,16], it has been suggested that parasites can also have a powerful role in shaping animal personalities [17–19]. Notably, consistent behavioural syndromes have been reported for other factors critical to fitness, such as predation risk and mating success [20], which are powerful drivers of many phenotypic traits. As a result, local parasite risk/pressure may also act as a selective force on personality traits and behavioural syndromes [19], and could particularly influence the adaptiveness of personality traits associated with high life-history productivity [18].
Beyond establishing whether personalities predict host susceptibility to parasitism, studying the contribution of such behavioural consistency/predictability to individual variation in disease resistance/tolerance is important to understand infection dynamics and patterns. For instance, this may aid in elucidating the high degree of macroparasite aggregation seen within most host populations [21], with many individuals not infected, or harbouring low parasite burdens. The role of host behaviour has been cited as a potential explanation for this heterogeneous distribution among hosts [22,23]; however, consistent differences in individual behaviour (personalities) have not been well studied to date. Given the wide-ranging implications on the evolution of personalities and disease dynamics, experimental studies are now required that explicitly examine the potential of personality traits to influence infection susceptibility [18].
There are five major animal personality traits that are well recognized in the literature [2]: boldness–shyness (reaction to threats), exploration–avoidance (reaction to novel stimuli), activity (general level of activity), aggressiveness (agonistic reaction to conspecifics) and sociability (non-aggressive reaction to conspecific presence). The ‘proactive–reactive axis’ [24] is often used to combine these separate traits into a general personality type, with proactive individuals tending to be more bold, exploratory, active and aggressive. For species with effective anti-parasite behaviours [25,26], innate behavioural differences could influence individual susceptibility to infections [19], but such behaviourally mediated parasite resistance strategies probably only involve a specific set of the personality traits above. In particular, boldness–shyness, exploration–avoidance and activity may be expected to play especially important roles in anti-parasite behavioural strategies, and should thus be consistent within individuals across different contexts if they also provide other advantages (e.g. energy gain).
Here, we examined how variation in host personality can affect disease risk by investigating individual larval amphibian activity level and exploratory behaviour in three different contexts: control (no stimuli), exposure to a novel food source and response to parasite infectious stages (in a common and widespread trematode, Echinoparyphium spp., known to cause host pathology). Our goal was to establish whether these two behaviours were correlated with one another, and also consistent both within and among contexts such that they constituted a syndrome. In particular, we wished to determine if activity and exploration in the control and novel food situations were predictive of host responses to parasite exposure and, ultimately, the level of host infection. Given that tadpoles can evade or brush off/remove trematode parasite free-swimming infectious stages, and an inability to perform such behaviours results in significantly higher infection [27,28], we expected that high levels of both host activity and exploratory behaviour, corresponding to proactive individuals, would be negatively correlated with parasite load.
2. Material and methods
(a). Tadpole maintenance
Four clutches of wood frog (Lithobates sylvaticus) eggs were locally collected from a single pond in May 2011. Eggs were kept in separate aerated aquaria filled with 10 l of dechlorinated water until hatching. Tadpoles were then maintained on a 14 L : 10 D cycle at 20°C and fed crushed fish-flake food ad libitum. After reaching Gosner developmental stage 25/26 [29], 26 tadpoles were haphazardly chosen from among the aquaria for the experiment and kept individually in 2 l containers holding 1 l of dechlorinated water. Tadpoles were not fed for the 24 h period preceding each round of recording described below.
(b). Parasite use
Tadpoles were exposed to free-swimming infective stages (cercariae) of the parasitic trematode Echinoparyphium spp., which routinely infect wood frog larvae [30]. Cercariae were obtained as previously detailed [31] after local collection of aquatic gastropods that serve as first intermediate hosts (Stagnicola elodes here), and were identified based on the presence of 43 collar spines [32]. Cercariae of Echinoparyphium spp. emerge from the snail and seek out a suitable second intermediate host (including larval amphibians), wherein they develop into cysts (metacercariae) within the kidneys after migrating up the cloaca. After infected tadpoles/frogs are ingested by the definitive host (various birds or mammals for Echinoparyphium), the metacercariae develop into adult worms and reproduce [32]. Immediately prior to each morning or afternoon recording session, cercariae from three to four infected snails were pooled together and then 15 cercariae each were aliquoted into 150 µl microcentrifuge tubes containing 100 µl of dechlorinated water. This ensured that all tadpoles were exposed to cercariae less than 4 h old, well within the 8 h window of maximum parasite infectivity typical for this group of trematodes [32].
(c). Behavioural recording
We recorded tadpole behaviour in three different conditions: control (no stimuli present), novel food source and parasite exposure. A 48 h period elapsed among each of the three recording conditions for each individual. Of the 26 tadpoles, half were recorded in the control condition first, followed by the novel food condition, while this was reversed for the other half. The parasite condition was always last owing to the expectation that infected individuals would behave differently in the other two recording conditions compared with those not yet infected [26,33], thus biasing the data. Tadpole behaviour was recorded digitally with an Everio GZ-MS250 video camera (JVC, Yokohama, Japan) mounted on a tripod such that an overhead view was obtained.
Tadpoles were recorded two at a time using separate set-ups divided by a partition. The two members of each pair were simultaneously subjected to different recording conditions (with the exception of parasite exposure). Each individual was placed in a clear 20 × 15 cm plastic container containing 500 ml of dechlorinated water for a 15 min acclimation period before each individual recording session began. Sheets demarcating the recording arena into three equal-sized ‘zones’ (left, middle and right) were placed underneath the containers. A cardboard blind was then put in place such that the video camera was accessible but tadpoles were not able to see the investigators. After the acclimation period, the video camera was switched on for 15 min either corresponding to the control condition for one of the tadpole pair or the novel food condition for the other. For the latter, a standard-sized rabbit food pellet (1 cm length) was added at the beginning of the 15 min recording period with a long set of forceps to reach around the blind. Because tadpoles had only been fed floating fish-flake food until this point, the rabbit food pellet resting at the bottom of the container represented a different and novel type of food. Notably, reaction to novel food is regularly used as a measure of neophobia in many behavioural studies, and neophobia limits exploratory behaviour [34]. Placement of the food pellet in the left zone (zone 1) for the tadpole in the food condition of a pair was followed by pellet placement in the right zone (zone 3) for the tadpole in the food condition in the subsequent pair, with continuing rotation among pairs. After 48 h, the same tadpoles were once again recorded as above in the opposite condition (e.g. novel food condition if previously recorded in the control condition). Tadpoles were returned to their individual containers and fed fish-flake food after each recording session. After each completed recording session, the containers were washed and dried prior to re-use such that none were used twice in a single day.
Forty-eight hours after the second recording session, all tadpoles were subject to the third condition, in which 15 cercariae were added to each container by agitating the contents of each individual microcentrifuge tube and adding the entire contents to the same zone in which they had previously received a food pellet. This was done so that tadpoles were unlikely to have a tendency to avoid the zone where food was previously absent, thereby avoiding cercariae a priori. Tadpoles were once again recorded for 15 min. After completion of all three recording sessions, tadpoles were euthanized in a buffered solution of the anaesthetic MS-222 and dissected to count the number of cysts in each individual.
(d). Behavioural analysis
From viewing the recordings, we noted whether tadpoles were active or not (swimming), as well as their zone location, every 20 s for each of the 15 min recording sessions. We then calculated the percentage of time sampling points during which tadpoles were active and present in the same zone as the novel stimulus or parasite introduction (exploratory behaviour), respectively. As such, we gathered data representative of three of the five major animal personality traits described earlier: exploration–avoidance (reaction to novel stimuli—new food source here), boldness–shyness (reaction to threats—parasite infection here) and activity (general level of activity in each of the three conditions).
(e). Statistical analysis
As a behavioural syndrome is defined as a correlation, and the critical statistical test is whether a correlation between behaviours is significantly different from zero [20], we tested for overall correlations among activity and exploration both within and across the three recording conditions with Pearson's correlation tests using arcsine-transformed data. We then used a generalized linear model (GLM with normal distribution and identity link function) with subject number as a categorical factor and log-transformed mass as a covariate in order to determine whether variation within individuals for both behaviours across treatments was less than between individuals, which would indicate high individual consistency in response to the same stimuli. A linear regression was used to test the relationship between log-transformed cyst abundance (infection intensity) and log-transformed mass. We tested for an effect of treatment order on infection intensity and both behavioural measures using a GLM. As treatment order had no significant effect on either behaviour or infection intensity, it was not considered further (see below). We then used generalized linear mixed models (GLMMs with normal distribution and identity link function) to generate models predicting infection intensity in all three recording conditions with activity level and time in stimulus zone as fixed factors, assigning subject number and mass as random factors. The best model was chosen by using the lowest Akaike information criterion value corrected for small sample size (AICc). Models with AICc values greater than 10 above the minimum were deemed to have no support. All analyses were conducted using SPSS v. 19.0.
3. Results
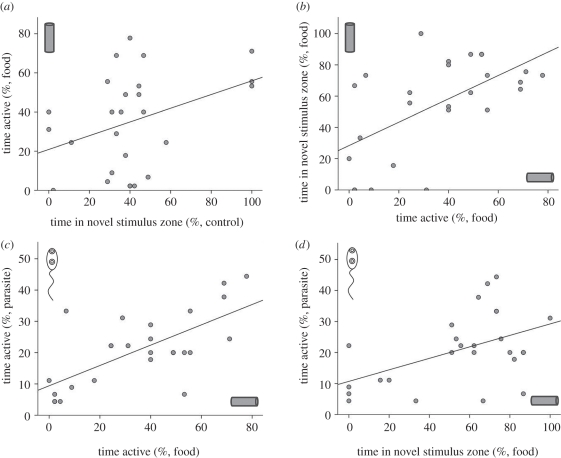
Individual tadpoles showed consistency in their activity level across the three recording conditions, with a significant difference among subjects (Pearson χ2 = 2.416, d.f. = 52, p = 0.046); however, tadpoles were not consistent in their time spent in the stimulus zone (Pearson χ2 = 6.047, d.f. = 52, p = 0.116). Of the 26 tadpoles, 35 per cent decreased their activity levels in both the novel food and parasite conditions compared with the control, while 42 per cent increased their activity in both conditions compared with the control. Significant positive overall correlations were found between the two behavioural measures among and within conditions, as well as for the same behaviour between conditions (figure 1). There was a significant overall relationship between activity level in the novel food condition and exploratory behaviour in the control condition (r = 0.394, p = 0.047), as well as activity levels in the novel food and parasite recording conditions (r = 0.690, p < 0.001), and activity in the parasite condition and exploratory behaviour in the food condition (r = 0.456, p = 0.019). In addition, there was one significant correlation within a recording condition, with a positive relationship between activity and exploratory behaviour in the novel food condition (r = 0.512, p = 0.007).
Figure 1.
Scatterplots of statistically significant relationships (using Pearson's correlations) involving individual tadpole activity level (percentage of time points swimming) and exploratory behaviour (percentage of time in same zone as stimulus) within and among recording conditions: (a) exploration in control condition and activity in food condition, (b) activity and exploration in novel food condition, (c) activity in novel food and parasite conditions, (d) exploration in novel food condition and activity in parasite condition. Note: linear regression best lines of fit are only to illustrate the nature of the relationships.
Treatment order did not have a significant overall effect (λ = 0.709, F7,18 = 1.057, p = 0.429). The relationship between tadpole mass and infection intensity was also not significant (adjusted R2 = −0.041, F1,24 = 0.026, p = 0.873). Overall mean parasite cyst abundance was 1.58 + 0.385 (s.e.), with a range of 0–8 cysts observed across the 26 individual tadpoles (eight were uninfected).
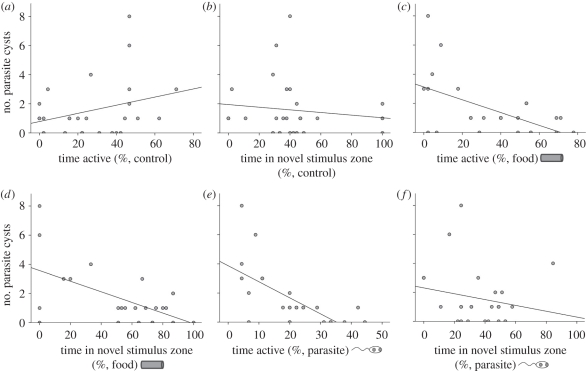
The best overall model predicting host parasite load included both activity and exploratory behaviour in the parasite condition (AICc = 45.644). Activity in the parasite condition as a lone predictor generated the second best model (AICc = 46.026), but the model with exploration only in this condition was not retained (AICc = 58.260). The models generated using activity level and exploratory behaviour, respectively, in the novel food condition were also retained (AICc = 54.676; AICc = 55.299), as was the model combining both of these predictors (AICc = 53.329). Less active tadpoles tended to have higher parasite loads, as did those that spent less time in the same zone as the novel food stimulus (figure 2). None of the models using the behavioural measures from the control condition as predictors of infection intensity were retained (table 1).
Figure 2.
Scatterplots illustrating relationships between individual tadpole infection intensity (number of parasite cysts) and either activity level (percentage of time points swimming) or exploratory behaviour (percentage of time in same zone as stimulus) among recording conditions: (a) activity in control condition, (b) exploration in control condition, (c) activity in novel food condition, (d) exploration in novel food condition, (e) activity in parasite condition and (f) exploration in parasite condition. Panels (c–e) represent significant behavioural predictors of infection intensity resulting from generalized linear mixed models. Linear regression best lines of fit are only to illustrate the nature of the relationships.
Table 1.
Results of the generalized linear mixed models predicting parasite infection intensity with host activity level and exploratory behaviour in the control, novel food and parasite conditions as fixed factors (subject number and mass included as random factors). AICc = Akaike information criterion corrected for small sample size, d.f. = degrees of freedom. The overall best model based on the lowest AICc value is indicated with italics.
| predictor(s) | AICc | d.f. | F | p |
|---|---|---|---|---|
| control condition | ||||
| activity | 57.785 | 1,24 | 0.877 | 0.356 |
| exploration | 59.666 | 1,24 | 0.320 | 0.577 |
| activity + exploration | 58.210 | 2,23 | 0.477 | 0.627 |
| novel food condition | ||||
| activity | 54.676a | 1,24 | 4.807 | 0.038 |
| exploration | 55.299a | 1,24 | 4.977 | 0.035 |
| activity + exploration | 53.329a | 2,23 | 3.318 | 0.054 |
| parasite exposure condition | ||||
| activity | 46.026a | 1,24 | 14.601 | 0.001 |
| exploration | 58.260 | 1,24 | 0.334 | 0.569 |
| activity + exploration | 45.644 | 2,23 | 6.997 | 0.004 |
aDenotes retained models with AICc values less than or equal to 10 above the minimum.
4. Discussion
Our results indicate that larval wood frogs exhibit behavioural syndromes based on the observed correlations within/among behaviours [1] and that these behavioural consistencies are important for susceptibility to parasite infection. There was consistency within individuals with respect to activity level and overall differences among individuals in their patterns of activity, reaction to a novel food source and reaction to parasite exposure. These correspond to the major traits of activity, exploration–avoidance and boldness–shyness, respectively, thus demonstrating that wood frog tadpoles have distinct ‘personalities’ as reported for a variety of animal taxa [5,20]. Activity level in response to the novel food source was a strong predictor of activity level when confronted with parasites, as was time spent in close proximity to the novel food (i.e. exploratory behaviour). Saliently, tadpoles tended to either increase their activity level in both the novel food and parasite conditions compared with the control or show a decrease in both. Individuals were not consistent in their time spent in the stimulus zone across conditions, but this is not surprising given that the parasite infectious stages are mobile. In addition, there was a significant positive correlation between activity in the novel food condition and time spent near/exploiting the novel food. This agrees with the general personality type corresponding to the proactive–reactive axis [24] described earlier, with ‘proactive’ tadpoles tending to be more bold, active and exploratory in different situations. Only one behaviour in the control condition (time in stimulus zone) showed a significant correlation (with activity in novel food condition), suggesting that ‘reactive’ individuals were not simply generally lethargic. While we cannot currently speculate about the stability of this particular behavioural syndrome in wood frog larvae through time, even short-term behavioural correlations can be ecologically important [35]. Given the short period of parasite exposure here, resulting in relatively low infection intensities compared with those observed in the field, the stability of such a syndrome requires further study as the link between the proactive–reactive axis and infection intensity may be more pronounced when parasites have limited opportunity.
Importantly, personality differences here were strong predictors of infection intensity and provide the first empirical demonstration that host behaviours in other contexts are related to behaviours mitigating infection risk and, ultimately, host parasite load. While previous studies have linked behavioural syndromes with patterns of parasitism by using already infected individuals [11,12], here we show that pre-existing general personality differences in animals can influence individual susceptibility to infection. Individuals with high levels of activity in both the novel food and the parasite conditions had lower infection levels, as did those who explored the novel food source, such that more proactive tadpoles were less susceptible to infection. High levels of activity and exploratory behaviour have been suggested to increase an individual's chances of infection with novel parasites if they readily approach novel entities in their environment, and such individuals may also be exposed to a wider array of parasites if they range more widely [17]. However, more active and exploratory individuals had lower parasite loads in the present study, which is probably attributable to the effective anti-parasite behaviours reported for larval amphibians in response to trematode cercariae, such as parasite evasion and removal [27,28]. Consequently, certain individuals may be more susceptible to infections simply based on their general personality type.
Alternatively, individuals with high activity and exploration levels may have been in superior condition to begin with and better able to resist/tolerate parasite infection through non-behavioural mechanisms, particularly given the links between immunity and the proactive–reactive axis [24]. We do not know if proactive tadpoles here also had an advantage through superior non-behavioural resistance mechanisms; however, studies examining behavioural influences on disease susceptibility should ideally employ both immunological assays and controlled exposure experiments to investigate the immunocompetence of different behavioural types [17].
We might expect most larval amphibians to show strong anti-parasite behaviours in response to trematode cercariae, given that these parasites are highly prevalent in most amphibian populations (including wood frogs), causing considerable mortality and pathology [36], and behavioural strategies have been demonstrated to be highly effective across tadpole and trematode species [27,28]. But why the positive relationships between responses to a novel food source and the threat of parasitism observed here? A major explanatory hypothesis for the existence of behavioural syndromes is that certain combinations of behavioural traits are greater than others with respect to overall fitness payoffs [1]. In this scenario, consistently high levels of activity and exploratory behaviour across different contexts may provide greater benefits than alternative combinations. For example, high levels of activity, boldness and exploration may provide additional advantages by increasing the chances of discovering/dominating important resources in heterogeneous environments [7]. Larval amphibians provide an excellent model to examine behavioural syndromes, including parasitism owing to the nature of, and variation among, their life histories.
Many animals may face a trade-off between behavioural traits that confer an advantage in one context but incur a cost in another. For example, personality traits associated with energy intake are expected to reach a maximum when they not only additionally reduce the cost of parasitism but predators are absent [18]. The adaptiveness of personality traits associated with energy intake and use (those promoting fast growth/high fecundity), which can contribute to active, bold and exploratory behaviours, may also affect an individual's parasite exposure and resistance [18], suggesting a link between the two benefits. Because wood frog tadpoles most commonly inhabit ephemeral ponds and face the threat of the pond drying before completing metamorphosis, these larvae are particularly sensitive to their foraging and growth rates [37]. As such, a behavioural syndrome in which larval wood frogs consistently exhibit high levels of activity, boldness and exploration in different contexts could be selected for by conferring dual benefits—maximal energy acquisition and decreased risk of trematode parasitism. Such syndromes may be particularly likely if behavioural correlations are mirrored by those at the genetic level [6,38]. Future studies should examine whether amphibian species or populations living in environments with high predation and parasitism risk are generally more likely to exhibit such behavioural syndromes/personalities than those living in low predation and parasitism areas [18].
Including diseases in studies of personalities/behavioural syndromes will aid in clarifying the ecological circumstances in which parasite-associated behaviours may be important, as well as situations in which pathogens can influence the evolution and stability of such syndromes, similar to the influences of habitat stability and predation [39]. Further examinations of how individual personality relates to disease risk will also be needed in order to determine the importance of behavioural syndromes for pathogen distribution within and among populations. This is particularly important because theoretical models have shown that a heterogeneous parasite distribution among hosts can have significant consequences for host–parasite population dynamics [40]. Consequently, host populations experiencing selection pressure for or against certain personality types/behavioural syndromes may experience different risks and consequences of disease, particularly if their ecological circumstances are changing (e.g. introduced predators, increased risk of pathogen contact).
Acknowledgements
All procedures were carried out under the approval of the Brandon University Animal Care Committee.
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) and from the Brandon University Research Council to J.K., as well as an NSERC Undergraduate Student Research Assistantship (USRA) to J.C.R.
References
- 1.Sih A., Bell A. M., Johnson J. C. 2004. Behavioural syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378 10.1016/j.tree.2004.04.009 (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 2.Réale D., Reader S. M., Sol D., McDougall P. T., Dingemanse N. J. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318 10.1111/j.1469-185X.2007.00010.x (doi:10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 3.Briffa M., Rundle S. D., Fryer A. 2008. Comparing the strength of behavioural plasticity and consistency across situations: animal personalities in the hermit crab Pagurus bernhardus. Proc. R. Soc. B 275, 1305–1311 10.1098/rspb.2008.0025 (doi:10.1098/rspb.2008.0025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kortet R., Hedrick A. 2007. A behavioural syndrome in the field cricket Gryllus integer: intrasexual aggression is correlated with activity in a novel environment. Biol. J. Linn. Soc. 91, 475–482 10.1111/j.1095-8312.2007.00812.x (doi:10.1111/j.1095-8312.2007.00812.x) [DOI] [Google Scholar]
- 5.Dingemanse N. J., Réale D. 2005. Natural selection and animal personality. Behaviour 142, 1165–1190 10.1163/156853905774539445 (doi:10.1163/156853905774539445) [DOI] [Google Scholar]
- 6.Bell A. M., Aubin-Horth N. 2010. What can whole genome expression data tell us about the ecology and evolution of personality? Phil. Trans. R. Soc. B 365, 4001–4012 10.1098/rstb.2010.0185 (doi:10.1098/rstb.2010.0185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf M., van Doorn G. S., Leimar O., Weissing F. J. 2007. Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–585 10.1038/nature05835 (doi:10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- 8.Lafferty K. D. 2006. Can the common brain parasite, Toxoplasma gondii, influence human culture? Proc. R. Soc. B 273, 2749–2755 10.1098/rspb.2006.3641 (doi:10.1098/rspb.2006.3641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ezenwa A. 2004. Host social behaviour and parasitic infection: a multifactorial approach. Behav. Ecol. 15, 446–454 10.1093/beheco/arh028 (doi:10.1093/beheco/arh028) [DOI] [Google Scholar]
- 10.Loys Richards E., van Oosterhout C., Cable J. 2010. Sex-specific differences in shoaling affect parasite transmission in guppies. PLoS ONE 10, e13285. 10.1371/journal.pone.0013285 (doi:10.1371/journal.pone.0013285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson D. S., Coleman K., Clark A. B., Biederman L. 1993. Shy–bold continuum in pumpkinseed sunfish (Lepomis gibbosus): an ecological study of a psychological trait. J. Comp. Psychol. 107, 250–260 10.1037/0735-7036.107.3.250 (doi:10.1037/0735-7036.107.3.250) [DOI] [Google Scholar]
- 12.Boyer N., Réale D., Marmet J., Pisanu B., Chapuis L. 2010. Personality, space use and tick load in an introduced population of Siberian chipmunks Tanias sibiricus. J. Anim. Ecol. 79, 538–547 10.1111/j.1365-2656.2010.01659.x (doi:10.1111/j.1365-2656.2010.01659.x) [DOI] [PubMed] [Google Scholar]
- 13.Hart B. L. 1997. Behavioural defence. In Host–parasite evolution: general principle and avian models (eds Clayton D. H., Moore J.), pp. 59–77 Oxford, UK: Oxford University Press [Google Scholar]
- 14.Hart B. L. 1990. Behavioural adaptations to pathogens and parasites: five strategies. Neurosci. Biobehav. Rev. 14, 273–294 10.1016/S0149-7634(05)80038-7 (doi:10.1016/S0149-7634(05)80038-7) [DOI] [PubMed] [Google Scholar]
- 15.Raffel T. R., Martin L. B., Rohr J. R. 2008. Parasites as predators: unifying natural enemy ecology. Trends Ecol. Evol. 23, 610–618 10.1016/j.tree.2008.06.015 (doi:10.1016/j.tree.2008.06.015) [DOI] [PubMed] [Google Scholar]
- 16.Rohr J. R., Swan A., Raffel T. R., Hudson P. J. 2009. Parasites, info-disruption, and the ecology of fear. Oecologia 159, 447–454 10.1007/s00442-008-1208-6 (doi:10.1007/s00442-008-1208-6) [DOI] [PubMed] [Google Scholar]
- 17.Barber I., Dingemanse N. J. 2010. Parasitism and the evolutionary ecology of animal personality. Phil. Trans. R. Soc. B 365, 4077–4088 10.1098/rstb.2010.0182 (doi:10.1098/rstb.2010.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kortet R., Hedrick A. V., Vainikka A. 2010. Parasitism, predation and the evolution of animal personalities. Ecol. Lett. 13, 1449–1458 10.1111/j.1461-0248.2010.01536.x (doi:10.1111/j.1461-0248.2010.01536.x) [DOI] [PubMed] [Google Scholar]
- 19.Coats J., Poulin R., Nakagawa S. 2010. The consequences of parasitic infections for host behavioural correlations and repeatability. Behaviour 147, 367–382 10.1163/000579509X12574307194101 (doi:10.1163/000579509X12574307194101) [DOI] [Google Scholar]
- 20.Sih A., Bell A. M. 2008. Insights for behavioural ecology from behavioural syndromes. Adv. Stud. Behav. 38, 227–281 10.1016/S0065-3454(08)00005-3 (doi:10.1016/S0065-3454(08)00005-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw D. J., Grenfell B. T., Dobson A. P. 1998. Patterns of macroparasite aggregation in wildlife host populations. Parasitology 117, 597–610 10.1017/S0031182098003448 (doi:10.1017/S0031182098003448) [DOI] [PubMed] [Google Scholar]
- 22.Poulin R., Rau M. E., Curtis M. A. 1991. Infection of brook trout fry, Salvelinus fontinalis, by ectoparasitic copepods: the role of host behaviour and initial parasite load. Anim. Behav. 41, 467–476 10.1016/S0003-3472(05)80849-8 (doi:10.1016/S0003-3472(05)80849-8) [DOI] [Google Scholar]
- 23.Brunner J. L., Ostfeld R. S. 2008. Multiple causes of variable tick burdens on small-mammal hosts. Ecology 89, 2259–2272 10.1890/07-0665.1 (doi:10.1890/07-0665.1) [DOI] [PubMed] [Google Scholar]
- 24.Koolhaas J. M. 2008. Coping styles and immunity in animals: making sense of individual variation. Brain Behav. Immun 22, 662–667 10.1016/j.bbi.2007.11.006 (doi:10.1016/j.bbi.2007.11.006) [DOI] [PubMed] [Google Scholar]
- 25.Behringer D. C., Butler M. J., Shields J. D. 2006. Avoidance of disease by social lobsters. Nature 441, 421. 10.1038/441421a (doi:10.1038/441421a) [DOI] [PubMed] [Google Scholar]
- 26.Moore J. 2002. Parasites and the behaviour of animals. Oxford, UK: Oxford University Press [Google Scholar]
- 27.Koprivnikar J., Forbes M. R., Baker R. L. 2006. On the efficiency of antiparasite behaviour: a case study of tadpole susceptibility to cercariae of Echinostoma trivolvis. Can. J. Zool. 84, 1623–1629 10.1139/z06-158 (doi:10.1139/z06-158) [DOI] [Google Scholar]
- 28.Daly E. W., Johnson P. T. J. 2011. Beyond immunity: quantifying the effects of host anti-parasite behaviour on parasite transmission. Oecologia 165, 1043–1050 10.1007/s00442-010-1778-y (doi:10.1007/s00442-010-1778-y) [DOI] [PubMed] [Google Scholar]
- 29.Gosner N. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16, 183–190 [Google Scholar]
- 30.Belden L. K. 2006. Impact of eutrophication on wood frog, Rana sylvatica, tadpoles infected with Echinostoma trivolvis cercariae. Can. J. Zool. 84, 1315–1321 10.1139/z06-119 (doi:10.1139/z06-119) [DOI] [Google Scholar]
- 31.Koprivnikar J., Forbes M. R., Baker R. L. 2008. Larval amphibian growth and development under varying density: are parasitized individuals poor competitors? Oecologia 155, 641–649 10.1007/s00442-007-0937-2 (doi:10.1007/s00442-007-0937-2) [DOI] [PubMed] [Google Scholar]
- 32.Fried B. 2001. Biology of echinostomes except Echinostoma. Adv. Parasitol. 49, 163–210 10.1016/S0065-308X(01)49040-3 (doi:10.1016/S0065-308X(01)49040-3) [DOI] [PubMed] [Google Scholar]
- 33.Poulin R. 2010. Parasite manipulation of host behaviour: an update and frequently asked questions. Adv. Stud. Behav. 41, 151–186 10.1016/S0065-3454(10)41005-0 (doi:10.1016/S0065-3454(10)41005-0) [DOI] [Google Scholar]
- 34.Minderman J., Reid J. M., Hughes M., Denny M. J. H., Hogg S., Evans P. G. H., Whittingham M. J. 2010. Novel environment exploration and home range size in starlings Sturnus vulgaris. Behav. Ecol. 21, 1321–1329 10.1093/beheco/arq151 (doi:10.1093/beheco/arq151) [DOI] [Google Scholar]
- 35.Luttbeg B., Sih A. 2010. Risk, resources and state-dependent adaptive behavioural syndromes. Phil. Trans. R. Soc. B 365, 3977–3990 10.1098/rstb.2010.0207 (doi:10.1098/rstb.2010.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson P. T. J., McKenzie V. J. 2008. Emerging helminthiases in amphibians: lessons from Ribeiroia and Echinostoma. In The biology of echinostomes (eds Fried B., Toledo R.), pp. 249–280 Berlin, Germany: Springer [Google Scholar]
- 37.Fraker M. E. 2010. Risk assessment and anti-predator behavior of wood frog (Rana sylvatica) tadpoles: a comparison with green frog (Rana clamitans) tadpoles. J. Herpetol. 44, 390–398 10.1670/09-033.1 (doi:10.1670/09-033.1) [DOI] [Google Scholar]
- 38.Dochtermann N. A. 2011. Testing Cheverud's conjecture for behavioural correlations and behavioural syndromes. Evolution 65, 1814–1820 10.1111/j.1558-5646.2011.01264.x (doi:10.1111/j.1558-5646.2011.01264.x) [DOI] [PubMed] [Google Scholar]
- 39.Dingemanse N. J., Wolf M. 2010. Recent models for adaptive personality differences: a review. Phil. Trans. R. Soc. B 365, 3947–3958 10.1098/rstb.2010.0221 (doi:10.1098/rstb.2010.0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson R. M., May R. M. 1979. Population biology of infectious diseases: part I. Nature 280, 361–367 10.1038/280361a0 (doi:10.1038/280361a0) [DOI] [PubMed] [Google Scholar]