Abstract
Women with BRCA1/2 mutations have a significantly higher lifetime risk of developing breast or ovarian cancer. We suggest that female mutation carriers may have improved fitness owing to enhanced fertility relative to non-carriers. Here we show that women who are carriers of BRCA1/2 mutations living in natural fertility conditions have excess fertility as well as excess post-reproductive mortality in relation to controls. Individuals who tested positive for BRCA1/2 mutations who linked into multi-generational pedigrees within the Utah Population Database were used to identify putative obligate carriers. We find that women born before 1930 who are mutation carriers have significantly more children than controls and have excess post-reproductive mortality risks. They also have shorter birth intervals and end child-bearing later than controls. For contemporary women tested directly for BRCA1/2 mutations, an era when modern contraceptives are available, differences in fertility and mortality persist but are attenuated. Our findings suggest the need to re-examine the wider role played by BRCA1/2 mutations. Elevated fertility of female mutation carriers indicates that they are more fecund despite their elevated post-reproductive mortality risks.
Keywords: fertility, mortality, BRCA1, BRCA2, pleiotropy, telomeres
1. Introduction
Women with BRCA1 or BRCA2 mutations have an estimated 40 to 85 per cent lifetime risk of developing breast cancer and 16 to 64 per cent risk of ovarian cancer [1–3]. These mutations are relatively prevalent, raising the possibility that these mutations may have beneficial effects despite the significant excess cancer risk. Could women with these mutations, whose breast and ovarian cancers arise typically after menopause, bear more children and have a longer reproductive interval? No studies have been conducted that focus exclusively on whether fertility as well as survival differences exist between female BRCA1/2 mutation carriers and non-carriers under natural fertility conditions, and whether these differences persist when modern contraception is available (see the studies of Moslehi et al. [4] and Mai et al. [5] that share some important features of this analysis).
The purpose of this study is to address two fundamental issues. First, what mechanisms may explain why BRCA1 and BRCA2 mutations persist in human populations given their adverse health consequences? Genetic variants have been shown to have pleiotropic functions, with beneficial effects possibly outweighing their adverse effects [6]. Excess mortality (due primarily to breast and ovarian cancer) risks that occur among mutation carriers could be adequate to result in strong negative selection [7]. We hypothesize that while BRCA1/2 gene mutations increase cancer incidence and mortality in middle and later adulthood, mutation carriers may have enhanced reproductive fitness because of elevated fertility rates, which may compensate for their excess levels of post-menopausal mortality. Second, we also hypothesize that the fertility effects of being a BRCA1 or BRCA2 mutation carrier are different in periods when no modern contraception available compared with periods where individuals may be making family planning decisions based on predictive genetic risk information heretofore unavailable. We hypothesize that family cancer history and the advent of genetic testing, along with the availability of effective family planning methods, lead to lower levels of fertility for female BRCA1/2 mutation carriers in relation to non-carriers. These hypotheses are tested using a unique resource, the Utah Population Database (UPDB), which identifies putative obligate carriers of BRCA1 or BRCA2 mutations whose descendants were tested directly for these mutations.
2. Study design
BRCA1/2 mutation carriers were identified from two longitudinal studies. The first was a large, prospective study that analysed the fertility behaviours and attitudes of BRCA1 mutation carriers. Participants of this study were members of a large Utah multi-generational pedigree or kindred (K2082) with an identified mutation at the BRCA1 locus. To increase our sample size and include carriers of deleterious mutations in both BRCA1 and BRCA2, participants from the High-Risk Breast Cancer Clinic (HRBCC) at the University of Utah's Huntsman Cancer Institute (HCI) were also selected. The HRBCC is a research resource for individuals with a family history of breast and ovarian cancer. A detailed description of methods, eligibility criteria and protocols for both studies has been described elsewhere [1,8]. There were 133 female mutation carriers from 49 kindreds (each with a unique founder) selected for this study based on the availability of their genetic test results from these two sources. Genetic testing for individuals in K2082 and the HRBCC were performed by Clinical Laboratory Improvement Act-approved laboratories. Tests for a few initial enrollees were done by the University of Utah's DNA Diagnostic Laboratory, but nearly all the remainder were conducted by Myriad Genetics [1,9,10] after these individuals were provided with extensive genetic counselling and gave their informed consent. Subjects were then classified as a BRCA1 or BRCA2 mutation carrier. Both studies were approved by the University of Utah's Institutional Review Board and the Resource for Genetic and Epidemiologic Research.
Information about each carrier's relatives was obtained from the UPDB. UPDB is a population-based resource used for biomedical research. UPDB holds data on approximately seven million individuals derived from genealogical, demographic and medical records spanning the past two centuries [11,12]. Linked genealogical and birth certificate records are contained within the UPDB and permit the identification of multi-generational pedigrees that range from 2 to 11 generations. Data from the Utah Cancer Registry, Idaho Cancer Registry and Utah death certificates are linked to the family history (genealogical) records to construct an extensive biodemographic research database.
Pedigree information of carriers was used to identify ancestors who are putative mutation carriers. Mutation status of ancestors in the UPDB was derived based on pedigree position and relationship to multiple tested carriers. Families with multiple tested mutation carriers were reviewed to identify the transmission of the mutation through the pedigree. This was done by first identifying two or more individuals who were directly tested and found to be carriers of the same BRCA1/2 mutation. The closest common ancestor (i.e. founder) between the two (or more) tested individuals was then identified based on the UPDB. All individuals that connect the founder and the tested individuals were identified as putative obligate mutation carriers.
Several selection criteria were used to identify the sample from the UPDB. Each individual was required to have complete information on birth dates, fertility history and age at last birth. The UPDB contains comprehensive birth history information, but no data exist for pregnancies and stillbirths. The few individuals who were members of a polygamous relationship were excluded. To focus the assessment of fertility on monogamous unions, only individuals who were married once are included. Individuals who never married or had incomplete spouse information were excluded. All offspring were required to have complete birthdates and to have been born in Utah or Idaho. The birth years before 1930 were used to approximate an era when women would have limited access to modern birth control (e.g. oral contraceptives) until their mid-30s at the earliest. Tested carriers born after 1975 were also not selected as a result of their severely censored fertility experience.
The first step in selecting controls from the UPDB was to identify founders for the 49 HRBCC/K2082 kindreds with a known mutation. Any descendants of these 49 founders were excluded from consideration as a control. Controls were selected by matching birth year of the mutation carriers. We restricted the analysis to parous individuals. This specification was used because the study design requires that all putative obligate carriers had at least one child survive to reproductive age. In other words, since there needs to be an unbroken chain of descendants connecting the founders to tested individuals among the obligate carriers, it was necessary to impose the same pedigree structure on the controls. Controls, like the carriers, were also required to have one spouse and complete fertility information.
The final sample of 181 carriers comprised carriers born before 1930 (obligate n = 48, directly tested n = 11) and tested female carriers (n = 122) born between 1930 and 1975, and 15 : 1 matched controls (n = 885 controls for the pre-1930 carriers and n = 1830 controls for the post-1930 women). Drawing on the UPDB, the potential pool of population-based controls (as well as mutation carriers) were those who were born before 1975, had no ancestors or descendants who were carriers, and, like the carriers, were once married with complete spouse information, had at least one child where the mother's age at first birth was known and gave birth to all children in Utah or Idaho. A control is unlikely to be a carrier given that none of their tested descendants are carriers and they themselves were not descendants of obligate carriers. If a carrier was incorrectly counted as a control, this misclassification would create a conservative bias. This protocol resulted in a pool of 101 023 potential controls that excluded 5536 descendants of the BRCA1 or BRCA2 founders. Women were restricted to survive to at least age 45 in order to observe completed (or nearly completed) fertility. We also report post-reproductive mortality risks associated with the BRCA1 and BRCA2 mutations. This survival restriction excludes 12 per cent of carriers and 5 per cent of controls, a difference that might again yield conservative results.
Two supporting complementary analyses were also conducted. The first, and the most historical, examined female fertility of the BRCA1/2 mutation founders (n = 49) with 15 : 1 matched (by birth year) controls (n = 733). This analysis (hereafter the ‘founder’ analysis) was conducted to assess fertility at the earliest possible point where natural fertility conditions prevailed. The second, and the most contemporary, considered women who enrolled at the HRBCC and completed questionnaires that asked about their reproductive history. They are from high-risk breast cancer families who enrolled at the HRBCC between 1994 and 2006, all of whom were tested for BRCA1/2 mutations. Given that contemporary women have substantially lower fertility, it is more informative to assess whether these women differed in their ability to conceive according to their mutation status, information obtained from the HRBCC questionnaire and unavailable for the larger sample of carriers in the UPDB.
(a). Statistical methods
We examined several measures of fertility. The first indicator is the number of children ever born (CEB). To examine the relationship between CEB and mutation status we used ordinary least squares (OLS) as well as Poisson regression (results were very similar; accordingly, we show here the OLS regressions results; Poisson results are given in the electronic supplementary material). This was done for both the full sample and the founder analysis. The availability of effective birth control methods was considered by performing separate regressions for carriers born before 1930 and born during 1930–1974. The year 1930 was selected because women born after that date would be fecund at the time modern family planning methods, notably exogenous hormones, became available. Mutation status was represented by a dummy variable, carrier (=1) versus control (=0). The other covariates used in all fertility models were birth year, age at first birth, and, when appropriate, age at marriage and the number of offspring who died as children. A dummy variable that describes whether a woman was an active member of the Church of Jesus Christ of Latter-day Saints (LDS, or Mormon) status was used in the pre-1930 models. A second fertility model was estimated to assess whether there was effect modification due to historical time. This was done by introducing a two-way interaction between mutation status and birth year (or whether born before or after 1930).
The effect of the subjects' parental fertility patterns on CEB was also considered, since a subject's parents' fertility might be associated with both the subject's mutation status and fertility. The number of siblings was therefore included in the regressions. The addition of parental fertility patterns did not alter the effect of mutation status and was therefore excluded from the models shown.
We investigated whether carriers were more likely to exceed the average number of children for their cohort. The association between fertility and mutation status was estimated by multiple logistic regression. The mean number of children for the sample was 3.73, so subjects with four or more children were classified as having ‘more’ children. The dependent variable was coded 1 if the subjects had four or more children; all other subjects were coded 0.
Additional measures of reproductive behaviours are examined that reflect underlying fecundity (particularly for those born during the pre-1930 era), as well as efforts to limit fertility through family planning (primarily for those born in 1930 or later). Moreover, we sought to avoid any artefacts that might produce an association between fertility and mutation status. In particular, it is possible that contemporary individuals chose to be tested genetically (with some testing positive) because they come from large families where the consequences of the test results could be particularly significant. If seeking genetic testing is linked to family size, then pedigrees containing mutation-positive persons may also contain putative obligate carriers with greater fertility. Accordingly, we have expanded the measures of fertility that are less sensitive to this potential limitation: age at first birth, first birth interval, average birth interval (mean length of time between all consecutive births with adjustments for twinning) and age at last birth. While all of these measures are related to CEB, they do provide additional assessments of fecundity, particularly for those bearing children before the advent of modern contraception. An examination of these characteristics also provides an opportunity to isolate the specific demographic components of fertility through which BRCA1/2 mutations may be operating. These additional outcomes are all continuous variables and are analysed using OLS regression.
To assess HRBCC women with respect to the effects of BRCA1/2 on difficulties becoming pregnant, we estimated logistic regressions for the dependent variable (1 = yes, 0 = no) based on the following question: ‘Did you ever try for one straight year or more to become pregnant and, during that time, not become pregnant?’ We analysed all HRBCC participants between ages 18 and 64 (n = 419) who answered this question. Covariates included in the model are age at interview, birth control usage, total live births at time of interview and marital status.
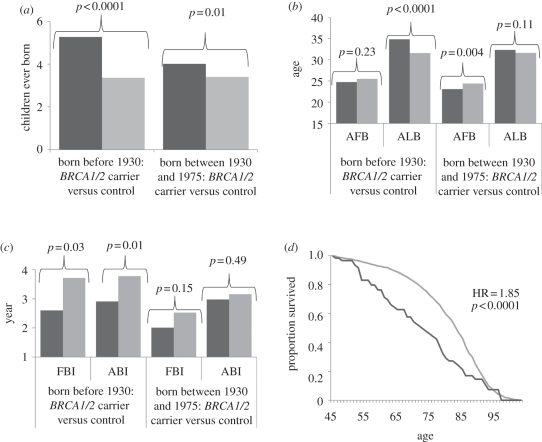
Mortality differences between BRCA1/2 carriers and controls for survival past age 45 are presented as shown in figure 1d (see electronic supplementary material, table S1), based on Cox proportional hazard regression analyses for persons born before 1930. They show significant excess mortality risks associated with being a carrier of a BRCA1 or BRCA2 mutation.
Figure 1.
(a) Effects of BRCA1/2 mutation status on number of children ever born, based on ordinary least squares (OLS) regression controlling for birth year, age at first birth and number of deceased children (who died by age 5). (b) Effects of BRCA1/2 mutation status on age at first birth (AFB) and age at last birth (ALB), based on OLS regressions controlling for birth year and age at marriage (and AFB for the ALB model). (c) Effects of BRCA1/2 mutation status on first birth interval (FBI) and average birth interval (ABI), based on OLS regressions controlling for birth year and age at first marriage. (d) Survival probabilities past age 45 by BRCA1/2 mutation status for women born before 1930, based on Cox proportional hazard regressions controlling for birth year. Models for women born before 1930 also controlled for LDS status. Dark grey bars or lines denote carrier and light grey bars or lines denote control.
3. Results
Descriptive statistics are displayed in table 1 and show that mutation carriers had approximately two more children (CEB) than controls. For example, controls born before 1930 had a mean CEB of 4.19 while carriers from the same era had 6.22.
Table 1.
Descriptive statistics.
| variables |
BRCA1/2 mutation carriers |
controls |
||||
|---|---|---|---|---|---|---|
| n | mean | s.d. | n | mean | s.d. | |
| born before 1930 | ||||||
| birth year | 59 | 1906.78 | 17.72 | 885 | 1906.78 | 17.58 |
| death year (if applicable) | 52 | 1974.50 | 24.25 | 716 | 1981.90 | 21.51 |
| year of first marriage | 47 | 1929.70 | 18.36 | 850 | 1928.23 | 17.53 |
| year of first birth | 59 | 1930.22 | 18.67 | 885 | 1930.96 | 18.49 |
| age at first marriage | 47 | 21.77 | 3.34 | 850 | 21.40 | 3.53 |
| age at first birth | 59 | 23.44 | 3.31 | 885 | 24.18 | 4.80 |
| age at last birth | 59 | 36.76 | 5.15 | 885 | 33.94 | 6.27 |
| first birth interval (years) | 47 | 1.68 | 1.49 | 850 | 2.82 | 3.38 |
| average birth interval (years) | 59 | 2.69 | 1.10 | 877 | 3.64 | 2.62 |
| number deceased children | 59 | 0.39 | 0.87 | 885 | 0.35 | 0.78 |
| children ever born | 59 | 6.22 | 2.74 | 885 | 4.19 | 2.68 |
| devout Mormon (dummy) | 59 | 0.76 | 0.43 | 885 | 0.83 | 0.38 |
| born 1930–1974 | ||||||
| birth year | 122 | 1949.10 | 9.43 | 1830 | 1949.10 | 9.39 |
| death year (if applicable) | 22 | 1997.41 | 6.67 | 102 | 2001.74 | 5.57 |
| year of first marriage | 63 | 1971.03 | 11.67 | 1670 | 1971.06 | 11.71 |
| year of first birth | 122 | 1972.19 | 10.10 | 1830 | 1973.52 | 11.59 |
| age at first marriage | 63 | 21.08 | 4.21 | 1670 | 22.00 | 4.45 |
| age at first birth | 122 | 23.09 | 3.51 | 1830 | 24.42 | 5.16 |
| age at last birth | 122 | 31.70 | 5.37 | 1830 | 31.72 | 5.45 |
| first birth interval (years) | 63 | 2.29 | 3.00 | 1670 | 2.50 | 2.87 |
| average birth interval (years) | 120 | 2.98 | 1.29 | 1824 | 3.17 | 2.15 |
| number deceased children | 122 | 0.03 | 0.22 | 1830 | 0.07 | 0.29 |
| children ever born | 122 | 4.13 | 1.82 | 1830 | 3.40 | 1.75 |
Figure 1a and electronic supplementary material, table S2 report the results of OLS regressions showing the effects of mutation status on CEB. There is a significant association between mutation status and CEB for pre-1930 carriers who have an average of 1.91 more children than controls. Excess and significant levels of CEB are also detected for post-1930 carriers, though the increase is attenuated to 0.61 CEB. The decline in the fertility effect of carrier status before and after 1930 is statistically significant (p < 0.001), a result that is largely attributable to the effects of BRCA1 carriers specifically. The significant fertility-enhancing effects of BRCA1/2 mutations are also detected based on the founder sample (electronic supplementary material, table S3): carriers have 1.17 more children than controls (p < 0.01) and are 2.67 times more likely to have four or more children (95% CI: 1.26–5.68).
To address the possibility that these fertility influences are attributable to unique mutations found among specific founders [10], we identified 23 distinct mutations for those born prior to 1930 and 37 for the more recent cohort (the two birth cohorts share 20 mutations; see electronic supplementary material, table S4). The most frequent mutation, c.3937C>T [13,14], is associated with K2082, with the remaining mutations comprising very small percentages; the next most frequent mutations comprised approximately 10 per cent of their respective cohorts (c.213-11T>G for the pre-1930 cohort, c.2035A>T for the later cohort). To address the possibility that members of K2082 may be dominating the results, we excluded their members and re-estimated our models. For pre-1930 carriers (42 carriers and 630 controls), the effects were again significant (1.76 more CEB, p < 0.001); a significant (albeit attenuated) effect was also detected for tested women (0.51 more CEB, p < 0.005; electronic supplementary material, table S2).
Logistic regressions were also estimated where the dependent variable measures whether an individual had four or more children. In relation to controls, pre-1930 carriers were approximately 3.6 times (95% confidence interval (CI): 1.85–7.15) more likely to have four or more children, whereas tested carriers born after 1930 were approximately 2.04 times more likely (95% CI: 1.37–3.02; see electronic supplementary material, table S5).
The impact of carrier status on the timing and pacing of child-bearing is shown in figure 1b,c (electronic supplementary material, table S6). For pre-1930 carriers, their fertility is marked by significantly shorter first and average birth intervals, as well as later ages at last birth. No influence of mutation status was detected for an early age at first birth. Post-1930 carriers show an earlier age at first birth; average and first birth intervals are shorter and age at last birth later; but all are insignificant.
For contemporary high-risk women under age 65 seen at the HRBCC, we compared BRCA1/2 mutation carriers with non-carriers with respect to their difficulty getting pregnant when intending to do so, according to the HRBCC questionnaire. No differences were detected between these two groups (OR = 0.904, 95% CI 0.52–1.58), and this null result did not vary by BRCA1 and BRCA2 mutation status (BRCA1: OR = 1.03, 95% CI: 0.55–1.93; OR = 0.74, 95% CI: 0.35–1.54; electronic supplementary material, table S7). These results suggest that comparisons of contemporary women's fertility by mutation status may be difficult when women's reproductive plans are strongly affected by choice.
4. Discussion
Our findings indicate that female mutation carriers bear more children, have shorter birth intervals and reproduce later in life than matched controls, a difference that is large initially when effective contraception is absent. Mutation carriers also have excess mortality after age 45 in relation to controls. This finding is probably owing to mortality from breast and ovarian cancer in BRCA1 mutation carriers, and cancers of the breast, ovary, pancreas and melanoma in BRCA2 mutation carriers. For those with death certificates in the pre-1930 cohort, carriers (n = 33) had 18 per cent who died from breast cancer and 27 per cent from ovarian cancer. Among controls (n = 621), the figures were 3 per cent and 1 per cent, respectively, estimates very close to those for the general population (www.cancer.org). Mai et al. [5] suggest that other causes of death are also elevated among mutation carriers.
What are the mechanisms that connect BRCA1 mutations to female fertility? Recent studies suggested that BRCA1/2 mutations limit embryogenesis [15–18], suggesting impaired reproductive fitness. Murine models have shown that homozygous deletions of BRCA1/2 result in embryonic lethality [16], while heterozygous mice developed normally and were fertile. Homozygous BRCA1/2 human embryos have been shown to spontaneously abort [3]. Pal et al. [19] argued that numerous mechanisms alter the process of cell cycle and division and DNA repair, and given that many are affected by BRCA proteins, BRCA1/2 mutations may limit rather than promote reproduction.
These predictions have not, however, been supported by the human data. Indeed, three recent investigations examining this association show that mutation status has no effect on female fertility. Pal et al. [19] asked whether female fertility was affected among carriers of BRCA1/2 mutations in relation to family controls, all in a contemporary population. They found that parity was the same between carriers and non-carriers. Friedman et al. [3] found no differences between carriers and non-carriers in terms of spontaneous abortions. Moslehi et al. [4] compared the fertility of mutation carriers and non-carriers with ovarian cancer and controls based on a largely contemporary sample of Ashkenazi Jewish women (born after 1930). They concluded that there was no evidence that BRCA mutations affected female fertility.
What mechanisms may be involved in a positive association between BRCA1/2 mutations and fertility? We suggest that an association between telomeres and BRCA mutations are consistent with the findings reported here (see also [19]). This proposed mechanism relies on (at least) two linkages: one between BRCA mutations and telomere length, and another between telomere length and fertility. With respect to the former, French et al. [20] reported that the disruption of BRCA1 may result in telomere lengthening (see also [21]). They also confirmed that the overexpression of BRCA1 limits telomerase activity and reduces telomere length. Their results suggest a possible role of BRCA1 mutations in protecting telomeres. Ballal et al. [22] also found that BRCA1 overexpression is associated with telomere shortening (for an exception, see [23]).
While previous studies have shown that longer telomeres are associated with increases in longevity [24] as well as reductions in the risk of major causes of death (except cancer), early evidence suggests that longer telomeres play a role in enhancing reproduction. Keefe et al. [25,26] note that telomerase, the enzyme that maintains telomere length, is not active in oocytes (but is during the blastocyst stage) so oocytes have their full telomere length at the earliest point of development. They argue that the lengthy period between foetal life and ovulation in mid-life would expose oocytes to the effects of reactive oxygen that would shorten telomeres. Accordingly, telomeres in oocytes would probably shorten with increasing age owing to the combined effects of late ovulation and the prolonged interval before ovulation. Keefe et al. examined these mechanisms by shortening telomeres in mice, which produced a phenotype comparable with age-related oocyte dysfunction in women. They also examined eggs donated by women aged 25–42 undergoing in vitro fertilization (IVF). Telomere lengths were longer in eggs from women who conceived than in those from women who did not after IVF. No women conceived with a mean telomere length from spare eggs shorter than 6.3 kb. They concluded that shorter telomere lengths in eggs predicted conception rates in women undergoing IVF. Earlier, Aydos et al. [27] studied telomere lengths of 37 females aged 50, and found a positive association between reproductive lifespan and telomere length.
The association between telomere length and reproductive ageing was also analysed by Hanna et al. [28], who compared women with recurrent miscarriages (RMs), premature ovarian failure (POF) and two control groups. RM women had significantly shorter age-adjusted average telomeres than controls [28]. Women with POF had longer age-adjusted mean telomeres for one of the two control groups, an inconsistency probably owing to the very small number of POF subjects, as the authors point out. The telomere length differences between RM and control women (who had viable pregnancies later in life) are consistent with the hypothesis that telomere length affects the rate of reproductive ageing in women. Our results on shorter first and average birth intervals are consistent with Hanna et al.'s finding.
Previous evidence indicates that fertility may be elevated among mutation carriers. A study investigating the effect of parity on breast cancer risk found that women with a BRCA2 mutation had elevated CEB (mean CEB: carrier = 3.3, negative = 3.0, control = 3.2), though statistical testing was not done [29]. Jernstrom et al. [30] examined the association between parity and cancer risk in mutation carriers and found a significantly higher mean number of births for carriers (1.6) versus controls (1.4).
In these studies, subjects had access to modern contraception, making it difficult to assess endogenous differences in fecundity between carriers and non-carriers. Knowledge of genetic testing status has been shown previously to affect family planning as our present findings suggest. Carriers, and those who chose not to be tested or did not know their genetic testing status, were less likely to want additional children than non-carriers [31]. Therefore, once effective contraception is accessible, BRCA mutation carriers may avail themselves of these family planning methods, allowing them to reduce fertility and in turn reduce the potential risk of burdening their children with cancer. Recent fertility differences may also be the result of women following recommended preventive guidelines for mutation carriers. In a study of behavioural differences 2 years after genetic testing, 46 per cent of carriers had obtained bilateral oophorectomies [32]. A family history of cancer has also been found to influence the decision for prophylactic surgery independent of genetic testing [31]. These findings are consistent with the idea that fertility of tested carriers (i.e. among contemporary women) may be reduced as a result of family history of breast/ovarian cancer.
Finally, Pavard & Metcalf [7] examined evolutionary selection of BRCA1 mutations and concluded that BRCA1 alleles may have experienced negative selection throughout human history. Although breast and ovarian cancer typically arise after menopause, sufficient numbers of incident cases occur before that age. They consider (but do not dismiss) whether antagonistic pleiotropy may be an explanation for the persistent (though rare) allele frequencies for deleterious BRCA1 mutations, though citing some empirical support for it [7]. They ‘show that BRCA1 mutations are subject to strong negative selection. BRCA1 is therefore not a good candidate for mutation accumulation’. (p. 7 of [7]). They indicate that for antagonistic pleiotropy to be plausible, positive selection on alleles leading to improved survival or fertility at younger ages must be substantial enough to offset the negative selection on survival at later ages. The specific role of fertility as part of this argument was not directly addressed in their study since their analysis explicitly assumes that BRCA mutation carriers and non-carriers do not differ with respect to fertility, and that fertility does not affect the risk of cancer for those with the mutation (p. 4 of [7]). Our results would suggest that the former assumption is not supported. With respect to the latter assumption, there is evidence that increasing fertility may mitigate the cancer risk of BRCA mutations [33,34]. This may explain the relatively lower levels of cancer incidence among mutation carriers in the more distant past [22,25]. Accordingly, some susceptibility alleles may have faced lower levels of negative selection in earlier environments and allowed them to have higher allele frequencies in the population. It is worth noting that a number of mutations identified in the sample (e.g. c.4065_4068delTCAA, c.1175_1214del40, c.5266dupC, c.6275_6276delTT, c.6486_6489delACAA, c.3937C>T) have been characterized as having been present 8–170 generations earlier [13,14]. Additional work remains to establish how ancient all the mutations are in this sample.
The higher number of CEB and higher mortality rate of BRCA1/2 mutation carriers may reflect the antagonistic pleiotropic features of these mutations. This raises a number of clinical issues in that the very individuals who carry these mutations are most likely to transmit them, given their larger family sizes. The fertility that may be elevated among mutation carriers may also offer some protection for mothers from subsequent cancer risk.
Acknowledgements
This work was supported by National Institutes of Health grant AG022095 (The Utah Study of Fertility, Longevity and Ageing). We wish to thank the Huntsman Cancer Foundation for database support provided to the Pedigree and Population Resource of the HCI, University of Utah. We also thank Dr Richard Cawthon, Dr David Goldgar and Vickie Venne for their comments, and Dr Jeffrey Botkin at the University of Utah School of Medicine and Alison Fraser, Diana Lane Reed, and Deb Ma at the HCI for valuable assistance in managing the data. Partial support for all datasets within the UPDB was provided by the University of Utah HCI and the HCI Cancer Centre Support Grant, P30 CA42014 from National Cancer Institute.
References
- 1.Botkin J. R., Croyle R. T., Smith K. R., Baty B. J., Lerman C., Goldgar D. E., Ward J. M., Flick B. J., Nash J. E. 1996. A model protocol for evaluating the behavioral and psychosocial effects of BRCA1 testing. J. Natl Cancer Inst. 88, 872–882 10.1093/jnci/88.13.872 (doi:10.1093/jnci/88.13.872) [DOI] [PubMed] [Google Scholar]
- 2.Easton D. F., Ford D., Bishop D. T. 1995. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am. J. Hum. Genet. 56, 265–271 [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman E., et al. 2006. Spontaneous and therapeutic abortions and the risk of breast cancer among BRCA mutation carriers. Breast Cancer Res. 8, R15. 10.1186/bcr1387 (doi:10.1186/bcr1387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moslehi R., Singh R., Lessner L., Friedman J. M. 2010. Impact of BRCA mutations on female fertility and offspring sex ratio. Am. J. Hum. Biol. 22, 201–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mai P. L., Chatterjee N., Hartge P., Tucker M., Brody L., Struewing J. P., Wacholder S., Toland A. E. 2009. Potential excess mortality in BRCA1/2 mutation carriers beyond breast, ovarian, prostate, and pancreatic cancers, and melanoma. PLoS ONE 4, e4812. 10.1371/journal.pone.0004812 (doi:10.1371/journal.pone.0004812) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams G. C. 1957. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411 10.2307/2406060 (doi:10.2307/2406060) [DOI] [Google Scholar]
- 7.Pavard S., Metcalf C. J. 2007. Negative selection on BRCA1 susceptibility alleles sheds light on the population genetics of late-onset diseases and aging theory. PLoS ONE 2, e1206. 10.1371/journal.pone.0001206 (doi:10.1371/journal.pone.0001206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frost C., Venne V., Cunningham D., Gerritsen-McKane R. 2004. Decision making with uncertain information: learning from women in a high risk breast cancer clinic. J. Genet. Couns. 13, 221. 10.1023/B:JOGC.0000027958.02383.a9 (doi:10.1023/B:JOGC.0000027958.02383.a9) [DOI] [PubMed] [Google Scholar]
- 9.John E. M., et al. 2004. The Breast Cancer Family Registry: an infrastructure for cooperative multinational, interdisciplinary and translational studies of the genetic epidemiology of breast cancer. Breast Cancer Res. 6, R375–R389 10.1186/bcr801 (doi:10.1186/bcr801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank T. S., et al. 2002. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J. Clin. Oncol. 20, 1480–1490 10.1200/JCO.20.6.1480 (doi:10.1200/JCO.20.6.1480) [DOI] [PubMed] [Google Scholar]
- 11.Bean L., Mineau G., Anderton D. 1990. Fertility change on the American frontier: adaptation and innovation. Berkeley, CA: University of California Press [Google Scholar]
- 12.Wylie J. E., Mineau G. P. 2003. Biomedical databases: protecting privacy and promoting research. Trends Biotechnol. 21, 113–116 10.1016/S0167-7799(02)00039-2 (doi:10.1016/S0167-7799(02)00039-2) [DOI] [PubMed] [Google Scholar]
- 13.Ferla R., et al. 2007. Founder mutations in BRCA1 and BRCA2 genes. Ann. Oncol. 18(Suppl. 6), vi93–vi98 10.1093/annonc/mdm234 (doi:10.1093/annonc/mdm234) [DOI] [PubMed] [Google Scholar]
- 14.Neuhausen S. L., et al. 1996. Haplotype and phenotype analysis of six recurrent BRCA1 mutations in 61 families: results of an international study. Am. J. Hum. Genet. 58, 271–280 [PMC free article] [PubMed] [Google Scholar]
- 15.Giscard d'Estaing S., Perrin D., Lenoir G. M., Guerin J. F., Dante R. 2005. Upregulation of the BRCA1 gene in human germ cells and in preimplantation embryos. Fertil. Steril. 84, 785–788 10.1016/j.fertnstert.2005.02.037 (doi:10.1016/j.fertnstert.2005.02.037) [DOI] [PubMed] [Google Scholar]
- 16.Liu C. Y., Flesken-Nikitin A., Li S., Zeng Y., Lee W. H. 1996. Inactivation of the mouse BRCA1 gene leads to failure in the morphogenesis of the egg cylinder in early postimplantation development. Genes Dev. 10, 1835–1843 10.1101/gad.10.14.1835 (doi:10.1101/gad.10.14.1835) [DOI] [PubMed] [Google Scholar]
- 17.Scully R., Livingston D. M. 2000. In search of the tumour-suppressor functions of BRCA1 and BRCA2. Nature 408, 429–432 10.1038/35044000 (doi:10.1038/35044000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de la Hoya M., Fernandez J. M., Tosar A., Godino J., Sanchez de Abajo A., Vidart J. A., Pérez-Segura P., Díaz-Rubio E. 2003. Association between BRCA1 mutations and ratio of female to male births in offspring of families with breast cancer, ovarian cancer, or both. JAMA 290, 929–931 10.1001/jama.290.7.929 (doi:10.1001/jama.290.7.929) [DOI] [PubMed] [Google Scholar]
- 19.Pal T., Keefe D., Sun P., Narod S. A. 2010. Fertility in women with BRCA mutations: a case-control study. Fertil. Steril. 93, 1805–1808 10.1016/j.fertnstert.2008.12.052 (doi:10.1016/j.fertnstert.2008.12.052) [DOI] [PubMed] [Google Scholar]
- 20.French J. D., Dunn J., Smart C. E., Manning N., Brown M. A. 2006. Disruption of BRCA1 function results in telomere lengthening and increased anaphase bridge formation in immortalized cell lines. Genes Chromosomes Cancer 45, 277–289 10.1002/gcc.20290 (doi:10.1002/gcc.20290) [DOI] [PubMed] [Google Scholar]
- 21.Xiong J., et al. 2003. BRCA1 inhibition of telomerase activity in cultured cells. Mol. Cell Biol. 23, 8668–8690 10.1128/MCB.23.23.8668-8690.2003 (doi:10.1128/MCB.23.23.8668-8690.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ballal R. D., Saha T., Fan S., Haddad B. R., Rosen E. M. 2009. BRCA1 localization to the telomere and its loss from the telomere in response to DNA damage. J. Biol. Chem. 284, 36 083–36 098 10.1074/jbc.M109.025825 (doi:10.1074/jbc.M109.025825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McPherson J. P., et al. 2006. A role for Brca1 in chromosome end maintenance. Hum. Mol. Genet. 15, 831–838 10.1093/hmg/ddl002 (doi:10.1093/hmg/ddl002) [DOI] [PubMed] [Google Scholar]
- 24.Cawthon R. M., Smith K. R., O'Brien E., Sivatchenko A., Kerber R. A. 2003. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361, 393–395 10.1016/S0140-6736(03)12384-7 (doi:10.1016/S0140-6736(03)12384-7) [DOI] [PubMed] [Google Scholar]
- 25.Keefe D. L., Franco S., Liu L., Trimarchi J., Cao B., Weitzen S., Agarwal S., Blasco M. A. 2005. Telomere length predicts embryo fragmentation after in vitro fertilization in women: toward a telomere theory of reproductive aging in women. Am. J. Obstet. Gynecol. 192, 1256–1260 10.1016/j.ajog.2005.01.036 (doi:10.1016/j.ajog.2005.01.036) [DOI] [PubMed] [Google Scholar]
- 26.Keefe D. L., Marquard K., Liu L. 2006. The telomere theory of reproductive senescence in women. Curr. Opin. Obstet. Gynecol. 18, 280–285 10.1097/01.gco.0000193019.05686.49 (doi:10.1097/01.gco.0000193019.05686.49) [DOI] [PubMed] [Google Scholar]
- 27.Aydos S. E., Elhan A. H., Tukun A. 2005. Is telomere length one of the determinants of reproductive life span? Arch. Gynecol. Obstet. 272, 113–116 10.1007/s00404-004-0690-2 (doi:10.1007/s00404-004-0690-2) [DOI] [PubMed] [Google Scholar]
- 28.Hanna C. W., Bretherick K. L., Gair J. L., Fluker M. R., Stephenson M. D., Robinson W. P. 2009. Telomere length and reproductive aging. Hum. Reprod. 24, 1206–1211 10.1093/humrep/dep007 (doi:10.1093/humrep/dep007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tryggvadottir L., Olafsdottir E., Gudlaugsdottir S., Thorlacius S., Jonasson J., Tulinius H., Eyfjord J. E. 2003. BRCA2 mutation carriers, reproductive factors and breast cancer risk. Breast Cancer Res. 5, R121–R128 10.1186/bcr619 (doi:10.1186/bcr619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jernstrom H., et al. 1999. Pregnancy and risk of early breast cancer in carriers of BRCA1 and BRCA2. Lancet 354, 1846–1850 10.1016/S0140-6736(99)04336-6 (doi:10.1016/S0140-6736(99)04336-6) [DOI] [PubMed] [Google Scholar]
- 31.Smith K. R., Ellington L., Chan A. Y., Croyle R. T., Botkin J. R. 2004. Fertility intentions following testing for a BRCA1 gene mutation. Cancer Epidemiol. Biomarkers Prev. 13, 733–740 [PubMed] [Google Scholar]
- 32.Botkin J. R., et al. 2003. Genetic testing for a BRCA1 mutation: prophylactic surgery and screening behavior in women 2 years post testing. Am. J. Med. Genet. A 118, 201–209 10.1002/ajmg.a.10102 (doi:10.1002/ajmg.a.10102) [DOI] [PubMed] [Google Scholar]
- 33.Narod S. A. 2001. Hormonal prevention of hereditary breast cancer. Ann. NY Acad. Sci. 952, 36–43 10.1111/j.1749-6632.2001.tb02726.x (doi:10.1111/j.1749-6632.2001.tb02726.x) [DOI] [PubMed] [Google Scholar]
- 34.Cullinane C. A. 2005. Effect of pregnancy as a risk factor for breast cancer in BRCA1/BRCA2 mutation carriers. Int. J. Cancer 117, 988–991 10.1002/ijc.21273 (doi:10.1002/ijc.21273) [DOI] [PubMed] [Google Scholar]