Abstract
Meiosis in triploids faces the seemingly insuperable difficulty of dividing an odd number of chromosome sets by two. Triploid vertebrates usually circumvent this problem through either asexuality or some forms of hybridogenesis, including meiotic hybridogenesis that involve a reproductive community of different ploidy levels and genome composition. Batura toads (Bufo baturae; 3n = 33 chromosomes), however, present an all-triploid sexual reproduction. This hybrid species has two genome copies carrying a nucleolus-organizing region (NOR+) on chromosome 6, and a third copy without it (NOR−). Males only produce haploid NOR+ sperm, while ova are diploid, containing one NOR+ and one NOR− set. Here, we conduct sibship analyses with co-dominant microsatellite markers so as (i) to confirm the purely clonal and maternal transmission of the NOR− set, and (ii) to demonstrate Mendelian segregation and recombination of the NOR+ sets in both sexes. This new reproductive mode in vertebrates (‘pre-equalizing hybrid meiosis’) offers an ideal opportunity to study the evolution of non-recombining genomes. Elucidating the mechanisms that allow simultaneous transmission of two genomes, one of Mendelian, the other of clonal inheritance, might shed light on the general processes that regulate meiosis in vertebrates.
Keywords: biased genome transmission, clonal, Mendelian segregation, recombination, triploid vertebrate, Bufo viridis subgroup
1. Introduction
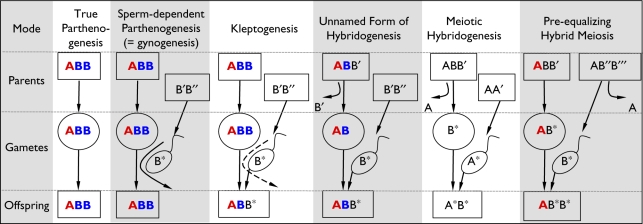
According to Mendel's second Law [1], alleles of different genes assort independently of one another during gamete formation. In sexually reproducing species, random transmission of paternal and maternal genomes is achieved by the independent segregation of chromosomes during meiosis. Some animal genomes, however, display transmission biases, often according to parent of origin, and usually as a consequence of ancient hybridization [2]. Hybrid lineages of ants, for instance, carry two independently evolving genomes, which are transmitted either meiotically [3] or clonally [4,5]. Similar processes occur in hybridogenetic vertebrates: in diploid hybridogenesis, one genome is transmitted clonally through the hybrid lineage, while the other is transmitted sexually by one of its parental species [6]. In meiotic hybridogenesis, both of the hybridizing genomes can be transmitted sexually, through crosses between diploid and triploid hybrids of different genomic compositions (figure 1; [14,16]).
Figure 1.
Reproductive modes of triploid vertebrates. Shown are the parental, gametic and offspring genomes (rows) under different reproductive modes (columns). A, B: genomes of different parental species. Bold coloured symbols indicate clonally transmitted copies, while thin black symbols with superscripts indicate different (recombined) copies. True parthenogenesis: clonal (males absent), restricted to reptiles [7,8]; Sperm-dependent parthenogenesis (i.e. gynogenesis): clonal, embryogenesis requires trigger from allospecific sperm that is not incorporated (rare ‘paternal leakage’ might incorporate subgenomic amounts of paternal DNA), occurs in teleost fishes and urodelan amphibians [9]; Kleptogenesis: females acquire full or partial genomes from their mates by a not fully understood mechanism, allowing them to purge genomes from deleterious alleles (here BB); described from urodelan amphibians [10]; Unnamed form of hybridogenesis: clonal diploid eggs are fertilized by sperm from a recombining sexual species that can be diploid or triploid (as in meiotic hybridogenesis); occurs in anuran amphibians and teleost fishes [11–13]; Meiotic hybridogenesis: may occur in triploid males and/or females; found in teleost fishes and anuran amphibians [14,15]; ploidy elevation of the diploid offspring, which might produce diploid hybrid gametes, can occur in the next generation (becoming then e.g. ABB′) to restore triploidy (similar to preceding form of hybridogenesis); Pre-equalizing hybrid meiosis: occurring in Batura toads: Both sexes are triploid and exhibit Mendelian segregation and recombination in the B genomes (equivalent to NOR+; this paper), while the A genome (i.e. NOR−) is clonally transmitted by the mother.
Bisexual reproduction of pure triploids is constrained because of the problem of equally distributing three chromosome sets in meiosis [17], for a review see [18]. Hybrids in Poeciliopis, for instance, are hybridogenetic in their diploid forms, but become gynogenetic as triploids [19,20]. As an alternative to gynogenesis or parthenogenesis (figure 1; [7–9,21]), some triploid vertebrates combine clonal and sexual elements in their reproductive modes—e.g. kleptogenesis or different forms of hybridogenesis [10,22–24], including meiotic hybridogenesis, which requires a reproductive community of different ploidy levels and genome composition.
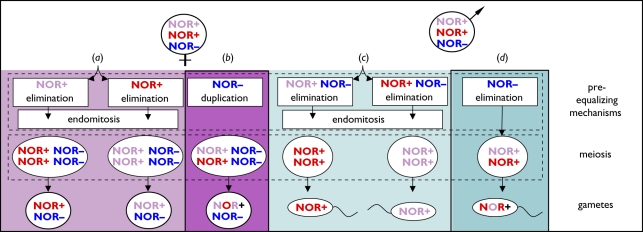
In this context, Batura toads (Bufo baturae) are exceptional in being sexually reproducing triploids [25]. This species of hybrid origin inhabits the high mountains of northern Pakistan (greater than 1500 m a.s.l.). Its genome (3n = 33; [25,26]) is composed of two chromosome sets carrying a nucleolus-organizing region (NOR+) on chromosome 6, and another set without such a region (NOR−; figure 2). Males only produce haploid NOR+ sperm, suggesting elimination of the NOR− set (11 chromosomes) before the onset of meiosis. In contrast, ova are diploid (2n = 22) with one NOR+ and one NOR− sets. Immature oocytes exhibit 22 lampbrush chromosome bivalents [25].
Figure 2.
Scheme of the reproductive system in triploid Batura toads with hypothetical mechanisms (a) and (b) for oogenesis and (c) and (d) for spermatogenesis. Blue NOR− symbol: unrecombined (clonal) chromosome set without NORs. Red or magenta NOR+ symbols: different NOR-carrying chromosome sets. Mixed red and magenta NOR+ symbols: recombined NOR-carrying sets. The mechanisms confirmed in the present study are framed.
Therefore, it has been assumed that the NOR− set undergoes purely maternal and clonal transmission. However, it remained unknown, whether and how the NOR+ sets recombine. For females, two hypotheses (figure 2) can be envisaged: (a) One NOR+ set is eliminated (either randomly or depending on parent of origin), followed by endomitotic auto-duplication of the two remaining sets. Meiosis thus only occurs between pseudo-bivalents [27], and produces one or at most two classes of otherwise clonal diploid ova. Alternatively (b), the NOR− set is auto-duplicated before meiosis, during which the two NOR+ sets recombine normally.
For males, similarly, a first hypothesis (figure 2c) is that the whole maternal complement (NOR+, NOR−) is eliminated, followed by paternal NOR+ duplication through pre-meiotic endomitosis. Meiotic pairings (NOR+/NOR+; [25]) would thus represent pseudo-bivalents, implying clonal transmission of one NOR+ set. Spermatocytes would comprise a single multi-locus genotype (or at most two, if NOR+ elimination were random). Alternatively (figure 2d), only the NOR− set is eliminated, and the two NOR+ sets undergo normal meiosis and recombination.
From multi-locus fingerprint data, Stöck et al. [25] identified several genotypes among the offspring from a single family. However, dominant multi-locus markers are not always straightforward to interpret, and thus shed little light on the underlying mechanisms. In the present paper, we performed sibship analyses with 15 co-dominant microsatellite loci to evaluate genome-wide patterns of transmission and segregation. Our results clearly confirm the purely clonal and maternal transmission of one set of chromosomes (NOR−), and show independent segregation and recombination of the two other sets (NOR+) in both males and females. This reshuffling of genetic material should allow efficient purging of the two sets of NOR+ chromosomes as in normal sexual reproduction. This is the first example of parallel clonal and meiotic transmission of chromosome sets within the same lineage of vertebrates.
2. Material and methods
Animals used in controlled breeding experiments were collected from three localities in northern Pakistan (electronic supplementary material, table S1) during three periods of fieldwork (June–July 1996, 1997 and 2000). We performed five breeding experiments involving triploid Batura toads. In addition, we crossed one Batura female with both a diploid Bufo variabilis male from Syria (2n = 22, with two NOR+ sets) and a tetraploid Bufo oblongus male from Iran (4n = 44, including two NOR+ and two NOR− sets; [28]). Twenty to 100 offspring were raised in tanks up to a larval length of 2–3 cm (Gosner-stages 30 to 38, [29]). A total of 85 tadpoles from the seven crosses were sampled for genetic analyses. Tadpoles were either karyotyped or their ploidy level determined by flow cytometry; DNA was extracted as described in Stöck et al. [30].
We tested a series of microsatellites markers from a genomic library enriched for repetitive elements from the Batura toad, some of which were previously used in other species (electronic supplementary material, table S2). Alleles were amplified, scored with GeneMapper v. 3.7 (Applied Biosystems), and named according to their lengths in base pairs as described [30]. Alleles from the NOR+ and NOR− sets, as well as null alleles (0), were identified from inheritance patterns (see §3). Linkage analyses were performed with Genepop (http://genepop.curtin.edu.au/; [31,32]) under default parameters, and potential linkage groups were checked by visual inspection. Given the manageable size of the dataset, progeny genotypes were also visually inspected for cases of recombination. The number of recombination events was normalized to the number of informative events per family, and departures from random segregation were tested for significance (using a χ2-test).
3. Results
(a). Inheritance patterns in Batura toads
A total of 15 microsatellites primer pairs amplified products in one or more families.
Five of them (D103, D105, D5, C224 and C203; electronic supplementary material, dataset S1) displayed up to three alleles per individual, which implies product amplification from both the NOR− and the two NOR+ sets. NOR− alleles were easily identified, being always homomorphic among offspring from a family, identical to the maternal copy, and different from the paternal one whenever parental copies differed (table 1 and electronic supplementary material, table S3 and dataset S1). Both NOR+ sets, by contrast, displayed biparental inheritance and Mendelian segregation (electronic supplementary material, table S3 and dataset S1). Each heterozygous parent transmitted its two alleles with equal probability (binomial tests). The 10 other markers presented a maximum of two alleles per individual, with biparental inheritance and Mendelian segregation, following expectations from meiotic NOR+ sets (electronic supplementary material, dataset S1).
Table 1.
Inheritance patterns of NOR− alleles at five loci (rows) in five 3n families (columns). Given are the numbers of offspring with maternal NOR− allele/numbers of informative events. ‘ni’ indicates not informative, (—) indicates analyses not performed. Across loci and families, we counted 139 cases of maternal inheritance out of 139 informative events. Full data are provided in electronic supplementary material, dataset S1.
| locus\family | 1 | 2 | 3 | 4 | 5 | all |
|---|---|---|---|---|---|---|
| D105 | 17/17 | 6/6 | ni | ni | 13/13 | 36/36 |
| C224 | 17/17 | ni | ni | ni | — | 17/17 |
| D5 | 15/15 | 6/6 | 25/25 | 10/10 | — | 56/56 |
| C203 | 17/17 | ni | ni | ni | 13/13 | 30/30 |
| D103 | ni | ni | ni | ni | — | ni |
| across loci | 66/66 | 12/12 | 25/25 | 10/10 | 26/26 | 139/139 |
Four linkage groups could be identified, involving two markers each (table 2). Out of 199 informative events, we detected a total of 25 cases of recombination (table 2), occurring in both sexes (electronic supplementary material, dataset S1 and table S3). All other pairs of markers were transmitted independently, generating a high diversity of multi-locus genotypes per family. Interestingly, the five markers amplifying a NOR− product were assigned to different linkage groups in the NOR+ genome (table 2 and electronic supplementary material, dataset S1), supporting genome-wide distribution of the NOR− markers.
Table 2.
Recombination patterns at four linkage groups. Rec., recombination observed; non-rec., no recombination observed. Expected values assuming independence are provided in italics. Total indicates total number of informative events over families and sexes.
| linkage group | rec. | non-rec. | total | rate | χ2 | p |
|---|---|---|---|---|---|---|
| D106/D124 | ||||||
| observed | 16 | 53 | 69 | 0.23 | 19.84 | <0.001 |
| expected | 34.5 | 34.5 | ||||
| C224/C123 | ||||||
| observed | 2 | 38 | 40 | 0.05 | 32.4 | <0.001 |
| expected | 20 | 20 | ||||
| C111/D103 | ||||||
| observed | 5 | 27 | 32 | 0.16 | 15.12 | <0.001 |
| expected | 16 | 16 | ||||
| D11/D107 | ||||||
| observed | 2 | 56 | 58 | 0.03 | 50.27 | <0.001 |
| expected | 29 | 29 | ||||
| total | 25 | 174 | 199 | |||
(b). Inter-ploidy crosses
Locus D105 could also be amplified from the progeny of a female B. baturae with (i) a diploid B. variabilis male (2n = 22, comprising two NOR+ sets) and (ii) a tetraploid B. oblongus male (4n = 44 including two NOR+ and two NOR− sets). All offspring sired by the B. variabilis father were triploid and inherited the maternal NOR− allele at locus D105, while the two NOR+ sets displayed biparental inheritance with Mendelian segregation in both parents. The offspring sired by the B. oblongus father were tetraploid, and presented four allelic copies at locus D105, corresponding, respectively, to two NOR− and two NOR+ sets. One NOR− allele was identical to the maternal copy, while the other was randomly inherited from the two paternal NOR− copies. The two NOR+ sets also showed biparental inheritance, with Mendelian segregation in both parents. Hence in both crossings, the Batura toad mother produced 2n oocytes with a clonally transmitted NOR− and a recombined NOR+. The B. variabilis male produced haploid sperm with a recombined NOR+, while the B. oblongus male produced diploid sperm with recombined NOR+ and NOR− sets (see also [25,33]).
4. Discussion
Our results show purely maternal and clonal transmission of all NOR− markers. Offspring inherited only the maternal copy at the five markers (D5, D103, D105, C224 and C203) that amplified a NOR− allele. As we show, these five markers are localized on different linkage groups on the NOR+ genome (electronic supplementary material, dataset S1), supporting the view that the whole NOR− set of chromosomes undergoes clonal and maternal transmission.
Furthermore, our results provide evidence for NOR+ recombination in both sexes. The 15 markers that amplified NOR+ alleles were assigned to 11 different linkage groups (corresponding to the haploid number of chromosomes in Batura toads), which displayed random and independent segregation in both sexes (electronic supplementary material, dataset S1). Heterozygous adults always transmitted their two alleles with equal probability, indicating random segregation of paternal and maternal NOR+ chromosome sets in both sexes (figure 2: pathways (b), (d)). In addition, recombination also occurred in both sexes, among loci from the same linkage groups (table 2).
Altogether, our results rule out hypothesis (a) for oogenesis (which assumes clonal production of NOR+/NOR− oocytes) and (c) for spermatogenesis (which assumes clonal production of NOR+ sperm). These findings raise important issues with respect to both the proximate mechanisms and evolutionary consequences associated with this unusual mode of reproduction.
(a). Proximate mechanisms
The NOR− genome of Batura toads is eliminated in males, but duplicated in females before meiosis. Premeiotic elimination and/or duplication of genomes have already been documented in hybridogenetic vertebrates, such as in the water frog Rana (Pelophylax) esculenta, a hybrid between Rana ridibunda (RR) and Rana lessonae (LL) [33,34]. When associated with R. ridibunda, R. esculenta females (RL) drop their paternal genome (R) from the germ line while doubling their L genome by pre-meiotic endomitosis. The ensuing meiosis thus involves completely homozygous pseudo-bivalents (LL), and produces non-recombined haploid (L) oocytes [35]. Mating with a R. ridibunda male then restores the RL genome. Hence, one set of chromosomes (R) recombines in the parental species, while the other set (L) is transmitted clonally by the hybrid.
Both sets of chromosomes recombine during meiotic hybridogenesis [15,36], which involves a reproductive community of hybrids of different ploidy levels and genomic compositions (RRL, LLR and RL). Triploids RRL drop their L genome from the germ line, and produce recombined haploid R gametes by normal meiosis. Triploids LLR similarly drop their R genome before meiosis, producing recombinant haploid L gametes. Finally, RL diploids form clonal diploid RL gametes after endomitosis [11]. Combining these gametes restores the original diploid and triploid genomes [36–38]. Similar mechanisms have been documented in several hybridogenetic teleost fishes [39–44].
Thus, the pre-meiotic elimination of NOR− in male Batura toads, followed by normal diploid meiosis of the two NOR+ sets, shares similarities with some processes occurring during meiotic hybridogenesis and other forms of hybridogenetic or kleptogenetic reproductive modes known from triploid vertebrates (figure 1). Similarly, the duplication of the NOR− in female Batura toads occurs through a gametogenetic mechanism (premeiotic endomitosis) that is well known from parthenogenetic and hybridogenetic vertebrates [23,45,46]. However, the Batura toad system seems unique among vertebrates in that (i) meiotic processes differ between sexes, and (ii) females simultaneously transmit one genome that is clonally duplicated, and another that undergoes normal meiosis. The closest system seems to be found in plants, such as heathers and dog roses, in which pollen only transmits a sexually reproducing genome, while ovules transmit clonally reproducing genomes in addition [47–49]. There is, however, no pre-meiotic duplication of clonal genomes in these cases. To our knowledge, auto-duplication of one entire chromosome set (NOR−) in the presence of a foreign diploid genome (NOR+/NOR+'; which remains pre-meiotically unchanged and is later transmitted in a Mendelian manner) has not been shown so far (figure 1).
(b). Evolutionary aspects
Batura toads display remarkably homogeneous (within-species) mitochondrial sequences [50], suggesting a unique and recent origin (though none of its potential parental species occurs within the species range, which encompasses three river drainages from northern Pakistan). Although triploids may directly arise from a cross between a diploid and a tetraploid parental species, the complex meiotic processes documented here (including the duplication of a whole genome in females and its elimination in males) might not have evolved right at the initial hybridization event. Intermediate steps may have included a period of hybrid interactions between lineages of different ploidy levels and genomic compositions, similar to the situation occurring now in northern Kyrgystan, where some triploid males, resulting from natural crosses between 2n Bufo turanensis mothers and 4n Bufo pewzowi fathers, backcross with females from either parental species [30]. Such mechanisms must be rare, however, owing to the low probability of meeting the complex genetic requirements necessary to achieve stable hybrid combinations of clonal and Mendelian genomes, as recently also hypothesized for old lineages of gynogenetic fish [51].
This reproductive mode also raises questions regarding selective processes and evolutionary fate. Polyploid (3n, 4n) lineages of green toads, which evolved several times independently, are clearly associated with harsh habitats [50]. Batura toads, in particular, live in extreme conditions of altitude and xericity [52]. One might speculate that the clonal reproduction of the NOR− genome allows preserving epistatic components of fitness, which may matter when selection stems from abiotic and predictably harsh environmental factors [53]. As a matter of fact, asexual lineages often occur in marginal habitats with more extreme conditions (colder, dryer, higher altitude and higher UV-radiation) than their sexual relatives [54–57]. An important question in this context is whether both genomes are expressed, and, if so, whether expression is differential (tissue-specific) as observed in allo-polyploid fishes [58] and plants [59].
This exceptional mode of NOR− inheritance should also have detrimental evolutionary consequences. First, its purely maternal transmission opens opportunities for genomic conflicts. We expect, in particular, feminizing factors to evolve on the matrilineal NOR−, to be then counter-balanced by masculinizing factors evolving on the biparentally transmitted NOR+. This might result in sex-ratio biases, as observed in the hybridogenetic R. esculenta, where the maternally transmitted clonal R genome harbours feminizing factors only [60]. Similarly, mutations that are deleterious only in males are not counter-selected and might accumulate, such as found in other maternally transmitted mitochondrial [61] or nuclear [62] genomes.
Second, the non-recombining NOR− set should progressively accumulate deleterious mutations, under the conjugate forces of enhanced drift, selective sweeps, background selection and Muller's ratchet [63–65], as happens to sex chromosomes (Y or W), and to the non-recombining genomes of hemiclonal vertebrates [66]. Batura toads, however, may have arisen too recently for such mutational meltdown or genomic conflict over sex determination to be detectable [25,50].
By contrast, the Mendelian segregation and recombination found in the NOR+ genome should prevent its evolutionary decay, ensuring the long-term evolutionary potential of Batura toads, as found in sexually reproducing vertebrates with normal meiosis.
Comparing gene sequences of the NOR− genome of B. baturae with those of its parental species, as well as those of tetraploid green toad lineages as B. oblongus and B. pewzowi, where the NOR− genomes also recombine according to cytological [28] and microsatellite (this study) evidence, might help gaining information, not only on the phylogenetic history of the NOR− set, but also on the patterns of selection occurring in this non-recombining genome. This might also allow investigating potential conflicts in sex-determination pathways, as well as possible intergenomic recombinations, such as observed in kleptogenetically reproducing Ambystoma [67].
5. Conclusions
The reproductive mode of B. baturae differs from those known so far in other vertebrates (figure 1) not only because the meiotic processes differ between sexes, but also because females display clonal and sexual reproduction simultaneously (pre-meiotic auto-duplication affects one chromosome set, while the others undergo normal meiosis). We hereby name this process ‘pre-equalizing hybrid meiosis’. Elucidating the mechanisms underlying these peculiarities might shed much light on the general processes that regulate meiosis in vertebrates. Batura toads also offer intriguing opportunities to compare evolutionary forces in recombining and non-recombining genomes within the same organism.
Acknowledgements
Research was carried out according to approved guidelines under the following permits: Bundesamt für Veterinärwesen BVET Nr. 1245/10, Bern, Switzerland; and Authorization No. 1798, Service de la consommation et des affaires vétérinaires, Canton de Vaud, Epalinges, Switzerland.
This work was in part supported by a research fellowship (Sto 493/1-2) from the Deutsche Forschungsgemeinschaft (DFG) and by a grant from the Fondation Agassiz of the University of Lausanne (1 July 2010) to M.St.; by the University of California, Berkeley (C.M.), funds to N.P. (Swiss National Science Foundation, grant 31003A-129894), and from the Deutsche Forschungsgemeinschaft, SFB 567 (MSch). We thank Robert Dressel, and Hendrik Veith for help in Pakistan; and Dunja K. Lamatsch for assistance with ploidy determination.
References
- 1.Mendel G. 1866. Versuche über Pflanzenhybriden [Experiments on plant hybridization]. Verh. Naturforsch. Ver. Brünn 4, 3–47 [In German.] [Google Scholar]
- 2.Keller L. 2010. Genetics: biased transmission of genomes according to parents of origin. Curr. Biol. 20, R601–R602 10.1016/j.cub.2010.05.048 (doi:10.1016/j.cub.2010.05.048) [DOI] [PubMed] [Google Scholar]
- 3.Helms Cahan S., Keller L. 2003. Complex hybrid origin of genetic caste determination in harvester ants. Nature 424, 306–309 10.1038/nature01744 (doi:10.1038/nature01744) [DOI] [PubMed] [Google Scholar]
- 4.Fournier D., Estoup A., Orivel J., Foucaud J., Jourdan H., Le Breton J., Keller L. 2005. Clonal reproduction by males and females in the little fire ant. Nature 435, 1230–1234 10.1038/nature03705 (doi:10.1038/nature03705) [DOI] [PubMed] [Google Scholar]
- 5.Kulmuni J., Seifert B., Pamilo P. 2010. Segregation distortion causes large-scale differences between male and female genomes in hybrid ants. Proc. Natl Acad. Sci. USA 107, 7371–7376 10.1073/pnas.0912409107 (doi:10.1073/pnas.0912409107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schultz R. J. 1969. Hybridization, unisexuality and polyploidy in the teleost Poeciliopsis (Poeciliidae) and other vertebrates. Am. Nat. 103, 605–619 10.1086/282629 (doi:10.1086/282629) [DOI] [Google Scholar]
- 7.Darevsky I. S. 1958. Natural parthenogenesis in certain subspecies of rock lizards, Lacerta saxicola Eversmann. Dokl. Akad. Nauk SSSR, Biol. Sci. 122, 730 [In Russian.] [Google Scholar]
- 8.Kearney M., Fujita M. K., Ridenour J. 2009. Lost sex in the reptiles: constraints and correlations. In Lost sex: the evolutionary biology of parthenogenesis (eds Schoen I., Martens K., van Dijk P.), pp. 447–474 Heidelberg, Germany: Springer [Google Scholar]
- 9.Beukeboom L. W., Vrijenhoek R. C. 1998. Evolutionary genetics and ecology of sperm-dependent parthenogenesis. J. Evol. Biol. 11, 755–782 10.1007/s000360050117 (doi:10.1007/s000360050117) [DOI] [Google Scholar]
- 10.Bogart J. P., Klemens M. W. 1997. Hybrids and genetic interactions of mole salamanders (Ambystoma jeffersonianum and A. laterale) (Amphibia: Caudata) in New York and New England. Am. Mus. Novit. 3, 1–78 [Google Scholar]
- 11.Polls-Pelaz M. 1994. Modes of gametogenesis among kleptons of the hybridogenetic water frog complex: an evolutionary synthesis. Zool. Polon. 39, 123–138 [Google Scholar]
- 12.Morishima K., Horie S. Y. E., Arai K. 2002. A cryptic clonal line of the loach Misgurnus anguillicaudatus (Teleostei: Cobitidae) evidenced by induced gynogenesis, interspecific hybridization, microsatellite genotyping and multilocus DNA fingerprinting. Zool. Sci. 19, 565–575 10.2108/zsj.19.565 (doi:10.2108/zsj.19.565) [DOI] [PubMed] [Google Scholar]
- 13.Oshima K., Morishima K., Yamaha E., Arai K. 2005. Reproductive capacity of triploid loaches obtained from Hokkaido Island, Japan. Ichthyol. Res. 52, 1–8 10.1007/s10228-004-0245-3 (doi:10.1007/s10228-004-0245-3) [DOI] [Google Scholar]
- 14.Alves M. J., Coelho M. M., Collares-Pereira M. J. 1998. Diversity in the reproductive modes of females of the Rutilus alburnoides complex (Teleostei, Cyprinidae): a way to avoid the genetic constraints of uniparentalism. Mol. Biol. Evol. 15, 1233–1242 [Google Scholar]
- 15.Günther R., Uzzell T., Berger L. 1979. Inheritance patterns in triploid Rana ‘esculenta’ (Amphibia, Salientia). Mitt. Zool. Mus. Berlin 55, 35–37 [Google Scholar]
- 16.Vinogradov A. E., Borkin L. J., Günther R., Rosanov J. M. 1990. Genome elimination in diploid and triploid Rana esculenta males: cytological evidence from DNA flow cytometry. Genome 33, 619–627 10.1139/g90-092 (doi:10.1139/g90-092) [DOI] [PubMed] [Google Scholar]
- 17.Charles J. S., Hamilton M. L., Petes T. D. 2010. Meiotic chromosome segregation in triploid strains of Saccharomyces cerevisiae. Genetics 18, 537–550 10.1534/genetics.110.121533 (doi:10.1534/genetics.110.121533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Comai L. 2005. The advantages and disadvantages of being polyploid. Nat. Rev. Gen. 6, 836–846 10.1038/nrg1711 (doi:10.1038/nrg1711) [DOI] [PubMed] [Google Scholar]
- 19.Schultz R. J. 1967. Gynogenesis and triploidy in the viviparous fish Poeciliopsis. Science 157, 1564–1567 10.1126/science.157.3796.1564 (doi:10.1126/science.157.3796.1564) [DOI] [PubMed] [Google Scholar]
- 20.Mateos M., Vrijenhoek R. C. 2005. Independent origins of allotriploidy in the fish genus Poeciliopsis. J. Hered. 96, 32–39 10.1093/jhered/esi010 (doi:10.1093/jhered/esi010) [DOI] [PubMed] [Google Scholar]
- 21.Monaco P., Rasch E., Balsano J. 1984. Apomictic reproduction in the Amazon molly, Poecilia formosa, and its triploid hybrids. In Evolutionary genetics of fishes (ed. Turner B.), pp. 311–318 New York, NY: Plenum Press [Google Scholar]
- 22.Bogart J., Bi K., Fu J., Noble D., Niedzwiecki J. 2007. Unisexual salamanders (genus Ambystoma) present a new reproductive mode for eukaryotes. Genome 50, 119–136 10.1139/G06-152 (doi:10.1139/G06-152) [DOI] [PubMed] [Google Scholar]
- 23.Avise J. 2008. Clonality. Oxford, UK: Oxford University Press [Google Scholar]
- 24.Lamatsch D., Stöck M. 2009. Sperm-dependent parthenogenesis and hybridogenesis in teleost fishes. In Lost sex: the evolutionary biology of parthenogenesis (eds Schoen I., Martens K., van Dijk P.), pp. 399–432 Heidelberg, Germany: Springer [Google Scholar]
- 25.Stöck M., et al. 2002. A bisexually reproducing all-triploid vertebrate. Nat. Genet. 30, 325–328 10.1038/ng839 (doi:10.1038/ng839) [DOI] [PubMed] [Google Scholar]
- 26.Stöck M., Schmid M., Steinlein C., Grosse R. 1999. Mosaicism in somatic triploid specimens of the Bufo viridis complex in the Karakoram with examination of calls, morphology and taxonomic conclusions. Ital. J. Zool. 66, 215–232 10.1080/11250009909356259 (doi:10.1080/11250009909356259) [DOI] [Google Scholar]
- 27.Uzzell T. 1970. Meiotic mechanisms of naturally occurring unisexual vertebrates. Am. Nat. 104, 433–445 10.1086/282678 (doi:10.1086/282678) [DOI] [Google Scholar]
- 28.Stöck M., Steinlein C., Lamatsch D. K., Schartl M., Schmid M. 2005. Multiple origins of tetraploid taxa in the Eurasian Bufo viridis subgroup. Genetica 124, 255–272 [DOI] [PubMed] [Google Scholar]
- 29.Gosner L. K. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16, 513–543 [Google Scholar]
- 30.Stöck M., Ustinova J., Lamatsch D. K., Schartl M., Perrin N., Moritz C. 2010. A vertebrate reproductive system involving three ploidy levels: hybrid origin of triploids in a contact zone of diploid and tetraploid Palearctic green toads (Bufo viridis subgroup). Evolution 64, 944–959 10.1111/j.1558-5646.2009.00876.x (doi:10.1111/j.1558-5646.2009.00876.x) [DOI] [PubMed] [Google Scholar]
- 31.Raymond M., Rousset F. 1995. Genepop (version 1.2): population genetics software for exact tests and ecumenicism. J. Hered. 86, 248–249 [Google Scholar]
- 32.Rousset F. 2008. Genepop'007: a complete reimplementation of the Genepop software for Windows and Linux. Mol. Ecol. Res. 8, 103–106 10.1111/j.1471-8286.2007.01931.x (doi:10.1111/j.1471-8286.2007.01931.x) [DOI] [PubMed] [Google Scholar]
- 33.Kauri H. 1954. Über die systematische Stellung der Europäischen Grünen Frösche Rana esculenta L. und Rana ridibunda Pall. [On the systematic position of the European green frogs Rana esculenta L. and Rana ridibunda Pall]. Acta Univ. Lund. N.F. 5, 1–30 [In German.] [Google Scholar]
- 34.Berger L. 1968. Morphology of the F1 generation of various crosses within Rana esculenta complex. Acta Zool. Cracov 13, 301–324 [Google Scholar]
- 35.Graf J.-D., Müller W. P. 1979. Experimental gynogenesis provides evidence of hybridogenetic reproduction in the Rana esculenta complex. Experientia 35, 1574–1576 10.1007/BF01953200 (doi:10.1007/BF01953200) [DOI] [PubMed] [Google Scholar]
- 36.Christiansen D. G., Reyer H. U. 2009. From clonal to sexual hybrids: genetic recombination via triploids in all-hybrid populations of water frogs. Evolution 63, 1754–1768 10.1111/j.1558-5646.2009.00673.x (doi:10.1111/j.1558-5646.2009.00673.x) [DOI] [PubMed] [Google Scholar]
- 37.Günther R. 1990. Die Wasserfrösche Europas [The water frogs of Europe]. Die Neue Brehm-Bücherei, vol. 600 [In German.] Wittenberg, Germany: Ziemsen [Google Scholar]
- 38.Christiansen D. G. 2009. Gamete types, sex determination and stable equilibria of all-hybrid populations of diploid and triploid edible frogs (Pelophylax esculentus). BMC Evol. Biol. 9, 135. 10.1186/1471-2148-9-135 (doi:10.1186/1471-2148-9-135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goddard K. A., Schultz R. J. 1993. Aclonal reproduction by polyploid members of the clonal hybrid species Phoxinus eos-neogaeus (Cyprinidae). Copeia 3, 650–660 10.2307/1447226 (doi:10.2307/1447226) [DOI] [Google Scholar]
- 40.Goddard K. A., Megwinoff O., Wessner L. L., Giaimo F. 1998. Confirmation of gynogenesis in Phoxinus eos-neogaeus (Pisces: Cyprinidae). J. Hered. 89, 151–157 10.1093/jhered/89.2.151 (doi:10.1093/jhered/89.2.151) [DOI] [Google Scholar]
- 41.Kim I. S., Lee E. H. 2000. Hybridization experiment of diploid-triploid cobitid fishes, Cobitis sinensis-longicorpus complex (Pisces: Cobitidae). Folia Zool. 49, 17–22 [Google Scholar]
- 42.Alves M. J., Coelho M. M., Collares-Pereira M. J. 2001. Evolution in action through hybridisation and polyploidy in an Iberian freshwater fish: a genetic review. Genetica 111, 375–385 10.1023/A:1013783029921 (doi:10.1023/A:1013783029921) [DOI] [PubMed] [Google Scholar]
- 43.Itono M., Morishima K., Fujimoto T., Bando E., Yamaha E., Arai K. 2006. Premeiotic endomitosis produces diploid eggs in the natural clone loach, Misgurnus anguillicaudatus (Teleostei: Cobitidae). J. Exp. Zool. 305A, 513–523 10.1002/jez.a.283 (doi:10.1002/jez.a.283) [DOI] [PubMed] [Google Scholar]
- 44.Morishima K., Yoshikawa H., Arai K. 2008. Meiotic hybridogenesis in triploid Misgurnus loach derived from a clonal lineage. Heredity 100, 581–586 10.1038/hdy.2008.17 (doi:10.1038/hdy.2008.17) [DOI] [PubMed] [Google Scholar]
- 45.Butlin R., Schön I., Griffiths H. I. 1998. Introduction to reproductive modes. In Sex and parthenogenesis: evolutionary ecology of reproductive modes in non-marine ostracodes (ed. Martens K.), pp. 1–24 Leiden, The Netherlands: Backhuys Publ [Google Scholar]
- 46.Lutes A. A., Neaves W. B., Baumann D. P., Wiegraebe W., Baumann P. 2010. Sister chromosome pairing maintains heterozygosity in parthenogenetic lizards. Nature 464, 283–287 10.1038/nature08818 (doi:10.1038/nature08818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith-White S. 1948. Polarised segregation in the pollen mother cells of a stable triploid. Heredity 2, 119–129 10.1038/hdy.1948.7 (doi:10.1038/hdy.1948.7) [DOI] [PubMed] [Google Scholar]
- 48.Werlemark G., Uggla M., Nybom H. 1999. Morphological and RAPD markers show a highly skewed distribution in a pair of reciprocal crosses between hemisexual dogrose species, Rosa sect. Caninae. Theor. Appl. Gen. 98, 557–563 10.1007/s001220051104 (doi:10.1007/s001220051104) [DOI] [Google Scholar]
- 49.Nybom H. 2007. Unique reproduction in dogroses (Rosa sect. Caninae) maintains successful and highly heterozygous genotypes. In Apomixis (eds Hörandl E., Grossniklaus U., van Dijk P. J., Sharbel T. F.), pp. 281–298 Koenigstein, Germany: Koeltz Scientific Books [Google Scholar]
- 50.Stöck M., Moritz C., Hickerson M., Frynta D., Dujsebayeva T., Eremchenko V., Macey J. R., Papenfuss T. J., Wake D. B. 2006. Evolution of mitochondrial relationships and biogeography of Palearctic green toads (Bufo viridis subgroup) with insights in their genomic plasticity. Mol. Phylogenet. Evol. 41, 663–689 10.1016/j.ympev.2006.05.026 (doi:10.1016/j.ympev.2006.05.026) [DOI] [PubMed] [Google Scholar]
- 51.Stöck M., Lampert K. P., Möller D., Schlupp I., Schartl M. 2010. Monophyletic origin of multiple clonal lineages in an asexual fish (Poecilia formosa). Mol. Ecol. 19, 5204–5215 10.1111/j.1365-294X.2010.04869.x (doi:10.1111/j.1365-294X.2010.04869.x) [DOI] [PubMed] [Google Scholar]
- 52.Litvinchuk S. N., et al. 2011. Influence of environmental conditions on the distribution of Central Asian green toads with three ploidy levels. J. Zool. Syst. Evol. Res. 9, 233–239 10.1111/j.1439-0469.2010.00612.x (doi:10.1111/j.1439-0469.2010.00612.x) [DOI] [Google Scholar]
- 53.Bell G. 1982. The Masterpiece of nature: the evolution and genetics of sexuality. London, UK: Croom Helm [Google Scholar]
- 54.Cuellar O. 1977. Animal parthenogenesis. Science 197, 837–843 10.1126/science.887925 (doi:10.1126/science.887925) [DOI] [PubMed] [Google Scholar]
- 55.Lynch M. 1984. Destabilizing hybridization, general purpose genotypes and geographic parthenogenesis. Quat. Rev. Biol. 59, 257–290 10.1086/413902 (doi:10.1086/413902) [DOI] [Google Scholar]
- 56.Kearney M. 2003. Why is sex so unpopular in the Australian desert? Trends Ecol. Evol. 18, 605–607 10.1016/j.tree.2003.09.021 (doi:10.1016/j.tree.2003.09.021) [DOI] [Google Scholar]
- 57.Vrijenhoek R. C., Davis Parker D., Jr 2009. Geographical parthenogenesis: general purpose genotypes and frozen niche variation. In Lost sex: the evolutionary biology of parthenogenesis (eds Schoen I., Martens K., van Dijk P.), pp. 99–131 Heidelberg, Germany: Springer [Google Scholar]
- 58.Pala I., Coelho M. M., Schartl M. 2008. Dosage compensation by gene-copy silencing in a triploid hybrid fish. Curr. Biol. 18, 1344–1348 10.1016/j.cub.2008.07.096 (doi:10.1016/j.cub.2008.07.096) [DOI] [PubMed] [Google Scholar]
- 59.Buggs R. J. A., et al. 2011. Transcriptomic shock generates evolutionary novelty in a newly formed, natural allopolyploid plant. Curr. Biol. 21, 551–556 10.1016/j.cub.2011.02.016 (doi:10.1016/j.cub.2011.02.016) [DOI] [PubMed] [Google Scholar]
- 60.Berger L., Uzzell T., Hotz H. 1988. Sex determination and sex ratios in western Palearctic water frogs: XX and XY female hybrids in the Pannonian Basin? Proc. Natl Acad. Sci. USA 140, 220–239 [Google Scholar]
- 61.Frank S. A., Hurst L. D. 1996. Mitochondria and male disease. Nature 383, 224. 10.1038/383224a0 (doi:10.1038/383224a0) [DOI] [PubMed] [Google Scholar]
- 62.Archetti M. 2005. Accumulation of deleterious mutations in the genome of hybridogenetic organisms. J. Theor. Biol. 234, 151–152 10.1016/j.jtbi.2004.11.019 (doi:10.1016/j.jtbi.2004.11.019) [DOI] [PubMed] [Google Scholar]
- 63.Muller H. 1964. The relation of recombination to mutational advance. Mut. Res. 1, 2–9 [DOI] [PubMed] [Google Scholar]
- 64.Felsenstein J. 1974. The evolutionary advantage of recombination. Genetics 78, 737–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Charlesworth B., Charlesworth D. 2000. The degeneration of Y chromosomes. Phil. Trans. R. Soc. Lond. B 355, 1563–1572 10.1098/rstb.2000.0717 (doi:10.1098/rstb.2000.0717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guex G. D., Hotz H., Semlitsch R. D. 2002. Deleterious alleles and differential viability in progeny of natural hemiclonal frogs. Evolution 56, 1036–1044 [DOI] [PubMed] [Google Scholar]
- 67.Bi K., Bogart J. P. 2006. Identification of intergenomic recombinations in unisexual salamanders of the genus Ambystoma by genomic in situ hybridization. Cytogenet. Genome Res. 112, 307–312 [DOI] [PubMed] [Google Scholar]