Abstract
A major challenge for the immune system is to control pathogens and stressed cells, such as infected or tumors cells, while sparing healthy self-cells. To achieve this tolerance to self, immune cells must recognize and differentiate “self” versus “nonself” and “self” versus “altered self.” In the absence of self-tolerance, cells of the adaptive immune system attack healthy cells and cause autoimmune diseases such as lupus, psoriasis, and type I diabetes. Mechanisms at work to ensure tolerance in the innate immune system are still poorly understood. Natural killer cells are innate immune lymphocytes, which have the capacity to kill cellular targets and produce cytokines without prior specific sensitization. Because of these intrinsic effector capacities, tolerance mechanisms must exist to prevent autoreactivity. Herein, we will review the present knowledge on NK cell tolerance.
Natural killer (NK) cells destroy pathogens and stressed cells; tolerance mechanisms prevent autoreactivity. NK cell self-tolerance relies on both MHC-I-dependent and -independent mechanisms.
Natural killer (NK) cells are bone marrow-derived lymphocytes involved in the control of microbial infections, tumor surveillance, hematopoietic allograft rejection, and pregnancy (Vivier et al. 2008, 2011). NK cell activation is controlled by multiple activating and inhibitory surface receptors. Among inhibitory receptors, the inhibitory killer cell immunoglobulinlike receptors (KIRs) in humans and their functional homologs Ly49 in the mouse bind classical major histocompatibility complex (MHC) class Ia molecules, whereas CD94/NKG2A heterodimers recognize nonclassical MHC class Ib molecules (i.e., HLA-E in humans and Qa-1b in mice) (Natarajan et al. 2002). Engagement of these surface receptors bearing intracytoplasmic tyrosine-based inhibitory motifs (ITIMs) triggers inhibitory pathways by recruiting tyrosine phosphatases such as the protein tyrosine phosphatases Src homology 2 (SH2) domain-containing protein tyrosine phosphatase 1 (SHP-1), SHP-2, or both (Burshtyn et al. 1996; Olcese et al. 1996; Bruhns et al. 2000; Vivier et al. 2004).
Among activating receptors, NKp46 and NKG2D are expressed on both human and mouse NK cells, whereas Ly49H, Ly49D, and NK1.1 (NKRP1-C) are expressed only in the mouse (Lanier 2008). These receptors associate with immunoreceptor tyrosine-based activation motif-bearing (ITAM) polypeptides such as CD3ζ, KARAP/DAP12, DAP10, or FcRγ to transduce activating signals. On interaction with encountering cells, the balance between these different signals will dictate NK cell response (Lanier 2005). As such, infected cells or tumor cells expressing low levels of surface MHC-I and overexpressing ligands for activating receptors will be killed by NK cells. The tuning of NK cell function is therefore essential to allow the efficient killing of these “stressed” cells and prevent reaction against endogenous healthy cells.
Initial observations by Kärre and colleagues revealed that wild-type (WT) NK cells kill cells lacking MHC-I expression, whereas MHC-I-sufficient cells are spared (Kärre et al. 1986). An underlying requirement for this “missing-self-recognition” model is that each NK cell expresses at least one self-MHC-I-specific receptor to be able to detect the presence/absence of self (Valiante et al. 1997). The discovery of MHC-specific inhibitory receptors provided the first explanation for NK cell tolerance. However, in-depth analysis of the mature NK cell population in normal humans or mice revealed that a substantial fraction of cells do not express any MHC-I-specific inhibitory receptors but are still tolerant to self (Table 1) (Fernandez et al. 2005; Kim et al. 2005; Anfossi et al. 2006). Therefore, NK cell self-tolerance relies on both MHC-I-dependent and -independent mechanisms.
Table 1.
NK cell reactivity analysis
| Model | Description | NK cell reactivity |
|---|---|---|
| WT | In C57BL/6 mice (H-2b) the majority of NK cells expresses at least one self-MHC class I-specific receptor (Ly49C, Ly49I, or NKG2A) and will therefore be educated through the interaction with MHC-I molecules. However, 10%–15% of the NK cells lack the expression of all three self-MHC-specific receptors (Fernandez et al. 2005; Kim et al. 2005). | Normal responsiveness of NK cells expressing self-MHC-I-specific receptors. NK cells, which lack these receptors, are hyporesponsive. MHC-I-deficient target cells are rejected. |
| MHC class I-deficient | Various models of MHC-I deficiency have been generated in the mouse owing to mutations (1) in β2-microglobulin (β2m) that is required for normal MHC-Ia and -Ib expression; (2) in the transporter associated with antigen processing (TAP), which is essential for normal MHC-I-peptide loading in the endoplasmic reticulum; and (3) in the KbDb class I molecules (Bix et al. 1991; Hoglund et al. 1991; Liao et al. 1991; Dorfman et al. 1997; Fernandez et al. 2005; Kim et al. 2005). | NK cells are hyporesponsive. MHC-I-deficient targets are not rejected. |
| MHC-I mosaic mouse | In C57BL/6 mice with mosaic expression of an H2Dd transgene, some cells express the transgene and others do not (Johansson et al. 1997). | No rejection of transgene-negative bone marrow or lymphoma grafts. |
| Expression of a single MHC-I molecule | The single chain trimer (SCT) MHC-I molecule, consisting of antigenic peptide-linker-β2m-linker-H2Kb heavy chain as a single polypeptide, binds only the Ly49C NK receptor. In an SCT-Kb transgenic mouse with KbDb and β2m deficiency, only one MHC molecule (H2Kb) is expressed and therefore only Ly49C+ NK cells can recognize self MHC-I (Kim et al. 2005). | Ly49C+ NK cells are more responsive than Ly49C− NK cells. |
| WT and MHC-I-deficient chimeras | WT, β2m-KO or a 1:1 mix of WT and β2m-KO fetal liver (Wu and Raulet 1997; Joncker et al. 2010) or bone marrow cells (Sun and Lanier 2008a) were used to reconstitute irradiated WT or β2m-KO hosts. | WT NK cells, which developed in an MHC-I-deficient host, cannot reject MHC-I-deficient grafts. In mixed chimeras WT NK cells also become hyporesponsive and do not reject the grafts whether or not the host was MHC-I deficient. |
| Adoptive transfer of WT NK cells into an MHC-I-deficient recipient | WT C57BL/6 splenocytes were transferred into irradiated β2m KO or control WT hosts. NK cells were analyzed 4 or 7–10 d after transfer (Joncker et al. 2010). | WT NK cells become hyporesponsive 7–10 d after transfer into MHC-I-deficient hosts. |
| Adoptive transfer of MHC-I-deficient NK cells into a WT recipient | β2m KO splenocytes were transferred into WT or β2m-KO (control) hosts and NK cells were analyzed 7 d later (Elliott et al. 2010; Joncker et al. 2010). | MHC-I-deficient NK cells acquire normal responsiveness when transferred into WT hosts. |
| Coculture of WT and MHC-I-deficient NK cells | β2m-KO and WT splenocytes were mixed at various ratios and stimulated on anti-NK1.1 antibody coated plates for 8 h in vitro (Elliott et al. 2010). | β2m-KO NK cells retain their hyporesponsive phenotype. |
| Transgenic human KIR/HLA expression on an MHC-I-deficient background | In the KbDbKO-TgKIR/HLA model, mouse MHC-I molecules are absent but all NK cells express the human MHC-I molecule HLA-CW3. In addition, all NK cells uniformly express KIR2DL3. The entire NK cell population can thus be educated through the interaction between KIR2DL3 and its cognate ligand HLA-CW3 (Sola et al. 2009). | Normal responsiveness |
| Transgenic expression of an NKG2D ligand | Constitutive or inducible Rae-1e transgene expression (ubiquitously or in the squamous epithelium) were obtained in various mouse models (Oppenheim et al. 2005). | Hyporesponsive |
| Transgenic expression of Ly49H ligand | Transgenic C57BL/6 mouse (m157-Tg) ubiquitously express m157, which is the murine cytomegalovirus-encoded ligand for the NK cell activating receptor Ly49H (Tripathy et al. 2008). m157 expressing chimeric mice were also generated by retroviral transduction of bone marrow stem cells with m157 (Sun and Lanier 2008b). | Ly49H+ NK cells become hyporesponsive. M157-Tg mice are more sensitive to MCMV and m157-Tg bone marrow (BM) is not rejected, whereas they are still able to reject MHC-I-deficient BM. |
| Adoptive transfer of WT NK cells into m157-Tg recipient | Mature WT spleen cells were transferred into m157-Tg mice and examined 9 d later (Tripathy et al. 2008). | Ly49H+ donor (WT) NK cells become hyporesponsive. |
| NKG2D-deficient mouse | Mice deficient for the activating receptor NKG2D were generated by targeting the Klrk1 locus (Zafirova et al. 2009). | Klrk1-deficient mice show enhanced NK cell-mediated resistance to MCMV infection and a general hyperresponsive phenotype. |
| MCMV infection of mixed BM chimeras of WT and MHC-I-deficient donors | Irradiated WT mice were reconstituted with a mix of WT and β2m-KO BM cells. 10 wk after reconstitution mice were infected with mouse cytomegalovirus (MCMV) (Sun and Lanier 2008a). | β2m-KO cells gradually declined over time in chimeric WT: β2m KO mice. β2m-KO cells are rapidly rejected by WT in chimeric mice following MCMV infection. |
| Normal individuals | In humans, “missing-self-recognition” is ensured by KIR inhibitory receptors recognizing self-MHC-I molecules. However, a fraction of NK cells lacks inhibitory KIR for self (Anfossi et al. 2006; Miller et al. 2007). | Normal responsiveness of NK cells expressing self-MHC-I-specific receptors.; NK cells which lack these receptors are hyporesponsive. |
| MHC class I-deficient patients | Patients with mutation in the transporters associated with antigen processing (TAP) lack MHC-1 expression (Zimmer et al. 1998; Furukawa et al. 1999; Vitale et al. 2002). | NK cells are hyporesponsive. |
EDUCATION OF NK CELLS BY MHC CLASS I
Besides its importance in the control of NK cell reactivity during interactions with potential target cells, the detection of self MHC-I through MHC-I-specific receptors was also shown to educate NK cells to acquire their full effector function (Fernandez et al. 2005; Kim et al. 2005). NK cells encountering educating cells expressing MHC-I receive a signal required to set the optimal NK cell reactivity threshold. Whether this MHC-I signal “arms”/“licenses” NK cells by increasing their reactivity, or rescues cells that would have been “disarmed”/rendered anergic by an overt stimulation is still under debate (Raulet 2006; Yokoyama and Kim 2006b; Brodin and Hoglund 2008). Because NK cells vary in the affinity and number of self-MHC-I inhibitory receptors they express, the strength of the educating signal varies from cell to cell (Brodin et al. 2008). It was shown that following education, NK cell reactivity increases with the number of different self-MHC-I-specific inhibitory receptors expressed (Brodin et al. 2009; Joncker et al. 2009). However, this amplified reactivity is subsequently balanced by the interaction of these inhibitory receptors with MHC-I molecules expressed on self-cells. As such, only NK cells that will be strongly inhibited by self MHC-I can gain maximal effector functions for subsequent interactions with target cells.
In humans and mice, several genes and multiple alleles encoding MHC-I-specific receptors have been identified (Parham 2005). The genes encoding these receptors and their MHC-I ligands are present on different chromosomes and segregate separately, prompting efforts to unveil the mechanisms by which NK cells are equipped with the set of inhibitory receptors that allow the detection of self-MHC-I molecules (Raulet et al. 2001; Yokoyama and Kim 2006a). One possibility would be that inhibitory receptors are expressed sequentially during NK cell development until the cognate self-MHC-I-specific receptor is acquired. Interactions between self MHC-I and its receptor would allow final NK cell maturation and educate NK cells to acquire full effector functions. The diversity of the inhibitory receptor repertoire among NK cells would be consistent with the random expression of the receptors before the acquisition of the self-MHC-I-specific receptor. The phenotypic heterogeneity of the NK cell pool would thus be the consequence of an ongoing maturation process: NK cells lacking inhibitory receptor would be the most immature ones as compared with those expressing self-MHC-I-specific inhibitory receptors. Transfer of sorted NK cells expressing only one type of inhibitory receptor into WT hosts revealed that these cells could indeed mature further to acquire other inhibitory receptors (Dorfman and Raulet 1998).
Multiple mouse models in which self-MHC expression was modified have highlighted the robustness of MHC-I-dependent tolerance. For instance, in KbDbKO-TgKIR/HLA mice on a C57BL/6 background, mouse MHC-I molecules are absent and replaced by the human MHC-I molecule, HLA-Cw3. In addition, all NK cells uniformly express the cognate human MHC-I-specific inhibitory receptor, KIR2DL3. In vivo rejection experiments showed that KbDbKO-TgKIR/HLA mice eliminate both KbDbKO and C57BL/6 splenocytes, whereas HLA-expressing cells are recognized as self and tolerated (Table 1) (Sola et al. 2009). This transgenic model revealed that NK cells could be reprogrammed to missing-self-recognition and thus adapt to an environment with a different “self.” The adaptability of NK cell MHC-I-dependent tolerance was also revealed in models with a heterogeneous self MHC-I expression. In the mosaic C57BL/6 mouse model in which some cells express the H2Dd transgene and others do not, there is no autoreactivity against cells that lack transgene expression (Johansson et al. 1997) (Table 1). Similarly, results from mixed WT and β2m-deficient BM chimeras showed that wild-type NK cells are hyporesponsive and tolerant to MHC class I-deficient cells in the chimeras (Table 1) (Wu and Raulet 1997).
MECHANISMS OF MHC-I-INDEPENDENT TOLERANCE
According to the missing-self hypothesis, NK cells that cannot be kept in check by MHC-I recognition will become activated. However, in normal humans and mice, a sizable fraction of NK cells achieves self-tolerance without expressing self-MHC-I-specific inhibitory receptors (Fernandez et al. 2005; Kim et al. 2005; Anfossi et al. 2006). This situation is exacerbated in mice or humans lacking MHC-I molecules (Zimmer et al. 1998). In these individuals, NK cells develop normally but fail to exert detectable autoimmunity or to kill MHC-I-deficient cells in vivo or in vitro. The fact that primary cells isolated from MHC-I-deficient mice were killed by WT NK cells supported the existence of an active mechanism of NK cell tolerance preventing the recognition of MHC-I-deficient cells as missing-self targets in MHC-I-deficient individuals (Bix et al. 1991; Liao et al. 1991).
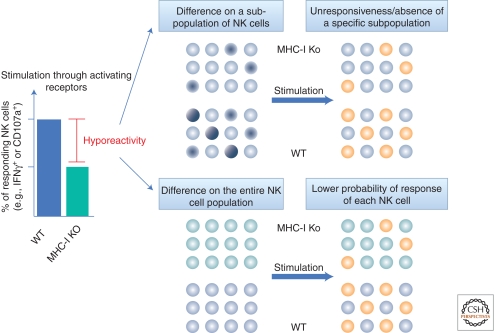
A first explanation for this MHC-I-independent tolerance was provided by the comparison of NK cell reactivity between WT and MHC-I-deficient individuals. NK cells raised in an MHC-I-deficient environment do not kill tumor targets as efficiently as WT individuals do. Stimulation with a cell-free system using activating receptor cross-linking with plate-bound antibodies revealed a reduction in the frequency of intracellular IFN-γ-positive or CD107a-expressing cells (a marker present in lytic granules that is surface exposed when NK cells degranulate) among the MHC-I-deficient NK cell population as compared with the WT NK cell population (Fernandez et al. 2005; Kim et al. 2005). This impaired response was also observed in WT individuals when comparing the fraction of NK cells lacking self-MHC-I-specific inhibitory receptors with educated NK cells expressing self-MHC-I-specific inhibitory receptors (Fernandez et al. 2005; Kim et al. 2005; Anfossi et al. 2006). Calcium release assays following activating receptor cross-linking showed that the very early stages of the activating signaling pathway are affected (Guia et al. 2011). In contrast, stimuli that bypass the proximal activation signal can equally stimulate educated and hyporesponsive NK cells, suggesting that the defect is upstream of the signals induced by phorbol myristate acetate (PMA) and ionomycin. In all the aforementioned read outs, the term hyporesponsive has been used to describe a decrease in the percentage of responding NK cells among the entire NK cell population. Two scenarios could lead to the hyporesponsive phenotype of uneducated NK cells: (1) either a specific responding subpopulation is absent or rendered unresponsive in the absence of MHC-I education or (2) the activating threshold of each NK cell is modified so that the probability of response is lower in uneducated as compared with MHC-I-educated NK cells (Fig. 1).
Figure 1.
Models of NK cell hyporeactivity. NK cells that are unable to interact with self MHC-I molecules (green bar), either because they were raised in an MHC-I-deficient environment or because they lack MHC-I-specific inhibitory receptors, are hyporesponsive to activating stimulations (graph) when compared with MHC-I-educated NK cells (blue bar). This diminished percentage of responding NK cells (orange circles) among the entire population in the absence of prior MHC-I-driven education could result from (top panel) the absence or the complete unresponsiveness of a given subpopulation (dark blue circles) or (bottom panel) from a lower probability of response of each NK cell.
The first hypothesis has been carefully studied and some modifications of the inhibitory Ly49 repertoire as well as changes in the percentage of NK cells expressing some specific maturation markers have been reported (Guia et al. 2011). However, none of these observations could entirely account for the hyporesponsive phenotype of NK cells from MHC-I-deficient individuals. This first possibility also implies that the small fraction of responding NK cells in the MHC-I-deficient population would have gained function via MHC-I-independent education signals.
In the second hypothesis, changes occur in the entire population to modulate the responsiveness of each NK cell. Yet, initial observations showed that the hyporesponsiveness was not correlated with changes in the surface expression level of activating receptors (Liao et al. 1991; Zimmer et al. 1998; Fernandez et al. 2005; Kim et al. 2005; Anfossi et al. 2006), and recent microarray analysis did not reveal the presence of any major transcriptional modification between educated and hyporesponsive NK cells (Guia et al. 2011). However, analysis of the receptor confinement at the plasma membrane of NK cells has offered new insight into the molecular mechanisms governing NK cell responsiveness. Fluorescence correlation spectroscopy experiments were recently conducted to monitor the confinement of activating and inhibitory receptors on MHC-I-educated and hyporesponsive primary NK cells. Using this dynamic nanoscopic approach, it was found that both activating and inhibitory receptors are confined in actin-dependent meshworks at the membrane of hyporesponsive NK cells. In contrast, in responsive NK cells, which were educated in an MHC-I-sufficient environment, activating receptors are not associated with these meshwork structures, and instead localize in membrane nanodomains. In contrast, the confinement of inhibitory receptors at the plasma membrane is unchanged between educated and hyporesponsive NK cells. These findings indicate that the confinement of activating receptors at the plasma membrane is associated with the control of NK cell reactivity and is therefore involved in the establishment/maintenance of self-tolerance (Fig. 2). The mechanisms by which MHC-I recognition via MHC-I-specific inhibitory receptors leads to the relocalization of activating receptors remain unknown. Nevertheless, NK cell education has been shown to require intact ITIMs, whereas SHP-1 was dispensable (Kim et al. 2005). As MHC-I-specific inhibitory receptors can recruit both SHP-1 and SHP-2 (Olcese et al. 1996), these results are still consistent with a possible involvement of protein tyrosine phosphatases in NK cell education. According to this scenario, the tyrosine phosphatase activity linked to ITIMs (SHP-1 and/or SHP-2) would act on the guanine exchange factor Vav-1, leading in turn to an alteration in the activity of the Rho GTPase RAC-1, which is known to promote actin polymerization (Riteau et al. 2003). This hypothesis is further supported by a recent study showing that inhibitory signaling blocks activating receptor clustering via cytoskeleton rearrangement in human NK cell lines (Abeyweera et al. 2011).
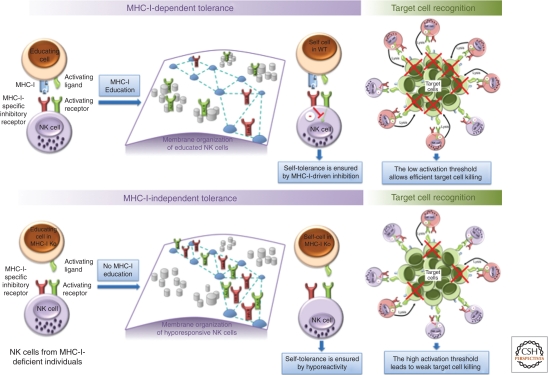
Figure 2.
Activating receptor confinement at the plasma membrane controls NK cell reactivity threshold to ensure self-tolerance. (Top panel) NK cells that are able to recognize self MHC-I will be educated and activating receptors (Act R, in green) will localize in nanodomains (gray cylinders), whereas inhibitory receptors (Inh R, in red) will be confined in actin-dependent meshwork structures (blue dashed lines) at the plasma membrane. If these educated NK cells encounter self-cells, MHC-I recognition by self-MHC-I-specific inhibitory receptors prevents NK cell activation and ensures tolerance. In the presence of target cells (green cells, expressing low amounts of MHC-I molecules and high amounts of activating ligands) the confinement of activating receptors in nanodomains prone to signal transduction favors NK cell activation (pink cells) and optimize target cell killing (green cell with red cross). In WT cells lacking MHC-I-specific inhibitory receptors or in NK cells from MHC-I-deficient individuals (bottom panel), no MHC-I education operates and both activating and inhibitory receptors will localize in actin-dependent meshwork structures at the plasma membrane of these uneducated NK cells. This organization will impede NK cell activating signaling pathway, thus increasing NK cell activation threshold. This hyporeactivity avoids NK cell activation by self-cells but also decreases target cell killing efficiency at steady state.
The tuning of NK cell activation thresholds has been so far mostly considered as the sole consequence of the inhibitory input received during MHC-I-driven education, but mice lacking activating receptors or engineered to express ligands for one particular activating receptor have highlighted the existence of MHC-independent education mechanisms. Zafirova and colleagues (Zafirova et al. 2009) showed that in the absence of the activating receptor, NKG2D, NK cells become hyperresponsive and NKG2D-deficient mice show enhanced resistance to mouse cytomegalovirus (MCMV) infection (Table 1). In contrast, the continuous engagement of the activating receptor Ly49H through the expression of its viral m157 ligand during development renders Ly49H+ NK cells hyporesponsive to activating stimulations and therefore tolerant to self-cells expressing the m157 activating ligand (Table 1) (Sun and Lanier 2008b; Tripathy et al. 2008). Similarly, sustained engagement of MICA, one of the ligands for NKG2D, leads to NKG2D silencing and general NK cell hyporesponsiveness (Table 1) (Oppenheim et al. 2005). Collectively, these data show that prolonged exposure of NK cells to one particular activating ligand not only impairs the cognate activating receptor but also affects several other activating receptors even if they function with other signaling adaptors (Coudert et al. 2008). Of note, the induction of NK cell tolerance via self-specific activating receptors was independent of self-specific inhibitory receptors (Tripathy et al. 2008). Analysis of the membrane organization of these hyporesponsive NK cells would be interesting to find out whether activating receptors and inhibitory receptors use the same kind of mechanisms to adjust the overall NK cell reactivity.
MAINTAINING NK CELL TOLERANCE
Recent experiments have revealed that mature NK cells can be reset and become tolerant if transferred into a new microenvironment with a different “self.” Indeed, it has been shown that mature WT NK cells transferred into MHC-I-deficient hosts do not become autoreactive but instead acquire a state of hyporesponsiveness and thus become tolerant (Joncker et al. 2010). Likewise, WT Ly49H+ NK cells adoptively transferred into m157-transgenic mice (ubiquitously expressing the virally encoded Ly49H ligand, m157) become hyporesponsive (Tripathy et al. 2008). Reciprocally, MHC-I-deficient NK cells transferred into WT hosts acquire an increased reactivity. Specifically, cells expressing inhibitory receptors for the host MHC-I molecules become educated (Elliott et al. 2010; Joncker et al. 2010). This gain-of-function will be balanced by inhibitory signals to avoid autoreactivity in the host. How NK cell resetting operates and the cells/factors involved are largely unknown but the in vitro coculture of WT and MHC-I-deficient splenocytes for a few hours was not sufficient to modify NK cell reactivity. In contrast, in mixed WT:MHC-I-deficient BM chimeras, MHC-I-deficient NK cells do not acquire responsiveness, and WT NK cells instead become hyporesponsive and tolerant to self-cells lacking MHC-I (Wu and Raulet 1997; Joncker et al. 2010). Therefore, when inconsistent education signals are encountered during maturation, mechanisms inducing hyporeactivity would be favored.
It has also been suggested that the expression of MHC-I on the NK cell itself could act in cis and directly educate NK cells (Doucey et al. 2004; Chalifour et al. 2009), but this cis-engagement is not a dominant element in promoting NK cell competence. Indeed, NK cells expressing MHC-I can become hyporeactive when they develop with MHC-I-deficient cells or if transferred into an MHC-I-deficient host (Wu and Raulet 1997; Elliott et al. 2010; Joncker et al. 2010).
For T and B cells, tolerance is established by key selective events during maturation, and the reactivity threshold is set in the mature population. The maintenance of T and B cell self-tolerance in the mature population at steady state is facilitated by this central tolerance mechanism and maintained in the periphery by regulatory T cells, by the capacity to eliminate potentially autoreactive cells by clonal deletion, or by converting them into an anergic state. The recent experiments using mature NK cell transfer showed that besides the induction of hyporesponsiveness that resembles the anergic state of T cells, mature NK cells can also modify their activation threshold to acquire greater reactivity. These observations underline the plasticity of the NK cell compartment and suggest that education occurs constantly during the lifetime of the NK cell. NK cells can thus rapidly switch from MHC-I-dependent to MHC-I-independent mechanisms and vice versa to maximize the likelihood of ensuring self-tolerance in any conditions.
BREAKING NK CELL TOLERANCE
Recent studies suggest that NK cells can promote type 1 diabetes (Poirot et al. 2004; Gur et al. 2010) but, to our knowledge, shown cases of true NK cell autoimmunity have not been reported either in humans or in mice (Schleinitz et al. 2010). Despite correlations between changes in the number and function of NK cells and clinical manifestations of autoimmune diseases such as systemic lupus erythematosus (SLE), NK cells have not been shown to mediate direct cell or tissue damage in these diseases. It remains possible that NK cells participate to autoimmune diseases via indirect mechanisms by promoting or preventing the exposure of autoantigens or by targeting the adaptive autoimmune response (e.g., DC) (Schleinitz et al. 2010). Yet, the fact that mature NK cells can swiftly adapt to a new environment, the complex combination of signals required to trigger full NK cell activation (e.g., overexpression of activating ligands coupled to a down-modulation of inhibitory signals), and the small size of the NK cell population might also be among the main reasons explaining why NK cell direct autoreactivity has not been detected.
However, there is a noticeable exception in the mouse to the lack of true cases of NK cell-mediated autoimmunity. Sun and Lanier have reported that MCMV infection of mixed WT:MHC-I-deficient chimeric mice leads to an NK cell-mediated rejection of host MHC-I-deficient hematopoietic cells (Table 1) (Sun and Lanier 2008a). In these mixed background conditions, acute inflammation driven by infection could thus break preestablished tolerance. Along these lines, a mechanism of tolerance based on the generation of a fraction of hyporesponsive cells could be detrimental if these cells were irreversibly committed to this low-responder phenotype. However, in WT mice infected with MCMV, the hyporesponsive NK cell fraction is converted to a higher state of responsiveness and thus plays a major role during the protective response (Sun and Lanier 2008a). Likewise, infection of MHC-I-deficient mice showed protection similar to that of WT mice (Tay et al. 1995). The hyporesponsive phenotype of NK cells that are unable to interact with MHC-I molecules must therefore contribute to the maintenance of self-tolerance at steady state, but this phenotype can be overcome to provide protective immunity under inflammatory conditions.
CONCLUDING REMARKS
The concept of NK cell tolerance emerged with the study of MHC-I-deficient humans and mice, and the discovery that a fraction of NK cells is unable to sense self-MHC-I molecules in WT conditions and should, according to the missing-self hypothesis, be autoreactive. However, the autoreactive potential of NK cells has never been proven in the absence of pathogen (Sun and Lanier 2008a). All experimental approaches have instead revealed the high degree of plasticity of the NK cell compartment and rather support a model of “adaptive” tolerance based on a constant sensing of the NK cell microenvironment (Table 1). This adaptability obviously raises a lot of questions as to the ability of NK cells to attack cancer cells without being reset by the tumor environment. The duration of the education provided by host cells and the time during which NK cells are exposed to altered-self or nonself cells seem essential for the outcome of the response. If MHC-I-deficient cells develop together with WT cells in chimeras for several weeks, they are tolerated. Conversely, MHC-I-deficient cells are rejected 24 h after in vivo injection into WT mice (Wu and Raulet 1997; Sola et al. 2009; Joncker et al. 2010). Targeting the right kinetics could thus be crucial for the development of new treatments to increase NK cell reactivity. Along these lines, in vivo infusion of blocking antibodies against inhibitory receptors in different mouse models was shown to increase the elimination of MHC-I+ syngenic tumors without affecting NK cell education (Koh et al. 2001, 2002, 2003; Sola et al. 2009).
The central role of MHC-I recognition to ensure NK cell education and tolerance has been widely accepted but the relevance of the “missing-self” recognition in physiological conditions still needs to be addressed. In addition, one could challenge the current concept by asking whether the hyporesponsiveness of uneducated NK cells is truly relevant in pathophysiological conditions. Indeed, according to the functional read outs used to assess NK cell function ex vivo, a significant proportion of responding NK cells is still present in the NK cell population described as hyporesponsive. These cells could be potentially autoreactive implying that other mechanisms exist to prevent their uncontrolled activation.
The fact that NK cells were initially described by their “natural killer” phenotype suggested that NK cells bore a high intrinsic autoreactive potential and the discovery of MHC-I inhibition and education has provided an explanation for the absence of autoreactivity of these cytotoxic cells. But the concept of “natural” killer has now been challenged by multiple data sets indicating that environmental factors (e.g., cytokines) and accessory cells are required to fully activate NK cell function. These extrinsic factors could be key regulators to avoid the inappropriate activation of NK cells. Further studies are thus required to decipher the diversity of the mechanisms regulating NK cell reactivity and to fully understand how NK cell tolerance is achieved in physiological conditions.
ACKNOWLEDGMENTS
The authors thank all the members of the laboratory for their help and valuable discussions. B.N.J. is supported by a fellowship from the Axa Research Fund. E.V. is supported by grants from the European Research Council (ERC Advanced grants), Agence Nationale de la Recherche (ANR), Ligue Nationale contre le Cancer (Equipe labellisée “La Ligue”), and institutional grants from Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, and Université de la Méditerranée to the Centre d’Immunologie de Marseille-Luminy. E.V. is a scholar of the Institut Universitaire de France.
CONFLICT OF INTEREST DISCLOSURE
E.V. is cofounder and shareholder of Innate-Pharma.
Footnotes
Editors: Diane Mathis and Alexander Y. Rudensky
Additional Perspectives on Immune Tolerance available at www.cshperspectives.org
REFERENCES
- Abeyweera TP, Merino E, Huse M 2011. Inhibitory signaling blocks activating receptor clustering and induces cytoskeletal retraction in natural killer cells. J Cell Biol 192: 675–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, et al. 2006. Human NK cell education by inhibitory receptors for MHC class I. Immunity 25: 331–342 [DOI] [PubMed] [Google Scholar]
- Bix M, Liao N-S, Zijlstra M, Loring J, Jaenisch R, Raulet D 1991. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature 349: 329–331 [DOI] [PubMed] [Google Scholar]
- Brodin P, Hoglund P 2008. Beyond licensing and disarming: A quantitative view on NK-cell education. Eur J Immunol 38: 2934–2937 [DOI] [PubMed] [Google Scholar]
- Brodin P, Lakshmikanth T, Johansson S, Karre K, Hoglund P 2008. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood 113: 2434–2441 [DOI] [PubMed] [Google Scholar]
- Brodin P, Karre K, Hoglund P 2009. NK cell education: Not an on-off switch but a tunable rheostat. Trends Immunol 30: 143–149 [DOI] [PubMed] [Google Scholar]
- Bruhns P, Vely F, Malbec O, Fridman WH, Vivier E, Daeron M 2000. Molecular basis of the recruitment of the SH2 domain-containing Inositol 5-phosphatases SHIP1 and SHIP2 by Fcγ RIIB. J Biol Chem 275: 37357–37364 [DOI] [PubMed] [Google Scholar]
- Burshtyn DN, Scharenberg AM, Wagtmann N, Rajagopalan S, Berrada K, Yi T, Kinet J-P, Long EO 1996. Recruitment of tyrosine phosphatase HCP by the killer cell inhibitory receptor. Immunity 4: 77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalifour A, Scarpellino L, Back J, Brodin P, Devevre E, Gros F, Levy F, Leclercq G, Hoglund P, Beermann F, et al. 2009. A Role for cis interaction between the inhibitory Ly49A receptor and MHC class I for natural killer cell education. Immunity 30: 337–347 [DOI] [PubMed] [Google Scholar]
- Coudert JD, Scarpellino L, Gros F, Vivier E, Held W 2008. Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood 111: 3571–3578 [DOI] [PubMed] [Google Scholar]
- Dorfman JR, Raulet DH 1998. Acquisition of Ly49 receptor expression by developing natural killer cells. J Exp Med 187: 609–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman JR, Zerrahn J, Coles MC, Raulet DH 1997. The basis for self-tolerance of natural killer cells in beta2-microglobulin- and TAP-1- mice. J Immunol 159: 5219–5225 [PubMed] [Google Scholar]
- Doucey MA, Scarpellino L, Zimmer J, Guillaume P, Luescher IF, Bron C, Held W 2004. Cis association of Ly49A with MHC class I restricts natural killer cell inhibition. Nat Immunol 5: 328–336 [DOI] [PubMed] [Google Scholar]
- Elliott JM, Wahle JA, Yokoyama WM 2010. MHC class I-deficient natural killer cells acquire a licensed phenotype after transfer into an MHC class I-sufficient environment. J Exp Med 207: 2073–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH 2005. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood 105: 4416–4423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa H, Yabe T, Watanabe K, Miyamoto R, Miki A, Akaza T, Tadokoro K, Tohma S, Inoue T, Yamamoto K, et al. 1999. Tolerance of NK and LAK activity for HLA class I-deficient targets in a TAP1-deficient patient (bare lymphocyte syndrome type I). Hum Immunol 60: 32–40 [DOI] [PubMed] [Google Scholar]
- Guia S, Jaeger BN, Piatek S, Mailfert S, Trombik T, Fenis A, Chevrier N, Walzer T, Kerdiles YM, Marguet D, et al. 2011. Confinement of activating receptors at the plasma membrane controls natural killer cell tolerance. Sci Signal 4: ra21. [DOI] [PubMed] [Google Scholar]
- Gur C, Porgador A, Elboim M, Gazit R, Mizrahi S, Stern-Ginossar N, Achdout H, Ghadially H, Dor Y, Nir T, et al. 2010. The activating receptor NKp46 is essential for the development of type 1 diabetes. Nat Immunol 11: 121–128 [DOI] [PubMed] [Google Scholar]
- Hoglund P, Ohlen C, Carbone E, Franksson L, Ljunggren HG, Latour A, Koller B, Karre K 1991. Recognition of beta 2-microglobulin-negative (beta 2m-) T-cell blasts by natural killer cells from normal but not from beta 2m- mice: Nonresponsiveness controlled by beta 2m- bone marrow in chimeric mice. Proc Natl Acad Sci 88: 10332–10336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson MH, Bieberich C, Jay G, Karre K, Hoglund P 1997. Natural killer cell tolerance in mice with mosaic expression of major histocompatibility complex class I transgene. J Exp Med 186: 353–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joncker NT, Fernandez NC, Treiner E, Vivier E, Raulet DH 2009. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: The rheostat model. J Immunol 182: 4572–4580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joncker NT, Shifrin N, Delebecque F, Raulet DH 2010. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J Exp Med 207: 2065–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärre K, Ljunggren HG, Piontek G, Kiessling R 1986. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defense strategy. Nature 319: 675–678 [DOI] [PubMed] [Google Scholar]
- Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, et al. 2005. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature 436: 709–713 [DOI] [PubMed] [Google Scholar]
- Koh CY, Blazar BR, George T, Welniak LA, Capitini CM, Raziuddin A, Murphy WJ, Bennett M 2001. Augmentation of antitumor effects by NK cell inhibitory receptor blockade in vitro and in vivo. Blood 97: 3132–3137 [DOI] [PubMed] [Google Scholar]
- Koh CY, Raziuddin A, Welniak LA, Blazar BR, Bennett M, Murphy WJ 2002. NK inhibitory-receptor blockade for purging of leukemia: Effects on hematopoietic reconstitution. Biol Blood Marrow Tr 8: 17–25 [DOI] [PubMed] [Google Scholar]
- Koh CY, Ortaldo JR, Blazar BR, Bennett M, Murphy WJ 2003. NK-cell purging of leukemia: Superior antitumor effects of NK cells H2 allogeneic to the tumor and augmentation with inhibitory receptor blockade. Blood 102: 4067–4075 [DOI] [PubMed] [Google Scholar]
- Lanier LL 2005. NK cell recognition. Annu Rev Immunol 23: 225–274 [DOI] [PubMed] [Google Scholar]
- Lannier LL 2008. Up on the tightrope: Natural killer cell activation and inhibition. Nat Immunol 9: 495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao N-S, Bix M, Zilstra M, Jaenish R, Raulet D 1991. MHC class I deficiency: Susceptibility to natural killer (NK) cells and impaired NK activity. Science 253: 199–202 [DOI] [PubMed] [Google Scholar]
- Miller JS, Cooley S, Parham P, Farag SS, Verneris MR, McQueen KL, Guethlein LA, Trachtenberg EA, Haagenson M, Horowitz MM, et al. 2007. Missing KIR-ligands is associated with less relapse and increased graft versus host disease (GVHD) following unrelated donor allogeneic HCT. Blood 109: 5058–5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan K, Dimasi N, Wang J, Mariuzza RA, Margulies DH 2002. Structure and function of natural killer cell receptors: Multiple molecular solutions to self, nonself discrimination. Annu Rev Immunol 20: 853–885 [DOI] [PubMed] [Google Scholar]
- Olcese L, Lang P, Vély F, Cambiaggi A, Marguet D, Blery M, Hippen KL, Biassoni R, Moretta A, Moretta L, et al. 1996. Human and mouse killer-cell inhibitory receptors recruit PTP1C and PTP1D protein tyrosine phosphatases. J Immunol 156: 4531–4534 [PubMed] [Google Scholar]
- Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, Girardi M, Hayday AC 2005. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol 6: 928–937 [DOI] [PubMed] [Google Scholar]
- Parham P 2005. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol 5: 201–214 [DOI] [PubMed] [Google Scholar]
- Poirot L, Benoist C, Mathis D 2004. Natural killer cells distinguish innocuous and destructive forms of pancreatic islet autoimmunity. Proc Natl Acad Sci 101: 8102–8107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet DH 2006. Missing self recognition and self tolerance of natural killer (NK) cells. Semin Immunol 18: 145–150 [DOI] [PubMed] [Google Scholar]
- Raulet DH, Vance RE, McMahon CW 2001. Regulation of the natural killer cell receptor repertoire. Annu Rev Immunol 19: 291–330 [DOI] [PubMed] [Google Scholar]
- Riteau B, Barber DF, Long EO 2003. Vav1 phosphorylation is induced by β2 integrin engagement on natural killer cells upstream of actin cytoskeleton and lipid raft reorganization. J Exp Med 198: 469–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleinitz N, Vely F, Harle JR, Vivier E 2010. Natural killer cells in human autoimmune diseases. Immunology 131: 451–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola C, Andre P, Lemmers C, Fuseri N, Bonnafous C, Blery M, Wagtmann NR, Romagne F, Vivier E, Ugolini S 2009. Genetic and antibody-mediated reprogramming of natural killer cell missing-self recognition in vivo. Proc Natl Acad Sci 106: 12879–12884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Lanier LL 2008a. Cutting edge: Viral infection breaks NK cell tolerance to “missing self”. J Immunol 181: 7453–7457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Lanier LL 2008b. Tolerance of NK cells encountering their viral ligand during development. J Exp Med 205: 1819–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay CH, Welsh RM, Brutkiewicz RR 1995. NK cell response to viral infections in β2-microglobulin-deficient mice. J Immunol 154: 780–789 [PubMed] [Google Scholar]
- Tripathy SK, Keyel PA, Yang L, Pingel JT, Cheng TP, Schneeberger A, Yokoyama WM 2008. Continuous engagement of a self-specific activation receptor induces NK cell tolerance. J Exp Med 205: 1829–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiante NM, Lienert K, Shilling HG, Smits BJ, Parham P 1997. Killer cell receptors: Keeping pace with MHC class I evolution. Immunol Rev 155: 155–164 [DOI] [PubMed] [Google Scholar]
- Vitale M, Zimmer J, Castriconi R, Hanau D, Donato L, Bottino C, Moretta L, de la Salle H, Moretta A 2002. Analysis of natural killer cells in TAP2-deficient patients: Expression of functional triggering receptors and evidence for the existence of inhibitory receptor(s) that prevent lysis of normal autologous cells. Blood 99: 1723–1729 [DOI] [PubMed] [Google Scholar]
- Vivier E, Nunes JA, Vely F 2004. Natural killer cell signaling pathways. Science 306: 1517–1519 [DOI] [PubMed] [Google Scholar]
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S 2008. Functions of natural killer cells. Nat Immunol 9: 503–510 [DOI] [PubMed] [Google Scholar]
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S 2011. Innate or adaptive immunity? The example of natural killer cells. Science 331: 44–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M-F, Raulet DH 1997. Class I-deficient hemopoietic cells and nonhemopoietic cells dominantly induce unresponsiveness of natural killer cells to class I-deficient bone marrow cell grafts. J Immunol 158: 1628–1633 [PubMed] [Google Scholar]
- Yokoyama WM, Kim S 2006a. How do natural killer cells find self to achieve tolerance? Immunity 24: 249–257 [DOI] [PubMed] [Google Scholar]
- Yokoyama WM, Kim S 2006b. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol Rev 214: 143–154 [DOI] [PubMed] [Google Scholar]
- Zafirova B, Mandaric S, Antulov R, Krmpotic A, Jonsson H, Yokoyama WM, Jonjic S, Polic B 2009. Altered NK cell development and enhanced NK cell-mediated resistance to mouse cytomegalovirus in NKG2D-deficient mice. Immunity 31: 270–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer J, Donato L, Hanau D, Cazenave JP, Tongio MM, Moretta A, Salle H 1998. Activity and phenotype of natural killer cells in peptide transporter (TAP)-deficient patients (type I bare lymphocyte syndrome). J Exp Med 187: 117–122 [DOI] [PMC free article] [PubMed] [Google Scholar]