Abstract
Social castes of eusocial insects may have arisen through an evolutionary modification of an ancestral reproductive ground plan, such that some adults emerge from development physiologically primed to specialize on reproduction (queens) and others on maternal care expressed as allo-maternal behaviour (workers). This hypothesis predicts that variation in reproductive physiology should emerge from ontogeny and underlie division of labour. To test these predictions, we identified physiological links to division of labour in a facultatively eusocial sweat bee, Megalopta genalis. Queens are larger, have larger ovaries and have higher vitellogenin titres than workers. We then compared queens and workers with their solitary counterparts—solitary reproductive females and dispersing nest foundresses—to investigate physiological variation as a factor in caste evolution. Within dyads, body size and ovary development were the best predictors of behavioural class. Queens and dispersers are larger, with larger ovaries than their solitary counterparts. Finally, we raised bees in social isolation to investigate the influence of ontogeny on physiological variation. Body size and ovary development among isolated females were highly variable, and linked to differences in vitellogenin titres. As these are key physiological predictors of social caste, our results provide evidence for developmental caste-biasing in a facultatively eusocial bee.
Keywords: Megalopta genalis, social evolution, division of labour, ground plan, caste determination, vitellogenin
1. Introduction
One of the distinguishing characteristics of eusocial insects is separation of individuals into reproductive castes (i.e. queens and workers). Queens and workers are behaviourally representative of two phases of a solitary reproductive cycle: (i) reproductive (egg-laying) and (ii) brood care. A growing body of evidence suggests that eusociality evolved through developmental decoupling of this ancestral reproductive ‘ground plan’ [1–5]. Variation stemming from ontogenetic influences could result in some adults being more physiologically primed for reproduction, while others are biased towards allo-maternal behaviour. This variation could provide a substrate for selection to act upon in the evolutionary origins of reproductive castes by influencing reproductive behaviour. If developmental shifts in ancestral character states provide a basis for queen and worker caste evolution, we would expect to find physiological underpinnings for reproductive caste in species that exhibit the simplest forms of sociality. We further expect variation in these physiological traits to be influenced by individual ontogeny.
The role of development in caste differentiation is well studied among species that are obligately eusocial [6–13]. Although these studies have been extremely valuable, insights into how solitary ground plans were first modified may be obscured by the novel evolutionary pressures arising from obligate reproductive castes [14–20]. Thus, species that still exhibit incipient forms of sociality are particularly important to study because they are more representative of the ancestral solitary state [5,21–23]. Such species are characterized by small colonies with division of labour and non-morphological castes that specialize on either reproduction or foraging [24,25]. Caste determination in these species is generally considered to occur in the adult stage and be highly influenced by dominance interactions, whereby differences in reproductive physiology (e.g. ovary development) are regarded as the result of overt suppression in response to aggression from a larger, dominant queen [26]. Accordingly, physiological correlates of ovary development, such as vitellogenin (Vg) protein titres, have not been studied in species with flexible social organization. This approach overlooks potential organizational effects of the nutritional, endocrine and molecular ontogenetic environments on the adult phenotype, and precludes a complete understanding of eusocial evolution [27–30]. Here, we measure Vg titres in conjunction with social behaviour, body size and reproductive physiology for the first time in an incipiently social species, the facultatively eusocial halictid bee Megalopta genalis (Halictidae).
We tested the hypothesis that variation in physiological traits arising from development served as a platform for caste evolution among eusocial insects by first identifying morphological and physiological correlates of reproductive caste among M. genalis queens and workers. We then investigated whether caste differences could arise from a decoupling of reproductive and maternal behaviour from an ancestral solitary ground plan by considering how these parameters vary in comparison across (i) solitary and queen reproductive females, and (ii) dispersing females versus philopatric workers. Finally, we reared females in isolation from the social environment to quantify the variation in these parameters that emerge from developmental processes. Our findings suggest ontogenetic caste-biasing in M. genalis, and support hypotheses of variation stemming from development as a mechanistic basis for eusocial evolution.
2. Study organism
The neotropical bee M. genalis has a facultative social organization where eusocial and solitary nests are temporally and spatially contemporaneous within a single well-studied population on Barro Colorado Island (BCI), in the Republic of Panama [31,32]. Social and solitary nests represent long-term alternative reproductive phenotypes, rather than temporary ontogenetic phases of a single life cycle [32–34]. Females (both future gynes and workers) and males are produced asynchronously and continuously throughout the tropical dry season and early wet season [35]. We were therefore able to evaluate the physiological and developmental dynamics of sociality without seasonality or environmental variability as confounding factors.
New nests are initiated by a single foundress, and 25 to 50 per cent of foundresses maintain this solitary life history by producing only sons in their first brood of offspring, thereby lacking potential workers [31–34]. This is likely to be an alternative reproductive strategy, rather than the result of sperm limitation, because solitary females have mated, occasionally produce daughters in subsequent broods, and have, on average, equal reproductive output compared with social queens [32]. The male-biased sex ratio of the first brood in solitary nests is not necessarily a later adaptation to a social life history. Split sex ratios occur in other solitary bees, and may be expected from a theoretical standpoint [36–38].
The alternative (social) strategy is to lay both male and female eggs in the first brood, with most of the latter remaining in their natal nests as non-reproductive workers [32]. Upon the emergence of workers, queens cease nearly all foraging, exhibit dominance behaviour and are fed by workers through trophallaxis [33,34]. Dispersing gynes are apparently produced in subsequent broods, with the help of workers [32].
The behaviour of dispersers and solitary females is representative of an ancestral solitary ground plan in that they disperse from their natal nest, initiate nests of their own and then begin a cycle of cell building, cell provisioning, egg laying and defending their developing offspring. Reproductive females that recruit workers are defined as queens because they cease cell building and provisioning to specialize on egg laying. Workers are also derived from this solitary life cycle in that they forego dispersal, nest founding and egg laying to specialize on cell building and provisioning behaviour.
3. Methods
(a). Source of bees for physiological and morphological comparison
All fieldwork and sample collections were carried out on BCI in 2008 and 2009 [31–33]. We included five groups of bees for comparison in our study: queens, workers, solitary reproductive females (‘solitary females’), new nest foundresses (‘dispersers’) and females reared in social isolation (‘isolated females’). Queens and solitary females are nest foundresses that have had at least one offspring emerge (electronic supplementary material, methods M1). They are further classified as queens or solitary females, depending on whether they retained at least one worker. Dispersers are future queens or solitary females, depending on whether they eventually produce workers. They differ from queens and solitary females in that they have not yet laid eggs and are probably younger (table 1). Workers are presumably similar in age to dispersers, but have remained in their natal nest as subordinate helpers, rather than dispersing to found a nest of their own. We made four sets of comparisons: (i) queens versus workers, (ii) solitary females versus queens, (iii) dispersers versus workers, and (iv) isolated females versus dispersers and workers. With the exception of queens and workers, each comparison used sets of similarly aged bees. Queens, solitary females and workers were collected from observation nests in the field, with detailed information about each individual's life history (electronic supplementary material, methods M1 and figure F1). We classified females emerging in observation nests as workers if they remained in their natal nests and foraged (electronic supplementary material, methods M1). Dispersers were collected from newly initiated natural nests, prior to laying eggs (electronic supplementary material, methods M2).
Table 1.
Source of bees for morphological and physiological comparisons.
| group | n | age (days) | source |
|---|---|---|---|
| queens | 23 | 61–108 | post-reproductive observation nests |
| solitary females | 15 | 62–107 | post-reproductive observation nests |
| workers | 23 | 5–38 | post-reproductive observation nests |
| dispersers | 21 | similar to workersa | new natural nests |
| isolated females | 93 | 10 | reared in cages from pupal stage |
aMegalopta genalis are typically less than 10 days old when they disperse from their natal nests. We collected dispersers from natural nests that had been newly initiated within 1 to 9 ± 3 days. Therefore, these bees are probably similar in age to workers.
Our methods ensured that each observation nest foundress had equal opportunity to remain solitary or develop a social nest. We reared individual female pupae collected from natural nests in tissue culture trays, and placed them in standardized observation nests within one day of eclosion, thereby eliminating variation in social interactions with natal nest mates and nest quality. These females, upon later producing adult offspring, were classified as either queens (with at least one active worker at the time of collection) or solitary females (without workers; electronic supplementary material, methods M1). Seasonal and local resource effects were unlikely to explain differences in social organization, as there were no differences in eclosion date for solitary females and social queens (χ2 = 1.47, p = 0.69, n = 38) nor the date their first offspring emerged (χ2 = 0.96, p = 0.81, n = 37), and social and solitary nests developed in close proximity (less than 1 m apart). We collected queens and solitary foundresses across a range of ages to ensure we captured age-related variation in our samples (queens: mean = 81 days, range = 61–108 days; solitary females: mean = 84 days, range = 62–107 days). Variation in physiology and morphology among solitary females and queens is therefore unlikely, owing to differences in nest quality, spatial and temporal resource availability, early adult experience or age at the time of collection. We also collected workers across a range of ages.
While it is likely that most of the foundresses we collected from new natural nests were young dispersers (based on ovary dissections), and therefore within the same age range as workers (mean age = 17 days, range = 5–38 days; table 1), it is possible that some were secondary dispersers whose original nests had been destroyed or usurped.
Differences between established workers and dispersers could reflect variation in both development and adult experience. In order to isolate the range of developmental sources of variation among the castes, we compared known workers and dispersers with isolated females. Female pupae were collected from natural nests and reared in plastic cages kept under ambient forest conditions in complete darkness (while still developing) or very dim light (after eclosion) to replicate light conditions experienced in natural nests (electronic supplementary material, methods M3). This experiment was replicated in 2008 and 2009. In 2008, females (n = 50) were randomly assigned to a collection group: 1, 4 or 8 days post-eclosion. In 2009, we collected all bees (n = 93) 10 days post-eclosion, when they were similar in age to new workers and recent dispersers. We used only 2009 10-day-old isolated females for comparison with workers and dispersers.
All collections were made within a five-week time period (26 April 2008–31 May 2008 and 23 April 2009–24 May 2009) to minimize potential seasonal effects (electronic supplementary material, methods M4). Megalopta genalis are crepuscular foragers [31]. We therefore completed all collections between 1330 and 1700 to minimize circadian effects, and to ensure that all bees would be present in the nest. The contents of each nest were recorded upon collection.
(b). Morphological and physiological measurements
We measured head width, ovary size, maximum oocyte length, and assayed Vg titres and mating status for every bee, except for ovary size in isolated females that were 1 or 4 days old. Previous data indicated 1–4 day old bees do not have detectable ovary development. Head width is a reliable estimate of body size [39–41], and is correlated with other body size metrics, such as intertegular width and dry weight [35,39] (electronic supplementary material, methods M5). Mating status was also assessed by noting the presence or absence of spermatozoa in the spermatheca, except when the spermatheca was damaged during dissection. Ovary size was measured as the total area (right + left) and maximum oocyte length as the longest single oocyte from digital photographs [39,40,42] (electronic supplementary material, methods M5). Oocyte length was highly correlated with ovary size in all groups (r2 = 0.95, p < 0.0001, n = 173), and we therefore present only ovary size results. To control for differences in body size, we calculated an ovarian index (ovary size/head width) to investigate the amount of ovary development that is independent of body size. Vg is a yolk precursor protein necessary for oogenesis among insects [43–47]. Modification of Vg function has been identified as an important mechanism governing complex forms of division of labour within highly eusocial honeybees, suggesting that selection on Vg function has been on-going throughout eusocial evolution [14,16–18,48]. Vg titres were assayed relative to β-galactosidase standard curves with sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), following Nelson et al. [18] (electronic supplementary material, methods M6). Western blots were used to confirm Vg band identity (electronic supplementary material, methods M6 and figure F2).
(c). Statistical analysis
All statistical analyses were in Stata (v. 9.2). We compared head width, ovary size, size-scaled ovarian index and Vg titre between queens and workers, queens and solitary females, and dispersers and workers, with non-parametric Mann–Whitney U-tests. Differences in sample sizes between each metric are due to ovary damage during dissection or poor preservation of some protein samples. We used reverse step-wise linear regression to investigate the influence of head width and Vg on ovary size. We also identified which factors (body size, ovary size, ovarian index or Vg) were important predictors of social behaviour with a reverse step-wise logistic regression, where social classification (queen versus solitary female or disperser versus worker) was the binary outcome. Head width was fixed in this model, because unlike ovary size and Vg titres, head width is determined at eclosion and cannot be affected by socio-ecological context during adulthood. We investigated changes in Vg titres as a function of early post-eclosion ageing with a Kruskal–Wallis test and a Levene's test for unequal variance among 2008 isolated females. The relationship between Vg titre and age was also assessed with linear regression in this group. K-means clustering was used to separate 2009 isolated females into two groups based on ovarian development, to further analyse the relationship between ovary development and Vg [49]. All p-values are two-tailed and adjusted by a Bonferroni correction factor when multiple tests are involved. All means are presented ± 1 standard deviation.
4. Results
(a). Morphological and physiological correlates of division of labour
Queens and workers significantly differed in each measured parameter (see electronic supplementary material, table T1 for means ± s.d.). Head width was larger for queens than workers (Z = 3.20, p = 0.001, n = 46). Queens also had larger ovaries than workers (Z = 5.61, p < 0.0001, n = 46), even when scaled for body size (Z = 5.55, p < 0.0001, n = 46). Vg titres were higher in queens than in workers (Z = 2.67, p = 0.008, n = 43).
(b). Queens versus solitary females
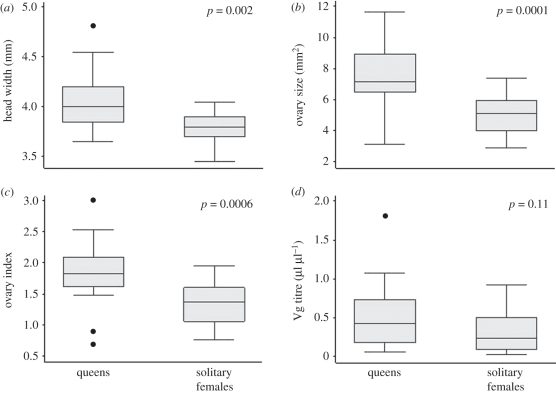
Queens had larger heads (Z = 3.09, p = 0.002, n = 38) and larger ovaries (Z = 3.84, p = 0.0001, n = 38) than reproductive solitary females (figure 1a,b and electronic supplementary material, table T1). Differences in the ovarian index scaled for body size were also significant (Z = 3.45, p < 0.0006, n = 38; figure 1c and electronic supplementary material, table T1). Vg titres, however, did not differ between queens and solitary females (Z = 1.59, p = 0.11, n = 36; figure 1d and electronic supplementary material, table T1), and head width was not a significant predictor of Vg titre (F = 1.38, r2 = 0.04, p = 0.25, n = 36). All queens (n = 22) and solitary females (n = 13) were inseminated. In a step-wise reverse linear regression, neither head width nor Vg titre were significant predictors of ovarian development among queens and solitary females (F = 0.01, r2 = 0, p = 0.99, n = 36).
Figure 1.
Morphological and physiological differences between queens and solitary females from observation nests. (a) Head width; (b) ovary size; (c) size-scaled ovary index; and (d) haemolymph Vg titre. p-values are from a Mann–Whitney U-test.
Head width (Z = 2.57, p = 0.01) and the size-scaled ovarian index (Z = 2.54, p = 0.01), but not ovary size or Vg, were significant predictors of social phenotype (solitary female versus queen) in a reverse step-wise logistic regression, which was highly significant overall (χ2 = 27.41, p < 0.0001, n = 36), and classified queens and solitary females with 86 per cent accuracy. Despite differences in morphology and physiology, there was no difference in the age or date at which queens and solitary females laid their first egg (oviposition age: queens = 25.87 ± 8.10 days, solitary females = 28.80 ± 9.99, Z = −1.14, p = 0.25, n = 38; date of oviposition: χ2 = 0.65, p = 0.89).
(c). Workers versus dispersers
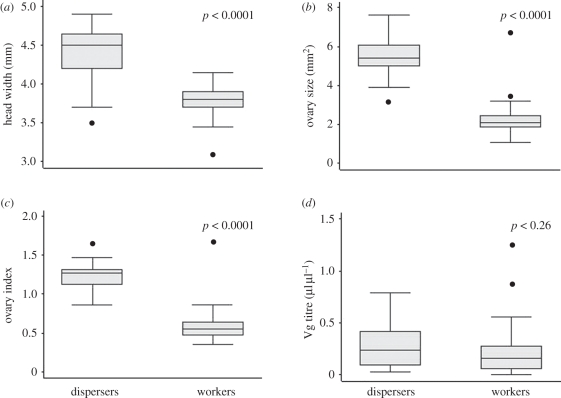
Dispersers had significantly larger heads (Z = 4.51, p < 0.0001, n = 44) and larger ovaries (Z = 5.20, p < 0.0001, n = 44) than workers (figure 2a,b and electronic supplementary material, table T1). Differences in ovary development were significant, even after scaling for body size (Z = 5.16, p < 0.0001, n = 44; figure 2c and electronic supplementary material, table T1). Vg titres did not differ significantly among dispersers and workers (Z = 1.12, p = 0.26, n = 41; figure 2d and electronic supplementary material, table T1), and head width was not a strong predictor of Vg (F = 3.75, r2 = 0.09, p = 0.06, n = 41). Most (15 out of 17) dispersers were inseminated. The two uninseminated dispersers came from 1–3-day-old nests. In contrast, only 1 of 22 workers was inseminated (frequency of mating in dispersers versus workers: χ2 = 29.86, p < 0.0001). This worker also had a large oocyte, was not foraging much, and may have been in the process of superseding her queen, who was 106 days old [32]. In a reverse step-wise regression, head width (t = 7.44, p < 0.001), but not Vg titre, was a significant predictor of ovary size among dispersers and workers (F = 55.30, p < 0.0001, n = 41, r2 = 0.58).
Figure 2.
Morphological and physiological differences between natural dispersers and workers from observation nests. (a) Head width; (b) ovary size; (c) size-scaled ovary index; and (d) haemolymph Vg titre. p-values are from a Mann–Whitney U-test.
In a reverse step-wise logistic regression, head width and the size-scaled ovarian index, but not ovary size or Vg, were significant predictors of disperser and worker phenotype (overall model: χ2 = 37.62, p < 0.0001, r2 = 0.66, n = 41; head: Z = 1.93, p = 0.05; ovarian index: Z = 2.79, p = 0.005), and the model classified females as workers or dispersers with 95 per cent accuracy.
(d). Socially isolated females
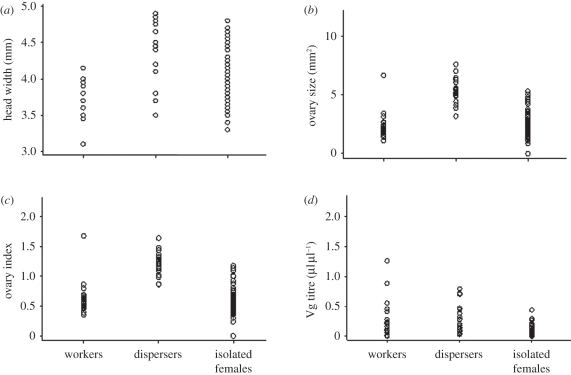
Head-width distributions of 10-day-old socially isolated females overlapped with the distributions of dispersers and workers (Z = 0.59, p = 0.56, n = 137; figure 3 and electronic supplementary material, table T1). Ovary size, the size-scaled ovarian index and Vg titres were significantly lower in 10-day-old isolated females than in dispersers and workers (ovary: Z = −4.21, p < 0.0001, n = 136; ovarian index: Z = −5.00, p < 0.0001, n = 134; Vg: Z = −5.58, p < 0.0001, n = 117; electronic supplementary material, table T1). Head width and Vg titre were significant predictors of ovary size (overall model: F = 29.51, r2 = 0.46, n = 72, p < 0.0001; head: t = 5.03, p < 0.0001; Vg: t = 4.00, p < 0.0001).
Figure 3.
Morphological and physiological variation among workers, dispersers and similarly aged females reared in social isolation. Each circle represents one individual. (a) Head width; (b) ovary size; (c) size-scaled ovary index; and (d) haemolymph Vg titre.
Despite having significantly lower medians, the range of ovary size in 10-day-old isolated females covered 70 per cent of the range found among known dispersers and workers. Visual inspection of figure 3 confirms that there is a great deal of variation in head width among socially isolated females, but variance in ovary development among isolated females is greater than can be explained by body size alone. Most (86%) dispersers had a size-scaled ovary index larger than 1, while all except a single worker had an ovary index of less than 1 (χ2 = 29.62, p < 0.0001, n = 44; figure 3). This worker had also mated and was possibly in the process of usurping her queen. In contrast, 10 per cent of socially isolated females had a size-scaled ovary index greater than 1. We used a k-means function to cluster isolated females into two groups based on the size-scaled ovarian index (o/h1 = 0.92 ± 0.16, n = 13; o/h2 = 0.49 ± 0.11, n = 77). The group with increased ovary activation had significantly higher levels of circulating Vg (Vg1 = 0.13 ± 0.14, n = 13; Vg2 = 0.04 ± 0.06, n = 59; Z = 2.75, p = 0.006, n = 72).
Among 2008 isolated females, Vg titres increased in relative quantity and variance with age (1, 4 and 8 days; linear regression: F = 11.27, r2 = 0.19, p = 0.002, n = 50; Levene's test of variance: W0 = 10.75, p = 0.0001, n = 50). This age association is driven largely by a significant difference in Vg titre between newly emerged and 4-day-old bees, suggesting Vg increases shortly after emergence (Z = −2.64, p = 0.008, n = 39). Vg titres also increased with age among all the 2009 individuals of known caste and isolated bees (F = 48.32, r2 = 0.24, p < 0.0001, n = 155), indicating that the relationship between age and Vg was consistent between years, and between cage-reared and wild-caught bees.
(e). Ovary development independent of body size
Unlike for the queen and solitary female group, head width was a significant predictor of ovarian development among dispersers, workers and the socially isolated females. This suggests that body size is a more defining feature of caste in this species because it could limit ovary size. We therefore further investigated the roles of body size and ovary size. Within the entire dataset, head width predicted only 4 per cent of the variance in ovary size (F = 7.49, p = 0.007, r2 = 0.04, n = 172). The females with the five largest ovary sizes were all queens. Their head widths ranged between 3.65 and 4.20 mm (3.65, 3.85, 3.85, 3.9, 4.2 mm), but the head widths of the largest bees in the study ranged from 4.80 to 4.90 mm. Among the other castes, 87 per cent of solitary females, 95 per cent of dispersers and 78 per cent of workers had head widths of 3.65 mm or smaller. The bee with the largest ovaries (11.62 mm) had a head width of 3.90 mm. Among the other castes, 27 per cent of solitary females, 86 per cent of dispersers and 35 per cent of workers had head widths of 3.90 mm or smaller. This indicates that body size does not functionally constrain ovary development. Social castes are tightly and independently linked to both body size and ovary development.
5. Discussion
Species with incipient and flexible social organization provide a powerful tool for understanding the evolutionary origins of eusociality. However, most research has been focused on ultimate (rather than proximate) explanations for social behaviour. Thus, mechanisms that are important in social behaviour among highly eusocial species, such as the caste-biasing role of developmental nutrition, have been understudied among less complex social species. While it is clear that caste determination among incipiently eusocial bees is multi-factorial, our results represent the first example of developmental caste-biasing in a facultatively eusocial bee. By identifying the physiological and morphological correlates of reproductive caste among queens and workers, and comparing them in representatives of their solitary counterparts (solitary reproductive females and dispersing nest foundresses), we were able to investigate how variation in physiological traits can provide a substrate for selection to act on fitness payoffs associated with each resulting phenotype in eusocial evolution. We were further able to demonstrate that some of this variation arises during individual development, as is true among species exhibiting highly complex eusociality. This suggests that developmental pathways associated with derived systems for the division of labour have evolved through exploitation of existing sources of variation in ancestral species. This supports the hypothesis that developmental decoupling of the ancestral reproductive life history of a solitary insect may underlie the evolution of eusocial castes.
(a). Morphological and physiological underpinnings of division of labour
Our study is the first quantitative assessment of variation in Vg titres in a halictid bee, which represents an origin of eusociality independent of the better-studied corbiculate bees [50,51]. Queens have significantly higher Vg titres than workers. Queens are also significantly larger and have more ovary activation than workers, although there is a considerable overlap between these groups [39,40]. Therefore, despite exhibiting high levels of behavioural plasticity, M. genalis castes are characterized by physiological differences that reflect their reproductive behaviour.
(b). Evolution of castes from a solitary ground plan
The flexible social organization of M. genalis provides an excellent opportunity to make direct comparisons between social castes (queens and workers) and their hypothesized ancestral solitary counterparts (solitary females and dispersers). Despite being similar in age and reproductive status, queens were larger in body size and had more ovarian development than solitary females. This pattern also held for dispersers and workers. For both dyads, differences in ovarian development were significant, even when scaled for body size, and this size-independent measure of ovary development was a significant predictor of behavioural class (for further discussion of body size, see electronic supplementary material, discussion D1). These patterns are consistent with a decoupling of a solitary reproductive ground plan, whereby queens specialize on reproduction (owing to their dependence on workers) and dispersers gear up to be reproductive, but do not participate in brood care. Workers and solitary females, on the other hand, demarcate at least a portion of their time and resources to brood care, and are thus expected to be in a less-activated state of reproductive physiology. Alternative explanations for differences in ovary development are not mutually exclusive of this decoupling (electronic supplementary material, discussion D1).
From the experimental design, the potential sources of initial variation among queens and solitary females were genetic variation and larval nutrition. Genetic variation is heretofore untested. Differences in ovarian traits at the time of collection may or may not have existed prior to the onset of egg-laying and offspring emergence, and our data cannot distinguish between cause and consequence of the social strategies. Although social phenotype is associated with ovary development and body size, this does not reflect differences in fecundity or overall condition. Body size is not a significant predictor of ovary development or fecundity within reproductive females [32,40], and there was not a significant difference in the age at which queens and solitary females laid their first egg. Furthermore, Vg titres did not significantly differ among solitary females and social queens. Vg levels can be an indicator of nutritional status [52], because they are synthesized in fat bodies, and mediated through nutrient-sensing pathways [20,47,53]. It is therefore unlikely that solitary females were in overall poorer condition, compared with social queens. Results of a tandem study confirm that these solitary females achieved equal reproductive success rate to social queens [32]. This suggests that there are intrinsic differences between social queens and solitary females [32].
These differences are also reflected among dispersers and workers, and could be influenced by organizational effects during development and/or from early adult experience prior to dispersal. Body size is determined prior to adulthood, which suggests that some different characteristic of social phenotype emerge during development. It is also likely, however, that small females are more easily dominated by their queens, which leads to suppression of ovary development and a tendency to work rather than disperse via post-emergence experience [54].
(c). Developmental caste-biasing
A role for larval nutrition on divergence in social phenotype (workers versus future dispersers) is well supported in wasps [27,55–60] (but see [61,62]). Fewer studies have tested this hypothesis among incipiently eusocial bees (but see [63–65]), despite independent origins of sociality in wasps and bees. The relationship between larval nutrition and adult reproductive behaviour has never been studied in solitary wasps or bees. Subordinate and sterile sweat bee workers can become reproductively active if their queen disappears [26,32,40,66,67]. This observation has led to the general consensus that social competition is the major caste determinant among primitively eusocial bees, and has deflected attention from the possible influence of developmental factors in shaping social roles. A worker's capacity to replace a missing queen does not, however, exclude the possibility that developmental processes influenced the initial trajectory towards becoming a worker or a dispersing reproductive.
Evidence from isolated females suggests that some of the variation in social phenotype among adults may arise prior to adulthood. Both body size and ovary development were the significant classifiers of social behaviour in each of our comparisons (queens versus solitary females and dispersers versus workers). While body size is fixed prior to adulthood, ovary development is highly affected by social context [40]. Nonetheless, some females showed significant reproductive development, despite being isolated from all reproductive, environmental and social stimuli. Moreover, some failed to show reproductive development despite a lack of social constraint. The amount and nutritional content of provisions consumed during the larval feeding stage of development is likely to influence Vg titres through metabolic pathways [68–71], and adult body size. The quantity and protein content of larval provisions provided to M. genalis daughters vary significantly more than those provided to males. This variation is indicative of maternal manipulation, and suggests that there may be some degree of caste-biasing stemming from factors associated with larval feeding [35]. This is not deterministic, as in ants and honeybees, but does suggest a conserved mechanism for eusocial evolution.
Isolated females were the only group for which the Vg titre was a significant predictor of ovary size. This indicates that social context may mask the organizational effects of other developmental processes, when they are not studied independently. The variation in an initial increase in Vg immediately following eclosion (as observed between newly emerged and 4-day-old cage-reared females and an increase in variance from 1–8 days) may be highly influenced by maternal manipulation of larval nutrition. This could jump-start ovarian development in well-nourished offspring, in that these offspring would have vitellogenin available for oogenesis earlier than their less well-nourished conspecifics.
The differences in Vg titres between queens and workers were not found among their solitary counterparts. It was surprising to find similar Vg levels between dispersers and workers, given the differences in their reproductive status and ovary development. Mating is known to elevate Vg synthesis and trigger oogenesis in honeybees [46], but this is apparently not true for M. genalis. Although dispersers had not begun laying eggs, nearly all (89%) were inseminated. Similar Vg levels among dispersers and workers may reflect a relationship between age and Vg titre, as workers and dispersers probably had similar age distributions. Detectable levels of Vg have also been found among sterile honeybee and stingless bee workers, in which Vg has evolved new functions [46,72]. In the case of species with flexible social organization, such as M. genalis, maintenance of circulating Vg may be beneficial such that workers are primed to become reproductively active in the event that their queen disappears.
6. Conclusions
Many M. genalis females exhibit a solitary life history, which is their presumed ancestral state. By contrast, there are also many females that become social queens or workers, reflective of a derived eusocial life history. Observed differences in body size, ovarian development and Vg production among solitary females, queens, workers, dispersers and females raised in isolation implicate larval nutrition and possibly maternal manipulation as playing a role in caste-biasing in M. genalis, complementing other factors such as social competition among nest-mates. Such direct comparisons between representatives expressing ancestral and derived behavioural states of sociality provide valuable insight into the mechanistic bases of social evolution, and show the importance of including both proximate and ultimate factors in investigations of social evolution.
Acknowledgements
K.M.K. was supported by a Short Term Fellowship from STRI, a 10-week Graduate Fellowship and a Pre-doctoral Fellowship from the Smithsonian Institution, a Holmes O. Miller grant and a George Bartholomew fellowship from UCLA EEB, a Joan Wright Goodman award from American Women in Science, a GAANN fellowship from US Department of Education, a graduate Mentor fellowship from the UCLA Graduate Division, a graduate fellowship from the Centre for Society and Genetics, and an NSF Doctoral Dissertation Improvement Grant. K.M.K. and P.N. were also supported by NSF grant IOS-0642085. A.R.S. was supported by grant COL 06-030 from the Secretaria Nacional de Ciencia, Tecnología e Innovación (SENACYT) of Panama to W.T.W. and A.R.S., a fellowship to A.R.S. from the NSF International Research Fellowship Programme, and a Smithsonian Institution Post-doctoral fellowship. K.E.I. was supported by an NSF Doctoral Dissertation Improvement Grant and a STRI-Arizona State University Post-doctoral award. G.V.A. was supported by RCN nos 180504, 185306 and 191699, Wissenschaftskolleg zu Berlin, and the PEW Charitable Trust. Additional support was provided by general research funds from STRI to W.T.W. We would like to thank Julian Medina Gutierrez, Dyana La Rosa, Sandra Bernal, Damien Jose Ramirez Garcia and Timothy Alvey for assistance in the field, and Ting-Yan Chan for assistance in measuring bees in the laboratory. Oris Acevedo, Belkys Jimenez and Orelis Arosemena and the rest of the STRI staff provided valuable logistic support. Research on BCI was conducted with permission from the Autoridad Nacional del Ambiente under permit no. SEX/A-34-09, in accordance with the laws of the Republic of Panama. We thank Simon Tierney and two anonymous reviewers for useful comments on previous versions of this manuscript.
References
- 1.Hunt J. H., Amdam G. V. 2005. Bivoltinism as an antecedent to eusociality in the paper wasp genus Polistes. Science 308, 264–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amdam G. V., Norberg K., Fondrk M. K., Page R. E., Jr 2004. Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honey bees. Proc. Natl Acad. Sci. USA 101, 11 350–11 355 10.1073/pnas.0403073101 (doi:10.1073/pnas.0403073101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.West-Eberhard M. J. 1987. Flexible strategy and social evolution. In Animal societies: theories and facts (eds Ito Y., Brown J. L., Kikkawa J.), pp. 35–51 Tokyo, Japan: Japan Scientific Societies Press [Google Scholar]
- 4.West-Eberhard M. J. 1996. Wasp societies as microcosms for the study of development and evolution. In Natural history and evolution of paper wasps (eds Turillazzi S., West Eberhard M. J.), pp. 290–317 Oxford, UK: Oxford University Press [Google Scholar]
- 5.Linksvayer T. A., Wade M. J. 2005. The evolutionary origin and elaboration of sociality in the aculeate Hymenoptera: maternal effects, sib-social effects, and heterochrony. Q. Rev. Biol. 80, 317–336 10.1086/432266 (doi:10.1086/432266) [DOI] [PubMed] [Google Scholar]
- 6.Corona M., Estrada E., Zurita M. 1999. Differential expression of mitochondrial genes between queens and workers during caste determination in the honeybee Apis mellifera. J. Exp. Biol. 202, 929–938 [DOI] [PubMed] [Google Scholar]
- 7.Evans J. D., Wheeler D. E. 1999. Differential gene expression between developing queens and workers in the honey bee, Apis mellifera. Proc. Natl Acad. Sci. USA 96, 5575–5580 10.1073/pnas.96.10.5575 (doi:10.1073/pnas.96.10.5575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi Y., Lo N., Miyata H., Kitade O. 2007. Sex-linked genetic influence on caste determination in a termite. Science 318, 985–987 10.1126/science.1146711 (doi:10.1126/science.1146711) [DOI] [PubMed] [Google Scholar]
- 9.Hughes W. O. H., Sumner S., Van Borm S., Boomsma J. J. 2003. Worker caste polymorphism has a genetic basis in Acromyrmex leaf-cutting ants. Proc. Natl Acad. Sci. USA 100, 9394–9397 10.1073/pnas.1633701100 (doi:10.1073/pnas.1633701100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linksvayer T. A., Wade M. J., Gordon D. M. 2006. Genetic caste determination in harvester ants: possible origin and maintenance by cyto-nuclear epistasis. Ecology 87, 2185–2193 10.1890/0012-9658(2006)87[2185:GCDIHA]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[2185:GCDIHA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 11.Pereboom J. J. M., Jordan W. C., Sumner S., Hammond R. L., Bourke A. F. G. 2005. Differential gene expression in queen-worker caste determination in bumble-bees. Proc. R. Soc. B 272, 1145–1152 10.1098/rspb.2005.3060 (doi:10.1098/rspb.2005.3060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wheeler D. E., Buck N., Evans J. D. 2006. Expression of insulin pathway genes during the period of caste determination in the honey bee, Apis mellifera. Insect Mol. Biol. 15, 597–602 10.1111/j.1365-2583.2006.00681.x (doi:10.1111/j.1365-2583.2006.00681.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maleszka R. 2008. Epigenetic integration of environmental and genomic signals in honey bees. Epigenetics 3, 188–192 10.4161/epi.3.4.6697 (doi:10.4161/epi.3.4.6697) [DOI] [PubMed] [Google Scholar]
- 14.Amdam G., Nilsen K. A., Norberg K., Fondrk M. K., Hartfelder K. 2007. Variation in endocrine signaling underlies variation in social life history. Am. Nat. 170, 37–46 10.1086/518183 (doi:10.1086/518183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amdam G. V., Csondes A., Fondrk M. K., Page R. E. 2006. Complex social behaviour derived from maternal reproductive traits. Nature 439, 76–78 10.1038/nature04340 (doi:10.1038/nature04340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amdam G. V., Norberg K., Hagen A., Omholt S. W. 2003. Social exploitation of vitellogenin. Proc. Natl Acad. Sci. USA 100, 1799–1802 10.1073/pnas.0333979100 (doi:10.1073/pnas.0333979100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amdam G. V., Omholt S. W. 2003. The hive bee to forager transition in honeybee colonies: the double repressor hypothesis. J. Theor. Biol. 223, 451–464 10.1016/S0022-5193(03)00121-8 (doi:10.1016/S0022-5193(03)00121-8) [DOI] [PubMed] [Google Scholar]
- 18.Nelson C. M., Ihle K. E., Fondrk M. K., Page R. E., Amdam G. V. 2007. The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol. 5, e62. 10.1371/journal.pbio.0050062 (doi:10.1371/journal.pbio.0050062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page R. E., Amdam G. V. 2007. The making of a social insect: developmental architectures of social design. BioEssays 29, 334–343 10.1002/bies.20549 (doi:10.1002/bies.20549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel A., Fondrk M. K., Kaftanoglu O., Emore C., Hunt G., Frederick K., Amdam G. V., Michalak P. 2007. The making of a queen: TOR pathway is a key player in diphenic caste development. PLoS ONE, 2e509. 10.1371/journal.pone.0000509 (doi:10.1371/journal.pone.0000509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wcislo W. T. 1997. Behavioral environments of sweat bees (Halictinae) in relation to variability in social organization. In Social behavior in insects and arachnids (eds Choe J. C., Crespi B. J.), pp. 316–332 Cambridge, UK: Cambridge University Press [Google Scholar]
- 22.Schwarz M. P., Richards M. H., Danforth B. N. 2007. Changing paradigms in insect social evolution: insights from Halictine and Allodapine bees. Annu. Rev. Entomol. 52, 127–150 10.1146/annurev.ento.51.110104.150950 (doi:10.1146/annurev.ento.51.110104.150950) [DOI] [PubMed] [Google Scholar]
- 23.Nowak M. A., Tarnita C. E., Wilson E. O. 2010. The evolution of eusociality. Nature 466, 1057–1062 10.1038/nature09205 (doi:10.1038/nature09205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson B. R., Linksvayer T. A. 2010. Deconstructing the superorganism: social physiology, groundplans, and sociogenomics. Q. Rev. Biol. 85, 57–79 10.1086/650290 (doi:10.1086/650290) [DOI] [PubMed] [Google Scholar]
- 25.Sherman P. W., Lacey E. A., Reeve H. K., Keller L. 1995. The eusociality continuum. Behav. Ecol. 6, 102–108 10.1093/beheco/6.1.102 (doi:10.1093/beheco/6.1.102) [DOI] [Google Scholar]
- 26.Michener C. D. 1990. Reproduction and castes in social halictine bees. In Social insects: an evolutionary approach to castes and reproduction (ed. Engels W.). New York, NY: Springer [Google Scholar]
- 27.O'Donnell S. 1998. Reproductive caste determination in eusocial wasps (Hymenoptera: Vespidae). Annu. Rev. Entomol. 43, 323–346 10.1146/annurev.ento.43.1.323 (doi:10.1146/annurev.ento.43.1.323) [DOI] [PubMed] [Google Scholar]
- 28.Keller L. 2009. Adaptation and the genetics of social behaviour. Phil. Trans. R. Soc. B 364, 3209–3216 10.1098/rstb.2009.0108 (doi:10.1098/rstb.2009.0108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.West-Eberhard M. J. 2003. Developmental plasticity and evolution. Oxford, UK: Oxford University Press [Google Scholar]
- 30.Wcislo W. T. 2000. Environmental hierarchy, behavioral contexts, and social evolution in insects. In Ecologia e comportameno de insetos (eds Martins R. P., Lewinsohn T. M., Barbeitos M. S.), pp. 49–84 Rio de Janeiro, Brasil: PPGE-UFRJ [Google Scholar]
- 31.Wcislo W. T., Arneson L., Roesch K., Gonzalez V., Smith A., Fernandez H. 2004. The evolution of nocturnal behaviour in sweat bees, Megalopta genalis and M. ecuadoria (Hymenoptera: Halictidae): an escape from competitors and enemies? Biol. J. Linn. Soc. 83, 377–387 10.1111/j.1095-8312.2004.00399.x (doi:10.1111/j.1095-8312.2004.00399.x) [DOI] [Google Scholar]
- 32.Kapheim K. M. 2010. Maternal effects and the evolution of sociality in a facultatively eusocial sweat bee, Megalopta genalis. PhD dissertation, University of California, Los Angeles, CA [Google Scholar]
- 33.Smith A. R., Wcislo W. T., O'Donnell S. 2007. Survival and productivity benefits to social nesting in the sweat bee Megalopta genalis (Hymenoptera: Halictidae). Behav. Ecol. Sociobiol. 61, 1111–1120 10.1007/s00265-006-0344-4 (doi:10.1007/s00265-006-0344-4) [DOI] [Google Scholar]
- 34.Wcislo W. T., Gonzalez V. H. 2006. Social and ecological contexts of trophallaxis in facultatively social sweat bees, Megalopta genalis and M. ecuadoria (Hymenoptera, Halictidae). Insect Sociaux 53, 220–225 10.1007/s00040-005-0861-6 (doi:10.1007/s00040-005-0861-6) [DOI] [Google Scholar]
- 35.Kapheim K., Bernal S., Smith A. R., Nonacs P., Wcislo W. 2011. Support for maternal manipulation of developmental nutrition in a facultatively eusocial bee, Megalopta genalis (Halictidae). Behav. Ecol. Sociobiol. 65, 1179–1190 10.1007/s00265-010-1131-9 (doi:10.1007/s00265-010-1131-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frank S. A. 1995. Sex allocation in solitary bees and wasps. Am. Nat. 146, 316–323 10.1086/285802 (doi:10.1086/285802) [DOI] [Google Scholar]
- 37.Charnov E. L. 1982. The theory of sex allocation. Princeton, NJ: Princeton University Press [Google Scholar]
- 38.Rosenheim J. A., Nonacs P., Mangel M. 1996. Sex ratios and multifaceted parental investment. Am. Nat. 148, 501–535 10.1086/285937 (doi:10.1086/285937) [DOI] [Google Scholar]
- 39.Smith A. R., Wcislo W., O'Donnell S. 2008. Body size shapes caste expression, and cleptoparasitism reduces body size in the facultatively eusocial bees Megalopta (Hymenoptera: Halictidae). J. Insect Behav. 21, 394–406 10.1007/s10905-008-9136-1 (doi:10.1007/s10905-008-9136-1) [DOI] [Google Scholar]
- 40.Smith A. R., Kapheim K. M., O'Donnell S., Wcislo W. T. 2009. Social competition but not subfertility leads to a division of labour in the facultatively social sweat bee Megalopta genalis (Hymenoptera: Halictidae). Anim. Behav. 78, 1043–1050 10.1016/j.anbehav.2009.06.032 (doi:10.1016/j.anbehav.2009.06.032) [DOI] [Google Scholar]
- 41.Tierney S. M., Gonzales-Ojeda T., Wcislo W. T. 2008. Nesting biology and social behavior of Xenochlora bees (Hymenoptera: Halictidae: Augochlorini). J. Kansas Entomol. Soc. 81, 61–72 10.2317/JKES-704.24.1 (doi:10.2317/JKES-704.24.1) [DOI] [Google Scholar]
- 42.Smith A. R., Seid M. A., Jiménez L. C., Wcislo W. T. 2010. Socially induced brain development in a facultatively eusocial sweat bee Megalopta genalis (Halictidae). Proc. R. Soc. B 277, 2157–2163 10.1098/rspb.2010.0269 (doi:10.1098/rspb.2010.0269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sappington T. W., Raikhel A S. 1998. Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem. Mol. Biol. 28, 277–300 10.1016/S0965-1748(97)00110-0 (doi:10.1016/S0965-1748(97)00110-0) [DOI] [PubMed] [Google Scholar]
- 44.Tufail M., Takeda M. 2008. Molecular characteristics of insect vitellogenins. J. Insect Physiol. 54, 1447–1458 10.1016/j.jinsphys.2008.08.007 (doi:10.1016/j.jinsphys.2008.08.007) [DOI] [PubMed] [Google Scholar]
- 45.Ziegler R., Van Antwerpen R. 2006. Lipid uptake by insect oocytes. Insect Biochem. Mol. Biol. 36, 264–272 10.1016/j.ibmb.2006.01.014 (doi:10.1016/j.ibmb.2006.01.014) [DOI] [PubMed] [Google Scholar]
- 46.Engels W. 1974. Occurrence and significance of vitellogenins in female castes of social Hymenoptera. Am. Zool. 14, 1229–1237 [Google Scholar]
- 47.Byrne B. M., Gruber M., Ab G. 1989. The evolution of egg yolk proteins. Prog. Biophys. Mol. Biol. 53, 33–69 10.1016/0079-6107(89)90005-9 (doi:10.1016/0079-6107(89)90005-9) [DOI] [PubMed] [Google Scholar]
- 48.Guidugli K. R., Nascimento A. M., Amdam G. V., Barchuk A. R., Omholt S., Simoes Z. L. P., Hartfelder K. 2005. Vitellogenin regulates hormonal dynamics in the worker caste of a eusocial insect. FEBS Lett. 579, 4961–4965 10.1016/j.febslet.2005.07.085 (doi:10.1016/j.febslet.2005.07.085) [DOI] [PubMed] [Google Scholar]
- 49.MacQueen J. 1967. Some methods for classification and analysis of multivariate observations. In Proc. Fifth Berkeley Symp. Mathematical Statistics and Probability (Berkeley, California, 1965/66), pp. 281–297 Berkeley, CA: University of California Press [Google Scholar]
- 50.Brady S. G., Sipes S., Pearson A., Danforth B. N. 2006. Recent and simultaneous origins of eusociality in halictid bees. Proc. R. Soc. B 273, 1643–1649 10.1098/rspb.2006.3496 (doi:10.1098/rspb.2006.3496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Danforth B. N. 2002. Evolution of sociality in a primitively eusocial lineage of bees. Proc. Natl Acad. Sci. USA 99, 286–290 10.1073/pnas.012387999 (doi:10.1073/pnas.012387999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toth A., Bilof K., Henshaw M., Hunt J., Robinson G. 2009. Lipid stores, ovary development, and brain gene expression in Polistes metricus females. Insectes Sociaux 56, 77–84 10.1007/s00040-008-1041-2 (doi:10.1007/s00040-008-1041-2) [DOI] [Google Scholar]
- 53.Roma G. C., Bueno O. C., Camargo-Mathias M. I. 2010. Morpho-physiological analysis of the insect fat body: a review. Micron 41, 395–401 10.1016/j.micron.2009.12.007 (doi:10.1016/j.micron.2009.12.007) [DOI] [PubMed] [Google Scholar]
- 54.Michener C. D., Brothers D. J. 1974. Were workers of eusocial Hymenoptera initially altruistic or oppressed? Proc. Natl Acad. Sci. USA 71, 671–674 10.1073/pnas.71.3.671 (doi:10.1073/pnas.71.3.671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keeping M. G. 2002. Reproductive and worker castes in the primitively eusocial wasp Belonogaster petiolata (DeGeer) (Hymenoptera: Vespidae): evidence for pre-imaginal differentiation. J. Insect Physiol. 48, 867–879 10.1016/S0022-1910(02)00156-7 (doi:10.1016/S0022-1910(02)00156-7) [DOI] [PubMed] [Google Scholar]
- 56.Gadagkar R., Bhagavan S., Chandrashekara K., Vinutha C. 1991. The role of larval nutrition in preimaginal biasing of caste in the primitively eusocial wasp Ropalidia marginata (Hymenoptera, Vespidae). Ecol. Entomol. 16, 435–440 10.1111/j.1365-2311.1991.tb00236.x (doi:10.1111/j.1365-2311.1991.tb00236.x) [DOI] [Google Scholar]
- 57.Gadagkar R., Vinutha C., Shanubhogue A., Gore A. P. 1988. Pre-imaginal biasing of caste in a primitively eusocial insect. Proc. R. Soc. Lond. B 233, 175–189 10.1098/rspb.1988.0017 (doi:10.1098/rspb.1988.0017) [DOI] [Google Scholar]
- 58.Murakami A. S. N., Shima S. N. 2006. Nutritional and social hierarchy establishment of the primitively eusocial wasp Mischocyttarus cassununga (Hymenoptera, Vespidae, Mischocyttarini) and related aspects. Sociobiology 48, 184–207 [Google Scholar]
- 59.Hunt J. H., Wolschin F., Henshaw M. T., Newman T. C., Toth A. L., Amdam G. V. 2010. Differential gene expression and protein abundance evince ontogenetic bias toward castes in a primitively eusocial wasp. PLoS ONE 5, e10674. 10.1371/journal.pone.0010674 (doi:10.1371/journal.pone.0010674) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hunt J. H., Kensinger B. J., Kossuth J. A., Henshaw M. T., Norberg K., Wolschin F., Amdam G. V. 2007. A diapause pathway underlies the gyne phenotype in Polistes wasps, revealing an evolutionary route to caste-containing insect societies. Proc. Natl Acad. Sci. USA 104, 14 020–14 025 10.1073/pnas.0705660104 (doi:10.1073/pnas.0705660104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sullivan J. D., Strassmann J. E. 1984. Physical variability among nest foundresses in the polygynous social wasp, Polistes annularis. Behav. Ecol. Sociobiol. 15, 249–256 10.1007/BF00292986 (doi:10.1007/BF00292986) [DOI] [Google Scholar]
- 62.Karsai I., Hunt J. H. 2002. Food quantity affect traits of offspring in the paper wasp Polistes metricus (Hymenoptera: Vespidae). Environ. Entomol. 31, 99–106 10.1603/0046-225X-31.1.99 (doi:10.1603/0046-225X-31.1.99) [DOI] [Google Scholar]
- 63.Bell W. J. 1973. Factors controlling initiation of vitellogenesis in a primitively social bee, Lasioglossum zephyrum (Hymenoptera: Halictidae). Insectes Sociaux 20, 253–260 10.1007/BF02223194 (doi:10.1007/BF02223194) [DOI] [Google Scholar]
- 64.Richards M. H., von Wettberg E. J., Rutgers A. C. 2003. A novel social polymorphism in a primitively eusocial bee. Proc. Natl Acad. Sci. USA 100, 7175–7180 10.1073/pnas.1030738100 (doi:10.1073/pnas.1030738100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Richards M. H., Packer L. 1994. Trophic aspects of caste determination in Halictus ligatus, a primitively eusocial sweat bee. Behav. Ecol. Sociobiol. 34, 385–391 10.1007/BF00167329 (doi:10.1007/BF00167329) [DOI] [Google Scholar]
- 66.Mueller U. G. 1996. Life history and social evolution of the primitively eusocial bee Augochlorella striata (Hymenoptera: Halictidae). J. Kansas Entomol. Soc. 69, 116–138 [Google Scholar]
- 67.Mueller U. G., Eickwort G. C., Aquadro C. F. 1994. DNA-fingerprinting analysis of parent–offspring conflict in a bee. Proc. Natl Acad. Sci. USA 91, 5143–5147 10.1073/pnas.91.11.5143 (doi:10.1073/pnas.91.11.5143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Amdam G. V., Page R. E. 2010. The developmental genetics and physiology of honeybee societies. Anim. Behav. 79, 973–980 10.1016/j.anbehav.2010.02.007 (doi:10.1016/j.anbehav.2010.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corona M., Velarde R. A., Remolina S., Moran-Lauter A., Wang Y., Hughes K. A., Robinson G. E. 2007. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc. Natl Acad. Sci. USA 104, 7128–7133 10.1073/pnas.0701909104 (doi:10.1073/pnas.0701909104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Münch D., Amdam G. V. 2010. The curious case of aging plasticity in honey bees. FEBS Lett. 584, 2496–2503 10.1016/j.febslet.2010.04.007 (doi:10.1016/j.febslet.2010.04.007) [DOI] [PubMed] [Google Scholar]
- 71.Parthasarathy R., Palli S. R. 2011. Molecular analysis of nutritional and hormonal regulation of female reproduction in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 41, 294–305 10.1016/j.ibmb.2011.01.006 (doi:10.1016/j.ibmb.2011.01.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dallacqua R., Simes Z., Bitondi M. 2007. Vitellogenin gene expression in stingless bee workers differing in egg-laying behavior. Insectes Sociaux 54, 70–76 10.1007/s00040-007-0913-1 (doi:10.1007/s00040-007-0913-1) [DOI] [Google Scholar]