Abstract
Fossilized compound eyes from the Cambrian, isolated and three-dimensionally preserved, provide remarkable insights into the lifestyle and habitat of their owners. The tiny stalked compound eyes described here probably possessed too few facets to form a proper image, but they represent a sophisticated system for detecting moving objects. The eyes are preserved as almost solid, mace-shaped blocks of phosphate, in which the original positions of the rhabdoms in one specimen are retained as deep cavities. Analysis of the optical axes reveals four visual areas, each with different properties in acuity of vision. They are surveyed by lenses directed forwards, laterally, backwards and inwards, respectively. The most intriguing of these is the putatively inwardly orientated zone, where the optical axes, like those orientated to the front, interfere with axes of the other eye of the contralateral side. The result is a three-dimensional visual net that covers not only the front, but extends also far laterally to either side. Thus, a moving object could be perceived by a two-dimensional coordinate (which is formed by two axes of those facets, one of the left and one of the right eye, which are orientated towards the moving object) in a wide three-dimensional space. This compound eye system enables small arthropods equipped with an eye of low acuity to estimate velocity, size or distance of possible food items efficiently. The eyes are interpreted as having been derived from individuals of the early crustacean Henningsmoenicaris scutula pointing to the existence of highly efficiently developed eyes in the early evolutionary lineage leading towards the modern Crustacea.
Keywords: Cambrian, compound eye, arthropoda, crustacea, Orsten, vision
1. Introduction
Stalked compound eyes of many arthropods, both ancient and recent, have a great potential for enhancing the available field of view. An especially sophisticated strategy has been discovered in an eye that is about one half a billion years old. This tiny eye, represented by six isolated stalked eyes, has been interpreted as belonging to individuals of the species Henningsmoenicaris scutula [1] ([2]; see also [3] for a detailed study of H. scutula), an early representative of the Crustacea, because no fragments of other species that could be the owner of these stalked eyes were found in the material. Based on its physiological properties, we here attempt to show how this tiny compound eye gathered information about its surroundings effectively, and we also discuss the habitat to which the animal was adapted.
2. Material and methods
‘Orsten’ refers to calcareous stinkstone concretions found in the Cambrian Alum Shale sequence of Sweden. Etching the Orsten nodules yielded assemblages of three-dimensionally preserved, secondarily phosphatized and mostly hollow-body microfossils of 0.1–2 mm in size (e.g. [4]; for general information and discussion of Orsten-type preservation see [5]).
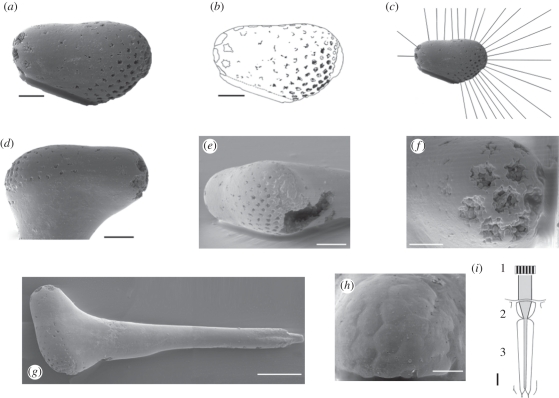
Six specimens of stalked eyes were available for study. The sizes of the whole specimens lie between 600 and approximately 1000 µm and the size of the ovoid visual eye structure lies between 260 and 405 µm. They all share a similar morphology and are from a single locality, the quarry of Gum, at the southwestern slope of Mt. Kinnekulle, Västergötland, Sweden (Agnostus pisiformis zone (formerly representing the lowermost zone 1 of the Furongian series, reconsidered as the uppermost, still unnamed series 3 Guzhangian stage, [6–8])). Detailed investigations of the structure and physiological properties of the eye were undertaken on the two most informative specimens (UB W383 and 384; figure 1a–h). Specimen UB W 383 (figure 1h) displays well-preserved hexagonal facets that give us an insight into the organization of their optical units.
Figure 1.
Stalked eyes belong to late developmental stages of Henningsmoenicaris scutula [1]. (a) UB W 384 eye in lateral view, showing rhabdom cavities. (b) Explanatory drawing of (a). (c) Bearings of optical axes in the vertical transection. (d–f) UB W 384 in (d) lateral, (e) frontal and (f) posterior aspects. (g) Whole stalked eye with mace-shaped head (left) and basal insertion to the joint right. (h) UB W 383 Facets in the eye. (i) Schematic of an apposition type ommatidium. 1. Contrasts inside the visual field of the ommatidium; 2. focusing dioptric apparatus; 3. rhabdom with sensory cells. Scale bars, 50 µm.
Compound eyes consist of more or less numerous individual units, the so-called ommatidia. In their most basal form, the so-called apposition eye, each ommatidium is composed of a distal dioptrical apparatus, which focuses the incident light onto a light-guiding structure, the so-called rhabdom, which is part of the sensory cells (figure 1i). It contains the visual pigments, which change their configuration as the light touches them. This process evokes an electrical signal that is processable by the sensory cells and the nervous system of the arthropod. Each of these units is separated by screening pigmentary cells. Thus, the distribution of light contrasts within the opening angle of one unit is combined onto the rhabdom and averaged to a medium ‘spot’ of light impression, rather than forming a real image inside each facet. In total, a compound eye of this type forms a mosaic-like image, and the acuity of vision of the arthropod depends mainly on the number of facets, as pixels define the acuity of a computer graphic. The rhabdoms represent the optical axes as the ray paths of each ommatidial unit, while in more advanced systems, as in superposition eyes, the rhabdoms are shorter; they terminate inside the compound eye, and the ray paths are more complicated. The apposition compound eye is the oldest type of compound eye, because more specialized types such as superposition eyes have been shown to be younger than the Carboniferous [9]. In apposition eyes, the rhabdoms are formed like pins that project deeply into the eye and UB W 384 possesses traces of the rhabdoms as cavities (figure 1a–g). Thus here, we consider the oldest identifiable functional type of compound eye—an apposition eye—so far known.
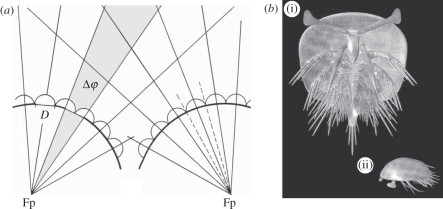
The analysis of the optical characters of the eye was attained by measurements and geometric constructions on the basis of scanning electron microscope (SEM) images of the eyes. The investigation of the optical axes is complex because of their three-dimensional arrangement. The orientation changes continuously, and thus it is possible to describe the character of this flow by a two-dimensional projection, a vertical transection through the visual surface. The lens distribution along this section characterizes well the active visual areas of this eye, as will be discussed later. Acuity depends essentially on, amongst other parameters, the interommatidial angle Δφ (figure 2a): the smaller Δφ is, the finer is the scanning pattern of the surroundings, and the higher the spatial resolution. The interommatidial angle was measured based on the optical axes. Because the specimens were fixed (glued) on stubs in the 1990s, not all Δφ or lens diameters D are available, and the measurements of the vertical cut must be taken for the adjacent areas representatively.
Figure 2.
(a) Definition of parameters used. Δφ, opening angle of the visual unit; D, lens diameter; Fp, optical axes intersecting at one point, here called: focal point. The optical axes of the ommatidia intersect in front of the ommatidia to form kite-shaped areas. (b) Reconstruction of H. scutula showing inferred position and orientation of the eye stalks. (i) Ventral aspect; (ii) lateral aspect.
The positions of the pair of eyes on the animal have been estimated based on the assumption that the distance between the insertion of the eyes is approximately half the length of the stalk, as can be taken from the reconstruction made by Haug et al. [3] from the fossil record of the earlier developmental stages of H. scutula. That the area of highest acuity of an eye is oriented to the front and/or laterally is almost trivial for a forwardly moving arthropod, scanning the field towards which it is moving.
3. Analyis of the visual system
The specimens of isolated eyes investigated herein have the shape of a mace comprising the slender eye stalk and the apical ovoid compound eye (figure 1g). In specimen UB W 384, the proximal end of the stalk shows a distinct constriction, probably the insertion to a joint, so the stalk was movable and represented here in its complete length. The smooth surface of the eye is indented by numerous irregular, somewhat star-shaped cavities with variable diameters. The largest of these cavities are located at the rounded end of the eye, the smallest at the wider end on the opposite side.
Orsten fossils are normally hollow-bodied, the preserved ‘shell’ resulting from incrustation, impregnation and mineralization by calcium phosphate (apatite) during early diagenesis, most probably was mediated by microbic bacteria. Sometimes, however, such phosphatization has been pervasive and the inside of the fossil became filled with phosphate, as in the pentsatomid figured by Maas et al. [5], fig. 4a,c and the stalked eyes found here. The rhabdoms, however, were less resistant; their positions are represented by elongated cavities, normal to the surface. The axial directions of the rhabdoms can thus be precisely reconstructed, indicating the ray paths inside the visual units, as their optical axes. As in extant arthropod apposition compound eyes, these traces of the rhabdoms intrude deeply into the entire eye.
Accordingly, the pattern of these cavities allows us to measure the direction of the ommatidial axes, since they stand normal and perpendicular to the local surface of the eye. As specimen UB W 383 (figure 1h) shows, the facets remain close to each other without any gaps, and are regularly hexagonal. The field of view has been measured between the most extreme optical axes, and is approximately 300°. If, as we believe, the stalk was movable, this already remarkably wide field of view would be enlarged even further.
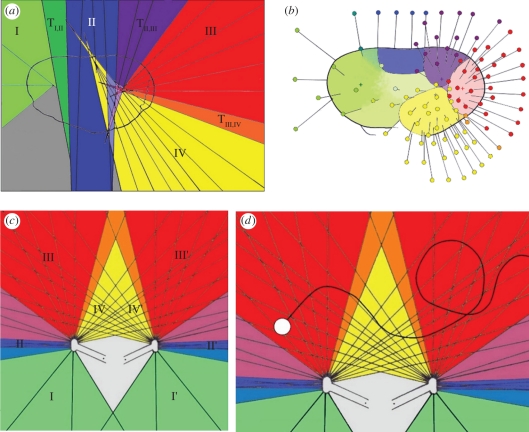
The analysis of the optical axes along the vertical transection through the visual surface (figure 1c) conspiciously shows that the axes of certain adjacent facets converge and form groups with intersecting optical axes inside the eye meeting at one point in common (figure 3a), while between these groups lie facets with axes of intermediate character. The axes of the intermediate facets do not have such a focal point and form a continuous transition to the orientations of the next group. In one group, the optical axes run almost parallel with each other. A three-dimensional reconstruction of the optical axes (figure 3b), as far as the fixation on the stubs allows, shows that adjacent visual facets of each area are in continuance to the groups of the vertical section, and form distinct areas also with transitional zones. The approximate intersections of their axes form more or less distinct focal areas inside of the eye.
Figure 3.
(a) Two-dimensional projection and areas of optical axes. I, II, III, IV areas of visual units with comparable function and character. The optical axes of each area have a single focal centre, where the axes intersect TI,II, TIII, IV, transitional areas with intermediate interommatidial angles. (b) Three-dimensional reconstruction of the orientation of the optical axes of the areas belonging to the groups I–IV. Crosses indicate the approximate positions of the focal centres inside of the eye. (c) Interaction of the ommatidial opening angles (morphological visual fields of each ommatidium) of both eyes. I, II, III, IV groups of ommatidal visual fields; I′, II′, III′, IV′ contralateral groups of ommatidal visual fields; grey area: dead zone without any visual perception. (d) O: moving object in the field of view. From the start to the moment of observation it crosses approximately 35 sectors, meaning approximately 35 coordinates given by particular couples of ommatidia.
In the vertical transection, the four recognized groups are characterized by different interommatidial angles. Group I has three ommatidia (along the transection) with a very large opening angle of Δφ = 39.8° ± 2.0° and an average ommatidial diameter of 40.4 ± 6.9 µm. One ommatidium lies in the intermediate zone between groups I and II. Group II consists of about four ommatidia (D = 25.8 ± 5.4 µm). Owing to the almost flat visual surface, the axes of these ommatidia run almost in parallel (Δφ: 0.75° ± 4.3°). This means that, under the given demands for a certain lens diameter to work efficiently under given light conditions, the finest pattern possible for scanning the surroundings is realized in this case. Furthermore, there are axes inside of group II crossing at a greater distance. Three ommatidia lie in the intermediate zone between groups II and III. Group III comprises six ommatidia. The axes of the ommatidia (D = 13.3 ± 2.1 µm) of group III are orientated like outwardly spreading beams having a median angle of Δφ = 10.8° ± 1.5°. A single ommatidium forms the intermediate zone between groups III and IV. Area IV comprises eight ommatidia. It is characterized by a comparatively small interommatidial angle of Δφ = 5.4° ± 1.53° and a median ommatidial diameter of 14.4 ± 3.8 µm.
Based on the properties of the distinguishable groups of differently organized optical axes of the eye, group III is interpreted as pointing to the front because it has a significantly higher resolution than the opposite end of the eye. Assuming that the arthropod was equipped with a pair of eyes, the resulting arrangement of the two eyes leads to overlapping fields of view of the areas of the two eyes to the front. Furthermore, especially within area IV, the optical axes of left and right eyes form a dense net of kite-shaped regions (figure 3c).
A ‘dead zone’ described by Burkhardt [10] as an area not being covered by any optical axes in the ‘normal’ (unmoved) position of the eye stalk is identified between and behind the left and right eyes (grey area in figure 3c). The spatial size of this dead zone of extant arthropods is usually correlated to the body [10], which the animals cannot see through. Based on the reconstructed ‘dead zone’ for the visual system investigated herein, the body of the eye bearer must have been at least 1.5 mm in length.
The ‘normal’ position of the eyes with regard to the body is shown in figure 2b. The wide ovoid shield covering the entire arthropod is restricted slightly at the front and thus gives space to the eyes to ‘peer’. The outermost position, when this is possible, was taken as the ‘normal’ position, when the main visual surface is directed laterally, able to scan the horizon, and the end with the higher acuity was orientated forwardly.
4. Discussion: the visual strategy of a small predator
Based on the strict geometric pattern of the half-a-billion-year-old eye of H. scutula investigated here, the functional aspects of vision in this species can be understood to a quite impressive degree. The organization of the four groups of different interommatidial angles reflects areas of different morphological acuity, corresponding to the width of the opening angle Δφ. The smaller Δφ, the finer the scanning patterns of the surroundings, and the higher the morphological resolution. Group III, representing its area of vision with its high acuity, was most probably oriented to the front. The large, more or less flat area of group II surveys the lateral part, probably the horizon, and a wide laterally extending environment, which is optimally scanned owing to the almost parallel optical axes resulting in the smallest Δφ possible. In this area, the slightly indented visual surface causes the intersection of the optical axes. This may be an artefact caused by fossilization, or the indentation may be regarded as a kind of ‘attempt’ to enable a slight stereoscopic view from this part of the eye. The part of the lowest acuity is the area around group I. It has the widest visual units, meaning that the visual units had a wide aperture to capture enough light to be able to function, in other words they had the highest sensitivity for capturing light. Possibly it was orientated backwards and slightly downwards, and thus able to survey the dark of the deeper sea below the animal. The function of the area adjacent to group IV is only interpretable when assuming the presence of a pair of these stalked eyes on this crustacean (see below).
A high number of small facets and low interommatidial angles, scanning the environment with a fine resolution, provide the animal with a good acuity of vision, but they require a high light intensity, as is illustrated in modern, diurnally active dragonflies, which have up to 20 000 facets (corresponding to 20 000 ‘pixels’ of their image) within each eye. When living in deeper regions or under a crepuscular lifestyle larger lenses would be necessary, capturing more light, but in the limited space available in a compound eye this would be at the cost of acuity. This ‘competition’ under ecological conditions leads to a compromise to achieve a maximum possible acuity with the lowest necessary amount of light. This is an optimization to the demands of life, which can be described by the so-called ‘eye parameter’ [11–15]. It was calculated as p = (½ × √3)D × Δφ µm rad) for hexagonally packed facets. D stands for the distances between the optical axes on the surface of the eye, as for the lens diameter, which is identical in densely packed compound eyes. The eye parameter p can be used for establishing the light conditions to which the animal was adapted, as has been undertaken with extant arthropods [11,13–15], for comparison.
p = 1.2 ± 0.95 (µm rad) indicates that H. scutula was a daylight-adapted diurnal arthropod, living probably close to the light-flooded surface, as many comparisons with extant arthropods demonstrate (compare [11,12]). Diurnal marine crustaceans have an eye parameter of about 2–4 µm rad, while diurnal terrestrial arthropods, not suffering from the absorption of light by the water, have an eye parameter of less than 2 µm rad, and the whole range of this parameter lies between values of less than 1 and more than 200 µm rad, following a logarithmic characteristic [11,12].
Although the lens diameter (and acuity) of the visual areas as shown is variable, the low standard variance of the eye parameter over all groups shows that the eye parameter seems to be balanced all over the eye (except for the specialized group I). Because the eye parameter is a measure for the light conditions to which the eyes are adapted, a balanced eye parameter all over the different functional areas of the eye indicates a stable adaptation to the light conditions mentioned above. Based on the reconstructed orientation of the eye, the entire visual field is reconstructed (figure 3). Most of these optical axes of both eyes interfere, which means that stereoscopic vision and depth perception are possible over a wide range. It also becomes evident that this stereoscopical view is extended far to the contralateral side of each eye.
Most surprising is the evaluation of the optical axes of the area of group IV. In many extant animals, where compound eyes are capable of stereoscopic vision, such an area is normally restricted widely to the front, as has been demonstrated for a large number of arthropods with stalked eyes [11,16]. Here in the fossil eye investigated, this zone of three-dimensional vision is, however, further extended very far to the contralateral side, mainly as the result of the extraordinary geometry of the eye, especially the position and arrangement of group IV. This area, owing to its far extension and its inward orientation, should have been able to survey the internal, proximal and contralateral fields of view very effectively.
One aspect of vision in apposition compound eyes, as seen above, is that within the field of view of any visual unit (corresponding to the opening angle of each ommatidium), no separate visual inputs can be differentiated. All visual inputs of one ommatidium are summed up to one average light signal, resulting in a mosaic-like image for the whole compound eye, and the range of any single facet is one informational unit, comparable to pixels in a digital graphic.
Yet, it is clear from the limited number of lenses (fewer than 200) of the fossil eye analysed here that it cannot have formed a highly differentiated and acute image. In this eye, the wide range of overlapping field of view, especially to the contralateral sides, must be interpreted as quite another principle of vision: here, each of the kite-shaped fields established from the intersections of these areas of vision (opening angles) of individual ommatidia (compare figures 2 and 4) stands for one optical informational unit—functioning like a coordinate system, as is common in compound eyes. The smaller are the kites as informational units, the finer is the acuity of vision. These fine acute fields of vision in arthropods are normally orientated to the front—but here are atypically extended over an enormously wide field towards the contralateral sides of the eyes. Accordingly, the eyes have a highly sensitive function: if an object is moving inside the net of optical axes (figure 3d), its movement from one sector to the next will be recognized immediately, because it changes from one point of the coordinate system to another. In other words, each time an object passes a line or a crossing point in this web, entering another informational unit (‘kite’), another pair of facets will recognize it, and its movement can be observed effectively. While two facets (one of the left eye and one of the right) always determine the position of an object spot inside the visual field of the animal, we find a dense network of optical axes. In the example of figure 3d, the moving object touches approximately 35 sectors, meaning approximately 35 coordinates, ensuring a highly efficient eye system for a creature searching for small moving objects and selectively catching food items, although not able to form acute images, owing to its small size and low number of facets (‘pixels’).
Even estimating distances is easy to achieve, as is judging the (relative) velocity and magnitude of an object, simply by comparing the sectors of the three-dimensional visual field. Because, as described before, a high acuity of vision is supported by a narrow web of informational units (small kites in figure 3), the acuity of the estimation of distances becomes less accurate the farther away the target of vision lies, because the size of the sectors increases with distance from the eye (compare figure 3).
If the eye stalks were moved outwards, the distance between both eye surfaces was wider and the size of the visual field would have been enhanced, but it would have a lower acuity, because the kites became wider. An enhancement of acuity would be achieved by the opposite assumptions. If the eyes approach by the inward-movement of the stalks, the stereoscopic field of view enters the internal blind zone, which may have been involved in controlling the feeding process when eating the captured prey, as the latter is reported for dragonflies or certain beetles [11]. The presumed optical situation, in principle, however, remains unchanged.
The dead zone is correlated with the size of the arthropod [10]. For the investigated eye, the body length of its owner can be estimated to have been at least about 1.5 mm, as the shape of the dead zone (figure 3c) correlates well with the shape of H. scutula (figure 2b). The largest complete specimen known of H. scutula is about 1 mm long, and it is known from fragments that this was not the final developmental stage, but that growth had probably continued subsequently [3]. A large trunk fragment, for example, indicates that individuals may have grown to at least 2.5 mm in length. On this basis, the investigated eyes could well be from H. scutula individuals that were 1.5 mm long. Yet, the stalk appears in developmental stage 8 and becomes progressively longer throughout ontogeny [3], and crustaceans plesiomorphically are equipped with compound eyes. In fact, H. scutula is the only known crustacean from the Orsten to have well-developed eye stalks, and the described eyes may have enabled H. scutula to actively grasp specific food items.
5. Conclusion
The tiny compound eye of H. scutula appears to be too small to establish an image-gathering function, as it has a low number of visual units. However, this eye is highly differentiated into four functionally different areas and may have enabled H. scutula to detect moving objects and also recognize their distances. Thus, H. scutula probably fed through catching selected food items, perhaps in a predatory lifestyle, but could easily watch out for predators itself, even when approaching from behind, and hide. Despite its small size and the inability to provide images of high acuity, the stalked eye of H. scutula represents a sophisticated visual system well-adapted to a specialized lifestyle, although it is almost half a billion years old.
Acknowledgements
This study would not have been possible without different sources of help for which we are very grateful. We thank Jean Vannier and Nigel Hughes, whose comments on earlier versions greatly improved the text, and Jean Vannier for reviewing the most recent manuscript. The Central Unit for Electron Microscopy, University of Ulm and its team supported our SEM work. We, furthermore, thank all people involved in writing free software programmes used in the course of this study, such as CombineZM and OpenOffice, and U. Falckenberg-Bongarts for her help with the illustrations. The German Science Foundation supported this study: J.T.H. under Wa-754/15-1 and C.C. under Wa-754/18-1.
References
- 1.Walossek D., Müller K. J. 1990. Upper Cambrian stem-lineage crustaceans and their bearing upon the monophyletic origin of crustacea and the position of Agnostus. Lethaia 23, 409–427 10.1111/j.1502-3931.1990.tb01373.x (doi:10.1111/j.1502-3931.1990.tb01373.x) [DOI] [Google Scholar]
- 2.Castellani C., Haug J. T., Haug C., Maas A., Schoenemann B., Waloszek D. In press Exceptionally well-preserved isolated eyes from the late Cambrian ‘Orsten’ faunal assemblages of Sweden. Palaeontology. [Google Scholar]
- 3.Haug J. T., Maas A., Waloszek D. 2010. Henningsmoenicaris scutula, Sandtorpia vestrogothiensis gen. et sp. nov. and heterochronic events in early crustacean evolution. Earth Environ. Sci. Trans. R. Soc. Edinb. 100, 311–350 10.1017/S1755691010008145 (doi:10.1017/S1755691010008145) [DOI] [Google Scholar]
- 4.Haug J. T., Maas A., Waloszek D. 2009. Ontogeny of two Cambrian stem crustaceans, †Goticaris longispinosa and †Cambropachycope clarksoni. Palaeontographica 289, 1–43 [Google Scholar]
- 5.Maas A., et al. 2006. The ‘Orsten’—more than a Cambrian Konservat-Lagerstätte yielding exceptional preservation. Palaeoworld 15, 266–282 10.1016/j.palwor.2006.10.005 (doi:10.1016/j.palwor.2006.10.005) [DOI] [Google Scholar]
- 6.Ahlberg P. 2003. Trilobites and intercontinental tie points in the Upper Cambrian of Scandinavia. Geol. Acta 1, 127–134 10.1344/105.000001599 (doi:10.1344/105.000001599) [DOI] [Google Scholar]
- 7.Terfelt F., Eriksson M. E., Ahlberg P., Babcock L. E. 2008. Furongian series (Cambrian) biostratigraphy of Scandinavia—a revision. Norwegian J. Geol. 88, 73–87 [Google Scholar]
- 8.Peng S., Yang X., Hughes N. C. 2008. The oldest known stalk-eyed trilobite, Parablackwelderia Kobayashi, 1942 (Damesellinae, Cambrian), and its occurrence in Shandong, China. J. Palaeontol. 82, 842–850 10.1666/07-123.1 (doi:10.1666/07-123.1) [DOI] [Google Scholar]
- 9.Gaten E. 1998. Eye structure and phylogeny: is there an insight? The evolution of superposition eyes in the Decapoda (Crustacea). Contrib. Zool. 67, 223–235 [Google Scholar]
- 10.Burkhardt D. 1973. Zum binokularen Entfernungssehen der Insekten. J. Comp. Physiol. A 87, 165–188 10.1007/BF01352159 (doi:10.1007/BF01352159) [DOI] [Google Scholar]
- 11.Horridge G. A. 1977. Insects which turn and look. Endeavour 1, 7–17 10.1016/0160-9327(77)90004-7 (doi:10.1016/0160-9327(77)90004-7) [DOI] [Google Scholar]
- 12.Land M. F. 1981. Optics and vision in invertebrates. In Vision in invertebrates. (Handbook of sensory physiology, vol. VII/6B) (ed. Autrum H.), pp. 471–592 Berlin, Germany: Springer [Google Scholar]
- 13.Snyder A. W. 1977. The acuity of compound eyes: physical limitations and design. J. Comp. Physiol. 116, 161–182 10.1007/BF00605401 (doi:10.1007/BF00605401) [DOI] [Google Scholar]
- 14.Snyder A. W. 1979. Physics of vision in compound eyes. In Vision in invertebrates. (Handbook of sensory physiology, vol. VII/6A) (ed. Autrum H.), pp. 225–313 Berlin, Germany: Springer [Google Scholar]
- 15.Snyder A. W., Stavenga D. G., Laughlin S. B. 1977. Spatial information capacity of compound eyes. J. Comp. Physiol. 116, 183–207 10.1007/BF00605402 (doi:10.1007/BF00605402) [DOI] [Google Scholar]
- 16.Burkhardt D., de la Motte I. 1983. How stalk-eyed flies eye stalk-eyed flies: observations and measurements of the eyes of Cyrtodiopsis whitei (Diopsidae, Diptera). J. Comp. Physiol. A 151, 407–421 10.1007/BF00605457 (doi:10.1007/BF00605457) [DOI] [Google Scholar]