Homoploid hybrid speciation occurs when a stable, fertile and reproductively isolated lineage results from hybridization between two distinct species, without a change in ploidy level. Reproductive isolation between a homoploid hybrid species and its parents is generally attained by chromosomal rearrangements, ecological divergence and/or spatial isolation from the parental species; these factors prevent the incipient hybrid species from being genetically swamped through mating with the parental species, and allow it to evolve as an independent lineage (Gross and Rieseberg, 2005). Homoploid hybrid species are useful for speciation studies because the parental (hybridizing) species are often extant, and provide a baseline against which the changes that accompany the speciation process are measured. In this issue, Brennan et al. (2012) quantify the degree of molecular and phenotypic divergence between a hybrid species and its progenitors. The results suggest that molecular and phenotypic divergence can each follow very different trajectories during the development of a new species, and raises the question of whether quantitative trait divergence is always higher than neutral marker divergence between a homoploid hybrid species and its parents.
Senecio squalidus, the homoploid hybrid species examined by Brennan et al. (2012) is one of the best-studied systems in the literature, and exemplifies the utility of homoploid hybrid species for questions about speciation. In the Senecio system, the history of the hybrid species is well documented (it originated roughly 300 years ago), and both the parental species (S. aethnensis and S. chrysanthemifolius) and original hybrid zone are still present in their native habitat (James and Abbott, 2005). The homoploid hybrid species is invasive, inhabiting urban environments such as vacant lots and roadsides in the British Isles (it was introduced by humans), whereas the parental species are found on the slopes of Mount Etna, Sicily. Despite these habitat differences, the hybrid species is either intermediate to, or is not divergent from, the parents for most of the 20 quantitative traits that were measured. In contrast, genetic differentiation between the homoploid hybrid species and the parental species (based on allozymes, microsatellites and indel markers) was fairly pronounced; FST was roughly 0.2 between the two parents, and between the hybrid species and the two parental species. Although the data were not amenable to a direct FST/QST comparison, principal coordinate analyses for both the phenotypic and molecular data showed much cleaner separation for the molecular markers.
As noted by Brennan et al. (2012), a higher level of molecular divergence compared with quantitative trait divergence is the opposite of expectations based on modes of evolution of the genetic underpinnings of quantitative traits compared with neutral molecular markers (McKay and Latta, 2002). In most studies, greater divergence is seen for quantitative traits that are exposed to natural selection than for neutral molecular markers, which (by definition), are not likely to be affected by adaptation to a new habitat. Why does the Senecio system defy expectations? One possible factor reducing the phenotypic divergence is the geographic isolation between the hybrid species and its parental species: the hybrid species is restricted to the British Isles and does not overlap with the range of its progenitors. Thus, the prominent phenotypic and ecological divergence seen in other homoploid hybrid species (for example, Rieseberg et al., 2003), where the hybrids and parental species are broadly sympatric, might not have been favored during the origin of S. squalidus. Conversely, the genetic distance between the hybrid species and the two progenitors may have been elevated by the extreme bottleneck and long period of relatively small population size experienced by S. squalidus upon its introduction into the British Isles, which would enhance the effects of genetic drift.
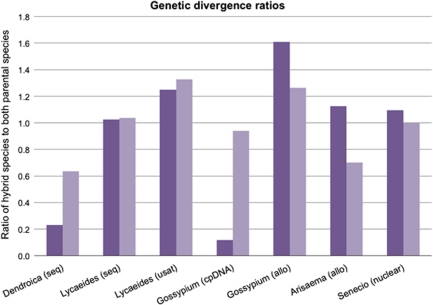
These findings are interesting, partially because they contradict some of our predictions about the rates of divergence of quantitative traits vs molecular markers, and partially because they highlight an absence of predictions about the genetic distance between a homoploid hybrid species and its direct progenitors (but see Chapman and Burke (2007) for a discussion about the genetic distance between the two parental species). What would be considered a normal level of genetic divergence between a homoploid hybrid species and its parents? The information required to answer this question does exist, as neutral molecular markers are often surveyed in studies of homoploid hybrid species, but the data are mostly used to determine the genetic structure of the parental and hybrid species, and genetic distances among the species are rarely reported. On the basis of a survey of reported genetic distances, it appears that it is not uncommon for the genetic distance between a homoploid hybrid species and one or both of its parental species to be equal to or greater than the genetic distance between the parental species, as indicated by a ratio of 1.0 or greater when the genetic distances are compared (Figure 1; Wendel et al., 1991; Maki and Murata, 2001; Gompert et al., 2006; Brelsford et al., 2011; Brennan et al., in press). In light of this, the similar levels of genetic distance between S. squalidus, S. aethnensis and S. chrysanthemifolius might be considered a fairly average status for a homoploid hybrid species and its parents. Obviously, more data are needed to evaluate this scenario—with only five hybrid species to compare (out of more than 30 known species in the literature), the sample size is not sufficient to draw broad conclusions. This paper represents a first step in producing a synthetic understanding of the patterns of, and reasons for, molecular and phenotypic divergence between homoploid hybrid species and their progenitors. Continuing this synthesis across a variety of different taxa is possible using both existing and new data, and it will be compelling to see what trends emerge.
Figure 1.
Ratios of the genetic distance between a homoploid hybrid species and its parental species to the genetic distance between parental species (for example, DH−P1/DP1−P2); the two columns for each genus represent the ratio for the hybrid species to each of the two parental species. Horizontal axis labels indicate the genus and the type of molecular marker in parentheses: seq, nuclear sequence; usat, microsatellite; cpDNA, chloroplast DNA; allo, allozyme; nuclear, combination of nuclear molecular markers. Taxa included are as follows (with the hybrid species listed first for each combination): Dendroica auduboni, D. nigrifrons, D. coronata; Lycaeides alpine form, L. idas, L. melissa; Gossypium bickii, G. sturtianum, G. australe/G. nelsonii; Arisaema ehimense, A. serratum, A. tosaense; Senecia squalidus, S. aethnensis, S. chrysanthemifolius. Note that allozyme data were reported as Nei's (Nei, 1972) genetic identity, and was converted to genetic distance using the standard formula (distance=−ln(identity)).
The author declares no conflict of interest.
References
- Brelsford A, Milá B, Irwin DE. Hybrid origin of Audubon's warbler. Mol Ecol. 2011;20:2380–2389. doi: 10.1111/j.1365-294X.2011.05055.x. [DOI] [PubMed] [Google Scholar]
- Brennan AC, Barker D, Hiscock SJ, Abbott RJ. Molecular genetic and quantitative trait divergence associated with recent homoploid hybrid speciation: a study of Senecio squalidus (Asteraceae) Heredity. 2012;108:87–95. doi: 10.1038/hdy.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman MA, Burke JM. Genetic divergence and hybrid speciation. Evolution. 2007;61:1773–1780. doi: 10.1111/j.1558-5646.2007.00134.x. [DOI] [PubMed] [Google Scholar]
- Gompert Z, Fordyce JA, Forister ML, Shapiro AM, Nice CC. Homoploid hybrid speciation in an extreme habitat. Science. 2006;314:1923–1925. doi: 10.1126/science.1135875. [DOI] [PubMed] [Google Scholar]
- Gross BL, Rieseberg LH. The ecological genetics of homoploid hybrid speciation. J Hered. 2005;96:241–252. doi: 10.1093/jhered/esi026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JK, Abbott RJ. Recent, allopatric, homoploid hybrid speciation: the origin of Seneciosqualidus (Asteraceae) in the British Isles from a hybrid zone on Mount Etna, Sicily. Evolution. 2005;59:2533–2547. [PubMed] [Google Scholar]
- Maki M, Murata J. Allozyme analysis of the hybrid origin of Arisaema ehimense (Araceae) Heredity. 2001;86:87–93. doi: 10.1046/j.1365-2540.2001.00813.x. [DOI] [PubMed] [Google Scholar]
- McKay JK, Latta RG. Adaptive population divergence: markers, QTL and traits. Trends Ecol Evol. 2002;17:285–291. [Google Scholar]
- Nei M. Genetic distance between populations. Am Naturalist. 1972;106:283–292. [Google Scholar]
- Rieseberg LH, Raymond O, Rosenthal DM, Lai Z, Livingstone K, Nakazato T, et al. Major ecological transitions in wild sunflowers facilitated by hybridization. Science. 2003;301:1211–1216. doi: 10.1126/science.1086949. [DOI] [PubMed] [Google Scholar]
- Wendel JF, Stewart JM, Rettig J. Molecular evidence for homoploid reticulate evolution among Australian species of Gossypium. Evolution. 1991;45:694–711. doi: 10.1111/j.1558-5646.1991.tb04339.x. [DOI] [PubMed] [Google Scholar]
Editor's suggested reading
- Kawakami T, trakosh SC, Zhen Y, Ungerer MC. Different scales of Ty1/copia-like retrotransposon proliferation in the genomes of three diploid hybrid sunflower species. Heredity. 2010;104:341–350. doi: 10.1038/hdy.2009.182. [DOI] [PubMed] [Google Scholar]
- Arnold ML, Ballerini ES, Brothers AN.2011Hybrid fitness, adaptation and evolutionary diversification: lessons learned from Louisiana Irises Hereditydoi: 10.1038/hdy.2011.65 [DOI] [PMC free article] [PubMed]
- Zakharov EV, Lobo NF, Nowak C, Hellmann JJ. Introgression as a likely cause of mtDNA paraphyly in two allopatric skippers (Lepidoptera: Hesperiidae) Heredity. 2009;102:590–599. doi: 10.1038/hdy.2009.26. [DOI] [PubMed] [Google Scholar]