Abstract
The impact of crop management and agricultural land use on the threat status of plants adapted to arable habitats was analysed using data from Red Lists of vascular plants assessed by national experts from 29 European countries. There was a positive relationship between national wheat yields and the numbers of rare, threatened or recently extinct arable plant species in each country. Variance in the relative proportions of species in different threat categories was significantly explained using a combination of fertilizer and herbicide use, with a greater percentage of the variance partitioned to fertilizers. Specialist species adapted to individual crops, such as flax, are among the most threatened. These species have declined across Europe in response to a reduction in the area grown for the crops on which they rely. The increased use of agro-chemicals, especially in central and northwestern Europe, has selected against a larger group of species adapted to habitats with intermediate fertility. There is an urgent need to implement successful conservation strategies to arrest the decline of this functionally distinct and increasingly threatened component of the European flora.
Keywords: rare weeds, agri-environment schemes, field margins, conservation, agro-ecosystems
1. Introduction
Vascular plants adapted to arable habitats are acknowledged to be among the most vulnerable groups in national floras to land-use change, particularly in western European states [1–4]. For example, in the UK, of the 30 plant species that have shown the greatest decline between the 1960s and 1990s, 60 per cent are associated with arable or other cultivated land [5,6] and 24 are listed as priority species on the UK Biodiversity Action Plan (www.ukbap.org.uk). However, the conservation status of arable plants is also increasingly raising concerns in eastern Europe, which tends to have less intensive agriculture [7–9]. Concomitant with national extinctions and increased threat to individual species, a reduction in the overall weed seed-bank has also been observed over recent decades in a number of European countries [10,11] as the abundance of common species has also declined [12].
Because the arable field is characterized by regular disturbance, the flora is dominated by annuals that rely on regular replenishment of the seed-bank for populations to persist. These plants are therefore particularly sensitive to changes in land use or management that reduce the proportion of the seed-bank germinating, seedling survival or the number of seeds per plant returning to the seed-bank [13]. A number of management changes, which impact on different stages of the plant life cycle, have been implicated in the decline of national arable plant populations. These include the shift from spring to autumn sowing, increased plant density and shading by the crop canopy, decreased crop diversity, increasing fertilizer and herbicide use [2,14,15], and more efficient seed cleaning [16]. While it is likely that there has been an abundance-based mechanism to the response of arable plants to agricultural intensification, with the most infrequent species disappearing first [17], there has also been a functional response. That is, changes in management have acted as filters on the weed community selecting against species with particular combinations of traits [18,19]. For example, the shift from spring to autumn sowing has reduced the regenerative niche for obligate spring-germinating species, such as Galeopsis angustifolia Ehrh. ex Hoffman and Valerianella dentata (L.) Pollich in the UK [14], and increased shading by the crop canopy has suppressed short species, such as Euphorbia exigua L. and certain Veronica species.
In response to national declines in arable plant diversity, as well as evidence of their value as a resource to higher trophic groups [20,21], a number of European nations have included options within subsidized agri-environment schemes that encourage the arable flora. These include conservation headlands and uncropped cultivated margins [22]. However, the value of these options to the conservation of arable plants has been constrained by the low uptake by farmers and limited geographical targeting to areas with high arable plant diversity [5,23–25]. There is therefore concern that European arable plants, as a group, will continue to decline, particularly as agricultural production in eastern Europe intensifies. This paper presents data on the threat status of arable plants from 29 European states, based on data from national Red Lists, in combination with local expert knowledge. As well as establishing a benchmark against which future national trends in arable plant diversity can be assessed at a European level, the data are analysed with respect to land-use and agricultural management statistics to address two questions. First, can explanatory variables be identified that predict the ranking of countries in terms of the numbers of arable species that are nationally rare or threatened? And, second, can the relative sensitivity of weed species to these variables be quantified?
2. Material and methods
(a). Data collection
An agricultural botanist was identified in each of 29 European countries and invited to complete a questionnaire. The experts were first asked to identify vascular plant species that are particularly associated with arable land and classified as recently extinct, critically endangered, endangered or vulnerable on their national Red List. In addition, a second list of species was requested from each country of arable plants that were either identified as ‘near threatened’ or did not appear on the national Red List but were known to be declining from on-going surveys or expert knowledge. These data were particularly valuable for states where the arable flora was traditionally under-represented in national vegetation surveys, such as in southern Europe, or where formal Red Lists were not available. Finally, information was requested on reasons behind national declines in arable plant diversity and any conservation practices being used to arrest these declines. For three countries from which completed questionnaires were not returned (Norway, Luxemburg and Ireland), the authors consulted the respective national Red Lists to obtain the data. In the case of Ireland, this was supplemented by data from an online consultation of nationally threatened plants hosted by the National Botanic Gardens of Ireland (http://www.botanicgardens.ie).
A database was compiled from completed questionnaires of all the plant species (sub-species were not included) that were identified as arable plants and were on the Red List or considered threatened in any European country. In addition, for each species, the wider European distribution was also obtained from the online Flora Europaea database (http://rbg-web2.rbge.org.uk/FE/fe.html), which was also used to standardize nomenclature. Each cell in the matrix of species × country was then assigned to a category: (1) species present in country but not on Red List or considered threatened, (2) species present in country and considered threatened but not listed as at least vulnerable on Red List, (3) species identified on Red List as vulnerable to critically endangered, and (4) species recently extinct. The relative threat status of each species was assessed using the following scoring system:
The following data on land-use and agricultural statistics for each European state in the survey were obtained from the FAOSTAT database of the UN Food and Agriculture Organization (http://www.fao.org): total land surface area, proportion of land in arable production and wheat yield for 2008 (the latest year for which a full dataset was available; table 1). Wheat was used as a representative crop to indicate the level of intensification as, in a previous analysis of correlates of agricultural statistics with farmland European bird populations, it was found to be the most widely grown crop and strongly correlated with the yields of other cereal types [26]. In addition, data were obtained on the two factors most commonly identified in the questionnaires as driving the national declines in arable plants: increased agro-chemical use and abandonment of arable land.
Table 1.
Land-use statistics for 29 European countries used to explain variance in the national threat status of arable plants. All data for 2008 unless indicated. Herbicide usage data calculated for all active ingredients registered for use on cereals. The dash symbols (—) indicate data not available.
| country | latitude | land area (1000 ha) | proportion arable land | loss of arable landb | wheat yield (t ha–1) | wheat fertilizer (kg N ha−1) | herbicide a.i. weight (1000 kg) | cereal area (1000 ha) | herbicide load (kg ha–1) | no. species present in country | no. species threatened/rare |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Austriaa | 47.3 | 8387 | 0.16 | −0.21 | 5.69 | 97 | 336 | 845 | 0.40 | 169 | 102 |
| Belgiuma | 50.8 | 3053 | 0.28 | −0.11 | 8.76 | 155 | 360 | 350 | 1.03 | 152 | 75 |
| Bulgaria | 43.0 | 11 100 | 0.28 | −1.98 | 4.17 | 60 | 128 | 1711 | 0.07 | 165 | 15 |
| Croatia | 45.2 | 5659 | 0.15 | −1.75 | 5.48 | — | 154 | 561 | 0.28 | 182 | 45 |
| Czech Republic | 49.8 | 7887 | 0.38 | −0.27 | 5.77 | 155 | 845 | 1561 | 0.54 | 166 | 84 |
| Denmarka | 56.0 | 4309 | 0.56 | −0.21 | 7.86 | 118 | 831 | 1513 | 0.55 | 115 | 56 |
| Estonia | 59.0 | 4523 | 0.13 | −3.16 | 3.18 | 80 | — | 309 | — | 90 | 22 |
| Finland | 64.0 | 33 842 | 0.07 | 0.14 | 3.64 | 85 | 706 | 1194 | 0.59 | 58 | 15 |
| Francea | 46.0 | 54 919 | 0.33 | 0.06 | 7.10 | 161 | 4978 | 9618 | 0.52 | 187 | 69 |
| Germanya | 51.0 | 35 711 | 0.33 | 0.07 | 8.09 | 150 | 5460 | 7038 | 0.78 | 183 | 131 |
| Greece | 39.0 | 13 196 | 0.16 | −2.04 | 2.95 | 55 | 168 | 1189 | 0.14 | 154 | 13 |
| Hungarya | 47.0 | 9303 | 0.49 | −0.38 | 4.98 | 70 | 321 | 2973 | 0.11 | 168 | 38 |
| Ireland | 53.0 | 7028 | 0.16 | 0.91 | 9.06 | 150 | — | 314 | — | 64 | 28 |
| Italy | 42.8 | 30 134 | 0.24 | −0.96 | 3.87 | 85 | 606 | 4038 | 0.15 | 183 | 18 |
| Latvia | 57.0 | 6456 | 0.18 | −1.14 | 3.86 | 75 | 118c | 544 | 0.22 | 90 | 27 |
| Lithuania | 56.0 | 6530 | 0.29 | −3.36 | 4.27 | 91 | 241c | 1022 | 0.24 | 90 | 17 |
| Luxemburg | 49.8 | 259 | 0.24 | −0.24 | 6.66 | — | — | 31 | — | 145 | 68 |
| The Netherlandsa | 52.3 | 4154 | 0.26 | 1.75 | 8.73 | 199 | 267 | 236 | 1.13 | 131 | 49 |
| Norway | 62.0 | 32 378 | 0.03 | −0.69 | 4.85 | 120 | — | 309 | — | 74 | 25 |
| Polanda | 52.0 | 31 268 | 0.40 | −1.10 | 4.07 | 91 | 2670 | 8599 | 0.31 | 150 | 17 |
| Portugala | 39.5 | 9209 | 0.11 | −3.66 | 2.30 | 90 | 122 | 364 | 0.33 | 125 | 1 |
| Romania | 46.0 | 23 839 | 0.37 | −0.47 | 3.42 | 40 | 443 | 5182 | 0.09 | 168 | 10 |
| Serbia | 44.0 | 8836 | 0.37 | — | 4.30 | — | 411 | 1905 | 0.22 | 185 | 16 |
| Slovakia | 48.7 | 4904 | 0.28 | −1.01 | 4.87 | 85 | 235 | 799 | 0.29 | 167 | 63 |
| Slovenia | 46.0 | 2027 | 0.09 | −0.60 | 4.53 | 90 | 42 | 107 | 0.39 | 185 | 56 |
| Spain | 40.0 | 50 537 | 0.25 | −1.07 | 3.25 | 85 | 2545 | 6685 | 0.38 | 169 | 11 |
| Swedena | 62.0 | 45 030 | 0.06 | −0.43 | 6.11 | 135 | 338 | 1078 | 0.31 | 107 | 33 |
| Switzerlanda | 47.0 | 4128 | 0.10 | −0.16 | 6.01 | 140 | 268 | 156 | 1.71 | 176 | 137 |
| UKa | 54.0 | 24 361 | 0.25 | −0.19 | 8.28 | 194 | 4372 | 3272 | 1.34 | 127 | 51 |
aSubsidized schemes available targeted at arable flora.
bCalculated as annual change in arable land area as percentage of 1993 baseline from linear regression fitted to years 1993–2008 (only 2000–2008 data available for Belgium and Luxemburg).
c2009 data.
Data on the rate of nitrogen fertilizer (kg ha–1) used in wheat in 2008 across Europe was obtained from a database held by Fertilizers Europe (previously the European Fertiliser Manufacturers Association; www.fertilizerseurope.com). There is not an equivalent common metric for herbicide inputs as rates will differ according to the products used and countries cannot strictly be compared like-for-like. However, by using the weight of all active ingredients used in cereals in 2008, this effect was minimized as it included a diversity of products. These data were obtained from a commercial database of herbicide usage across Europe (Amis Global, www.amisglobal.com) and used to calculate a herbicide ‘load’ for each country by dividing by the area of cereals grown obtained from the FAOSTAT database. The change in the amount of arable land in each country was calculated using data from FAOSTAT on arable areas between 1993 (the first year with data on all countries except Belgium and Luxemburg) and 2008. The amount of arable land in each year was expressed as a proportion of the 1993 baseline and a linear regression fitted to the data to calculate the slope or annual change.
(b). Statistical analysis
The completion of the questionnaire involved a degree of subjectivity in identifying which species on national Red Lists were particularly associated with arable habitats. To account for this variability in the assessment of habitat preference, the database was filtered to only include species that were identified as rare or threatened arable plants in at least three countries. This short list was used to analyse the relationship of land use and management with the proportion of the species present in each country that were identified as rare or threatened. For all subsets regression using generalized linear models (GLMs) was used to identify the model that explained the maximum variability in the proportion of rare or threatened species using only explanatory variables with p < 0.05. As well as total land area, proportion of arable land and wheat yield, the average latitude of each country was also included in the analysis. Because fertilizer and herbicide use were both significantly positively correlated with wheat yield (r = 0.86, p < 0.001 and r = 0.67, p < 0.001, respectively) and with each other (r = 0.78, p < 0.001), they were not included in the GLM. Using binomial distribution with a logit link function allowed the variability in the total number of species present in each country (ranging from 58 in Finland to 187 in France) to be accounted for. As opposed to a step-wise approach, all subset regression analysed all possible combinations of explanatory variables, using the adjusted R2 and Mallows Cp as criteria for comparing models.
The effect of fertilizer and herbicide use on the numbers of species in different threat categories was analysed separately using variance partitioning in a redundancy analysis (RDA) using CANOCO v. 4.5 software [27]. This enabled the proportion of variance explained by collinear variables to be quantified. The counts of species in each category were log-transformed and standardized by country, to construct a similarity matrix of relative proportions, and input into an RDA with fertilizer dose and herbicide load as explanatory variables. The variance between the countries that could be accounted for by herbicide or fertilizers alone was then tested by constraining the ordination using each variable in turn while including the other as a covariate and comparing with the analysis using both as explanatory variables. Data on fertilizers were not available for Croatia, Luxemburg and Serbia, and herbicide data were not available for the small markets of Estonia, Ireland, Luxemburg and Norway. In addition, only 2009 data were available on herbicides for Latvia and Lithuania. All of these countries were excluded from the RDA, leaving a total of 21.
Finally, a hypergeometric probability function was used to test whether any plant families were disproportionately represented in the short list of rare or threatened arable plants [28]. The function calculates the probability of a number of positive results from sampling without replacement using four parameters: N, size of population; K, number of items with the desired characteristic in the population; n, number of samples drawn; and x, number of successes in the sample. The total number of species present in the Flora Europaea (excluding Pteridophytes and Gymnosperms), 10 835, was input as N. For each family represented in the short list of rare or threatened arable plants, the total number of species in the Flora Europaea was obtained [29], n. K was calculated as the total number of species in the Flora Europaea that were on the rare or threatened arable plant list and x as the number in the family being analysed that were rare or threatened.
3. Results
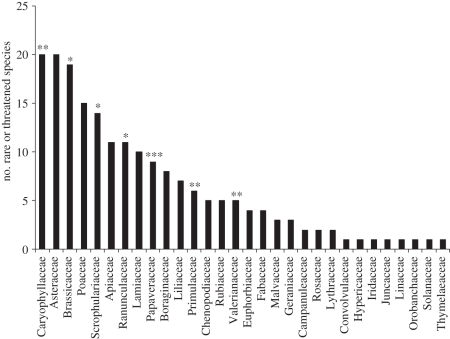
The database of rare or threatened arable plants contained 582 species. Of these, 193 species were either on the national Red Data Lists or considered threatened in at least three of the 29 European countries from which questionnaires were returned. The most common families represented in this short list were the Caryophyllaceae, Asteraceae and Brassicaceae, of which the Caryophyllaceae and Brassicaceae were significantly over-represented when compared with the European flora as a whole. This was also the case for a number of other families (figure 1), particularly the Papaveraceae. The most common genera were Veronica (eight species), Silene and Bromus (both six species). The factors most commonly identified as causing national declines in arable floras were increased use of agro-chemicals and the abandonment of marginal land, mentioned in 21 and 14 questionnaires, respectively. The latter was especially associated with eastern European countries. Decreasing crop diversity was the next most commonly cited factor (in 10 questionnaires), with particular emphasis placed on the decline of rye (Secale cereale L.) and flax (Linum usitatissimum L.) as major crops. Less commonly cited factors included irrigation, which was identified in the decline of species adapted to dry-land agriculture in Spain and Portugal, and loss of stubbles in the Czech Republic, with implications for species such as Stachys annua L.
Figure 1.
Numbers of species from each family represented in the short list of rare or threatened European arable plants (cited in questionnaires from at least three countries). The probability of the over-representation of each family in the list when compared with the European flora as a whole, calculated using a hypergeometric probability distribution, is indicated: *p < 0.05, **p < 0.01, ***p < 0.001.
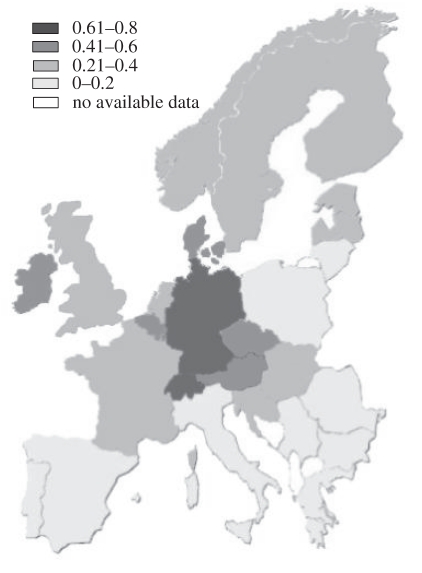
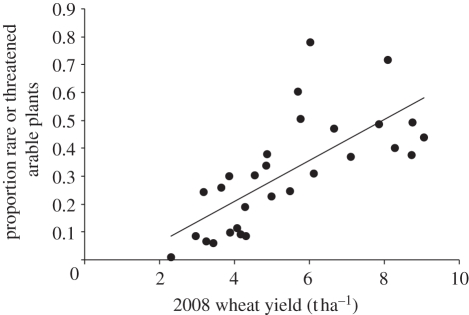
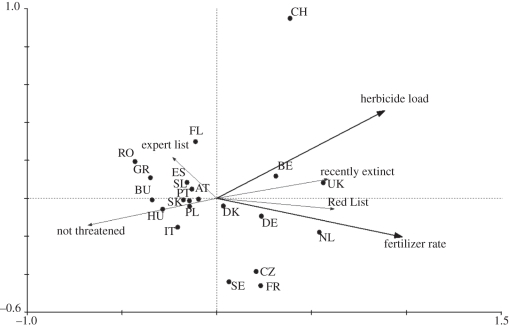
The comparison of countries in terms of the proportion of species on the short list that were present in the country and identified as rare, threatened or extinct revealed a concentration of countries in central or northwest Europe with higher numbers of species (figure 2). The GLM that explained the most variance between countries was wheat yield (R2 = 46.5, Cp = 2.2, p < 0.001), which had a positive relationship with the proportion of nationally rare or threatened arable plants (figure 3). The model was not significantly improved by the inclusion of any other explanatory variables. The only other variable that had a significant effect was loss of arable land (p = 0.001 when included as the only explanatory variable). However, this was negatively correlated with the proportion of nationally rare or threatened arable plants, indicating that intensification of crop production is the main threat to this group of plants. This conclusion was supported by the results of the RDA. When both fertilizer dose and herbicide load were included in the constrained analysis, 33.4 per cent of the total variance between countries in the relative proportions of species in the different threat categories was explained (p = 0.004; figure 4). When the effect of the two variables were analysed separately, including the other as a covariate, 8 per cent of the total variance was able to be partitioned to fertilizers alone and 2.8 per cent to herbicides alone. The close correlation between the two variables meant that the remaining 22.6 per cent could not be partitioned to either.
Figure 2.
Comparison of European countries in terms of the proportion of arable plant species occurring in the country (using the short list of 193) that were identified as rare or threatened.
Figure 3.
Relationship between national wheat yields and the proportion of arable plant species occurring in the country (using the short list of 193) that were identified as rare or threatened (y = 0.073x – 0.081, R2 = 0.51).
Figure 4.
RDA analysis using fertilizer dose and herbicide load as explanatory variables on 21 countries where comparable data were available. AT, Austria; BE, Belgium; BU, Bulgaria; CH, Switzerland; CZ, Czech Republic; DE, Germany; DK, Denmark; ES, Spain; FL, Finland; FR, France; GR, Greece; HU, Hungary; IT, Italy; NL, The Netherlands; PL, Poland; PT, Portugal; RO, Romania; SE, Sweden; SK, Slovakia; SL, Slovenia; UK, United Kingdom.
4. Discussion
The analysis of the threat status of European arable plants provides further evidence of the trend, established in numerous other studies, of the negative impact of increasing intensification of crop production on the biodiversity of agro-ecosystems [10,30–33]. The ranking of species according to their score (table 2) showed that those that are specialized to a single crop are particularly vulnerable, including some that have coevolved to mimic morphological or phenological characteristics of the crop [34–36]. These include the flax specialists Cuscuta epilinum L. and Silene linicola C. C. Gmelin, and cereal specialists including Bromus secalinus L. and Lolium remotum Schrank in rye, or Bromus grossus Desf. ex DC. in spelt. A number of these species, including S. linicola and B. grossus, are anecophytes with no known natural habitats outside the cultivated field and are endemic to Europe. Several other specific factors have been identified in the literature as being responsible for the decline of individual species, including improved seed cleaning for Agrostemma githago L. [37], the loss of stubbles for Stachys annua L. [38] and the drainage of wet depressions that are typically colonized by arable plants with higher moisture demand. These species have tended to decline or are already extinct across Europe, irrespective of the level of intensification, as a result of, for example, the reduction in the area of flax grown or the use of cleaner crop seed.
Table 2.
Species in short list of rare or threatened European arable plants (cited in questionnaires from at least three countries) ordered according to the species score. Only the top 48 species are presented (representing the upper quartile of the list).
| species | family | countries in which species is present | countries in which species is rare, threatened or recently extinct | score |
|---|---|---|---|---|
| Bromus grossus | Poaceae | 3 | 3 | 2.00 |
| Silene linicola | Caryophyllaceae | 6 | 4 | 1.83 |
| Logfia neglecta | Asteraceae | 5 | 3 | 1.80 |
| Cuscuta epilinum | Convolvulaceae | 25 | 16 | 1.64 |
| Agrostemma githago | Caryophyllaceae | 29 | 25 | 1.62 |
| Adonis flammea | Ranunculaceae | 18 | 16 | 1.61 |
| Spergularia segetalis | Caryophyllaceae | 9 | 5 | 1.56 |
| Adonis aestivalis | Ranunculaceae | 19 | 16 | 1.53 |
| Scandix pecten-veneris | Apiaceae | 24 | 17 | 1.46 |
| Lolium temulentum | Poaceae | 29 | 21 | 1.45 |
| Camelina alyssum | Brassicaceae | 25 | 16 | 1.44 |
| Vaccaria pyramidata | Caryophyllaceae | 23 | 16 | 1.35 |
| Linaria arvensis | Scrophulariaceae | 18 | 9 | 1.33 |
| Conringia orientalis | Brassicaceae | 20 | 13 | 1.25 |
| Lolium remotum | Poaceae | 28 | 14 | 1.21 |
| Asperula arvensis | Rubiaceae | 20 | 11 | 1.20 |
| Bupleurum rotundifolium | Apiaceae | 23 | 15 | 1.17 |
| Caucalis platycarpos | Apiaceae | 20 | 12 | 1.15 |
| Bromus secalinus | Poaceae | 28 | 19 | 1.14 |
| Galium tricornutum | Rubiaceae | 23 | 13 | 1.13 |
| Turgenia latifolia | Apiaceae | 20 | 10 | 1.10 |
| Ajuga chamaepitys | Lamiaceae | 21 | 12 | 1.10 |
| Arnoseris minima | Asteraceae | 23 | 12 | 1.09 |
| Androsace maxima | Primulaceae | 15 | 7 | 1.07 |
| Adonis annua | Ranunculaceae | 15 | 9 | 1.07 |
| Legousia speculum-veneris | Campanulaceae | 17 | 11 | 1.06 |
| Neslia paniculata | Brassicaceae | 24 | 13 | 1.04 |
| Legousia hybrida | Campanulaceae | 16 | 9 | 1.00 |
| Roemeria hybrida | Papaveraceae | 5 | 3 | 1.00 |
| Thymelaea passerina | Thymelaeaceae | 19 | 10 | 1.00 |
| Misopates orontium | Scrophulariaceae | 24 | 12 | 0.96 |
| Valerianella dentata | Valerianaceae | 23 | 12 | 0.96 |
| Nigella arvensis | Ranunculaceae | 18 | 9 | 0.94 |
| Adonis microcarpa | Ranunculaceae | 9 | 4 | 0.89 |
| Melampyrum arvense | Scrophulariaceae | 25 | 13 | 0.88 |
| Bifora radians | Apiaceae | 15 | 8 | 0.87 |
| Filago pyramidata | Asteraceae | 15 | 6 | 0.87 |
| Valerianella rimosa | Valerianaceae | 22 | 10 | 0.86 |
| Papaver argemone | Papaveraceae | 27 | 13 | 0.85 |
| Lathyrus aphaca | Fabaceae | 19 | 8 | 0.84 |
| Centaurea cyanus | Asteraceae | 29 | 14 | 0.83 |
| Anagallis minima | Primulaceae | 29 | 13 | 0.83 |
| Ranunculus arvensis | Ranunculaceae | 28 | 12 | 0.82 |
| Gagea arvensis | Liliaceae | 21 | 10 | 0.81 |
| Silene noctiflora | Caryophyllaceae | 26 | 13 | 0.81 |
| Hypochoeris glabra | Asteraceae | 28 | 10 | 0.79 |
| Kickxia elatine | Scrophulariaceae | 23 | 10 | 0.78 |
| Bromus arvensis | Poaceae | 27 | 13 | 0.78 |
However, of potentially greater concern for arable plant biodiversity at the national and continental scale is the more general trend towards the intensification of agriculture with the consequent biotic homogenization of the landscape [39,40]. The results presented in this paper support the conclusions of previous studies that eutrophication, either through atmospheric nitrogen deposition or fertilizers, is one of the major drivers of decreasing habitat heterogeneity and species loss [41–43], and that declining species are spread disproportionately across plant families, potentially contributing to the phylogenetic shift in the European flora [44]. Although greater variance in the threat status of arable plants between countries could be attributed to fertilizers alone as opposed to herbicides when they were included in the same analysis, it was not possible to fully separate the effects of the two factors. It is likely that they have acted in parallel, with herbicides reducing the overall niche for sustainable arable plant populations in the context of a functional filtering of species through increased fertility [17]. As well as in-field management drivers, at a regional and local scale, landscape factors such as field size, management of field margins and landscape complexity have also been shown to influence weed diversity [3,45,46]. It is likely that countries with less intensive agriculture would also have smaller fields and more complex landscapes, although it was not possible to obtain data on these finer-scale metrics with sufficient coverage to include them in the models used in this study. However, loss of field boundaries was identified as a driver of arable plant declines in seven questionnaires and field margins are an important refuge for declining arable plant species [1]. A consideration of the landscape context of conservation strategies will therefore be an important consideration at the regional scale.
As discussed above, the arable plants specialized to individual crops appear to be the most sensitive to changes in cropping patterns. If these species are removed from the list, the top of the ranking of species (table 2) is then dominated by species with a similar ecological strategy, reflected in a relatively short stature and/or a large seed, indicating a specific ecological response to the drivers of disturbance and fertility [47]. Increased seed size has implications for colonizing ability, being able to establish in less favourable environments [48] and competitive ability, particularly for below-ground resources [19,49]. Because of the allometric relationship between mature biomass and seed production [50], species with a larger seed will also be less fecund, making them less able to buffer the seedling mortality from herbicides. In addition, seed size has also been negatively correlated with persistence in the seed-bank [51], further selecting against these species as they are less able to exploit ephemeral opportunities for growth related to failures of weed control or crop rotation. A short stature will result in a low competitive ability in dense crop canopies, where increasing fertilizer use means nutrients are non-limiting and light is the main resource limiting growth [2,52]. As opposed to more characteristically stress-tolerant ruderals (sensu [53]), which may continue to persist in other disturbed, less productive environments such as coastal areas, species with a combination of short stature and large seed have been found to be adapted to habitats with intermediate fertility [19]—habitats that are declining most rapidly in response to increasing eutrophication of landscapes [17].
Any continent-wide analysis of the threat status of arable plants will be limited by the fact that the procedure of compiling Red Lists does not follow a uniform protocol across different countries but involves partly subjective assessment steps by experienced botanists or state agencies that may differ. This may partly explain the very high proportions of threatened or rare species reported for Switzerland and Germany, in contrast to countries such as The Netherlands and Belgium with similar floras and comparable levels of intensification. These former countries have particularly sensitive Red List criteria, where all species that have shown recent population declines in a significant part of the country are included. However, we expect this kind of bias to be restricted to a few central European states, as the Red List criteria are more similar in other countries.
In contrast to other taxa adapted to agro-ecosystems that have suffered declines in response to agricultural intensification, particularly birds [26,54], the rationale behind the conservation of arable plants is less straightforward. This group of plants is traditionally viewed as an impediment to crop production, and a number of the species on the list compiled in this study would at one time have been serious weeds. However, two reasons can be identified to argue for the preservation of these floral elements. First, the similarity in the autecology of the most vulnerable species indicates that the factors identified in this study are systematically removing a functionally distinct component of the fabric of agro-ecosystems. Many of these plants are now restricted to arable habitats, and continuing declines in cropped fields will therefore result in a loss of plant functional diversity at a national and continental scale, with possible consequences for the specialist fauna they support [55]. Second, the decline in diversity of arable plants has happened in parallel with a decrease in total abundance of plant resources in the agro-ecosystem [10,56] implicated in the decline of invertebrates and birds [20,30]. The loss of arable plant species from the environment is therefore indicative of a wider degradation of the agro-ecosystem. Any further erosion of plant functional diversity in the agricultural landscapes of Europe may also limit the adaptability of these ecosystems to future changes in climate or land management.
5. Conclusion
The study has identified a suite of plant species that are already extinct or particularly vulnerable at a European scale to the increasing intensification of agricultural production. Many of these plants are still relatively common in countries where agro-chemical inputs are modest compared with those with the highest wheat yields, but these countries have still observed declines in floral diversity in response to changes in the types of crops grown, abandonment of arable land or re-intensification of former marginal arable land for the production of biofuels/bioenergy [57]. We contend that threatened arable floras have an intrinsic ecological value that justifies measures to preserve them, and the habitats with which they are associated, in the agricultural landscape. This will inevitably involve establishing refuges on marginal land, generally characterized by less-fertile soils where crop competition and agro-chemical inputs are reduced [58]. Field margins in intensively cultivated landscapes, subsidized by national agri-environment schemes, will have an important role to play in this regard [1,59]. However, agri-environment options targeted at arable plants tend to be unpopular with farmers, and field margins are, by nature, ephemeral, and vulnerable to changes in subsidies and market forces. More extensive projects that identify the nationally important areas for arable plant communities and implement measures to conserve them on a landscape scale are therefore more likely to deliver a long-term solution [25,58].
Acknowledgements
Special thanks to all the people who filled in a questionnaire: Adriatik Çakalli (Albania), Thorsten Englisch (Austria), Marie Legast, Louis-Marie Delescaille, Wouter van Landuyt and François Henriet (Belgium), Antoaneta Petrova (Bulgaria), Nada Hulina (Croatia), Zdeňka Lososová (Czech Republic), Christian Andreasen and Peter Wind (Denmark), Tsipe Aavik, Jaan Liira and Elle Roosaluste (Estonia), Terho Hyvönen (Finland), Bruno Chauvel (France), Erwin Bergmeier (Greece), Gyula Pinke, Zita Dorner (Hungary), Stefano Tassinazo (Italy), Arben Mehmeti (Kosovo), Dace Piliksere (Latvia), Vytautas Pilipavicius, Valerijus Rasomavicius (Lithuania), Guy Colling (Luxemburg), David Kleijn (The Netherlands), Therese With Berge (Norway), Denise Fu Dostatny (Poland), Anabela Ferreira Belo (Portugal), Mihai Puşcaş and Erika Schneider (Romania), Alexey P. Seregin (Russia), Pal Boza, Goran Anackov and Milica Rat (Serbia), Pavol Eliáš jun. (Slovakia), Urban Šilc (Slovenia), Josep Antoni Conesa, Jordi Recasens and Aritz Royo (Spain), Mora Aronsson (Sweden), Katja Jacot (Switzerland) and Ivan Moysiyenko (Ukraine), and to Christian Pallière at Fertilizers Europe for contributing fertilizer data, and Bob Fairclough at Amis Global for the herbicide data. The authors express their sincere thanks to the Deutsche Bundesstiftung Umwelt (DBU) for financial support of the study.
References
- 1.Fried G., Petit S., Dessaint F., Reboud X. 2009. Arable weed decline in Northern France: crop edges as refugia for weed conservation? Biol. Conserv. 142, 238–243 10.1016/j.biocon.2008.09.029 (doi:10.1016/j.biocon.2008.09.029) [DOI] [Google Scholar]
- 2.Kleijn D., vanderVoort L. A. C. 1997. Conservation headlands for rare arable weeds: the effects of fertilizer application and light penetration on plant growth. Biol. Conserv. 81, 57–67 10.1016/S0006-3207(96)00153-X (doi:10.1016/S0006-3207(96)00153-X) [DOI] [Google Scholar]
- 3.Baessler C., Klotz S. 2006. Effects of changes in agricultural land-use on landscape structure and arable weed vegetation over the last 50 years. Agric. Ecosyst. Environ. 115, 43–50 10.1016/j.agee.2005.12.007 (doi:10.1016/j.agee.2005.12.007) [DOI] [Google Scholar]
- 4.Meyer S., Krause B., Wesche K., Leuschner C. 2010. Changes in Central German arable plant communities over the last 50 years: a semi-quantitative study. In Proc. of 15th EWRS Symp., Kaposvár, Hungary, 2010, pp. 135–136 Doorwerth, The Netherlands: EWRS. [Google Scholar]
- 5.Still K. S. 2007. A future for rare arable plants. Aspects Appl. Biol. 81, 175–182 [Google Scholar]
- 6.Preston C. D., Pearman D. A., Dines T. D. 2002. New atlas of the British and Irish Flora. Oxford, UK: Oxford University Press [Google Scholar]
- 7.Eliáš P., Eliáš P., Baranec T. 2005. The new Red List of Slovak endangered weeds, pp. 23–28 Nitra, Slovakia: Slovak University of Agriculture [Google Scholar]
- 8.Pinke G., Pál R., Botta-Dukat Z., Chytry M. 2009. Weed vegetation and its conservation value in three management systems of Hungarian winter cereals on base-rich soils. Weed Res. 49, 544–551 10.1111/j.1365-3180.2009.00730.x (doi:10.1111/j.1365-3180.2009.00730.x) [DOI] [Google Scholar]
- 9.Lososová Z. 2003. Estimating past distribution of vanishing weed vegetation in South Moravia. Preslia 75, 71–79 [Google Scholar]
- 10.Robinson R. A., Sutherland W. J. 2002. Post-war changes in arable farming and biodiversity in Great Britain. J. Appl. Ecol. 39, 157–176 10.1046/j.1365-2664.2002.00695.x (doi:10.1046/j.1365-2664.2002.00695.x) [DOI] [Google Scholar]
- 11.Squire G. R., et al. 2003. On the rationale and interpretation of the Farm Scale Evaluations of genetically modified herbicide-tolerant crops. Phil. Trans. R. Soc. Lond. B 358, 1779–1799 10.1098/rstb.2003.1403 (doi:10.1098/rstb.2003.1403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutman P. J. W., Storkey J., Martin H., Holland J. 2009. Abundance of weeds in arable fields in southern England in 2007/08. Aspects Appl. Biol. 91, 163–168 [Google Scholar]
- 13.Freckleton R. P., Watkinson A. R. 1998. Predicting the determinants of weed abundance: a model for the population dynamics of Chenopodium album in sugar beet. J. Appl. Ecol. 35, 904–920 10.1111/j.1365-2664.1998.tb00008.x (doi:10.1111/j.1365-2664.1998.tb00008.x) [DOI] [Google Scholar]
- 14.Wilson P., King M. 2003. The biology of arable plants. Arable plants: a field guide, p. 312 London, UK: Hanway Press [Google Scholar]
- 15.Moss S. R., Storkey J., Cussans J. W., Perryman S. A. M., Hewitt M. V. 2004. The Broadbalk long-term experiment at Rothamsted: what has it told us about weeds? Weed Sci. 52, 864–873 10.1614/WS-04-012R1 (doi:10.1614/WS-04-012R1) [DOI] [Google Scholar]
- 16.Firbank L. G., Watkinson A. R. 1986. Modeling the population dynamics of an arable weed and its effects upon crop yield. J. Appl. Ecol. 23, 147–159 10.2307/2403088 (doi:10.2307/2403088) [DOI] [Google Scholar]
- 17.Suding K. N., Collins S. L., Gough L., Clark C., Cleland E. E., Gross K. L., Milchunas D. G., Pennings S. 2005. Functional- and abundance-based mechanisms explain diversity loss due to N fertilization. Proc. Natl Acad. Sci. USA 102, 4387–4392 10.1073/pnas.0408648102 (doi:10.1073/pnas.0408648102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Booth B. D., Swanton C. J. 2002. Assembly theory applied to weed communities. Weed Sci. 50, 2–13 10.1614/0043-1745(2002)050[0002:AIATAT]2.0.CO;2 (doi:10.1614/0043-1745(2002)050[0002:AIATAT]2.0.CO;2) [DOI] [Google Scholar]
- 19.Storkey J., Moss S. R., Cussans J. W. 2010. Using assembly theory to explain changes in a weed flora in response to agricultural intensification. Weed Sci. 58, 39–46 10.1614/WS-09-096.1 (doi:10.1614/WS-09-096.1) [DOI] [Google Scholar]
- 20.Marshall E. J. P., Brown V. K., Boatman N. D., Lutman P. J. W., Squire G. R., Ward L. K. 2003. The role of weeds in supporting biological diversity within crop fields. Weed Res. 43, 77–89 10.1046/j.1365-3180.2003.00326.x (doi:10.1046/j.1365-3180.2003.00326.x) [DOI] [Google Scholar]
- 21.Storkey J., Westbury D. B. 2007. Managing arable weeds for biodiversity. Pest Manag Sci. 63, 517–523 10.1002/ps.1375 (doi:10.1002/ps.1375) [DOI] [PubMed] [Google Scholar]
- 22.Walker K. J., Critchley C. N. R., Sherwood A. J., Large R., Nuttall P., Hulmes S., Rose R., Mountford J. O. 2007. The conservation of arable plants on cereal field margins: an assessment of new agri-environment scheme options in England, UK. Biol. Conserv. 136, 260–270 10.1016/j.biocon.2006.11.026 (doi:10.1016/j.biocon.2006.11.026) [DOI] [Google Scholar]
- 23.Butler S. J., Brooks D., Feber R. E., Storkey J., Vickery J. A., Norris K. 2009. A cross-taxonomic index for quantifying the health of farmland biodiversity. J. Appl. Ecol. 46, 1154–1162 [Google Scholar]
- 24.Kleijn D., Sutherland W. J. 2003. How effective are European agri-environment schemes in conserving and promoting biodiversity? J. Appl. Ecol. 40, 947–969 10.1111/j.1365-2664.2003.00868.x (doi:10.1111/j.1365-2664.2003.00868.x) [DOI] [Google Scholar]
- 25.Wilson P. J. 2007. Important arable plant areas: criteria for the assessment of arable sites. Aspects Appl. Biol. 81, 183–189 [Google Scholar]
- 26.Donald P. F., Sanderson F. J., Burfield I. J., van Bommel F. P. J. 2006. Further evidence of continent-wide impacts of agricultural intensification on European farmland birds, 1990–2000. Agric. Ecosyst. Environ. 116, 189–196 10.1016/j.agee.2006.02.007 (doi:10.1016/j.agee.2006.02.007) [DOI] [Google Scholar]
- 27.Lepš J., Šmilauer P. 2003. Multivariate analysis of ecological data using CANOCO, pp. 60–75 Cambridge, UK: Cambridge University Press [Google Scholar]
- 28.Pilgrim E. S., Crawley M. J., Dolphin K. 2004. Patterns of rarity in the native British flora. Biol. Conserv. 120, 161–170 10.1016/j.biocon.2004.02.008 (doi:10.1016/j.biocon.2004.02.008) [DOI] [Google Scholar]
- 29.Webb D. A. 1978. Flora Europaea: a retrospect. Taxon 27, 3–14 10.2307/1220472 (doi:10.2307/1220472) [DOI] [Google Scholar]
- 30.Chamberlain D. E., Fuller R. J., Bunce R. G. H., Duckworth J. C., Shrubb M. 2000. Changes in the abundance of farmland birds in relation to the timing of agricultural intensification in England and Wales. J. Appl. Ecol. 37, 771–788 10.1046/j.1365-2664.2000.00548.x (doi:10.1046/j.1365-2664.2000.00548.x) [DOI] [Google Scholar]
- 31.Krebs J. R., Wilson J. D., Bradbury R. B., Siriwardena G. M. 1999. The second silent spring? Nature 400, 611–612 10.1038/23127 (doi:10.1038/23127) [DOI] [Google Scholar]
- 32.Stoate C., Báldi A., Beja P., Boatman N. D., Herzon I., van Doorn A., de Snoo G. R., Rakosy L., Ramwell C. 2009. Ecological impacts of early 21st century agricultural change in Europe: a review. J. Environ. Manag. 91, 22–46 10.1016/j.jenvman.2009.07.005 (doi:10.1016/j.jenvman.2009.07.005) [DOI] [PubMed] [Google Scholar]
- 33.Firbank L. G., Petit S., Smart S., Blain A., Fuller R. J. 2008. Assessing the impacts of agricultural intensification on biodiversity: a British perspective. Phil. Trans. R. Soc. B 363, 777–787 10.1098/rstb.2007.2183 (doi:10.1098/rstb.2007.2183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrett S. C. H. 1983. Crop mimicry in weeds. Econ. Bot. 37, 255–282 10.1007/BF02858881 (doi:10.1007/BF02858881) [DOI] [Google Scholar]
- 35.Baker H. G. 1974. The evolution of weeds. Annu. Rev. Ecol. Syst. 5, 1–24 10.1146/annurev.es.05.110174.000245 (doi:10.1146/annurev.es.05.110174.000245) [DOI] [Google Scholar]
- 36.Harlan J. R. 1965. Possible role of weed races in evolution of cultivated plants. Euphytica 14, 173–176 10.1007/BF00038984 (doi:10.1007/BF00038984) [DOI] [Google Scholar]
- 37.Firbank L. G. 1988. Agrostemma githago L. J. Ecol. 76, 1232–1246 10.2307/2260645 (doi:10.2307/2260645) [DOI] [Google Scholar]
- 38.Pinke G., Pál R. 2009. Floristic composition and conservation value of the stubble-field weed community, dominated by Stachys annua in western Hungary. Biologia 64, 279–291 10.2478/s11756-009-0035-5 (doi:10.2478/s11756-009-0035-5) [DOI] [Google Scholar]
- 39.Benton T. G., Vickery J. A., Wilson J. D. 2003. Farmland biodiversity: is habitat heterogeneity the key? Trends Ecol. Evol. 18, 182–188 10.1016/S0169-5347(03)00011-9 (doi:10.1016/S0169-5347(03)00011-9) [DOI] [Google Scholar]
- 40.Smart S. M., Thompson K., Marrs R. H., Le Duc M. G., Maskell L. C., Firbank L. G. 2006. Biotic homogenization and changes in species diversity across human-modified ecosystems. Proc. R. Soc. B 273, 2659–2665 10.1098/rspb.2006.3630 (doi:10.1098/rspb.2006.3630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kleijn D., et al. 2009. On the relationship between farmland biodiversity and land-use intensity in Europe. Proc. R. Soc. B 276, 903–909 10.1098/rspb.2008.1509 (doi:10.1098/rspb.2008.1509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevens C. J., et al. 2010. Nitrogen deposition threatens species richness of grasslands across Europe. Environ. Pollut. 158, 2940–2945 10.1016/j.envpol.2010.06.006 (doi:10.1016/j.envpol.2010.06.006) [DOI] [PubMed] [Google Scholar]
- 43.Maskell L. C., Smart S. M., Bullock J. M., Thompson K., Stevens C. J. 2010. Nitrogen deposition causes widespread loss of species richness in British habitats. Glob. Change Biol. 16, 671–679 10.1111/j.1365-2486.2009.02022.x (doi:10.1111/j.1365-2486.2009.02022.x) [DOI] [Google Scholar]
- 44.Winter M., et al. 2009. Plant extinctions and introductions lead to phylogenetic and taxonomic homogenization of the European flora. Proc. Natl Acad. Sci. USA 106, 21 721–21 725 10.1073/pnas.0907088106 (doi:10.1073/pnas.0907088106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gabriel D., Thies C., Tscharntke T. 2005. Local diversity of arable weeds increases with landscape complexity. Perspect. Plant Ecol. Evol. Syst. 7, 85–93 10.1016/j.ppees.2005.04.001 (doi:10.1016/j.ppees.2005.04.001) [DOI] [Google Scholar]
- 46.Marshall E. J. P. 2009. The impact of landscape structure and sown grass margin strips on weed assemblages in arable crops and their boundaries. Weed Res. 49, 107–115 10.1111/j.1365-3180.2008.00670.x (doi:10.1111/j.1365-3180.2008.00670.x) [DOI] [Google Scholar]
- 47.Westoby M. 1998. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil 199, 213–227 10.1023/A:1004327224729 (doi:10.1023/A:1004327224729) [DOI] [Google Scholar]
- 48.Turnbull L. A., Coomes D., Hector A., Rees M. 2004. Seed mass and the competition/colonization trade-off: competitive interactions and spatial patterns in a guild of annual plants. J. Ecol. 92, 97–109 10.1111/j.1365-2745.2004.00856.x (doi:10.1111/j.1365-2745.2004.00856.x) [DOI] [Google Scholar]
- 49.Freckleton R. P., Watkinson A. R. 2001. Predicting competition coefficients for plant mixtures: reciprocity, transitivity and correlations with life-history traits. Ecol. Lett. 4, 348–357 10.1046/j.1461-0248.2001.00231.x (doi:10.1046/j.1461-0248.2001.00231.x) [DOI] [Google Scholar]
- 50.Sugiyama S., Bazzaz F. A. 1998. Size dependence of reproductive allocation: the influence of resource availability, competition and genetic identity. Funct. Ecol. 12, 280–288 10.1046/j.1365-2435.1998.00187.x (doi:10.1046/j.1365-2435.1998.00187.x) [DOI] [Google Scholar]
- 51.Thompson K., Band S. R., Hodgson J. G. 1993. Seed size and shape predict persistence in soil. Funct. Ecol. 7, 236–241 10.2307/2389893 (doi:10.2307/2389893) [DOI] [Google Scholar]
- 52.Gaudet C. L., Keddy P. A. 1988. A comparative approach to predicting competitive ability from plant traits. Nature 334, 242–243 10.1038/334242a0 (doi:10.1038/334242a0) [DOI] [Google Scholar]
- 53.Grime J. P., et al. 1997. Integrated screening validates primary axes of specialisation in plants. Oikos 79, 259–281 10.2307/3546011 (doi:10.2307/3546011) [DOI] [Google Scholar]
- 54.Butler S. J., Boccaccio L., Gregory R. D., Vorisek P., Norris K. 2010. Quantifying the impact of land-use change to European farmland bird populations. Agric. Ecosyst. Environ. 137, 348–357 10.1016/j.agee.2010.03.005 (doi:10.1016/j.agee.2010.03.005) [DOI] [Google Scholar]
- 55.Gibson R. H., Nelson I. L., Hopkins G. W., Hamlett B. J., Memmott J. 2006. Pollinator webs, plant communities and the conservation of rare plants: arable weeds as a case study. J. Appl. Ecol. 43, 246–257 10.1111/j.1365-2664.2006.01130.x (doi:10.1111/j.1365-2664.2006.01130.x) [DOI] [Google Scholar]
- 56.Potts G. R., Ewald J. A., Aebischer N. J. 2009. Long-term changes in the flora of the cereal ecosystem on the Sussex Downs, England, focusing on the years 1968–2005. J. Appl. Ecol. 47, 215–226 10.1111/j.1365-2664.2009.01742.x (doi:10.1111/j.1365-2664.2009.01742.x) [DOI] [Google Scholar]
- 57.Ammermann K. 2008. Cultivated biomass for energy production: effects on biodiversity and landscapes. Natur Landschaft 83, 103–110 [Google Scholar]
- 58.Meyer S., Wesche K., Leuschner C., van Elsen T., Metzner J. 2010. A new conservation strategy for arable weed vegetation in Germany: the project ‘100 fields for biodiversity’. Plant Breed. Seed Sci. 61, 25–34 10.2478/v10129-010-0009-3 (doi:10.2478/v10129-010-0009-3) [DOI] [Google Scholar]
- 59.Marshall E. J. P. 2002. Introducing field margin ecology in Europe. Agric. Ecosyst. Environ. 89, 1–4 10.1016/S0167-8809(01)00314-0 (doi:10.1016/S0167-8809(01)00314-0) [DOI] [Google Scholar]