Abstract
The discovery of the genetic causes of syndromic autism spectrum disorders and intellectual disabilities has greatly informed our understanding of the molecular pathways critical for normal synaptic function. The top-down approaches using human phenotypes and genetics helped identify causative genes and uncovered the broad spectrum of neuropsychiatric features that can result from various mutations in the same gene. Importantly, the human studies unveiled the exquisite sensitivity of cognitive function to precise levels of many diverse proteins. Bottom-up approaches applying molecular, biochemical, and neurophysiological studies to genetic models of these disorders revealed unsuspected pathogenic mechanisms and identified potential therapeutic targets. Moreover, studies in model organisms showed that symptoms of these devastating disorders can be reversed, which brings hope that affected individuals might benefit from interventions even after symptoms set in. Scientists predict that insights gained from studying these rare syndromic disorders will have an impact on the more common nonsyndromic autism and mild cognitive deficits.
Approximately 1% of the human population has an autism spectrum disorder (ASD). Key insight into synaptic function has been gained by studying the many genetic alterations that cause ASD.
It is estimated that ∼1% of the human population has an autism spectrum disorder (ASD). ASD has widely varied behavioral manifestations, severity, and comorbid conditions (hence the term “spectrum”), but those diagnosed with autism are characterized by impaired communication and reciprocal social interactions, and restricted and repetitive patterns of activities and interests (Baird et al. 2006). Approximately 70% of those diagnosed with autism also have intellectual disability (ID), and 25% have a seizure disorder (Tuchman and Rapin 2002). There is a strong genetic basis for autism, but the risk architecture is highly heterogeneous, and a large number of genes have been implicated (Abrahams and Geschwind 2008). This daunting phenotypic and etiologic complexity, shared by other major psychiatric illnesses, has slowed progress toward developing new therapies.
However, autism researchers are optimistic that the possibility of substantial progress may soon be realized (Krueger and Bear 2011). First, the genes have been discovered for numerous syndromic disorders that prominently feature ASD and ID. Second, these gene mutations have been reproduced in animal models that allow detailed examination of the underlying brain pathophysiology. Third, animal research has converged on altered synaptic function as a likely basis for impaired cognition and possibly ASD. Fourth, insights gained on how synapses function differently in the face of these mutations have suggested novel therapeutic interventions validated in preclinical models and that have shown promise in preliminary human clinical trials. Fifth, the fact that ASD and ID can be diagnosed in early childhood maximizes potential benefits of therapy because it can be started at a time when the brain is most plastic. Finally, animal studies using gene reactivation or pharmacological interventions suggest that substantial improvements can be seen even when treatments begin in adulthood (Ehninger et al. 2008b). Thus, a genetic diagnosis of a developmental brain disorder need not be a “life sentence” of permanent and inexorable mental disability.
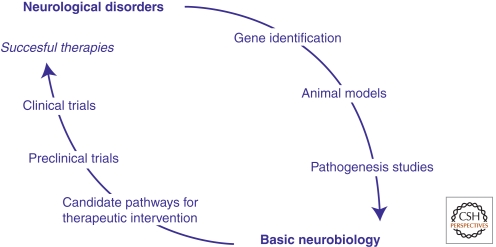
The path from gene discovery to novel treatment is outlined in Figure 1. This process often begins with astute clinical observations that some patients can be distinguished by a common set of phenotypic traits, thus defining a syndrome. Molecular genetic studies can then be undertaken to test the hypothesis that the syndrome has a genetic cause. In the event that disruption of a single gene or DNA segment causes the disease (i.e., a “highly penetrant” mutation), then it is possible to create an animal model (usually a mouse) that carries the same genetic disruption. Although the effects of the genetic lesion will likely manifest differently at the behavioral level in animals and humans because of differences in the complexity of the brains, it is reasonable to postulate that disruptions in elementary neuronal functions are likely to be shared. Understanding this neuronal pathophysiology is critical for identifying potential therapeutic targets. If these targets can be validated in the animal models, then chemistry ensues to generate molecules that can engage the target and satisfy the pharmacodynamic and pharmacokinetic drug requirements. If they are shown to be safe, drug candidates may then advance to human clinical trials. There are currently clinical trials ongoing in several single-gene syndromic disorders associated with ASD and ID. Most of these target alterations in synaptic signaling.
Figure 1.
The promise of molecular medicine in genetically defined disorders of brain development.
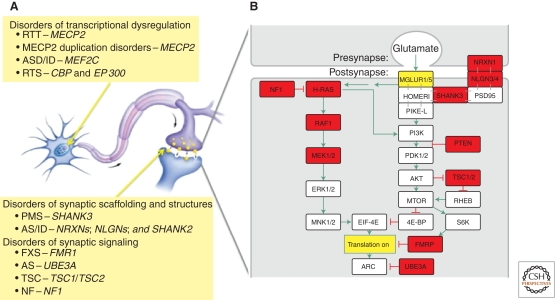
The notion that some ASD and associated ID represent “synapsopathies” (or “synaptopathies”) is supported by the preponderance of penetrant mutations in genes associated with synaptic structure and function. The most common single-gene mutations in ASD with ID are associated with fragile X syndrome (FMR1), tuberous sclerosis (TSC1, TSC2), neurofibromatosis (NF1), Angelman syndrome (UBE3A), Rett syndrome (MECP2), the PTEN hamartoma tumor syndrome, and Phelan-McDermid syndrome (SHANK3) (for review, see Betancur 2011). Rare mutations in the neuroligin (NLGN3, NLGN2) and neurexin (NRXN1) genes also cause autism (Jamain et al. 2003). Although this is by no means an exhaustive list of genes implicated in autism (and many await discovery), it is notable nonetheless that these highly penetrant mutations occur in genes that are critical regulators of synaptic function, and further, illuminate biochemical pathways that might be pathogenic in ASD and ID (Fig. 2).
Figure 2.
(A) Schematic of a neuron and axonal-dendritic synapse that depict examples of cellular localization of the various types of defects in ASD/ID. (B) A signaling pathway at the excitatory synapses that couples activity as registered by the release of glutamate to local control of protein synthesis. Disruption of the gene products indicated in the colored boxes greatly increases the risk of ASD/ID. Syndromic disorders with increased prevalence of ASD include Phelan-McDermid Syndrome (SHANK3); Noonan syndrome (RAF1, MEK1); Neurofibromatosis type 1 (NF1); Costello syndrome (H-Ras, MEK1); Cowden syndrome (PTEN); Cardio-facio-cutaneous (CFC) syndrome (MEK1/2); Tuberous sclerosis complex (TSC1/2); Fragile X syndrome (FMRP); Angelman syndrome (AS UBE3a); Rett syndrome (RTT–MeCP2); and Rubinstein-Taybi syndrome (RTS–CREB binding protein, p300). Rare, nonsyndromic ASDs include NLGN3/4 and NRXN1; ID/ASD: SHANK2.
In this article, we focus on a few syndromic disorders associated with ASD and ID that are characterized by penetrant mutations in genes that have been shown in animal models to disrupt synaptic function. Our goal is to highlight the similarities and differences in these syndromes and their underlying synaptic pathophysiology. Optimal synaptic function occurs within a narrow dynamic range along many dimensions, and it is not surprising that pathophysiology occurs at the edges of these spectra. What has come as a surprise, however, is that ASD and ID appear to be common consequences of disruptive mutations that cause synaptic pathophysiology at both ends of a spectrum. In other words, both “gain-of-function” and “loss-of-function” mutations can manifest in similar ways. Insights into the pathophysiology of ASD and ID have raised the possibility of therapeutic interventions to bring synapses into a normal operating range.
SINGLE GENE DISORDERS OF SYNAPTIC SCAFFOLDING AND STRUCTURE
Phelan-McDermid Syndrome and SHANK3 Disorders
Clinical Features and Phenotypic Spectrum
Phelan-McDermid syndrome (PMS) is a microdeletion syndrome of chromosome 22q13 characterized by severe neonatal hypotonia, some dysmorphic features, and global developmental delay. Patients have severe language delay or no speech; they have poor eye contact, decreased socialization, and stereotyped movements. Additional neurological phenotypes include teeth grinding, aggressive behavior, and seizures (Phelan et al. 2001; Phelan 2008; Dhar et al. 2010).
PMS results from deletions of variable length at the terminal region of the long arm of chromosome 22. Studies aimed at identifying the gene(s) critical for PMS phenotypes led to the identification of SH3 and Ankyrin-domain-containing protein (SHANK3) as the gene responsible for many neurological phenotypes. Bonaglia et al. (2001) were the first to identify disruption in SHANK3 (also known as ProSAP2) in an affected child with a translocation. Characterization of DNA from more than 60 additional patients revealed consistently that heterozygous loss of SHANK3 is responsible for the neurological phenotypes (Anderlid et al. 2002; Wilson et al. 2003; Dhar et al. 2010). The discovery that PMS is caused by haploinsufficiency of SHANK3 inspired investigators to evaluate this gene in other disorders, especially ASDs. Deletions spanning SHANK3 as well as protein-truncating mutations were discovered in patients with autism and ID (Durand et al. 2007; Moessner et al. 2007). Furthermore, duplications spanning SHANK3 have also been reported in a patient with Asperger and in two patients with ID and dysmorphic features (Durand et al. 2007; Okamoto et al. 2007). Recently, de novo mutations in SHANK3 were identified in patients with schizophrenia (Gauthier et al. 2010). Altogether, these data show that the phenotypes caused either by haploinsufficiency or duplication of SHANK3 include PMS, ASD with cognitive deficits, ID, and schizophrenia.
Pathophysiology
Biochemical and in vivo genetic studies are providing insight into the function of Shank3 and its paralogs. The Shank protein family, which includes Shank1, 2, and 3, are components of the postsynaptic density and interact with guanylate kinase–associated protein (also known as PSD-95-binding protein or SAPAP), Homer, cortactin-binding protein, and the somatostatin receptor (Du et al. 1998; Boeckers et al. 1999; Naisbitt et al. 1999; Tu et al. 1999; Yao et al. 1999; Zitzer et al. 1999). Shank proteins, also known as proline-rich synapse-associated proteins (ProSAPs), contain several protein–protein interaction domains. The amino termini contain five to six ankyrin repeats, followed by the Src homology 3 (SH3) domain and the PDZ domain. The carboxyl terminus contains several proline-rich clusters, a cortactin-binding domain, and a sterile α motif. Alternative splicing and alternative transcription start sites, as well as internal promoter regulation, result in Shank isoforms that lack another domain (Boeckers et al. 1999; Lim et al. 1999; Maunakea et al. 2010). All Shank proteins are expressed in the brain, especially in cortical and hippocampal neurons; however, the expression of different paralogs varies in other brain regions and tissues (Lim et al. 1999). Shank proteins localize to the PSD and stabilize PDS-95/SAPAP/Shank/Homer complexes. In addition, they recruit inositol 1, 4, 5-triphosphate (IP3) receptors and F-actin to the synapse, thereby enlarging dendritic spine heads and stabilizing them (Tu et al. 1998, 1999; Sala et al. 2001). Consistent with the important role of Shank proteins in synaptic function, overexpression of Shank1 in hippocampal neurons leads to increased maturation and size of dendritic spines (Sala et al. 2001). Furthermore, deletion of Shank1 in mice leads to smaller spines, thinner PSDs, and weakened synaptic transmission. Shank1-null mice displayed anxiety-like behavior, impaired contextual fear memory, and poor long-term retention of a spatial task despite the enhanced performance on this task (Hung et al. 2008).
In vitro and in vivo studies highlight the important role of Shank3 for synaptic function. Knockdown of Shank3 in hippocampal neurons cultured in vitro leads to reduced number and increased length of dendritic spines, whereas expression of Shank3 in aspiny cerebellar granule neurons is sufficient to induce functional dendritic spines (Roussignol et al. 2005). Two recent studies reported on the in vivo consequences of loss of Shank3 function in mice. Bozdagi et al. 2010 deleted the exons coding for the ankyrin repeat domain; thus, the mice lacked the full-length, long isoform of Shank3. Shank3+/− mice lacking the long isoform had reduced basal synaptic transmission in hippocampal CA1 neurons and decreased long-term potentiation (LTP). The finding of decreased GluR1-positive puncta in the stratum radiatum suggests altered AMPA receptor trafficking (Bozdagi et al. 2010). In addition to these synaptic abnormalities, Shank3+/− males displayed reduced responses to female social cues but no deficits on additional social tasks (Bozdagi et al. 2010). Two additional Shank3 mutant mice have been characterized: one termed Shank3A in which the long isoform (Shank3α) is ablated by deletion of the ankyrin repeats, and a second termed Shank3B in which the PDZ domain is targeted, thus eliminating both the long α isoform and short Shank3β isoform (Peça et al. 2011). Shank3B−/− mice, in particular, groomed excessively, displayed anxiety-like behaviors, and showed decreased social interactions. Analysis of striatal synaptic extracts from Shank3B−/− mice revealed decreased levels of several PSD proteins including SAPAP3, Homer, PSD-93, GluR2, NR2A, and NR2B. In addition to the biochemical changes, the thickness and length of the PSD were reduced, and there was a significant reduction of spine density. Finally, these postsynaptic changes accompanied reduced excitatory synaptic transmission in the striatum (Peça et al. 2011). Although Shank3B+/− mutants need to be studied further to relate the behavioral, synaptic, and biochemical changes to the PMS, the findings from both the Shank3+/− and Shank3B−/− mice point to disruption of glutamatergic signaling and AMPA-mediated transmission in both models and raise the possibility that enhancement of AMPA transmission might be helpful in disorders resulting from haploinsufficiency of SHANK3.
Disorders Due to Dysfunction of Neuroligins and Neurexins
Clinical Features and Phenotypic Spectrum
Mutations in the X-linked NEUROLIGIN3 and NEUROLIGIN4 (NLGN3 and NLGN4) were among the first ASD-causing mutations to be identified. A missense mutation causing R451C substitution within a highly conserved region in NLGN3 was detected in two male siblings: one with autism, severe intellectual disability, and seizures; and his brother with Asperger syndrome. Their mother, a carrier, did not manifest any phenotypes, which is typical of many X-linked disorders (Jamain et al. 2003). The mutations in NLGN4 cause a frameshift and early truncation of the protein. The first identified NLGN4 mutation occurred in a male with autism, in his brother with Asperger syndrome, and in the asymptomatic carrier mother (Jamain et al. 2003). Other truncating mutations were identified in all affected members of a large family with X-linked ID (Laumonnier et al. 2004) and in two male siblings; one with autism, ID, and a motor tic, and another with attention deficit hyperactivity, cognitive deficits, and Tourette syndrome diagnosis (Lawson-Yuen et al. 2008).
A de novo single-base substitution near the promoter region of NLGN4 has been reported in one male with autism and profound ID. The investigators suspect that this mutation leads to increased NLGN4 mRNA levels (at least in lymphoblast lines), raising the possibility that NLGN4 overexpression is also detrimental (Daoud et al. 2009). Thus, mutations in NLGN3 and NLGN4 cause a wide range of social and cognitive abnormalities ranging from Asperger autism to severe cognitive deficits and tics. These discoveries inspired studies to determine if NLGN receptors and neurexins are mutated in ASD. The first abnormalities involving NEUREXIN1 (NRXN1) were identified in two individuals with ASD and balanced chromosomal abnormalities (Kim et al. 2008). Subsequently, another abnormality was detected in a boy with dysmorphic features, cognitive deficits, and autistic features who had a large deletion that included the part of NRXN1 that codes for the α-promoter and exons 1–5, leaving the downstream β-promoter intact (Zahir et al. 2008). Compound heterozygosity for deletion and stop mutations (S979X) in NRXN1 have been reported in a patient with autistic behaviors, severe ID, hyperbreathing, and some dysmorphic features (Zweier et al. 2009).
Several deletions and duplications of NRXN1 have been reported in patients with schizophrenia. Although some copy number variants (CNVs) were reported in controls, their higher prevalence in schizophrenia and ASD/ID, as well as occasional truncating mutations in NRXN1, suggest that haploinsufficiency of NRXN1 confers a risk of ID, ASD, or schizophrenia (Rujescu et al. 2009; Ching et al. 2010; Gauthier et al. 2011). A single frameshifting mutation in NRXN2 has been reported in a patient with ASD and in his father, who has language delay (Gauthier et al. 2011).
Pathophysiology
NRXNs and NLGNs are synaptic cell adhesion molecules whose critical role in synaptic function has been well established. Three genes—NRXN1, NRXN2, and NRXN3—encode for mammalian NRXNs, each generating two isoforms, α and β, from independent promoters. The α-isoform is longer and contains six extracellular Laminin, NRXN, sex-hormone binding globulin domains (LNS) and three epidermal growth factor-like domains, whereas the β-isoform contains a single LNS domain (Tabuchi and Sudhof 2002). Complex alternative splicing generates thousands of splice variants (Missler and Sudhof 1998). NLGNs are endogenous ligands for NRXN and are encoded by four genes in humans: NLGN 1, 2, 3, and 4. They undergo alternative splicing at one (NLGN2, 3, and 4) or two (NLGN1) sites. Both NRXNs and NLGNs have single transmembrane domains and short cytoplasmic domains containing PDZ-binding motifs at the carboxyl terminus (Hata et al. 1996; Irie et al. 1997). NRXNs and NLGNs form a trans-synaptic complex believed to organize the presynaptic and postsynaptic compartments through various interactions with proteins like CASK, MAGUK, and PSD95 (Sudhof 2008). Although evidence about the functional roles of NLGNs and NRXNs initially came from coculturing experiments showing that expression of these proteins in nonneuronal cells can induce presynaptic and postsynaptic specialization, respectively (Scheiffele et al. 2000; Graf et al. 2004; Nam and Chen 2005), genetic studies in mice underscored the importance of these molecules for synaptic function. Mice lacking Nlgn1, 2, and 3 have normal synapse numbers and ultrastructure, but die perinatally from respiratory failure. Neurophysiological studies revealed that glutamatergic and GABAergic/glycinergic synaptic transmission is impaired in the respiratory centers of the triple null animals (Varoqueaux et al. 2006). Interestingly, mice carrying a single deletion or double knockout of these genes are viable. The finding that Nlgn1 deficiency impairs N-methyl-d-aspartate (NMDA) receptor signaling, whereas Nlgn2 deficiency causes deficits in inhibitory synaptic transmission, is consistent with the localization of NLGN1 and 2 to excitatory and inhibitory synapses, respectively (Chubykin et al. 2007). The data also argue that the lethality of the triple null animals is due to synthetic effects rather than functional redundancy. Loss of NLGN 3 causes some features that are typically seen in ASD patients. Nlgn3-null mice displayed impaired ultrasound vocalization and altered social memory, possibly owing to olfactory deficiency. The animals, however, performed as well as wild types on direct social interactions and did not have learning deficits or stereotypies (Radyushkin et al. 2009).
Mice carrying a knockin allele with the R451C substitution (discovered in a single ASD patient) displayed impaired social interactions, enhanced spatial learning, and had increased levels of vesicular inhibitory amino acid transporter without evidence of an increased number of synapses. In addition, the R451C knockin mice had increased inhibitory synaptic transmission. In contrast, the Nlgn3-null mice did not manifest such changes, supporting the proposal that the R451C substitution causes behavioral and neurophysiological phenotypes by a gain-of-function mechanism (Tabuchi et al. 2007). A deletion of an NLGN4 ortholog in mice caused impaired social interactions and decreased ultrasonic vocalization, consistent with the human studies illustrating that loss-of-function mutations cause ASD and ID (Jamain et al. 2008).
Studies of mice lacking NRXNs are complicated by the existence of various isoforms. Mice lacking α-NRXN isoforms through targeting of the α-isoforms in all three Neurexin genes (Nrxn1, 2, and 3) die prenatally because of breathing difficulties. The individual Nrxn knockout mice live longer but are also compromised and die at different times postnatally. Synapse numbers (except for minor decline in inhibitory synapse number) and ultrastructural studies did not reveal major abnormalities. Synaptic function, however, was impaired, as evident by decreased spontaneous as well as evoked neurotransmitter release in both the neocortex and the brain stem of α-NRXN-deficient mice. Presynaptic Ca2+ channel function was compromised in the α-NRXN-null mice based on decreased presynaptic Ca2+ currents, especially N-type Ca2+ currents (Missler et al. 2003).
These data illustrate that the α-NRXNs, like their ligands the NLGNs, are essential for proper synapse assembly and function, but not for the initial formation of synapses. Exactly how haploinsufficiency of NRXN1 causes the ASD/ID and schizophrenia spectrum phenotypes must await additional studies, but it is certain to involve some synaptic dysfunction.
SINGLE-GENE DISORDERS OF SYNAPTIC SIGNALING
Fragile X Syndrome
Clinical Features and Phenotypic Spectrum
In 1943, Martin and Bell described a family in which intellectual deficits (then reported as mental retardation) segregated as an X-linked trait (Martin and Bell 1943). Lubs subsequently observed a constriction on the long arm of the X chromosome in some males with ID. Moreover, Lubs reported that the affected males had macroorchidism (large testes); large, low-set ears; asymmetric facial features, and large hands (Lubs 1969; von Reyn et al. 1978). After mapping of this chromosomal variation to Xq27.3 (Harrison et al. 1983) and confirming the fragile site in many families segregating ID, the disorder became known as fragile X syndrome (FXS). Affected males have moderate to severe ID, speech delay, and motor abnormalities. Common psychiatric features include hyperactivity, anxiety, and severe autism spectrum phenotypes. Many males with FXS display gaze avoidance and stereotyped repetitive behaviors, and insist on sameness. Connective tissue abnormalities are common and include joint hyperextensibility, mitral valve prolapse, and mild dilation of the aorta (Hagerman et al. 1984). About one-third of the females with FXS have some ID.
Postmortem neuropathological studies revealed structural abnormalities of dendritic spines (Comery et al. 1997; Irwin et al. 2000). Specifically, there is an increase in spine-like protrusions on apical and basal dendrites in fragile X cerebral cortex, raising the possibility of failure of the normal spine elimination that typically occurs during postnatal development. Since the discovery that expansions of a CGG repeat in the fragile X mental retardation gene (FMR1) causes FXS (Fu et al. 1991; Verkerk et al. 1991), additional phenotypes were uncovered in premutation carriers. Specifically, males (and, to a lesser degree, females) carrying premutation alleles developed a late-onset neurodegenerative disorder known as fragile X–associated tremor/ataxias syndrome (FXTAS) (Hagerman and Hagerman 2004). About 25% of females carrying a premutation allele develop premature ovarian failure (Allingham-Hawkins et al. 1999).
Pathophysiology
The protein product of the FMR1 gene, called FMRP (fragile X mental retardation protein), is enriched in neurons and binds to mRNAs both in the soma and the dendrites. Several functional roles have been proposed, but the evidence is now quite compelling that FMRP serves as a negative regulator of translation of many mRNA transcripts (O’Donnell and Warren 2002). Mouse and fly fragile X models, in which the genes homologous to FMR1 have been knocked out, have been instrumental in understanding the pathophysiology of the disease (Bhogal and Jongens 2010). Consistent with the notion of FMRP as a translational repressor, an increased rate of basal protein synthesis is observed in the hippocampus of Fmr1-null mice (Qin et al. 2005; Osterweil et al. 2010).
Important synaptic regulators of protein synthesis are metabotropic glutamate receptors 1 and 5 (mGluR1 and mGluR5) (Greenough et al. 2001). These receptors appear to be part of a molecular machine that ensures that the supply of synaptic proteins keeps up with demand as registered by the release of glutamate at excitatory synapses. Partial inhibition of mGluR5 can restore wild-type levels of protein synthesis in the Fmr1-null mice (Aschrafi et al. 2005; Dolen et al. 2007; Gross et al. 2010; Osterweil et al. 2010). The theory that excessive protein synthesis downstream from mGluR5 is pathogenic in fragile X (Bear et al. 2004) has now been extensively tested and validated in both mouse and fly models (Krueger and Bear 2011). Based on the strength of these preclinical findings, negative mGluR5 regulators recently entered human clinical trials for fragile X syndrome (Levenga et al. 2010). Preliminary results appear promising, and larger trials are planned (Jacquemont et al. 2011). Additional exploratory trials are being conducted using minocycline, an inhibitor of the matrix metalloproteinase MMP-9, which is regulated by mGluR5 and FMRP and is overexpressed in the Fmr1-null mouse (Bilousova et al. 2009; Wang et al. 2010).
Another consistent finding in animal models of fragile X is evidence for impaired GABAergic inhibition (Levenga et al. 2010). This impairment could be a consequence of excessive protein synthesis during development or a parallel pathophysiologic process. In any case, some fragile X phenotypes that respond to mGluR5 inhibitors have also been ameliorated by administering compounds that activate GABA receptors, both in the mouse (Pacey et al. 2009; Olmos-Serrano et al. 2010) and the fly (Chang et al. 2008) models. The selective GABAB receptor agonist R-baclofen has recently attracted interest as a potential therapeutic in FXS, because it both decreases neuronal excitability via activation of dendritic potassium conductances and directly inhibits the synaptic release of glutamate (and therefore, indirectly, mGluR5 activation) (Kohl and Paulsen 2010).
Angelman Syndrome
Clinical Features and Phenotypic Spectrum
Angelman syndrome (AS) is characterized by intellectual and developmental disabilities, balance problems, severe language deficits, and seizures. These children display various behavioral problems, including hand-flapping movements that led Angelman to describe them as “puppet children” (Angelman 1965). Bower and Jeavons described the syndrome as “happy puppet syndrome” because of the happy disposition, frequent smiles, and unexplained bouts of laughter (Bower and Jeavons 1967). Additional features include hyperactivity, sleep abnormalities, tongue protrusion, and some dysmorphic features like a prominent mandible and wide mouth (Clayton-Smith 1993). Pathologically, the only consistent deficit is the small brain size. The majority of AS cases are caused by maternal deletions of 15q11-q13, whereas 2%–5% result from paternal uniparental disomy of 15q11-13. Imprinting defects involving the bipartite imprinting center in 15q11-13 also account for some cases of AS (Magenis et al. 1987; Nicholls et al. 1989; Ohta et al. 1999). The culprit gene in 15q11-13 is the ubiquitin E3 ligase (UBE3A) (Kishino et al. 1997; Matsuura et al. 1997).
Pathophysiology
Several synaptic deficits were described in the Ube3a knockout mouse model of AS (Philpot et al. 2010), including reduced density and strength of excitatory synapses and, at later developmental stages, reduced functional inhibition. Striking synaptic plasticity phenotypes include a severe impairment in NMDA receptor-dependent long-term depression (LTD), an increased threshold for long-term potentiation (LTP), and a loss of experience-dependent visual cortical plasticity (Yashiro et al. 2009; Sato and Stryker 2010). This synaptic rigidity will likely account for ID in AS.
Because Ube3a directs proteolysis via the proteasome, the deficits in synaptic function and plasticity are believed to be related to increased levels of Ube3a target proteins. Although there are many potential targets, the search is narrowed by the important discovery that the pathogenic proteins appear to be regulated by neural activity (Yashiro et al. 2009). Reducing visual cortical activity by housing the mice in complete darkness restored normal LTD and LTP in the Ube3a-null mice. One activity-regulated protein that is rapidly reduced in visual cortex by dark exposure is Arc, critical for glutamate receptor trafficking at synapses (Shepherd and Bear 2011). Recent work has shown that Arc is a Ube3a substrate and is overexpressed in the null mice. Increased expression of Arc in the absence of Ube3a leads to internalization of AMPA receptors and impaired excitatory synaptic transmission (Greer et al. 2010). Arc is translated downstream from activation of mGluR5 and regulated by FMRP. Thus, excessive or poorly regulated expression of Arc could be a convergence point in the pathophysiology of FXS and AS (Kelleher and Bear 2008). It is of great interest to know if inhibitors of mGluR5 correct mutant phenotypes in the Angelman mouse model.
Another important observation is that boosting the activity of αCaMKII (calcium-calmodulin-dependent protein kinase II) by mutating an inhibitory phosphorylation site can rescue LTP and learning deficits in the Ube3a mutant mice (van Woerden et al. 2007). Increased expression (Neve and Bear 1989) and recruitment of αCaMKII to synapses via the NR2B subunit of NMDA receptors in the visual cortex (Chen and Bear 2007) are known consequences of dark exposure. Thus, strategies to increase phosphorylation of αCaMKII substrates (which include AMPA receptors) may represent another therapeutic approach to AS.
It is noteworthy that maternal duplication of the 15q11-q13 region occurs in ∼1% of individuals with nonsyndromic autism (Cook et al. 1998; Christian et al. 2008). Overexpression of UBE3A and consequent reductions in ARC could contribute to the pathophysiology, because an Arc knockout mouse shows impairments in synaptic plasticity that are similar to those reported in the Ube3a-null mice (McCurry et al. 2010). Regardless of the cause, duplications and deletions of 15q11-q13 provide another example of how ASD and ID (and defective synaptic plasticity) can be a common consequence of both increased and decreased gene dosage.
Tuberous Sclerosis Complex
Clinical Features and Phenotypic Spectrum
Tuberous sclerosis complex (TSC) is a neurocutaneous dominantly inherited multisystem disorder characterized by the presence of benign tumors (hamartomas) that occur in many organs, but most notably the brain, skin, eyes, kidneys, and heart (Curatolo et al. 2008). These hamartomas represent overgrown cells from the tissue within which they reside. Friedrich von Recklinghausen was the first to describe TSC in 1862, but it was Désiré-Magloire Bourneville who in the 1880s coined the term “sclérose tubéreuse” after discovering sclerotic tubers in postmortem tissues from patients with epilepsy and ID (Bourneville 1880; Jay 1999). The neurological phenotypic spectrum of TSC is quite variable, ranging from patients with normal intellect and no seizures to those with severe ID and medically refractory seizures. The neurological manifestations, however, are the most devastating features of the disease and represent the most common causes of morbidity and mortality. Seizures are the most common neurological problem occurring in 80%–90% of the patients (Curatolo et al. 2008). Intellectual disability and autism spectrum phenotypes occur in ∼50% of the patients (Allingham-Hawkins et al. 1999). Nonnervous tissue abnormalities and complications include renal angiomyolipomas and renal cysts, cardiac rhabdomyomas, retinal hamartomas, enamel pits in permanent teeth, arterial aneurysms, and skin abnormalities such as hypomelanotic macules and angiofibromas. Neuropathological lesions include subependermal nodules that are typically along the walls of the lateral ventricles, giant astrocytomas that might grow into the ventricles and cause obstruction and hydrocephalus, and cortical tubers that contain abnormally large neurons and glia. Treatment is usually symptomatic, and life expectancy is reduced by complications of seizures.
TSC is caused by mutations in two distinct genes: TSC1 and TSC2. Mutations in TSC1 cause up to 30% of familial cases and 15% of sporadic causes, whereas mutations in TSC2 account for up to 50% of sporadic causes and a high percentage of familial cases (Crino et al. 2006; Curatolo et al. 2008).
Pathophysiology
The proteins encoded by TSC1 and TSC2, harmartin (TSC1) and tuberin (TSC2), form a heterodimeric complex that responds to numerous intracellular signals to regulate (via an intermediary called Rheb for “Ras homolog enriched in brain”) the protein kinase mTOR (mammalian target of rapamycin) residing in the protein complex mTORC1. TSC1/2 complex functions as a negative regulator of mTOR (see Fig. 2). Relief from TSC1/2 repression of mTOR by upstream signaling (e.g., PI3 kinase acting through PDK1 and AKT) stimulates cell growth and proliferation, for example, in response to growth factors and nutrients. Homozygous silencing mutations of either TSC1 or TSC2 are embryonic lethal. Humans born with the disease typically have heterozygous truncating germline mutations in either TSC1 or TSC2. It is believed that hamartomas are caused by a “second hit” that disables the functional allele (so-called loss of heterozygosity) or otherwise impairs the remaining TSC protein, causing uncontrolled cell growth (Orlova and Crino 2010; Han and Sahin 2011).
It is not surprising that brain development would be altered by tuber growth and seizures during early life (Numis et al. 2011). However, there is a growing appreciation that substantial brain dysfunction occurs independently of tumor formation and epilepsy (de Vries 2010). This hypothesis received a major boost with the development of rodent models of TSC. Heterozygous null mutations of Tsc1 or Tsc2 were both shown to cause cognitive and synaptic impairments in the absence of gross neuropathology or seizures (von der Brelie et al. 2006; Goorden et al. 2007; Ehninger et al. 2008a; Nie et al. 2010). One particularly interesting phenotype reported in the Tsc2+/− mouse is an enhancement of late-phase LTP (Ehninger et al. 2008a). Persistent LTP requires synthesis of synaptic proteins that might be increased in abundance owing to excess mTOR activity.
Treatment of Tsc mutant mice with the immunosuppressive drug rapamycin, an mTOR inhibitor, ameliorates several phenotypes (Meikle et al. 2008; Ehninger and Silva 2011; Tsai and Sahin 2011). Of particular interest are the findings that adult-onset treatment can correct the late-phase LTP phenotype as well as learning deficits (Ehninger et al. 2008a). This suggests that some synaptic and behavioral phenotypes in TSC, like FXS and Rett syndrome (see below), are due to ongoing pathophysiological processes rather than an irreversible derailment of development. These pathophysiological changes may be corrected with drugs.
It is noteworthy that the pathogenesis of FXS, AS, and TSC involves regulation of synaptic protein abundance and turnover. In FXS this is due to derepression of translation of FMRP-target mRNAs; in AS this is due to reduced proteolysis of UBE3a-target proteins; and in TSC this is due to derepression of the protein kinase mTOR that stimulates mRNA translation. An appealing idea is that overlapping behavioral manifestations of these diseases, such as ASD, seizures, and ID, may be related to impaired regulation of synaptic protein abundance (Kelleher and Bear 2008). The question remains, however, as to the degree to which these disorders share pathophysiological changes and respond to similar treatment approaches. Indeed, the excessive protein synthesis in the Fmr1-null hippocampus, surprisingly, is not corrected by treatment with the mTOR inhibitor rapamycin (Osterweil et al. 2010).
SINGLE GENE DISORDERS OF TRANSCRIPTIONAL DYSREGULATION
Rett Syndrome and MECP2 Disorders
Clinical Features and Phenotypic Spectrum
Rett syndrome (RTT) is a neurological disorder that manifests in females during early childhood. The disorder was originally described by Andreas Rett (Rett 1966), but it became recognized in the medical community when Hagberg and colleagues published a description of 35 cases in 1983 (Hagberg et al. 1983). Girls with Rett appear to develop normally up to 6–18 mo of age, but head growth typically decelerates, leading to microcephaly by the second or third year of life. Patients lose purposeful use of their hands and develop stereotypic hand wringing or washing movements. Several autistic features including loss of language, social withdrawal, lack of eye-to-eye contact, expressionless face, and lack of response to social cues appear after 2 yr of age. Cognitive deficits and motor abnormalities including ataxia, apraxia, and rigidity become apparent between 3 and 5 yr of age. Autonomic abnormalities such as vasomotor disturbances (cold hands and feet) and abnormal breathing patterns (hyperventilation alternating with hypopnea during wakefulness) occur in early childhood. Seizures often start around 4 yr of age but tend to decrease in severity in adulthood. Similarly, some social behaviors improve around 10 yr of age or shortly after (Hagberg 1995; Chahrour and Zoghbi 2007; Neul et al. 2010). Additional features include sleep disturbances, teeth grinding, screaming fits, anxiety, osteopenia, scoliosis, severe constipation, and cardiac abnormalities (including tachycardia, prolonged corrected QT interval, and sinus bradycardia). As patients get older, some develop Parkinsonian features (Hagberg 2005). Many patients can live into their sixth and seventh decades without evidence of further progression of disease.
The discovery that mutations in the X-linked methyl-CpG-binding protein 2 (MECP2) that encodes MeCP2 cause RTT syndrome (Amir et al. 1999) affirmed that this mostly (99%) sporadic disorder has a genetic basis and that the majority of the cases (up to 97% of classic RTT) are caused by mutations in this gene (Neul et al. 2008). Importantly, the discovery of MECP2 as the RTT-causing gene revealed that mutations in this gene cause a broad spectrum of neuropsychiatric phenotypes. In females, the phenotypes might range from mild learning disabilities to autism, tremors, and anxiety. This is mainly due to variation in patterns of X-chromosome inactivation (XCI) (Christodoulou and Ho 1993; Zappella et al. 2001; Huppke et al. 2006; Lasalle and Yasui 2009).
Males with MECP2 mutations have a broad spectrum of phenotypes depending on the severity of the mutations. Null alleles cause severe encephalopathy, motor deficits, breathing abnormalities, seizures, autonomic dysfunction, and early lethality by 2 yr of age. Mutations that partially compromise the function of the protein (such as late truncating mutations or hypomorphic missense alleles) lead to a variety of phenotypes such as juvenile-onset schizophrenia, bipolar disorders, autism, and Parkinsonian features, all typically accompanied by some degree of cognitive deficits and tremors (Christodoulou and Ho 1993; Cohen et al. 2002; Klauck et al. 2002). It is remarkable that mutations in MECP2 can result in symptoms of almost all neuropsychiatric disorders ranging from subtle cognitive deficits to autism, anxiety, ataxia, seizures, Parkinsonism, schizophrenia, bipolar, and a plethora of autonomic nervous system abnormalities.
The finding that duplications and triplications spanning the MECP2 locus in Xq28 cause a progressive neurological syndrome highlighted the sensitivity of the nervous tissue to precise levels of this protein (Meins et al. 2005; Van Esch et al. 2005). Although the duplications and triplications span multiple genes in Xq28, the finding that the minimal region of overlap spans MECP2 and the interleukin receptor–associated kinase 1 and that the isolated doubling of MeCP2 levels in mice causes most features of the Xq28 syndrome (Collins et al. 2004; Del Gaudio et al. 2006) pinpointed MECP2 as the mediator of the duplication syndrome phenotypes. Males typically have hypotonia and developmental delay, manifesting first as autism then progressing to severe motor and cognitive impairments, epilepsy, tremors, RTT-like features, and premature lethality (Van Esch et al. 2005; Ramocki et al. 2009).
Female phenotypes include anxiety, depression, broad autism phenotypes, and RTT-like symptoms due to variations in patterns of XCI (Ramocki et al. 2009; Ramocki et al. 2010; Grasshoff et al. 2011). The breadth of the phenotypes associated with either lost or increased levels of MeCP2 and the significant overlap in the phenotypes in these two disorders raised questions about the mechanisms mediating these disorders, which were subsequently clarified through the study of animal models.
Pathophysiology
MeCP2 is a nuclear protein that binds to methylated cytosines (Lewis et al. 1992) and is a member of a methyl-CpG-binding protein family (Hendrich and Bird 1998). Cell-based studies showed that MeCP2 interacts with histone deacetylase–containing complexes and represses transcription (Jones et al. 1998; Nan et al. 1998). The findings that gene expression changes are subtle in mouse models of RTT, and, surprisingly, several genes are down-regulated upon loss of MeCP2 but are increased upon its overexpression, suggested that this protein is not a classical transcriptional repressor (Tudor et al. 2002; Nuber et al. 2005; Jordan et al. 2007; Chahrour et al. 2008). Indeed, MeCP2 binds to the promoter regions of several genes that are repressed upon loss of MeCP2 and are activated by its presence, including brain-derived neurotrophic factor (BDNF) and several other neuronal genes (Chen et al. 2003; Martinowich et al. 2003; Yasui et al. 2007; Chahrour et al. 2008). In addition to the gene-specific binding, chromatin-immunoprecipitation (ChIP) studies revealed that MeCP2 binds widely throughout the genome (Yasui et al. 2007; Skene et al. 2010) and is as abundant as one molecule for every two nucleosomes (Skene et al. 2010). The findings that MeCP2 and H1 compete for DNA binding sites support the hypothesis that MeCP2 might act as an alternative linker histone (Ghosh et al. 2010; Skene et al. 2010). However, how such competition causes the specific Rett and MeCP2 duplication phenotypes and the root of the very specific opposing gene expression changes are not clear at this time. Additional molecular and biochemical studies are required to gain better insight into the mechanism by which MeCP2 affects gene expression in vivo. While the molecular functions of MeCP2 are being elucidated, the neurobiological consequences of either loss or gain of this protein have been extensively studied, and such studies have provided important insight into the pathogenesis of RTT, MECP2 duplications, and the role of certain neurons in mediating specific phenotypes associated with MeCP2 dysfunction.
MeCP2 is abundant in neurons, and its levels increase postnatally as neurons mature (Shahbazian et al. 2002b; Balmer et al. 2003; Kishi and Macklis 2004). Recent studies revealed that MeCP2 is also expressed in glia—albeit at lower levels than neurons—and that glia lacking MeCP2 fail to support dendritic morphology of either wild-type or Mecp2-null neurons (Ballas et al. 2009; Maezawa et al. 2009; Kifayathullah et al. 2010; Maezawa and Jin 2010). These studies raised the possibility that some noncell-autonomous effects from glia contribute to RTT pathogenesis. Although Mecp2-null glia might cause or aggravate some of the neuronal phenotypes, several studies, however, have shown that loss of MeCP2 specifically from neurons is sufficient to compromise function and to cause one or more RTT phenotypes.
Mice lacking functional MeCP2 reproduce features of RTT (Chen et al. 2001; Guy et al. 2001; Shahbazian et al. 2002a; Pelka et al. 2006). Despite the devastating neurological phenotypes, the brain appears normal, with the exception of microcephaly, decrease in dendritic spine density, and dendritic swelling (Belichenko et al. 2009). Mice expressing twice the normal levels of MeCP2 reproduce human duplication syndrome features, but without much evidence of major pathological changes (Collins et al. 2004). Evidence for synaptic abnormalities came from various neurophysiological studies using both RTT and duplication models. RTT model studies revealed reduced LTP and impaired synaptic plasticity (Asaka et al. 2006; Moretti et al. 2006), whereas doubling MeCP2 caused enhanced LTP (Collins et al. 2004). Studies using autaptic preparations from hippocampal glutamatergic neurons revealed that neurons lacking MeCP2 have decreased excitatory postsynaptic currents (EPSCs), whereas those having excess MeCP2 had increased EPSCs, most likely caused by the decrease and increase in the number of glutamatergic synapses in these mutants, respectively (Chao et al. 2007). Using human induced pluripotent stem cells (iPSCs) from RTT patients, Marchetto et al. (2010) showed that neurons derived from RTT-iPSCs were smaller, had fewer synapses, and had altered activity-dependent calcium transients and decreased spontaneous postsynaptic currents. A reduction in EPSCs and excitatory synaptic strength was also detected using cortical slices from Mecp2-null mice (Dani et al. 2005). Additionally, defects in inhibitory synaptic transmission were detected in the cortex, hippocampus, and brain stem of Mecp2-null mutants (Dani et al. 2005; Medrihan et al. 2008; Zhang et al. 2008). Voltage-sensitive dye imaging revealed that area CA1 is hyperexcitable in Mecp2-null mice and that this hyperexcitability originates in the CA3 region (Calfa et al. 2011).
Deletion of MeCP2 from various neuronal types revealed that this protein is critical for the functional integrity of a diverse set of neurons. Loss of Mecp2 from forebrain glutamatergic neurons causes motor abnormalities, anxiety-like behavior, social abnormalities, and impaired learning (Gemelli et al. 2006), whereas a deletion in Sim1 hypothalamic neurons causes aggression, abnormal stress responses, and hyperphagia (Fyffe et al. 2008). Several additional cell-specific deletions caused partial phenotypes of RTT (Adachi et al. 2009; Samaco et al. 2009). A more recent study revealed that deletion of Mecp2 in GABAergic neurons reproduced most of the features of RTT (including the stereotyped behavior and premature lethality) and resulted in reduced GABA signaling based on findings of reduced inhibitory quantal size and mIPSCs (Chao et al. 2010). The extent of the reduction in glutamate decarboxylase (GAD) expression and GABA immunoreactivity in neurons was consistent with the degree of reduction in GABA signaling uncovered by the neurophysiological studies. A common theme observed in several of the Mecp2 conditional deletions is the cell-autonomous compromise to the biosynthetic enzymes critical for the neurotransmitters in the respective neurons (e.g., tyrosine hydroxylase in catecholamine neurons and GAD in inhibitory neurons) (Samaco et al. 2009; Chao et al. 2010). MeCP2 does bind the promoters of all of the genes encoding these enzymes as well as the promoter of various neuropeptides (e.g., Crh, Bdnf, and Sst) that are reduced in Mecp2-null mice. In contrast, these neuropeptides are increased in MeCP2-overexpressing mice, and there is more MeCP2 binding at their respective promoters. Although the way MeCP2 loss or gain regulates key neuronal genes is still not fully understood, doubling MeCP2 levels clearly causes disease by a gain-of-function (hyperfunction) mechanism.
The changes in biosynthetic enzymes and neuropeptides suggest that normal Mecp2 function is critical for activity-dependent gene transcription. This notion is supported by the finding that activity leads to phosphorylation of MeCP2 at S421 (Chen et al. 2003; Martinowich et al. 2003; Zhou et al. 2006). Although in cell-based assays, phosphorylation at S421 appeared to be critical for activity-dependent Bdnf transcription, additional in vivo studies are needed to determine the precise effect of S421 phosphorylation on gene transcription. Mutating S421 to an alanine causes a minor phenotype of hyperactivity. In contrast, phosphorylation of MeCP2 at serine 80 seems to be decreased by activity, and S80 mutation to alanine causes decrease in locomotor activity (Tao et al. 2009).
Although RTT is a devastating disorder, genetic and pharmacologic studies provide hope that the disease is reversible. Silencing of Mecp2 in mice using a lox-Stop-lox cassette causes RTT-like features to be reversed if the expression of MeCP2 is induced in adult animals (Giacometti et al. 2007; Guy et al. 2007). These results show that the neuronal connectivity is intact and the neurons and glia are not permanently damaged from MeCP2 loss. Tropea et al. (2009) showed that using a fragment of insulin-like growth factor (IGF-1) in systemic treatment of MeCP2 mutant mice prolonged their median survival by ∼5 wk and improved locomotive activity, breathing, and heart rates, and abolished the prolonged ocular dominance plasticity seen in Mecp2+/− mice. These findings show that the synaptic abnormalities seen in the RTT model are partially reversible using this pharmacological approach. Kline et al. (2010) evaluated the synaptic function of the nucleus tractus solitarius of Mecp2-null mice and found that the mEPSCs are reduced in these mice. Because this synaptic dysfunction was accompanied by decreased BDNF, they applied exogenous BDNF to the brain stem preparation and found that it rescued the synaptic defects (Kline et al. 2010). Another pathway that is altered in RTT is the AKT/mTOR signaling pathway, which is decreased before symptom onset as evidenced by significant reductions in levels of phosphorylated ribosomal protein S6 (rpS6) in neurons of Mecp2-null mice (Ricciardi et al. 2011). Interestingly, the RTT model shows a decrease in AKT/mTOR signaling in contrast with models of TSC and fragile X, in which AKT/mTOR activity is increased.
Although it is too early to determine the ideal therapeutic approach in RTT, several findings point to interesting candidates. The reductions of BDNF levels in Mecp2-null mice coupled with the amelioration of phenotypes when BDNF is used exogenously or genetically (Chang et al. 2006; Kline et al. 2010) raise the possibility that drugs that enhance BDNF signaling might rescue RTT phenotypes. The vast number of gene expression changes are sure to contribute to the various RTT phenotypes; thus, therapies targeting chromatin-remodeling proteins such as histone deacetylase or histone acetylase might prove useful. Further studies exploring IGF-1 as a potential therapy are worthy of consideration, especially given the reduction in AKT/mTOR activity. Lastly, the finding that many RTT phenotypes can be recapitulated by deleting Mecp2 in GABAergic neurons raises the possibility that enhancing GABA signaling might rescue several phenotypes.
CONCLUDING REMARKS
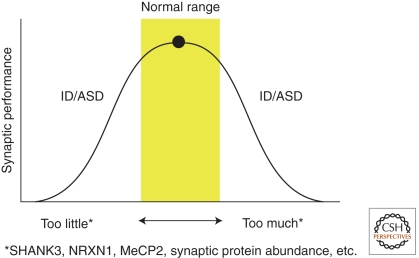
The study of syndromic autism and disorders associated with cognitive dysfunction has had a major impact on the fields of genetics, pediatrics, neurology, psychiatry, and neuroscience. Such human studies allowed the identification of the genetic bases of more than 100 ASD and ID disorders, permitted genetic counseling and early interventions for affected families, and uncovered novel mutational mechanisms. Examples of novel mutational mechanisms include genomic disorders involving gains and losses of segments of the genomes (Stankiewicz and Lupski 2010), dynamic mutations such as the trinucleotide expansions in the fragile X syndrome (Orr and Zoghbi 2007), and genomic uniparental disomy or imprinting disorders (Spence et al. 1988; Nicholls et al. 1989). From a neurobiological perspective, the gains from studying human patients have been enormous. First, we learned that either gains or losses of certain proteins or chromosomal segments (e.g., MECP2, SHANK3, and 15q11-13) could cause overlapping phenotypes, ID, motor abnormalities, or seizures (Fig. 3). In the case of MeCP2, this happens despite opposing molecular and synaptic changes, suggesting that the overlapping features might result from failure of homeostatic regulation of synaptic function. This clearly represents a challenge for developing therapies that might target more than one type of ID or autism spectrum disorder.
Figure 3.
Gain or loss of function of individual genes often yields an overlapping behavioral phenotype in humans that includes ASD and ID. The same appears to be true of a physiological process such as local synaptic protein synthesis, in which too much or too little can be manifest in similar ways. We propose that optimal synaptic function occurs within a limited dynamic range, and the pathophysiology at both ends of this range can cause autistic behavior and intellectual disability.
Disturbances in protein translation at the synapse is an example of a mechanism affecting synaptic function and homeostasis, which provides a therapeutic opportunity for more than one type of ASD/ID. Loss of FMRP, TSC1/2, PTEN, or Ube3a causes disturbances in translational control and impacts protein levels at the synapse. Thus, identifying therapies that affect translational control might prove useful for this group of disorders. Rapamycin and mGluR5 modulators are emerging as such potential candidates (Aschrafi et al. 2005; Dolen et al. 2007; Zhou et al. 2009; Gross et al. 2010; Osterweil et al. 2010; Ehninger and Silva 2011). Transcriptional dysregulation is another potential mechanism occurring in several disorders caused by loss or gain of function of nuclear factors such as MeCP2, CREB-binding protein (Petrij et al. 1995), EP300 (Roelfsema et al. 2005), or MEF2C (Le Meur et al. 2010) (Fig. 2). Drugs that target histone deacetylases, histone acetylases, or other chromatin-modifying proteins might prove useful in this class of disorders.
Key insight into neurobiology, especially the regulation of synaptic function and homeostasis, has been gained from the study of syndromic human disorders and their respective animal models. The identification of FMRP and elucidation of its role in translational control have uncovered a critical step downstream from the metabotropic glutamate receptors (Bear et al. 2004). Genetic studies involving selective deletions of Mecp2 in specific neurons permit analysis of the consequences of partial dysfunction of such neurons. This is best illustrated by the case of GABAergic neurons, where loss of MeCP2 in such neurons uncovered the neuropsychiatric consequences of partial reduction in GABA signaling (Chao et al. 2010). The diversity of phenotypes uncovered in human patients and the various alleles causing such diverse phenotypes are key for dissecting critical domains and residues within a protein and for attributing specific neuropsychiatric phenotypes to subtle physiological changes at the synapse.
Basic research and pathogenesis studies of autism and ID disorders in model organisms have been critical in advancing our understanding of several of these disorders. To date, many of the potential pathways that can be targeted therapeutically have been defined from such fundamental research. The fact that genetic studies have illustrated that some of these disorders can be reversible in mice is quite exciting and brings hope that, with effective therapies, affected children and young adults might gain skills and functionality. The new intense efforts through whole-genome sequencing of samples from nonsyndromic ASD and ID cases promise to uncover hundreds of new genes whose proteins or RNAs are likely to affect synaptic function. The challenge for neurobiologists is to dissect the mechanisms by which these gene products affect synaptic function and to identify points of convergence and shared pathways that could affect a group of disorders when modified. Children with ASD and ID have taught us more than any model system about the exquisite sensitivity of the synapse to subtle molecular perturbation. The knowledge we continue to gain from studying human disorders and from pursuing fundamental basic research on such disorders will, it is hoped, help develop effective therapies for the millions of children affected by these disorders and who have to endure their toll for decades.
Footnotes
Editors: Morgan Sheng, Bernardo Sabatini, and Thomas Südhof
Additional Perspectives on The Synapse available at www.cshperspectives.org
REFERENCES
- Abrahams BS, Geschwind DH 2008. Advances in autism genetics: On the threshold of a new neurobiology. Nat Rev Genet 9: 341–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi M, Autry AE, Covington HE III, Monteggia LM 2009. MeCP2-mediated transcription repression in the basolateral amygdala may underlie heightened anxiety in a mouse model of Rett syndrome. J Neurosci 29: 4218–4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, Holden JJ, Yang KT, Lee C, Hudson R, Gorwill H, Nolin SL, Glicksman A, et al. 1999. Fragile X premutation is a significant risk factor for premature ovarian failure: The International Collaborative POF in Fragile X study—preliminary data. Am J Med Genet 83: 322–325 [PMC free article] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY 1999. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 23: 185–188 [DOI] [PubMed] [Google Scholar]
- Anderlid BM, Schoumans J, Anneren G, Tapia-Paez I, Dumanski J, Blennow E, Nordenskjold M 2002. FISH-mapping of a 100-kb terminal 22q13 deletion. Hum Genet 110: 439–443 [DOI] [PubMed] [Google Scholar]
- Angelman H 1965. “Puppet children”: A report of three cases. Dev Med Child Neurol 7: 681–688 [DOI] [PubMed] [Google Scholar]
- Asaka Y, Jugloff DG, Zhang L, Eubanks JH, Fitzsimonds RM 2006. Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol Dis 21: 217–227 [DOI] [PubMed] [Google Scholar]
- Aschrafi A, Cunningham BA, Edelman GM, Vanderklish PW 2005. The fragile X mental retardation protein and group I metabotropic glutamate receptors regulate levels of mRNA granules in brain. Proc Natl Acad Sci 102: 2180–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird G, Simonoff E, Pickles A, Chandler S, Loucas T, Meldrum D, Charman T 2006. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: The Special Needs and Autism Project (SNAP). Lancet 368: 210–215 [DOI] [PubMed] [Google Scholar]
- Ballas N, Lioy DT, Grunseich C, Mandel G 2009. Non–cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology: A cellular model for Rett syndrome. Nat Neurosci 12: 311–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer D, Goldstine J, Rao YM, LaSalle JM 2003. Elevated methyl-CpG-binding protein 2 expression is acquired during postnatal human brain development and is correlated with alternative polyadenylation. J Mol Med 81: 61–68 [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST 2004. The mGluR theory of fragile X mental retardation. Trends Neurosci 27: 370–377 [DOI] [PubMed] [Google Scholar]
- Belichenko PV, Wright EE, Belichenko NP, Masliah E, Li HH, Mobley WC, Francke U 2009. Widespread changes in dendritic and axonal morphology in Mecp2-mutant mouse models of Rett syndrome: Evidence for disruption of neuronal networks. J Comp Neurol 514: 240–258 [DOI] [PubMed] [Google Scholar]
- Berkel S, Marshall CR, Weiss B, Howe J, Roeth R, Moog U, Endris V, Roberts W, Szatmari P, Pinto D, et al. 2010. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat Genet 42: 489–491 [DOI] [PubMed] [Google Scholar]
- Betancur C 2011. Etiological heterogeneity in autism spectrum disorders: More than 100 genetic and genomic disorders and still counting. Brain Res 1380: 42–77 [DOI] [PubMed] [Google Scholar]
- Bhogal B, Jongens TA 2010. Fragile X syndrome and model organisms: Identifying potential routes of therapeutic intervention. Dis Model Mech 3: 693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilousova TV, Dansie L, Ngo M, Aye J, Charles JR, Ethell DW, Ethell IM 2009. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet 46: 94–102 [DOI] [PubMed] [Google Scholar]
- Boeckers TM, Kreutz MR, Winter C, Zuschratter W, Smalla KH, Sanmarti-Vila L, Wex H, Langnaese K, Bockmann J, Garner CC, et al. 1999. Proline-rich synapse-associated protein-1/cortactin binding protein 1 (ProSAP1/CortBP1) is a PDZ-domain protein highly enriched in the postsynaptic density. J Neurosci 19: 6506–6518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaglia MC, Giorda R, Borgatti R, Felisari G, Gagliardi C, Selicorni A, Zuffardi O 2001. Disruption of the ProSAP2 gene in a t(12;22)(q24.1;q13.3) is associated with the 22q13.3 deletion syndrome. Am J Hum Genet 69: 261–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourneville D 1880. Sclérose tubéreuse des circonvolutions cérébrales: Idiotie et épilepsie hemiplégique. Arch Neurol 1: 81–89 [Google Scholar]
- Bower BD, Jeavons PM 1967. The “happy puppet” syndrome. Arch Dis Child 42: 298–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N, Kajiwara Y, Yang M, Katz AM, Scattoni ML, et al. 2010. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism 1: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfa G, Hablitz JJ, Pozzo-Miller L 2011. Network hyperexcitability in hippocampal slices from Mecp2 mutant mice revealed by voltage-sensitive dye imaging. J Neurophysiol 105: 1768–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Zoghbi HY 2007. The story of Rett syndrome: From clinic to neurobiology. Neuron 56: 422–437 [DOI] [PubMed] [Google Scholar]
- Chahrour M, Yung SY, Shaw C, Zhou X, Wong STC, Qin J, Zoghbi HY 2008. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320: 1224–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Khare G, Dani V, Nelson S, Jaenisch R 2006. The disease progression of Mecp2mutant mice is affected by the level of BDNF expression. Neuron 49: 341–348 [DOI] [PubMed] [Google Scholar]
- Chang S, Bray SM, Li Z, Zarnescu DC, He C, Jin P, Warren ST 2008. Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Nat Chem Biol 4: 256–263 [DOI] [PubMed] [Google Scholar]
- Chao HT, Zoghbi HY, Rosenmund C 2007. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron 56: 58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu HC, Heintz N, et al. 2010. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 468: 263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WS, Bear MF 2007. Activity-dependent regulation of NR2B translation contributes to metaplasticity in mouse visual cortex. Neuropharmacology 52: 200–214 [DOI] [PubMed] [Google Scholar]
- Chen RZ, Akbarian S, Tudor M, Jaenisch R 2001. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet 27: 327–331 [DOI] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME 2003. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science 302: 885–889 [DOI] [PubMed] [Google Scholar]
- Ching MS, Shen Y, Tan WH, Jeste SS, Morrow EM, Chen X, Mukaddes NM, Yoo SY, Hanson E, Hundley R, et al. 2010. Deletions of NRXN1 (neurexin-1) predispose to a wide spectrum of developmental disorders. Am J Med Genet B Neuropsychiatr Genet 153B: 937–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian SL, Brune CW, Sudi J, Kumar RA, Liu S, Karamohamed S, Badner JA, Matsui S, Conroy J, McQuaid D, et al. 2008. Novel submicroscopic chromosomal abnormalities detected in autism spectrum disorder. Biol Psychiatry 63: 1111–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou J, Ho G 1993. MECP2-related disorders. In Gene Reviews (Internet) (ed. Pgon RA, et al. ). University of Washington, Seattle [Google Scholar]
- Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Sudhof TC 2007. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron 54: 919–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton-Smith J 1993. Clinical research on Angelman syndrome in the United Kingdom: Observations on 82 affected individuals. Am J Med Genet 46: 12–15 [DOI] [PubMed] [Google Scholar]
- Cohen D, Lazar G, Couvert P, Desportes V, Lippe D, Mazet P, Heron D 2002. MECP2 mutation in a boy with language disorder and schizophrenia. Am J Psychiatry 159: 148–149 [DOI] [PubMed] [Google Scholar]
- Collins AL, Levenson JM, Vilaythong AP, Richman R, Armstrong DL, Noebels JL, Sweatt JD, Zoghbi HY 2004. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum Mol Genet 13: 2676–2689 [DOI] [PubMed] [Google Scholar]
- Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, Greenough WT 1997. Abnormal dendritic spines in fragile X knockout mice: Maturation and pruning deficits. Proc Natl Acad Sci 94: 5401–5404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook EH Jr, Courchesne RY, Cox NJ, Lord C, Gonen D, Guter SJ, Lincoln A, Nix K, Haas R, Leventhal BL, et al. 1998. Linkage-disequilibrium mapping of autistic disorder, with 15q11–13 markers. Am J Hum Genet 62: 1077–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crino PB, Nathanson KL, Henske EP 2006. The tuberous sclerosis complex. N Engl J Med 355: 1345–1356 [DOI] [PubMed] [Google Scholar]
- Curatolo P, Bombardieri R, Jozwiak S 2008. Tuberous sclerosis. Lancet 372: 657–668 [DOI] [PubMed] [Google Scholar]
- Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB 2005. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett Syndrome. Proc Natl Acad Sci 102: 12560–12565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoud H, Bonnet-Brilhault F, Vedrine S, Demattei MV, Vourc’h P, Bayou N, Andres CR, Barthelemy C, Laumonnier F, Briault S 2009. Autism and nonsyndromic mental retardation associated with a de novo mutation in the NLGN4Xgene promoter causing an increased expression level. Biol Psychiatry 66: 906–910 [DOI] [PubMed] [Google Scholar]
- Del Gaudio D, Fang P, Scaglia F, Ward PA, Craigen WJ, Glaze DG, Neul JL, Patel A, Lee JA, Irons M, et al. 2006. Increased MECP2 gene copy number as the result of genomic duplication in neurodevelopmentally delayed males. Genet Med 8: 784–792 [DOI] [PubMed] [Google Scholar]
- de Vries PJ 2010. Targeted treatments for cognitive and neurodevelopmental disorders in tuberous sclerosis complex. Neurotherapeutics 7: 275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar SU, del Gaudio D, German JR, Peters SU, Ou Z, Bader PI, Berg JS, Blazo M, Brown CW, Graham BH, et al. 2010. 22q13.3 deletion syndrome: Clinical and molecular analysis using array CGH. Am J Med Genet A 152A: 573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF 2007. Correction of fragile X syndrome in mice. Neuron 56: 955–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Weed SA, Xiong WC, Marshall TD, Parsons JT 1998. Identification of a novel cortactin SH3 domain-binding protein and its localization to growth cones of cultured neurons. Mol Cell Biol 18: 5838–5851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsater H, et al. 2007. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet 39: 25–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Silva AJ 2011. Rapamycin for treating tuberous sclerosis and autism spectrum disorders. Trends Mol Med 17: 78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ 2008a. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med 14: 843–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Li W, Fox K, Stryker MP, Silva AJ 2008b. Reversing neurodevelopmental disorders in adults. Neuron 60: 950–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YH, Kuhl DPA, Pizutti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJMH, Holden JJA, Fenwick RG Jr, Warren ST, et al. 1991. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell 67: 1047–1058 [DOI] [PubMed] [Google Scholar]
- Fyffe SL, Neul JL, Samaco RC, Chao HT, Ben-Shachar S, Moretti P, McGill BE, Goulding EH, Sullivan E, Tecott LH, et al. 2008. Deletion of Mecp2 in Sim1-expressing neurons reveals a critical role for MeCP2 in feeding behavior, aggression, and the response to stress. Neuron 59: 947–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier J, Champagne N, Lafrenière RG, Xiong L, Spiegelman D, Brustein E, Lapointe M, Peng H, Côté AB, Noreau A, et al. 2010. De novo mutations in the gene encoding the synaptic scaffolding protein SHANK3 in patients ascertained for schizophrenia. Proc Natl Acad Sci 107: 7863–7868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier J, Siddiqui TJ, Huashan P, Yokomaku D, Hamdan FF, Champagne N, Lapointe M, Spiegelman D, Noreau A, Lafreniere RG, et al. 2011. Truncating mutations in NRXN2 and NRXN1 in autism spectrum disorders and schizophrenia. Hum Genet 130: 563–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemelli T, Berton O, Nelson ED, Perrotti LI, Jaenisch R, Monteggia LM 2006. Postnatal loss of methyl-CpG binding protein 2 in the forebrain is sufficient to mediate behavioral aspects of Rett syndrome in mice. Biol Psychiatry 59: 468–476 [DOI] [PubMed] [Google Scholar]
- Ghosh RP, Horowitz-Scherer RA, Nikitina T, Shlyakhtenko LS, Woodcock CL 2010. MeCP2 binds cooperatively to its substrate and competes with histone H1 for chromatin binding sites. Mol Cell Biol 30: 4656–4670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacometti E, Luikenhuis S, Beard C, Jaenisch R 2007. Partial rescue of MeCP2 deficiency by postnatal activation of MeCP2. Proc Natl Acad Sci 104: 1931–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorden SM, van Woerden GM, van der Weerd L, Cheadle JP, Elgersma Y 2007. Cognitive deficits in Tsc1+/− mice in the absence of cerebral lesions and seizures. Ann Neurol 62: 648–655 [DOI] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM 2004. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 119: 1013–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasshoff U, Bonin M, Goehring I, Ekici A, Dufke A, Cremer K, Wagner N, Rossier E, Jauch A, Walter M, et al. 2011. De novo MECP2 duplication in two females with random X-inactivation and moderate mental retardation. Eur J Hum Genet 19: 507–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Klintsova AY, Irwin SA, Galvez R, Bates KE, Weiler IJ 2001. Synaptic regulation of protein synthesis and the fragile X protein. Proc Natl Acad Sci 98: 7101–7106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer PL, Hanayama R, Bloodgood BL, Mardinly AR, Lipton DM, Flavell SW, Kim TK, Griffith EC, Waldon Z, Maehr R, et al. 2010. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell 140: 704–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Nakamoto M, Yao X, Chan CB, Yim SY, Ye K, Warren ST, Bassell GJ 2010. Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome. J Neurosci 30: 10624–10638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A 2001. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet 27: 322–326 [DOI] [PubMed] [Google Scholar]
- Guy J, Gan J, Selfridge J, Cobb S, Bird A 2007. Reversal of neurological defects in a mouse model of Rett syndrome. Science 315: 1143–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg B 1995. Clinical delineation of Rett syndrome variants. Neuropediatrics 26: 62. [DOI] [PubMed] [Google Scholar]
- Hagberg B 2005. Rett syndrome: Long-term clinical follow-up experiences over four decades. J Child Neurol 20: 722–727 [DOI] [PubMed] [Google Scholar]
- Hagberg B, Aicardi J, Dias K, Ramos O 1983. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: Report of 35 cases. Ann Neurol 14: 471–479 [DOI] [PubMed] [Google Scholar]
- Hagerman PJ, Hagerman RJ 2004. Fragile X-associated tremor/ataxia syndrome (FXTAS). Ment Retard Dev Disabil Res Rev 10: 25–30 [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Van Housen K, Smith AC, McGavran L 1984. Consideration of connective tissue dysfunction in the fragile X syndrome. Am J Med Genet 17: 111–121 [DOI] [PubMed] [Google Scholar]
- Han JM, Sahin M 2011. TSC1/TSC2 signaling in the CNS. FEBS Lett 585: 973–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison CJ, Jack EM, Allen TD, Harris R 1983. The fragile X: A scanning electron microscope study. J Med Genet 20: 280–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata Y, Butz S, Sudhof TC 1996. CASK: A novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci 16: 2488–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrich B, Bird A 1998. Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol Cell Biol 18: 6538–6547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung AY, Futai K, Sala C, Valtschanoff JG, Ryu J, Woodworth MA, Kidd FL, Sung CC, Miyakawa T, Bear MF, et al. 2008. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci 28: 1697–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppke P, Maier EM, Warnke A, Brendel C, Laccone F, Gartner J 2006. Very mild cases of Rett syndrome with skewed X inactivation. J Med Genet 43: 814–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Sudhof TC 1995. Neuroligin 1: A splice site-specific ligand for β-neurexins. Cell 81: 435–443 [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Nguyen T, Sudhof TC 1996. Structures, alternative splicing, and neurexin binding of multiple neuroligins. J Biol Chem 271: 2676–2682 [DOI] [PubMed] [Google Scholar]
- Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl TW, Sudhof TC 1997. Binding of neuroligins to PSD-95. Science 277: 1511–1515 [DOI] [PubMed] [Google Scholar]
- Irwin SA, Galvez R, Greenough WT 2000. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex 10: 1038–1044 [DOI] [PubMed] [Google Scholar]
- Jacquemont S, Curie A, des Portes V, Torrioli MG, Berry-Kravis E, Hagerman RJ, Ramos FJ, Cornish K, He Y, Paulding C, et al. 2011. Epigenetic modification of the FMR1gene in fragile X syndrome is associated with differential response to the mGluR5 antagonist AFQ056. Sci Transl Med 3: 64ra61. [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, et al. 2003. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet 34: 27–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, et al. 2008. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci 105: 1710–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay V 1999. Tuberous sclerosis. Pediatr Dev Pathol 2: 197–198 [DOI] [PubMed] [Google Scholar]
- Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N, Strouboulis J, Wolffe AP 1998. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet 19: 187–191 [DOI] [PubMed] [Google Scholar]
- Jordan C, Li HH, Kwan HC, Francke U 2007. Cerebellar gene expression profiles of mouse models for Rett syndrome reveal novel MeCP2 targets. BMC Med Genet 8: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya A, Kitabatake Y, Sawa A 2008. Neurodevelopmental disturbance in the pathogenesis of major mental disorders. Brain Nerve 60: 445–452 [PubMed] [Google Scholar]
- Kelleher RJ III, Bear MF 2008. The autistic neuron: Troubled translation? Cell 135: 401–406 [DOI] [PubMed] [Google Scholar]
- Kifayathullah LA, Arunachalam JP, Bodda C, Agbemenyah HY, Laccone FA, Mannan AU 2010. MeCP2 mutant protein is expressed in astrocytes as well as in neurons and localizes in the nucleus. Cytogenet Genome Res 129: 290–297 [DOI] [PubMed] [Google Scholar]
- Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, Shen Y, Lally E, Weiss LA, Najm J, Kutsche K, et al. 2008. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet 82: 199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi N, Macklis JD 2004. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol Cell Neurosci 27: 306–321 [DOI] [PubMed] [Google Scholar]
- Kishino T, Lalande M, Wagstaff J 1997. UBE3A/E6-AP mutations cause Angelman syndrome. Nat Genet 15: 70–73 [DOI] [PubMed] [Google Scholar]
- Klauck SM, Lindsay S, Beyer KS, Splitt M, Burn J, Poustka A 2002. A mutation hot spot for nonspecific X-linked mental retardation in the MECP2 gene causes the PPM-X syndrome. Am J Hum Genet 70: 1034–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD, Ogier M, Kunze DL, Katz DM 2010. Exogenous brain-derived neurotrophic factor rescues synaptic dysfunction in Mecp2-null mice. J Neurosci 30: 5303–5310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl MM, Paulsen O 2010. The roles of GABAB receptors in cortical network activity. Adv Pharmacol 58: 205–229 [DOI] [PubMed] [Google Scholar]
- Krueger DD, Bear MF 2011. Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annu Rev Med 62: 411–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasalle JM, Yasui DH 2009. Evolving role of MeCP2 in Rett syndrome and autism. Epigenomics 1: 119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP, Raynaud M, Ronce N, Lemonnier E, Calvas P, et al. 2004. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet 74: 552–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson-Yuen A, Saldivar JS, Sommer S, Picker J 2008. Familial deletion within NLGN4 associated with autism and Tourette syndrome. Eur J Hum Genet 16: 614–618 [DOI] [PubMed] [Google Scholar]
- Le Meur N, Holder-Espinasse M, Jaillard S, Goldenberg A, Joriot S, Amati-Bonneau P, Guichet A, Barth M, Charollais A, Journel H, et al. 2010. MEF2C haploinsufficiency caused by either microdeletion of the 5q14.3 region or mutation is responsible for severe mental retardation with stereotypic movements, epilepsy and/or cerebral malformations. J Med Genet 47: 22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenga J, de Vrij FM, Oostra BA, Willemsen R 2010. Potential therapeutic interventions for fragile X syndrome. Trends Mol Med 16: 516–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Meehan RR, Henzel WJ, Maurer-Fogy I, Jeppesen P, Klein F, Bird A 1992. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell 69: 905–914 [DOI] [PubMed] [Google Scholar]
- Lim S, Naisbitt S, Yoon J, Hwang JI, Suh PG, Sheng M, Kim E 1999. Characterization of the Shank family of synaptic proteins. Multiple genes, alternative splicing, and differential expression in brain and development. J Biol Chem 274: 29510–29518 [DOI] [PubMed] [Google Scholar]
- Lubs HA 1969. A marker X chromosome. Am J Hum Genet 21: 231–244 [PMC free article] [PubMed] [Google Scholar]
- Maezawa I, Jin LW 2010. Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. J Neurosci 30: 5346–5356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I, Swanberg S, Harvey D, LaSalle JM, Jin LW 2009. Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions. J Neurosci 29: 5051–5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magenis RE, Brown MG, Lacy DA, Budden S, LaFranchi S 1987. Is Angelman syndrome an alternate result of del(15)(q11q13)? Am J Med Genet 28: 829–838 [DOI] [PubMed] [Google Scholar]
- Marchetto MC, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, Chen G, Gage FH, Muotri AR 2010. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 143: 527–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Bell J 1943. A pedigree of mental defect showing sex-linkage. Arch Neurol Psychiat 6: 154–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE 2003. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science 302: 890–893 [DOI] [PubMed] [Google Scholar]
- Matsuura T, Sutcliffe JS, Fang P, Galjaard RJ, Jiang YH, Benton CS, Rommens JM, Beaudet AL 1997. De novo truncating mutations in E6-AP ubiquitin-protein ligase gene (UBE3A) in Angelman syndrome. Nat Genet 15: 74–77 [DOI] [PubMed] [Google Scholar]
- Maunakea AK, Nagarajan RP, Bilenky M, Ballinger TJ, D'Souza C, Fouse SD, Johnson BE, Hong C, Nielsen C, Zhao Y, et al. 2010. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature 466: 253–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurry CL, Shepherd JD, Tropea D, Wang KH, Bear MF, Sur M 2010. Loss of Arc renders the visual cortex impervious to the effects of sensory experience or deprivation. Nat Neurosci 13: 450–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrihan L, Tantalaki E, Aramuni G, Sargsyan V, Dudanova I, Missler M, Zhang W 2008. Early defects of GABAergic synapses in the brain stem of a MeCP2 mouse model of Rett syndrome. J Neurophysiol 99: 112–121 [DOI] [PubMed] [Google Scholar]
- Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, Kwiatkowski DJ 2008. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: Effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci 28: 5422–5432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meins M, Lehmann J, Gerresheim F, Herchenbach J, Hagedorn M, Hameister K, Epplen JT 2005. Submicroscopic duplication in Xq28 causes increased expression of the MECP2 gene in a boy with severe mental retardation and features of Rett syndrome. J Med Genet 42: e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler M, Sudhof TC 1998. Neurexins: Three genes and 1001 products. Trends Genet 14: 20–26 [DOI] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Sudhof TC 2003. α-Neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature 423: 939–948 [DOI] [PubMed] [Google Scholar]
- Moessner R, Marshall CR, Sutcliffe JS, Skaug J, Pinto D, Vincent J, Zwaigenbaum L, Fernandez B, Roberts W, Szatmari P, et al. 2007. Contribution of SHANK3 mutations to autism spectrum disorder. Am J Hum Genet 81: 1289–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P, Levenson JM, Battaglia F, Atkinson R, Teague R, Antalffy B, Armstrong D, Arancio O, Sweatt JD, Zoghbi HY 2006. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J Neurosci 26: 319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, Weinberg RJ, Worley PF, Sheng M 1999. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron 23: 569–582 [DOI] [PubMed] [Google Scholar]
- Nam CI, Chen L 2005. Postsynaptic assembly induced by neurexin–neuroligin interaction and neurotransmitter. Proc Natl Acad Sci 102: 6137–6142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A 1998. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393: 386–389 [DOI] [PubMed] [Google Scholar]
- Neul JL, Fang P, Barrish J, Lane J, Caeg EB, Smith EO, Zoghbi H, Percy A, Glaze DG 2008. Specific mutations in methyl-CpG-binding protein 2 confer different severity in Rett syndrome. Neurology 70: 1313–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neul JL, Kaufmann WE, Glaze DG, Christodoulou J, Clarke AJ, Bahi-Buisson N, Leonard H, Bailey ME, Schanen NC, Zappella M, et al. 2010. Rett syndrome: Revised diagnostic criteria and nomenclature. Ann Neurol 68: 944–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve RL, Bear MF 1989. Visual experience regulates gene expression in the developing striate cortex. Proc Natl Acad Sci 86: 4781–4784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls RD, Knoll JHM, Butler MG, Karam S, Lalande M 1989. Genetic imprinting suggested by maternal heterodisomy in non-deletion Prader-Willi syndrome. Nature 342: 281–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie D, Di Nardo A, Han JM, Baharanyi H, Kramvis I, Huynh T, Dabora S, Codeluppi S, Pandolfi PP, Pasquale EB, et al. 2010. Tsc2–Rheb signaling regulates EphA-mediated axon guidance. Nat Neurosci 13: 163–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuber UA, Kriaucionis S, Roloff TC, Guy J, Selfridge J, Steinhoff C, Schulz R, Lipkowitz B, Ropers HH, Holmes MC, et al. 2005. Up-regulation of glucocorticoid-regulated genes in a mouse model of Rett syndrome. Hum Mol Genet 14: 2247–2256 [DOI] [PubMed] [Google Scholar]
- Numis AL, Major P, Montenegro MA, Muzykewicz DA, Pulsifer MB, Thiele EA 2011. Identification of risk factors for autism spectrum disorders in tuberous sclerosis complex. Neurology 76: 981–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell WT, Warren ST 2002. A decade of molecular studies of fragile X syndrome. Annu Rev Neurosci 25: 315–338 [DOI] [PubMed] [Google Scholar]
- Ohta T, Buiting K, Kokkonen H, McCandless S, Heeger S, Leisti H, Driscoll DJ, Cassidy SB, Horsthemke B, Nicholls RD 1999. Molecular mechanism of Angelman syndrome in two large families involves an imprinting mutation. Am J Hum Genet 64: 385–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto N, Kubota T, Nakamura Y, Murakami R, Nishikubo T, Tanaka I, Takahashi Y, Hayashi S, Imoto I, Inazawa J, et al. 2007. 22q13 microduplication in two patients with common clinical manifestations: A recognizable syndrome? Am J Med Genet A 143A: 2804–2809 [DOI] [PubMed] [Google Scholar]
- Olmos-Serrano JL, Paluszkiewicz SM, Martin BS, Kaufmann WE, Corbin JG, Huntsman MM 2010. Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome. J Neurosci 30: 9929–9938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova KA, Crino PB 2010. The tuberous sclerosis complex. Ann NY Acad Sci 1184: 87–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr HT, Zoghbi HY 2007. Trinucleotide repeat disorders. Annu Rev Neurosci 30: 575–621 [DOI] [PubMed] [Google Scholar]
- Osterweil EK, Krueger DD, Reinhold K, Bear MF 2010. Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J Neurosci 30: 15616–15627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacey LK, Heximer SP, Hampson DR 2009. Increased GABA(B) receptor-mediated signaling reduces the susceptibility of fragile X knockout mice to audiogenic seizures. Mol Pharmacol 76: 18–24 [DOI] [PubMed] [Google Scholar]
- Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G 2011. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 472: 437–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelka GJ, Watson CM, Radziewic T, Hayward M, Lahooti H, Christodoulou J, Tam PP 2006. Mecp2 deficiency is associated with learning and cognitive deficits and altered gene activity in the hippocampal region of mice. Brain 129: 887–898 [DOI] [PubMed] [Google Scholar]
- Petrij F, Giles RH, Dauwerse HG, Saris JJ, Hennekam RC, Masuno M, Tommerup N, van Ommen GJ, Goodman RH, Peters DJ, et al. 1995. Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature 376: 348–351 [DOI] [PubMed] [Google Scholar]
- Phelan MC 2008. Deletion 22q13.3 syndrome. Orphanet J Rare Dis 3: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan MC, Rogers RC, Saul RA, Stapleton GA, Sweet K, McDermid H, Shaw SR, Claytor J, Willis J, Kelly DP 2001. 22q13 deletion syndrome. Am J Med Genet 101: 91–99 [DOI] [PubMed] [Google Scholar]
- Philpot BD, Thompson CE, Franco L, Williams CA 2010. Angelman syndrome: Advancing the research frontier of neurodevelopmental disorders. J Neurodev Disord 3: 50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M, Kang J, Burlin TV, Jiang C, Smith CB 2005. Postadolescent changes in regional cerebral protein synthesis: An in vivo study in the Fmr1 null mouse. J Neurosci 25: 5087–5095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radyushkin K, Hammerschmidt K, Boretius S, Varoqueaux F, El-Kordi A, Ronnenberg A, Winter D, Frahm J, Fischer J, Brose N, et al. 2009. Neuroligin-3-deficient mice: Model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav 8: 416–425 [DOI] [PubMed] [Google Scholar]
- Ramocki MB, Peters SU, Tavyev YJ, Zhang F, Carvalho CM, Schaaf CP, Richman R, Fang P, Glaze DG, Lupski JR, et al. 2009. Autism and other neuropsychiatric symptoms are prevalent in individuals with MeCP2 duplication syndrome. Ann Neurol 66: 771–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramocki MB, Tavyev YJ, Peters SU 2010. The MECP2 duplication syndrome. Am J Med Genet A 152A: 1079–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rett A 1966. On an unusual brain atrophy syndrome in hyperammonemia in childhood. Wien Med Wochenschr 116: 723–726 [PubMed] [Google Scholar]
- Ricciardi S, Boggio EM, Grosso S, Lonetti G, Forlani G, Stefanelli G, Calcagno E, Morello N, Landsberger N, Biffo S, et al. 2011. Reduced AKT/mTOR signaling and protein synthesis dysregulation in a Rett syndrome animal model. Hum Mol Genet 20: 1182–1196 [DOI] [PubMed] [Google Scholar]
- Roelfsema JH, White SJ, Ariyurek Y, Bartholdi D, Niedrist D, Papadia F, Bacino CA, den Dunnen JT, van Ommen GJ, Breuning MH, et al. 2005. Genetic heterogeneity in Rubinstein-Taybi syndrome: Mutations in both the CBP and EP300 genes cause disease. Am J Hum Genet 76: 572–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussignol G, Ango F, Romorini S, Tu JC, Sala C, Worley PF, Bockaert J, Fagni L 2005. Shank expression is sufficient to induce functional dendritic spine synapses in aspiny neurons. J Neurosci 25: 3560–3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rujescu D, Ingason A, Cichon S, Pietilainen OP, Barnes MR, Toulopoulou T, Picchioni M, Vassos E, Ettinger U, Bramon E, et al. 2009. Disruption of the neurexin 1 gene is associated with schizophrenia. Hum Mol Genet 18: 988–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala C, Piech V, Wilson NR, Passafaro M, Liu G, Sheng M 2001. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron 31: 115–130 [DOI] [PubMed] [Google Scholar]
- Samaco RC, Mandel-Brehm C, Chao HT, Ward CS, Fyffe-Maricich SL, Ren J, Hyland K, Thaller C, Maricich SM, Humphreys P, et al. 2009. Loss of MeCP2 in aminergic neurons causes cell-autonomous defects in neurotransmitter synthesis and specific behavioral abnormalities. Proc Natl Acad Sci 106: 21966–21971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Stryker MP 2010. Genomic imprinting of experience-dependent cortical plasticity by the ubiquitin ligase gene Ube3a. Proc Natl Acad Sci 107: 5611–5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T 2000. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 101: 657–669 [DOI] [PubMed] [Google Scholar]
- Shahbazian M, Young J, Yuva-Paylor L, Spencer C, Antalffy B, Noebels J, Armstrong D, Paylor R, Zoghbi H 2002a. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron 35: 243–254 [DOI] [PubMed] [Google Scholar]
- Shahbazian MD, Antalffy B, Armstrong DL, Zoghbi HY 2002b. Insight into Rett syndrome: MeCP2 levels display tissue- and cell-specific differences and correlate with neuronal maturation. Hum Mol Genet 11: 115–124 [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Bear MF 2011. New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci 14: 279–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene PJ, Illingworth RS, Webb S, Kerr AR, James KD, Turner DJ, Andrews R, Bird AP 2010. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol Cell 37: 457–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JE, Perciaccante RG, Greig GM, Willard HF, Ledbetter DH, Hejtmancik JF, Pollack MS, O’Brien WE, Beaudet AL 1988. Uniparental disomy as a mechanism for human genetic disease. Am J Hum Genet 42: 217–226 [PMC free article] [PubMed] [Google Scholar]
- Stankiewicz P, Lupski JR 2010. Structural variation in the human genome and its role in disease. Annu Rev Med 61: 437–455 [DOI] [PubMed] [Google Scholar]
- Sudhof TC 2008. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455: 903–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Sudhof TC 2002. Structure and evolution of neurexin genes: Insight into the mechanism of alternative splicing. Genomics 79: 849–859 [DOI] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Sudhof TC 2007. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science 318: 71–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Hu K, Chang Q, Wu H, Sherman NE, Martinowich K, Klose RJ, Schanen C, Jaenisch R, Wang W, et al. 2009. Phosphorylation of MeCP2 at serine 80 regulates its chromatin association and neurological function. Proc Natl Acad Sci 106: 4882–4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea D, Giacometti E, Wilson NR, Beard C, McCurry C, Fu DD, Flannery R, Jaenisch R, Sur M 2009. Partial reversal of Rett Syndrome-like symptoms in MeCP2 mutant mice. Proc Natl Acad Sci 106: 2029–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai P, Sahin M 2011. Mechanisms of neurocognitive dysfunction and therapeutic considerations in tuberous sclerosis complex. Curr Opin Neurol 24: 106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF 1998. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron 21: 717–726 [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, et al. 1999. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron 23: 583–592 [DOI] [PubMed] [Google Scholar]
- Tuchman R, Rapin I 2002. Epilepsy in autism. Lancet Neurol 1: 352–358 [DOI] [PubMed] [Google Scholar]
- Tudor M, Akbarian S, Chen RZ, Jaenisch R 2002. Transcriptional profiling of a mouse model for Rett syndrome reveals subtle transcriptional changes in the brain. Proc Natl Acad Sci 99: 15536–15541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Esch H, Bauters M, Ignatius J, Jansen M, Raynaud M, Hollanders K, Lugtenberg D, Bienvenu T, Jensen LR, Gecz J, et al. 2005. Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. Am J Hum Genet 77: 442–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Woerden GM, Harris KD, Hojjati MR, Gustin RM, Qiu S, de Avila Freire R, Jiang YH, Elgersma Y, Weeber EJ 2007. Rescue of neurological deficits in a mouse model for Angelman syndrome by reduction of αCaMKII inhibitory phosphorylation. Nat Neurosci 10: 280–282 [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N 2006. Neuroligins determine synapse maturation and function. Neuron 51: 741–754 [DOI] [PubMed] [Google Scholar]
- Verkerk AJMH, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DPA, Pizutti A, Reiner O, Richards S, Victoria MF, Zhang R, et al. 1991. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65: 905–914 [DOI] [PubMed] [Google Scholar]
- von der Brelie C, Waltereit R, Zhang L, Beck H, Kirschstein T 2006. Impaired synaptic plasticity in a rat model of tuberous sclerosis. Eur J Neurosci 23: 686–692 [DOI] [PubMed] [Google Scholar]
- von Reyn CF, Roberts TM, Owen R, Beaver PC 1978. Infection of an infant with an adult Toxocara cati (Nematoda). J Pediatr 93: 247–249 [DOI] [PubMed] [Google Scholar]
- Wang LW, Berry-Kravis E, Hagerman RJ 2010. Fragile X: Leading the way for targeted treatments in autism. Neurotherapeutics 7: 264–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HL, Wong AC, Shaw SR, Tse WY, Stapleton GA, Phelan MC, Hu S, Marshall J, McDermid HE 2003. Molecular characterisation of the 22q13 deletion syndrome supports the role of haploinsufficiency of SHANK3/PROSAP2 in the major neurological symptoms. J Med Genet 40: 575–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao I, Hata Y, Hirao K, Deguchi M, Ide N, Takeuchi M, Takai Y 1999. Synamon, a novel neuronal protein interacting with synapse-associated protein 90/postsynaptic density-95-associated protein. J Biol Chem 274: 27463–27466 [DOI] [PubMed] [Google Scholar]
- Yashiro K, Riday TT, Condon KH, Roberts AC, Bernardo DR, Prakash R, Weinberg RJ, Ehlers MD, Philpot BD 2009. Ube3a is required for experience-dependent maturation of the neocortex. Nat Neurosci 12: 777–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui DH, Peddada S, Bieda MC, Vallero RO, Hogart A, Nagarajan RP, Thatcher KN, Farnham PJ, Lasalle JM 2007. Integrated epigenomic analyses of neuronal MeCP2 reveal a role for long-range interaction with active genes. Proc Natl Acad Sci 104: 19416–19421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahir FR, Baross A, Delaney AD, Eydoux P, Fernandes ND, Pugh T, Marra MA, Friedman JM 2008. A patient with vertebral, cognitive and behavioural abnormalities and a de novo deletion of NRXN1α. J Med Genet 45: 239–243 [DOI] [PubMed] [Google Scholar]
- Zappella M, Meloni I, Longo I, Hayek G, Renieri A 2001. Preserved speech variants of the Rett syndrome: Molecular and clinical analysis. Am J Med Genet 104: 14–22 [DOI] [PubMed] [Google Scholar]
- Zhang L, He J, Jugloff DG, Eubanks JH 2008. The MeCP2-null mouse hippocampus displays altered basal inhibitory rhythms and is prone to hyperexcitability. Hippocampus 18: 294–309 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Hong EJ, Cohen S, Zhao WN, Ho HY, Schmidt L, Chen WG, Lin Y, Savner E, Griffith EC, et al. 2006. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron 52: 255–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Blundell J, Ogawa S, Kwon CH, Zhang W, Sinton C, Powell CM, Parada LF 2009. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J Neurosci 29: 1773–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitzer H, Honck HH, Bachner D, Richter D, Kreienkamp HJ 1999. Somatostatin receptor interacting protein defines a novel family of multidomain proteins present in human and rodent brain. J Biol Chem 274: 32997–33001 [DOI] [PubMed] [Google Scholar]
- Zweier C, de Jong EK, Zweier M, Orrico A, Ousager LB, Collins AL, Bijlsma EK, Oortveld MA, Ekici AB, Reis A, et al. 2009. CNTNAP2 and NRXN1 are mutated in autosomal-recessive Pitt-Hopkins-like mental retardation and determine the level of a common synaptic protein in Drosophila. Am J Hum Genet 85: 655–666 [DOI] [PMC free article] [PubMed] [Google Scholar]