Abstract
Acute chest pain in hypertensive patients presenting to the emergency room constitute a wide spectrum of life threatening conditions including an acute aortic dissection. Acute Aortic syndromes constitute uncommon but lethal identities, with high morbidity and mortality requiring a high index of suspicion, appropriate diagnostic tools and urgent line of management. The authors are reporting a case of an elderly hypertensive lady, presenting with acute chest pain secondary to type B aortic dissection, which was missed on the initial presentation. The authors reviewed the current practice of diagnosing and managing acute aortic dissection.
Case Report
A 72 years old female patient presented to our emergency room, with central chest pain radiating to upper abdomen and both shoulders and backache for the last two weeks, exacerbated for the last three hours. Patient had no history of dyspnea, palpitation, diaphoresis, vomiting or any urinary symptoms. No history of trauma was noted. Two weeks earlier she had presented with similar chest pain and left flank pain, found to have microscopic hematuria and other basic investigations done at that time came to be normal, diagnosed and treated as esophagitis and left ureteric colic. Her pain had persisted with mild relief.
A review of her medical history revealed that she had a five year history of hypertension on regular angiotensin converting enzyme inhibitor, and no history of ischemic heart disease, stroke, hyperlipidemia, diabetes or peptic ulcer disease.
She did not smoke nor consume alcohol. An initial physical examination was normal, except for high blood pressure 180/110 mmHg. Her electrocardiography showed 0.5 mm ST segment elevation in V3 and V4 anterior leads. Patient was given chewable aspirin 300 mg, intravenous opiate, antacid and a dose of intravenous anti-ulcer agent (proton pump inhibitor).
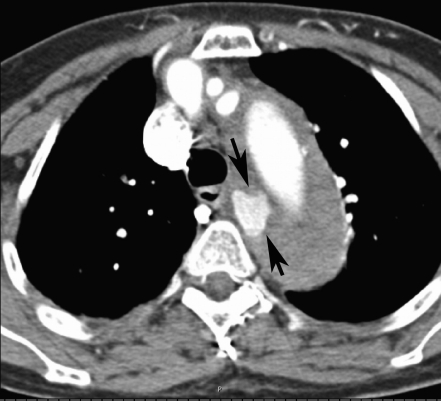
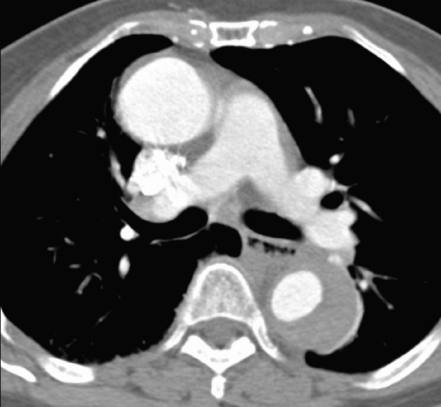
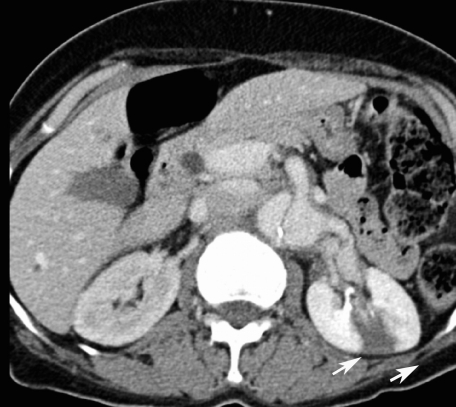
She was kept in the resuscitation area monitoring her pulse, blood pressure and on continuous electrocardiography monitor. Serial electrocardiography did not show any dynamic changes and serial cardiac enzymes were normal (troponin <0.01 ng/dl). Her laboratory tests revealed a normal full blood count, serum glucose, electrolytes, renal function, amylase and liver function test. Urine dipstick was normal. Chest X-Ray showed a widened mediastinum with double ring shadow (Figure 1). This was followed by a computed tomogram angiography (CTA) which revealed an extensive aortic dissection starting distal to the left subclavian artery, with a deep penetrating atheromatous ulcer arising at the medial wall of the descending aorta. The dissection is extending to the level of left renal artery and the left kidney was partially infarcted. The false lumen was identified to be thrombosed. The CTA findings are consistent with diagnosis of Type B Aortic dissection (Figure 2, 3 & 4).
Figure 1.
Plain radiograph of the chest shows wide mediastinum and double density over the aortic arch
Figure 2.
Axial enhanced CT image at level of the aortic arch shows thrombosed false lumen. A deep atheromatous ulcer seen arising from the medial wall of the arch. Note normal enhancing great vessels.
Figure 3.
Enhanced axial image at level of pulmonary artery. Note the thrombosed false lumen of the posterolateral aspect of the descending aorta. The ascending aorta appears normal.
Figure 4.
Axial enhanced image at level of the renal arteries demonstrating the intimal flap with displaced calcified plaque. The left renal and superior mesenteric arteries arise from the false lumen. There is a wedge shaped cortical hypodense lesion within the left kidney representing wedge infarct.
Patient was admitted to the intensive care unit and received intravenous boluses of beta-blocker and nitroprusside infusion to keep systolic blood pressure less than 110 mmHg, as well treated with intravenous opiates for her chest pain. She spent two days in the intensive care unit. Her renal functions remained normal and sent home on day seven of admission after further control of her blood pressure. Patient has been followed as an outpatient. Repeated computed tomogram angiography at six months and one year showed complete thrombosis of the false lumen with no false aneurysm formation and no change in the diameter of the aorta.
In summary, this elderly hypertensive lady has presented with an acute type B aortic dissection complicated with left renal infarction. The diagnosis was missed on the initial presentation and she was treated conservatively by controlling her blood pressure and chest pain. The left renal infarction was diagnosed late and her renal functions remained normal.
Discussion
Acute Aortic syndromes constitute an uncommon but an important cardiovascular emergency. Acute Aortic Dissection is a medical emergency with high morbidity and mortality requiring emergent diagnosis and therapy.1 Acute aortic dissection is the most common catastrophe of the aorta, with an incidence of 10 cases per 100 000 of population per year.2 Acute dissection remains a lethal disease, where 21% of patients died before hospital admission, 22.7% within 6 hours if left untreated, 50% within 24 hours, and 68% within the first week.3 This high mortality rate stresses the urgency for prompt diagnosis and initiation of appropriate therapy. Increased awareness of presenting symptoms and high index of suspicion are the key points in managing these conditions. Rapid advances in noninvasive imaging technology have facilitated the early diagnosis and proper management of patients presenting with acute chest, back or abdominal pain.
Acute aortic dissection is characterized by the development of an intimal flap separating the true and false lumen.1,3-5 The dissection can spread from the site of the intimal flap in either antegrade or retrograde fashion, involving side branches of the aorta and causing malperfusion syndrome by dynamic or static obstruction (from coronary arteries to the iliac arteries), tamponade or aortic insufficiency.5-7 There are a number of systems used to classify aortic dissections. DeBakey classification is based on the site of the intimal tear (entry site) and the extent of the dissecting hematoma. In DeBakey type I and type II dissection, the entry point is located in the ascending aorta, usually a few centimeters above the aortic valve. In type I dissection, the hematoma extends for a variable distance beyond the ascending aorta, while in type II the dissecting hematoma is confined to ascending aorta. In type III, the dissection originates in the descending aorta, typically just beyond the origin of the left subclavian artery and propagates antegrade into the descending aorta or rarely retrograde into the aortic arch and ascending aorta. Stanford classification has refined these types into the extent of the dissection rather than the entry site. Stanford type A dissection refers to all dissection that involve the ascending aorta and the entry site may be located anywhere along the aorta, while Stanford type B dissection is confined to the aorta distal to the left subclavian artery.1
Men are affected more frequently by acute dissection, with male to female ratio of 5:1. Hypertension is present in 70-80% of cases.5,8 Hypertension affects arterial wall composition causing intimal thickening, fibrosis, calcification and extracellular fatty acid deposition. The extracellular matrix undergoes an accelerated degradation, apoptosis, and elastolysis with hyalinization of collagen. These lead to intimal disruption, adventitial fibrosis, vessel obstruction and smooth muscle cells necrosis, creating aneurysms and dissections.9-11 Other risk factors include; bicuspid aortic valve, smoking, dyslipidemia, diabetes, pregnancy, cocaine use, turner syndrome, and connective tissue disorders like Marfan’s Syndrome and Ehlers-Danlos Syndrome.1,8,12 There is, as well, the iatrogenic aortic dissection associated with catheter interventions and after valve or aortic surgery.9,13
High index of suspicion is the key point in early diagnosis and management of acute dissection. Acute dissection can present with instantaneous chest, or back pain. The pain is sharp, severe and sometimes radiating and migrating in nature. History and signs of chronic hypertension, and signs of connective tissue syndrome might be present. Symptoms of specific malperfusion syndrome occur from the dissection related side branches’ occlusion or ischemia. Syncope might be present in 20% of patients with acute aortic dissection in the absence of a history of typical pain or neurological findings. Patients might present with stroke, myocardial infarction, heart failure, aortic valve regurgitation and finally cardiac tamponade, which may result in hypotension and syncope. Distal extension may present with paraplegia, visceral and renal ischemia and acute limb ischemia.
Physical examination should be thorough and complete, assessing patient’s cognitive functions, any signs of lateralization or stroke. Hemodynamic stability should be assessed looking for pulse deficits, assessing jugular venous pressure, paradoxical pulse and signs of shock. Pulse deficits gives important but uncommon clue for dissection, reported to be present in less than 20% of patients in the International Registry of Aortic Dissection (IRAD). A diastolic murmur for detection of aortic regurgitation occurs in only 40-50% of patients with proximal dissections.1,8,12
Patients with symptoms and signs of acute aortic dissection are usually missed, suspecting initially other conditions like acute coronary syndromes, pericarditis, aortic stenosis, pulmonary embolism, cholecystitis, bowel infarction and stroke. Missed diagnosis of acute aortic dissection is catastrophic and should be suspected in any patient presenting with unexplained syncope, chest pain, back pain, abdominal pain, stroke, pulse deficit, acute onset congestive heart failure, acute renal failure, acute paraplegia and acute limb ischemia.
Early diagnosis of acute aortic dissection depends mainly on a high index of suspicion. Physicians correctly suspect the entity in only 15% to 43% of the clinical presentations.3 Sometimes, it is an incidental finding while investigating for other pathology. The rapid and proper diagnosis of aortic dissection is essential given the fatality of the disease and the dramatic benefits from medical, surgical and endovascular interventions. The modalities available for diagnosing acute aortic dissection include chest radiography, contrast computed tomography, magnetic resonance imaging, trans-thoracic echocardiography, trans-esophageal echocardiography and conventional angiography. It is essential to confirm the diagnosis of acute aortic dissection and to give the extent of the dissection and the extent of life threatening complications like tamponade.
Chest radiography is suggestive but not diagnostic for aortic dissection. Widening of aortic silhouette is present in 60% to 90% of cases.8,12 Displacement of aortic calcification and pleural effusions might be present on chest radiography.14 Contrast-enhanced computed tomography (CT) is less invasive and has sensitivity of 83% and specificity of 87% to 100% in diagnosis of acute aortic dissection.15,16 CT scan was the most common diagnostic tool used in patients in the International Registry of Acute Aortic Dissection (IRAD).8,17 The recent advances in multi-slice CT technology made it easier and more rapid scanning times with improved spatial resolution and reduced helical artifacts. CT scan can identify intramural hematoma, intimal flaps, true and false lumens, thickening of the aortic wall with internal displacement of intimal calcifications.
Magnetic Resonance Imaging (MRI) is a dynamic noninvasive imaging technique that gives high resolution structural and functional information. It provides excellent anatomic details about heart and aorta; it can differentiate between true and false lumen by differentiating slow flowing blood and clot from high flowing blood. In addition, MRI can determine the presence of aortic insufficiency. MRI has 95% sensitivity and 100% specificity in diagnosing aortic dissection. Three dimensional gadolinium enhanced magnetic resonance angiography techniques allows rapid identification of thoracic and abdominal aorta and their branch vessels.
Trans-thoracic echocardiography (TTE) can evaluate the aortic root and arch, but the distal ascending aorta and descending aorta are not well visualized. TTE has sensitivity of 77% to 80% and specificity of 93% to 96%. Main technical limitations are narrow intercostal spaces, obesity, and emphysema. Trans-esophageal echocardiography (TEE) overcomes the limitations of TTE because of proximity of esophagus to the aorta. TEE has sensitivity of 98% and specificity of 95%.18,19 In contrary to CT and MRI, TEE has the advantage that it can be performed in unstable patients. A disadvantage of TEE is its limitation to visualize distal thoracic and abdominal aorta. TTE and TEE is operator dependant.
Conventional angiography which used to be the gold standard for diagnosing aortic dissection, has been replaced by axial imaging modalities. Aortography has a sensitivity of 86% to 88%, and specificity of 94% for diagnosing aortic dissection.20 False negative aortograms occurs when the false lumen does not opacify, both true and false lumen opacify at the same time, the intimal flap is not detected, or when the intramural hematoma is not detected.
Surgical therapy is the mainstay of treatment for type A dissections. While for type B dissection, surgery did not prove superiority over medical treatment, in fact it ends up with more morbidities and mortalities. Hence, medical treatment remains the option for uncomplicated Type B dissections. Surgical therapy, or nowadays the endovascular therapy, is indicated in type B whenever there are complications like aortic branch compromise (stroke, acute ischemic limb), malperfusion syndrome (Mesenteric, Renal ischemia), acutely developed aneurysm or rupture, and in case of uncontrollable pain or uncontrolled high blood pressure.
References
- 1.Mukherjee D, Eagle KA. Aortic dissection–an update. Curr Probl Cardiol 2005. Jun;30(6):287-325 10.1016/j.cpcardiol.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 2.Svensson LG, Crawford ES. Aortic dissection and aortic aneurysm surgery: clinical observations, experimental investigations, and statistical analyses. Part II. Curr Probl Surg 1992. Dec;29(12):913-1057 10.1016/0011-3840(92)90003-L [DOI] [PubMed] [Google Scholar]
- 3.Mészáros I, Mórocz J, Szlávi J, Schmidt J, Tornóci L, Nagy L, et al. Epidemiology and clinicopathology of aortic dissection. Chest 2000. May;117(5):1271-1278 10.1378/chest.117.5.1271 [DOI] [PubMed] [Google Scholar]
- 4.Prêtre R, Von Segesser LK. Aortic dissection. Lancet 1997. May;349(9063):1461-1464 10.1016/S0140-6736(96)09372-5 [DOI] [PubMed] [Google Scholar]
- 5.Hirst AE, Jr, Johns VJ, Jr, Kime SW., Jr Dissecting aneurysm of the aorta: a review of 505 cases. Medicine (Baltimore) 1958. Sep;37(3):217-279 10.1097/00005792-195809000-00003 [DOI] [PubMed] [Google Scholar]
- 6.Masuda Y, Takanashi K, Takasu J, Watanabe S. Natural history and prognosis of medical treatment for the patients with aortic dissections. Nippon Geka Gakkai Zasshi 1996. Oct;97(10):890-893 [PubMed] [Google Scholar]
- 7.Roberts CS, Roberts WC. Aortic dissection with the entrance tear in the descending thoracic aorta. Analysis of 40 necropsy patients. Ann Surg 1991. Apr;213(4):356-368 10.1097/00000658-199104000-00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000. Feb;283(7):897-903 10.1001/jama.283.7.897 [DOI] [PubMed] [Google Scholar]
- 9.von Kodolitsch Y, Aydin MA, Koschyk DH, Loose R, Schalwat I, Karck M, et al. Predictors of aneurysmal formation after surgical correction of aortic coarctation. J Am Coll Cardiol 2002. Feb;39(4):617-624 10.1016/S0735-1097(01)01784-3 [DOI] [PubMed] [Google Scholar]
- 10.Reed D, Reed C, Stemmermann G, Hayashi T. Are aortic aneurysms caused by atherosclerosis? Circulation 1992. Jan;85(1):205-211 [DOI] [PubMed] [Google Scholar]
- 11.Larson EW, Edwards WD. Risk factors for aortic dissection: a necropsy study of 161 cases. Am J Cardiol 1984. Mar;53(6):849-855 10.1016/0002-9149(84)90418-1 [DOI] [PubMed] [Google Scholar]
- 12.Spittell PC, Spittell JA, Jr, Joyce JW, Tajik AJ, Edwards WD, Schaff HV, et al. Clinical features and differential diagnosis of aortic dissection: experience with 236 cases (1980 through 1990). Mayo Clin Proc 1993. Jul;68(7):642-651 [DOI] [PubMed] [Google Scholar]
- 13.Januzzi JL, Sabatine MS, Eagle KA, Evangelista A, Bruckman D, Fattori R, et al. Latrogenic aortic dissection. Am J Cardiol 2002;89:263-266 . 10.1016/S0002-9149(01)02312-8 [DOI] [PubMed] [Google Scholar]
- 14.Barbant SD, Eisenberg MJ, Schiller NB. The diagnostic value of imaging techniques for aortic dissection. Am Heart J 1992. Aug;124(2):541-543 10.1016/0002-8703(92)90632-6 [DOI] [PubMed] [Google Scholar]
- 15.Hartnell G, Costello P. The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med 1993. Jun;328(22):1637-1638, author reply 1638 10.1056/NEJM199306033282213 [DOI] [PubMed] [Google Scholar]
- 16.Fisher ER, Stern EJ, Godwin JD, II, Otto CM, Johnson JA. Acute aortic dissection: typical and atypical imaging features. Radiographics 1994. Nov;14(6):1263-1271, discussion 1271-1274 [DOI] [PubMed] [Google Scholar]
- 17.Moore AG, Eagle KA, Bruckman D, Moon BS, Malouf JF, Fattori R, et al. Choice of computed tomography, transesophageal echocardiography, magnetic resonance imaging, and aortography in acute aortic dissection: International Registry of Acute Aortic Dissection (IRAD). Am J Cardiol 2002. May;89(10):1235-1238 10.1016/S0002-9149(02)02316-0 [DOI] [PubMed] [Google Scholar]
- 18.Erbel R, Engberding R, Daniel W, Roelandt J, Visser C, Rennollet H. Echocardiography in diagnosis of aortic dissection. Lancet 1989. Mar;1(8636):457-461 10.1016/S0140-6736(89)91364-0 [DOI] [PubMed] [Google Scholar]
- 19.Nienaber CA, Spielmann RP, von Kodolitsch Y, Siglow V, Piepho A, Jaup T, et al. Diagnosis of thoracic aortic dissection. Magnetic resonance imaging versus transesophageal echocardiography. Circulation 1992. Feb;85(2):434-447 [DOI] [PubMed] [Google Scholar]
- 20.Cigarroa JE, Isselbacher EM, DeSanctis RW, Eagle KA. Diagnostic imaging in the evaluation of suspected aortic dissection. Old standards and new directions. N Engl J Med 1993. Jan;328(1):35-43 10.1056/NEJM199301073280107 [DOI] [PubMed] [Google Scholar]