Abstract
The long-term isolation of South America during most of the Cenozoic produced a highly peculiar terrestrial vertebrate biota, with a wide array of mammal groups, among which caviomorph rodents and platyrrhine primates are Mid-Cenozoic immigrants. In the absence of indisputable pre-Oligocene South American rodents or primates, the mode, timing and biogeography of these extraordinary dispersals remained debated. Here, we describe South America's oldest known rodents, based on a new diverse caviomorph assemblage from the late Middle Eocene (approx. 41 Ma) of Peru, including five small rodents with three stem caviomorphs. Instead of being tied to the Eocene/Oligocene global cooling and drying episode (approx. 34 Ma), as previously considered, the arrival of caviomorphs and their initial radiation in South America probably occurred under much warmer and wetter conditions, around the Mid-Eocene Climatic Optimum. Our phylogenetic results reaffirm the African origin of South American rodents and support a trans-Atlantic dispersal of these mammals during Middle Eocene times. This discovery further extends the gap (approx. 15 Myr) between first appearances of rodents and primates in South America.
Keywords: South America, Hystricognathi, Mid-Eocene climatic optimum, phylogeny, Platyrrhini
1. Introduction
The origin and biogeographic history of South American caviomorph rodents (e.g. guinea pigs, chinchillas, capybara) and platyrrhine primates (e.g. marmosets, capuchins, spider monkeys) are hotly debated issues in mammal evolution [1–14]. Fossil-constrained molecular analyses for both groups suggest either a Cretaceous vicariance event related to the break-up of Gondwana or an Eocene dispersal to South America, probably from Africa [9,15]. In the absence of indisputable pre-Oligocene South American rodent or primate fossils [16–18], the mode, timing and biogeography of these dispersals have so far remained poorly constrained [5,13,19,20]. Until now, the earliest South American hystricognathous rodents described, from low-latitude Santa Rosa [21], Peru (latest Eocene–Oligocene [13,20]) and higher-latitude Tinguiririca [5,19], Chile (earliest Oligocene, approx. 32 Ma), primarily included crown-group caviomorphs, thereby suggesting a much earlier in situ initial radiation of the group.
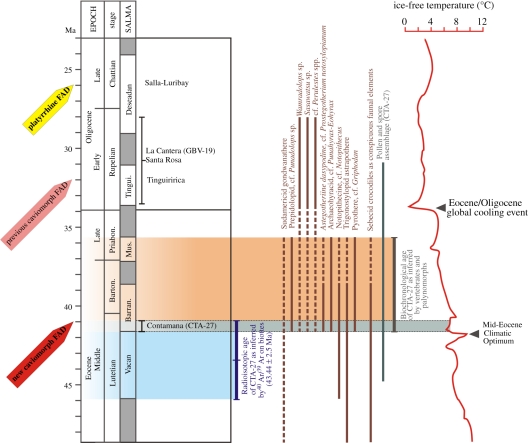
Here, we report the discovery of the oldest known South American caviomorphs, deriving from a 30-cm-thick lens of light-grey clay with irregular limestone concretions at the top of the Yahuarango Formation [22], designated CTA-27 (figure 1). A Middle Eocene age for CTA-27 (earlier than 40.94 Ma) is based on 40Ar/39Ar step heating of single biotite grains separated from a tuffaceous silt located 47 m above the fossiliferous level in the same section, and dated to 43.44 ± 2.5 Ma (see the electronic supplementary material). As summarized in figure 1, a late Middle Eocene age for CTA-27 (41.6–40.94 Ma) is further refined by mammalian biochronology: in CTA-27, the rodents were found in association with typical Middle to Late Eocene marsupials and endemic ungulates, such as the polydolopimorph Punadolops (Barrancan–Mustersan South American Land Mammal Age, SALMA [25]), an astegotheriine dasypodid armadillo close to Prostegotherium notostylopianum (Vacan–Barrancan SALMA [31]), a small archaeohyracid notoungulate related to Eohyrax (Barrancan) and Punahyrax (Barrancan–Mustersan [32]), a small pyrothere closely allied to Griphodon (Vacan–Mustersan), and an unidentified trigonostylopid astrapothere (Vacan–Mustersan), which consistently constrain a Barrancan–Mustersan biochronological range (41.6–35.8 Ma; figure 1; electronic supplementary material) for the locality. CTA-27 also has yielded a diversified, strictly continental palynoflora characteristic of the Middle Eocene to Early Oligocene (44.8–30.9 Ma; palynological zones T6-T10 [33]; figure 1 and electronic supplementary material). The flora indicates a tropical rainforest habitat, with no evidence of savannah vegetation.
Figure 1.
Stratigraphical range of CTA-27 locality (inferred by radioisotopy, vertebrate biochronology and palynostratigraphy) and Mid-Cenozoic global climate. The age of CTA-27 is bracketed between 41.6 Ma (base of the Gran Barranca Member [23,24]) and 40.94 Ma (youngest age provided by 40Ar/39Ar datings at CTA-29; electronic supplementary material, figure S6). Age of both key Palaeogene vertebrate localities and biotic events of South America are based on data from Flynn et al. [19], Goin et al. [25,26], Goin & Candela [27], Shockey et al. [28], López [29] and Vucetich et al. [13]. Global climate is inferred by the δ18O temperature scale of Zachos et al. [30] (red curve to the right), showing the Mid-Eocene Climatic Optimum by the time of deposition of CTA-27. Barran., Barrancan; Barton., Bartonian; FAD, first appearance datum; Mus., Mustersan; Priabon., Priabonian; SALMA, South American Land Mammal Age; Tingui., Tinguirirican.
The specimens described here are permanently stored in the Museum of Natural History in Lima, Peru (MUSM). These new rodents allow us to document the pattern and the timing of major events in early hystricognath evolution and biogeography.
2. Results
Systematic palaeontology
Placentalia Owen, 1837; Order Rodentia Bowdich, 1821; Infraorder Hystricognathi Tullberg, 1899; Parvorder Caviomorpha Wood, 1955.
Plesion Cachiyacuy, New Genus.
Type species. Cachiyacuy contamanensis, New Species.
Etymology. Contraction of Cachiyacu (Local River) and cuy, Quechua for guinea pig.
Generic Diagnosis. Rodents characterized by brachydont and bunolophodont teeth. Upper molars are pentalophodont with strong mesolophule and distinct and long metaloph. Differs from Eobranisamys, Branisamys, Eosallamys, Sallamys, Eoespina and Draconomys in showing a long metaloph not backwardly directed and connected to the posteroloph, but transverse and without lingual connection. Accessory, thin and short enamel crests may connect the metaloph to the posteroloph and/or to the mesolophule. Differs from Eobranisamys, Branisamys and Canaanimys in the absence of taeniodont pattern on upper teeth owing to the presence of a strong lingual protoloph. Differs from Eoincamys and Incamys in having brachydont instead of hypsodont teeth, pentalophodont instead of tetralophodont upper molars, thinner and transverse instead of strong and oblique crests, and in the absence of a taeniodont pattern on both upper and lower teeth (i.e. lingual protoloph and anterior arm of hypoconid lacking).
Other species. Cachiyacuy kummeli, New Species.
Formation and age. Top of the Yahuarango Formation [22], latest Lutetian in age (≈41 Ma).
Cachiyacuy contamanensis, New Species.
Etymology. Refers to the geographical provenance of the specimens, close to the city of Contamana.
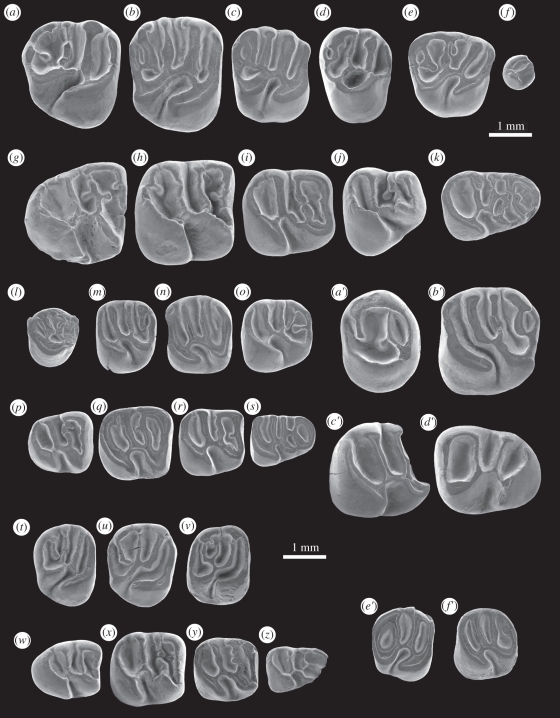
Holotype. MUSM 1871, a right M2 (figure 2b).
Figure 2.
Scanning electron microscope images (in occlusal view) and dimensions (length × width, in millimetres) of fossil caviomorph teeth from CTA-27. (a–k) Cachiyacuy contamanensis new gen. and sp.: (a) MUSM 1870, right (r) M3 (2.34 × 2.44); (b) MUSM 1871, r M2 (holotype; 2.22 × 2.69); (c) MUSM 1872, left (l) M1 (reversed; 2.07 × 2.22); (d) MUSM 1873, r P4 (1.82 × 2.38); (e) MUSM 1874, l DP4 (reversed; 2.03 × 1.98); (f) MUSM 1875, DP3 (0.77 × 0.82); (g) MUSM 1876, r M3 (2.46 × 2.24); (h) MUSM 1877, r M2 (2.44 × 2.36); (i) MUSM 1878, r M1 (2.15 × 1.99); (j) MUSM 1879, r P4 (1.99 × 2.0); (k) MUSM 1880, r DP4 (2.31 × 1.53). (l–s) Cachiyacuy kummeli new gen. and sp.: (l) MUSM 1881, broken r P4 (reversed; 1.16 × –); (m) MUSM 1882, l M1 (holotype; 1.45 × 1.63); (n) MUSM 1883, l M2 (1.69 × 1.87); (o) MUSM 1884, l M3 (1.67 × 1.71); (p) MUSM 1885, r M3 (1.6 × 1.47); (q) MUSM 1886, r M2 (1.83 × 1.67); (r) MUSM 1887, r M1 (1.59 × 1.54); (s) MUSM 1888, l DP4 (reversed; 1.53 × 1.2). (t–z) Canaanimys maquiensis new gen. and sp.: (t) MUSM 1889, r M3 (1.5 × 1.87); (u) MUSM 1890, r M2 (holotype; 1.63 × 1.95); (v) MUSM 1891, l M1 (reversed; 1.46 × 1.82); (w) MUSM 1892, l M3 (1.71 × 1.36); (x) MUSM 1893, l M2 (reversed; 1.8 × 1.79); (y) MUSM 1894, l M1 (reversed; 1.54 × 1.57); (z) MUSM 1895, broken r DP4 (−×1.07). (a′–d′) Eobranisamys sp.: (a′) MUSM 1896, l P4 (1.86 × 2.33); (b′) MUSM 1897, l M1 (2.39 × 2.49); (c′) MUSM 1898, broken r M2 (−×2.23); (d′) MUSM 1899, l M3 (2.47 × 2.06). (e′,f′) cf. Eoespina sp.: (e′) MUSM 1912, r M2 (1.48 × 1.85); (f′) MUSM 1913, l M2 (1.51 × 1.77).
Type locality. CTA-27 Locality, Loreto, Peru.
Diagnosis. Cachiyacuy contamanensis (body mass estimated at 80–120 g) is approximately 30 per cent larger than C. kummeli. Differs from C. kummeli in having upper molars with generally buccal cusps and styles more marked, and lower molars sometimes developing accessory enamel crests.
Cachiyacuy kummeli, New Species.
Etymology. Dedicated to Bernhard Kummel, the geologist who first extensively described the Cachiyacu section in the 1940s [22].
Holotype. MUSM 1882, a left M1 (figure 2m).
Type locality. CTA-27 Locality, Loreto, Peru.
Formation and age. Top of the Yahuarango Formation, latest Lutetian in age (approx. 41 Ma).
Diagnosis. Cachiyacuy kummeli (body mass estimated at 30–40 g) is approximately 30 per cent smaller than C. contamanensis. The molars have slightly thinner transverse crests and the cusp(id)s are more salient than in C. contamanensis.
Plesion Canaanimys, New Genus.
Type species. Canaanimys maquiensis, New Species.
Etymology. Combination of Canaan, name of a local Shipibo native community, and mys, Greek for mouse.
Generic diagnosis. As for the type species.
Canaanimys maquiensis, New Species.
Etymology. Specific epithet is for Maquía, the locality where the fossils were found.
Holotype. MUSM 1890, a right M2 (figure 2u).
Type locality. CTA-27 Locality, Loreto, Peru.
Formation and age. Top of the Yahuarango Formation [22], latest Lutetian in age (approx. 41 Ma).
Diagnosis. Tiny rodent (body mass estimated at approx. 40 g) characterized by brachydont and bunolophodont teeth. It differs from all other caviomorphs in having teeth with moderately low and sharp transverse crest(id)s, lower molars having generally incomplete metalophulid II, and pentalophodont upper molars with a well-developed metaloph that turns anteriorly (not posteriorly) and connects either to the mesolophule lingually or to the mesial extremity of the anterior arm of the hypocone—a primitive condition that is reminiscent of that found in stem Hystricognathi (e.g. Baluchimys, Protophiomys, Waslamys, Phiomys, Hodsahibia, Bugtimys and Ottomania). The lingual protoloph is either slightly developed or lacking (i.e. taeniodont pattern).
In addition, four teeth are referred to the cavioid Eobranisamys sp. (figure 2a′–d′) and 2 diminutive teeth to the octodontoid cf. Eoespina sp. (figure 2e′–f′). The teeth referred to Eobranisamys sp., notably the only upper molar (figure 2b′), display features reminiscent of upper molars of Eobranisamys romeropittmanae and of Eobranisamys riverai from Santa Rosa, Peru [21]. This is particularly evident in the development of a taeniodont (absence of lingual protoloph, i.e. hypoflexus and paraflexus are merged) and pentalophodont pattern, characterized by the presence of a strong and transverse mesolophule, and a strong and distinct metaloph, which is transverse and connected to the posteroloph. However, Eobranisamys sp. differs substantially from E. romeropittmanae in being about half the size, and from E. riverai in being 20 per cent smaller and in showing sharper crests and more salient cusps (plesiomorphic traits). The 2 diminutive M2s referred to cf. Eoespina sp. (figure 2e′–f′) resemble those of Eoespina woodi from Santa Rosa [21], in showing a tetralophodont pattern with strong and long mesolophule, antero-, proto- and posteroloph (no metaloph), and having a rounded crown outline in occlusal view. The specimens from CTA-27 differ in having less inflated cusps, and in showing a mesiodistally narrower and lingually constricted internal sinus (hypoflexus).
3. Discussion
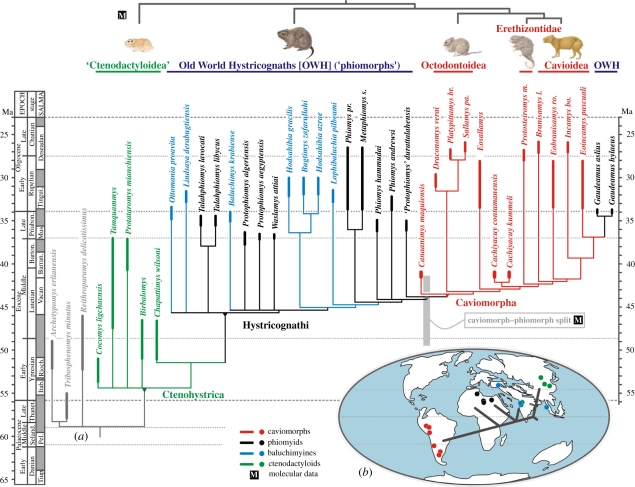
All five rodent taxa currently known from Contamana CTA-27 are remarkably small compared with subsequent caviomorphs (including those from Tinguiririca [5], Santa Rosa [21], La Cantera [13] and younger localities [4]), with a 30–120 g estimated adult body mass range: C. maquiensis (figure 2t–z), C. kummeli (figure 2l–s) and cf. Eospina sp. (figure 2e′–f′) were meadow vole-sized, while C. contamanensis (figure 2a–k) and Eobranisamys sp. (figure 2a′–d′) were small rat-sized (see the electronic supplementary material). Interestingly, this very small size distribution for the oldest known South American caviomorphs is comparable to that observed in Africa [11] for the earliest known radiation of the sister group: the phiomorphs [2,3,5,8,11]. In addition, the moderate morphological disparity among CTA-27 rodents suggests a short interval of caviomorph evolutionary history in South America prior to the Contamana record (figure 3a). Cachiyacuy, Canaanimys and cf. Eoespina share a similar dental bauplan with the earliest Afro-Asian hystricognaths (phiomyids [11] and baluchimyines [8]; latest Middle Eocene–Early Oligocene in age; figure 3a), including brachydonty, bunolophodonty, upper molar pentalophodonty [8], low crest obliquity and multi-serial subtype one to two incisor enamel microstructure [34]. This is exemplified by the striking dental resemblances between Canaanimys, Cachiyacuy and their Old World counterparts, such as Protophiomys and Baluchimys (for Canaanimys) and Phiomys and Metaphiomys (for Cachiyacuy; figure 3a). CTA-27 thus probably documents the earliest stages of caviomorph evolution (i.e. their first adaptive radiation in South America). However, the dental pattern of Canaanimys is somewhat paradoxical as it appears quite primitive in showing a metaloph configuration that is unique among caviomorphs (otherwise found only in basal phiomyids and baluchimyines), but also quite specialized in having taeniodont upper molars, as in advanced cavioid caviomorphs (figure 2t–v). Some subsequent Palaeogene cavioid caviomorphs (such as Eoincamys and Incamys) exhibit a highly specialized dental pattern, noticeably reminiscent of that of the coeval African phiomyid Gaudeamus [3,12,14]. The phylogenetic position of the latter as a derived caviomorph in the current analysis (figure 3a) is supported by dental characters such as hypsodonty or crest obliquity. These comparable dental patterns, highly specialized for Palaeogene rodents, are likely to reflect adaptive convergence rather than close phylogenetic affinities [14].
Figure 3.
Phylogeny and dispersal scenario of Palaeogene caviomorph rodents and related taxa. (a) Phylogeny of Contamana rodents among Palaeogene ctenohystricans (Ctenohystrica), hystricognaths (Hystricognathi) and caviomorphs (Caviomorpha). Strict consensus cladogram obtained with a maximum-parsimony PAUP (phylogenetic analysis using parsimony) heuristic search (see the electronic supplementary material). The consistency (CI), retention (RI) and homoplasy (HI) indices of the 13 shortest trees (859 steps) are 0.33, 0.63 and 0.68, respectively. Branch support of the concerned nodes (Bremer indices) appears in the electronic supplementary material, figure S2a. Contamana rodents included in this analysis (bold type) are stem caviomorphs (i.e. not referable to Octodontoidea, Erethizontidae or Cavioidea). cf. Eoespina sp. (not included, two teeth) is interpreted as a stem octodontoid. Eobranisamys sp. (four teeth) might be connected to the Eobranisamys romeropittmanae branch (Cavioidea), which suggests a late Middle Eocene age for the common ancestor of cavioids. Sister-group relationships between Gaudeamus (advanced phiomorph; Old World Hystricognath, OWH) and Eoincamys (caviomorph) probably document a striking convergent dental evolution [11,14], rather than independent dispersal from South America to Africa. Light-grey shaded bar illustrates molecular estimate ranges for the caviomorph–phiomorph split (CPS; 45.4 ± 4.1 Ma [9]), thus located behind the morphologically supported CPS node (grey triangle). On top, dark-grey tree sketches phylogenetic relationships among living ctenohystricans as supported by molecular data [9]. Barran., Barrancan; Barton., Bartonian; Itab., Itaboraian; Mus., Mustersan; Pel., Peligran; Priabon., Priabonian; Rioch., Riochican; SALMA, South American Land Mammal Age; Seland., Selandian; Thanet., Thanetian; Tingui., Tinguirirican; Species name abbreviations: bo., bolivianus; br., brachyodon; l., luribayensis; m., medianus; pa., pascuali; pr., paraphiomyoides; ro., romeropittmanae; s., schaubi. Rodent drawings by M. J. Orliac. See the electronic supplementary material for further details. (b) Palaeogeographic world map at 40 Ma and privileged phylogeographic sketch of Palaeogene hystricognath rodents, as inferred by both the consensus tree shown in (a) and the geographical range of concerned taxa (coloured circles; same colour codes as in (a)).
The dramatic global cooling and drying episode recorded around the Eocene–Oligocene transition (approx. 34 Ma; opening of Drake Passage and development of Antarctic ice sheet [30,35]) are usually regarded as having shaped major faunal changes in South America, including the arrival of caviomorphs [19,36]. The presence of caviomorph rodents in approximately 41-Ma-old sediments of Peruvian Amazonia shows that both their dispersal and initial radiation occurred instead during a much warmer and wetter period, around the Mid-Eocene Climatic Optimum [30,37] (figures 1 and 3a), which is consistent with the associated palynoflora (see electronic supplementary material, table S3). Such a view strongly supports the Middle Eocene caviomorph/phiomorph split (CPS; 45.4 ± 4.1 Ma; figure 3a), as estimated by molecular analyses [9,15]. Based on the present work and recent discoveries of stem hystricognaths in the Late Eocene of Asia [8] and Africa [11,14], the initial radiations of Old World phiomorphs and New World caviomorphs were apparently synchronous and rapid in both land masses (figure 3a). The inferred calibrated phylogeny of Palaeogene hystricognaths highlights a significant Middle Eocene gap in the Old World hystricognath record (figure 3a). Based on our phylogenetic analysis, Africa is the homeland for the last common ancestor of caviomorphs (figure 3b). The latest reconstructions of South Atlantic geometry, palaeowinds and palaeocurrents during the Middle Eocene [7] are consistent with trans-Atlantic sweepstakes dispersal of hystricognaths from Africa to South America [2,10].
An Asian origin for stem Hystricognathi and stem anthropoid primates has gained strong support in recent years [8,11,38,39]. Based on our phylogeny (figure 3a,b), stem hystricognathous rodents probably invaded Africa from Asia around the Early–Middle Eocene transition. However, at that time and/or by the time of the estimated CPS [9,15], only zegdoumyid anomaluroids are known from Africa, which are phylogenetically remote from the clade Hystricognathi [40]. The latter are not recorded in Africa before the latest Middle Eocene [8,11,41]. Similar timing and dispersal scenarios are proposed for stem anthropoid primates [38,39]. By contrast, the present discovery further extends the temporal gap between South America's first appearance datum of rodents (now greater than or equal to 41 Ma) and that of primates (Late Oligocene of Bolivia, approx. 26 Ma [16]), thus suggesting the possibility of distinct dispersal events from Africa for both groups, as hypothesized by molecular data [9].
Morphological and molecular evidence [17,18] presently provide no definitive answer as to whether platyrrhines underwent a radiation before Late Oligocene times, but the platyrrhine/catarrhine divergence is estimated to have occurred around the Middle–Late Eocene transition on molecular grounds [9]. This period records both the earliest African catarrhines/stem anthropoids (of Asian origin [39]) and the earliest South American caviomorphs (figure 3a). Africa may have played the role of a stopover for pioneer platyrrhines, within a ‘land-mass-hopping’ process between Asia and South America, as for caviomorphs several million years earlier (figure 3). Once again, it is not clear whether platyrrhines and catarrhines diverged in Asia or in Africa [39]. However, in the Old World, most Middle Eocene–Early Oligocene localities with hystricognaths also yield primates [8,11,14,39,41], a situation that strongly contrasts with the coeval South American fossil record. Moreover, based on palynological data [33,37] and current palaeoenvironmental reconstructions [42], low-latitude areas of South America were seemingly highly favourable to the survival and expansion of platyrrhine primates throughout Palaeogene times. The Palaeogene platyrrhine record in South America is so far restricted to the Late Oligocene deposits of the Salla-Luribay area in Bolivia [16,17], but it can be expected that other regions of South America hosted primates much before.
According to South America's fossil record, and given that middle- and high-latitude areas were extensively investigated in the last centuries [1,36] (i.e. much more than low-latitude regions, including western Amazonia [20]), the earliest caviomorphs seemingly expanded southward during the late Middle Eocene–Early Oligocene period, from low (Peruvian Amazonia; approx. 41 Ma or earlier) to middle (central Chile; approx. 32 Ma [5,19]) and then to high latitudes (Patagonian Argentina; approx. 30 Ma [13]). Such a southward shift, fully contradictory to the apparent northward expansion as deduced from the fossil record available 20 years ago, highlights the critical dependence of deep time dispersal scenarios on the fossil record, and shows how severely under-sampled the tropics are.
Acknowledgements
We thank the IRD-PeruPetro Convention Programme, the Canaan Shipibo Native Community and Maple Gas Peru S.A. for access to the field, J.-L. Hartenberger, F. Delsuc, M. Steiper and O. Bertrand for fruitful discussion, P. Baby, M. Roddaz, C. Gautheron, Y. Calderón and L. Quiroz for help with fieldwork, F. Leite and D. Carvajal for their work on pollen, and C. Cazevieille (Centre de Ressources en Imagerie Cellulaire, Montpellier) for access to a scanning electron microscope facility. We are much indebted to R. Cifelli for having edited this article, as well as to C. de Muizon and E. F. Seiffert for their constructive remarks on an earlier version of the manuscript. P.-O.A. was supported by CNRS ‘Eclipse 2’, CNRS ‘Paleo2’ and Toulouse University ‘SPAM’; L.M. and M.J.O. were supported by ANR-08-JCJC-0017 (PALASIAFRICA); G.B. and T.M. were supported by Alexander von Humboldt-Foundation, Bonn. Publication ISEM 2011-126.
References
- 1.Wood A. E., Patterson B. 1959. The rodents of the Deseadan Oligocene of Patagonia and the beginnings of South American rodent evolution. Bull. Mus. Comp. Zool. 120, 281–428 [Google Scholar]
- 2.Hoffstetter R. 1972. Origine et dispersion des Rongeurs Hystricognathes. C. R. Acad. Sci. (Paris) 274, 2867–2870 [Google Scholar]
- 3.Lavocat R. 1976. Rongeurs Caviomorphes de l'Oligocène de Bolivie: II Rongeurs du bassin Déséadien de Salla-Luribay. Palaeovertebrata 7, 15–90 [Google Scholar]
- 4.Patterson B., Wood A. E. 1982. Rodents from the Deseadan Oligocene of Bolivia and the relationship of Caviomorpha. Bull. Mus. Comp. Zool. 149, 372–543 [Google Scholar]
- 5.Wyss A. R., Flynn J. J., Norell M. A., Swisher C. C., II, Charrier R., Novacek M. J., McKenna M. C. 1993. South America's earliest rodent and recognition of a new interval of mammalian evolution. Nature 365, 434–437 10.1038/365434a0 (doi:10.1038/365434a0) [DOI] [Google Scholar]
- 6.Houle A. 1999. The origin of platyrrhines: an evaluation of the antarctic scenario and the floating island model. Am. J. Phys. Anthropol. 109, 541–559 (doi:10.1002/(SICI)1096-8644(199908)109:4<541::AID-AJPA9>3.0.CO;2-N) [DOI] [PubMed] [Google Scholar]
- 7.Huchon D., Douzery P. 2001. From the Old World to the New World: a molecular chronicle of the phylogeny and biogeography of hystricognath rodents. Mol. Phylogenet. Evol. 20, 238–251 10.1006/mpev.2001.0961 (doi:10.1006/mpev.2001.0961) [DOI] [PubMed] [Google Scholar]
- 8.Marivaux L., Welcomme J.-L., Vianey-Liaud M., Jaeger J. 2002. The role of Asia in the origin and diversification of hystricognathous rodents. Zool. Scrip. 31, 225–239 10.1046/j.1463-6409.2002.00074.x (doi:10.1046/j.1463-6409.2002.00074.x) [DOI] [Google Scholar]
- 9.Poux C., Chevret P., Huchon D., De Jong W. W., Douzery P. 2006. Arrival and diversification of caviomorph rodents and platyrrhine primates in South America. Syst. Biol. 55, 228–244 10.1080/10635150500481390 (doi:10.1080/10635150500481390) [DOI] [PubMed] [Google Scholar]
- 10.Bandoni de Oliveira F., Molina E. C., Marroig G. 2009. Paleogeography of the South Atlantic: a route for primates and rodents into the New World? In South American primates: comparative perspectives in the study of behavior, ecology, and conservation. Developments in Primatology: Progress and Prospects, vol 16 (eds Garber P. A., Estrada A., Bicca-Marques J. C., Heymann E. W., Strier K. B.), pp. 55–68 New York, NY: Springer + Business Media [Google Scholar]
- 11.Sallam H. M., Seiffert E. R., Steiper M. E., Simons E. L. 2009. Fossil and molecular evidence constrain scenarios for the early evolutionary and biogeographic history of hystricognathous rodents. Proc. Natl Acad. Sci. USA 106, 16 722–16 727 10.1073/pnas.0908702106 (doi:10.1073/pnas.0908702106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coster P., et al. 2010. Gaudeamus lavocati sp. nov. (Rodentia, Hystricognathi) from the early Oligocene of Zallah, Libya: first African caviomorph? Naturwissenschaften 97, 697–706 10.1007/s00114-010-0683-x (doi:10.1007/s00114-010-0683-x) [DOI] [PubMed] [Google Scholar]
- 13.Vucetich M. G., Vieytes E. C., Pérez M. E., Carlini A. A. 2010. The rodents from La Cantera and the early evolution of caviomorphs in South America. In The paleontology of Gran Barranca: evolution and environmental change through the Middle Cenozoic of Patagonia (eds Madden R. H., Carlini A. A., Vucetich M. G., Kay R. F.), pp. 193–205 Cambridge, UK: Cambridge University Press [Google Scholar]
- 14.Sallam H. M., Seiffert E. R., Simons E. L. 2011. Craniodental morphology and systematics of a new family of hystricognathous rodents (Gaudeamuridae) from the Late Eocene and Early Oligocene of Egypt. PLoS ONE 6, 1–29 10.1371/journal.pone.0016525 (doi:10.1371/journal.pone.0016525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Opazo J. C., Wildman D. E., Prychitko T., Johnson R. M., Goodman M. 2006. Phylogenetic relationships and divergence times among New World monkeys (Platyrrhini, Primates). Mol. Phylogenet. Evol. 40, 274–280 10.1016/j.ympev.2005.11.015 (doi:10.1016/j.ympev.2005.11.015) [DOI] [PubMed] [Google Scholar]
- 16.Kay R. F., MacFadden B. J., Madden R. H., Sandeman H., Anaya F. 1998. Revised age of the Salla beds, Bolivia, and its bearing on the age of the Deseadan South American Land Mammal ‘Age’. J. Vert. Paleontol. 18, 189–199 10.1080/02724634.1998.10011043 (doi:10.1080/02724634.1998.10011043) [DOI] [Google Scholar]
- 17.Kay R. F., Williams B. A., Anaya F. 2001. The adaptations of Branisella boliviana, the earliest South American monkey. In Reconstructing behavior in the primate fossil record (eds Plavcan J. M., van Schaik C., Kay R. F., Jungers W. L.), pp. 339–370 New York, NY: Plenum Publishers [Google Scholar]
- 18.Hodgson J. A., Sterner K. N., Matthews L. J., Burrell A. S., Jani R. A., Raaum R. L., Stewart C.-B., Disotell T. R. 2009. Successive radiations, not stasis, in the South American primate fauna. Proc. Natl Acad. Sci. USA 106, 5534–5539 10.1073/pnas.0810346106 (doi:10.1073/pnas.0810346106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flynn J. J., Wyss A. R., Croft D. A., Charrier R. 2003. The Tinguiririca fauna, Chile: biochronology, paleoecology, biogeography, and a new earliest Oligocene South American Land Mammal ‘Age’. Pal. Pal. Pal. 195, 229–259 [Google Scholar]
- 20.MacFadden B. J. 2006. Extinct mammalian biodiversity of the ancient New World tropics. Trends Ecol. Evol. 21, 157–165 [DOI] [PubMed] [Google Scholar]
- 21.Frailey C. D., Campbell K. E. 2004. Paleogene rodents from Amazonian Peru: the Santa Rosa local fauna. In The paleogene mammalian fauna of Santa Rosa, Amazonian Peru (ed. Campbell K. E.), pp. 71–130 Los Angeles, CA: Natural History Museum of Los Angeles County [Google Scholar]
- 22.Kummel B. 1948. Geological reconnaissance of Contamana region, Peru. GSA Bull. 59, 1217–1266 10.1130/0016-7606(1948)59[1217:GROTCR]2.0.CO;2 (doi:10.1130/0016-7606(1948)59[1217:GROTCR]2.0.CO;2) [DOI] [Google Scholar]
- 23.Legendre S. 1986. Analysis of mammalian communities from the Late Eocene and Oligocene of southern France. Palaeovertebrata 16, 191–212 [Google Scholar]
- 24.Ré G., Bellosi E. S., Heizler M., Vilas J. F., Madden R. H., Carlini A. A., Kay R. F., Vucetich M. G. 2010. A geochronology for the Sarmiento Formation at Gran Barranca. In The paleontology of Gran Barranca: evolution and environmental change through the Middle Cenozoic of Patagonia (eds Madden R. H., Carlini A. A., Vucetich M. G., Kay R. F.), pp. 46–58 Cambridge, UK: Cambridge University Press [Google Scholar]
- 25.Goin F. J., Candela A. M., López G. M. 1998. Middle Eocene marsupials from Antofagasta de la Sierra, north western Argentina. Geobios 31, 75–85 [Google Scholar]
- 26.Goin F. J., Abello M. A., Chornogubsky L. 2010. Middle Tertiary marsupials from central Patagonia (early Oligocene of Gran Barranca): understanding South America's Grande Coupure. In The paleontology of Gran Barranca: evolution and environmental change through the Middle Cenozoic of Patagonia (eds Madden R. H., Carlini A. A., Vucetich M. G., Kay R. F.), pp. 69–105 Cambridge, UK: Cambridge University Press [Google Scholar]
- 27.Goin F. J., Candela A. M. 2004. New paleogene marsupials from the Amazon Basin of eastern Peru. In The Paleogene mammalian fauna of Santa Rosa, Amazonian Peru (ed. Campbell K. E.), pp. 15–60 Los Angeles, CA: Natural History Museum of Los Angeles County [Google Scholar]
- 28.Shockey B. J., Hitz R., Bond M. 2004. Paleogene Notoungulates from the Amazon Basin of Peru. In The paleogene mammalian fauna of Santa Rosa, Amazonian Peru (ed. Campbell K. E.), pp. 61–70 Los Angeles, CA: Natural History Museum of Los Angeles County [Google Scholar]
- 29.López G. M. 2010. Divisaderan: land mammal age or local fauna? In The Paleontology of Gran Barranca: evolution and environmental change through the Middle Cenozoic of Patagonia (eds Madden R. H., Carlini A. A., Vucetich M. G., Kay R. F.), pp. 410–420 Cambridge, UK: Cambridge University Press [Google Scholar]
- 30.Zachos J. C., Dickens G. R., Zeebe R. E. 2008. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature 451, 279–283 10.1038/nature06588 (doi:10.1038/nature06588) [DOI] [PubMed] [Google Scholar]
- 31.Carlini A. A., Ciancio M. R., Scillato-Yané G. J. 2010. Middle Eocene-Early Miocene Dasypodidae (Xenarthra) of southern South America: faunal succession at Gran Barranca—biostratigraphy and paleoecology. In The paleontology of Gran Barranca: evolution and environmental change through the Middle Cenozoic of Patagonia (eds Madden R. H., Carlini A. A., Vucetich M. G., Kay R. F.), pp. 106–129 Cambridge, UK: Cambridge University Press [Google Scholar]
- 32.Reguero M. A., Croft D. A., López G. M., Alonso R. N. 2008. Eocene archaeohyracids (Mammalia, Notoungulata, Hegetotheria) from the Puna, northwest Argentina. J. S. Am. Earth Sci. 26, 225–233 10.1016/j.jsames.2008.05.004 (doi:10.1016/j.jsames.2008.05.004) [DOI] [Google Scholar]
- 33.Jaramillo C., Rueda M., Torres V. 2011. A Palynological zonation for the Cenozoic of the Llanos and Llanos Foothills of Colombia. Palynology 35, 46–84 10.1080/01916122.2010.515069 (doi:10.1080/01916122.2010.515069) [DOI] [Google Scholar]
- 34.Martin T. 1994. African origin of caviomorph rodents is indicated by incisor enamel microstructure. Paleobiology 20, 5–13 [Google Scholar]
- 35.Livermore R., Nankivell A., Eagles G., Morris P. 2005. Paleogene opening of Drake Passage. EPSL 236, 459–470 10.1016/j.epsl.2005.03.027 (doi:10.1016/j.epsl.2005.03.027) [DOI] [Google Scholar]
- 36.Flynn J. J., Wyss A. R. 1998. Recent advances in South American mammalian paleontology. Trends Ecol. Evol. 13, 449–454 [DOI] [PubMed] [Google Scholar]
- 37.Jaramillo C., Hoorn C., Silva S. A. F., Leite F., Herrera F., Quiroz L., Dino R., Antonioli L. 2010. Amazonia, landscape and species evolution (eds Hoorn C., Wesselingh F. P.), pp. 317–334 Hoboken, NJ: Blackwell [Google Scholar]
- 38.Tabuce R., et al. 2009. Anthropoid vs. strepsirhine status of the African Eocene primates Algeripithecus and Azibius: craniodental evidence. Proc. R. Soc. B 276, 4087–4094 10.1098/rspb.2009.1339 (doi:10.1098/rspb.2009.1339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaeger J.-J., et al. 2010. Late middle Eocene epoch of Libya yields earliest known radiation of African anthropoids. Nature 467, 1095–1098 10.1038/nature09425 (doi:10.1038/nature09425) [DOI] [PubMed] [Google Scholar]
- 40.Marivaux L., Adaci M., Bensalah M., Gomes Rodrigues H., Hautier L., Mahboubi M., Mebrouk F., Tabuce R., Vianey-Liaud M. 2011. Zegdoumyidae (Rodentia, Mammalia), stem anomaluroid rodents from the early to middle Eocene of Algeria (Gour Lazib, Western Sahara): new dental evidence. J. Syst. Palaeontol. (doi:10.1080/14772019.2011.562555) [Google Scholar]
- 41.Pickford M., Senut B., Morales J., Mein P., Sanchez I. M. 2008. Mammalia from the Lutetian of Namibia. Mem. Geol. Surv. Namibia 20, 465–514 [Google Scholar]
- 42.Hoorn C., et al. 2010. Amazonia through time: andean uplift, climate change, landscape evolution, and biodiversity. Science 330, 927–931 10.1126/science.1194585 (doi:10.1126/science.1194585) [DOI] [PubMed] [Google Scholar]