Abstract
Heart failure (HF) is a common and serious comorbidity of diabetes. Oxidative stress has been associated with the pathogenesis of chronic diabetic complications including cardiomyopathy. The ability of antioxidants to inhibit injury has raised the possibility of new therapeutic treatment for diabetic heart diseases. Riboflavin constitutes an essential nutrient for humans and animals and it is an important food additive. Riboflavin, a precursor of flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD), enhances the oxidative folding and subsequent secretion of proteins. The objective of this study was to investigate the cardioprotective effect of riboflavin in diabetic rats. Diabetes was induced in 30 rats by a single injection of streptozotocin (STZ) (70 mg /kg). Riboflavin (20 mg/kg) was orally administered to animals immediately after induction of diabetes and was continued for eight weeks. Rats were examined for diabetic cardiomyopathy by left ventricular (LV) remadynamic function. Myocardial oxidative stress was assessed by measuring the activity of superoxide dismutase (SOD), the level of malondialdehyde (MDA) as well as heme oxygenase-1 (HO-1) protein level. Myocardial connective tissue growth factor (CTGF) level was measured by Western blot in all rats at the end of the study. In the untreated diabetic rats, left ventricular systolic pressure (LVSP) rate of pressure rose (+dp/dt), and rate of pressure decay (−dp/dt) were depressed while left ventricular end-diastolic pressure (LVEDP) was increased, which indicated the reduced left ventricular contractility and slowing of left ventricular relaxation. The level of SOD decreased, CTGF and HO-1 protein expression and MDA content rose. Riboflavin treatment significantly improved left ventricular systolic and diastolic function in diabetic rats, there were persistent increases in significant activation of SOD and the level of HO-1 protein, and a decrease in the level of CTGF. These results suggest that riboflavin treatment ameliorates myocardial function and improves heart oxidant status, whereas raising myocardial HO-1 and decreasing myocardial CTGF levels have beneficial effects on diabetic cardiomyopathy.
Key words: riboflavin, diabetic cardiomyopathy, heme oxygenase-1.
Introduction
Cardiovascular disease is the most common serious complication of diabetes mellitus. Prominent defects of diabetic cardiomyopathy include the prolonged duration of contraction and relaxation1 and reduced cardiac compliance. Although diabetic cardiomyopathy has been the subject of intensive investigation over the last 30 years, the pathogenesis of diabetic cardiomyopathy is still far from being fully clarified. Various studies have reported that the circulating markers of myocardial damage, proinflammatory cytokines, calcium dysregulation, oxidative stress and atherogenic lipids are elevated in diabetic patients.2–9
Metabolically, the diabetic heart is characterized by diminished glucose utilization and increased fatty acid oxidation resulting in lipid accumulation in the myocardium.10–11 This myocardial lipotoxicity results in alterations in the inflammatory cytokine levels and evokes a cascade of disparaging changes that leads to cardiac damage.
Numerous studies have demonstrated that the pivotal mediator for the pathogenesis of diabetes and its cardiovascular complications is oxidative stress.12,13 The increase in the level of oxidative stress results from an increased generation of reactive oxygen species (ROS) or from a reduction in antioxidants, which was thought to contribute to the initiation and progression of cardiac dysfunction and remodeling of the extracellular matrix in the heart.14–17 ROS are continuously produced in most cells under physiological conditions, and their levels are regulated by a number of antioxidant enzymes, such as superoxide dismutase, glutathione peroxidase and catalase, as well as by other non-enzymatic antioxidants. The ability of antioxidants to inhibit these injuries has raised the possibility of new therapeutic treatment for diabetes and its complication. Heme oxygenase-1 (HO-1) is a ubiquitously expressed stress inducible enzyme that catabolizes heme into bilirubin, carbon monoxide (CO) and iron.18 The byproducts of heme catabolism exert pleiotropic cytoprotective effects in the heart. Bilirubin is a powerful antioxidant,19 and CO exerts vasodilatory, anti-inflammatory and anti-proliferative effects.20 On another level, antioxidant administration has been reported to show beneficial effects on parameters of oxidative stress and cardiovascular functions in experimental diabetes.21
Connective tissue growth factor (CTGF), cysteine-rich glycoproteins, is an important stimulant of fibrosis,22 and is currently suggested to be an important downstream amplifier of the effects of the profibrogenic master cytokine transforming growth factor (TGF)-β.23,24 For cardiac fibrotic responses to diabetes, attention has been paid recently to the role of CTGF, since several studies indicated that renal and cardiac fibrosis may not be fully dependent on TGF-β, but may be predominantly dependent on CTGF.25,26 Therefore, an ideal approach to prevent DCM may target reducing CTGF expression.
Riboflavin is a precursor of flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD), which serve as coenzymes for numerous oxidases and dehydrogenases in eukaryotic cells.27 For example, FAD-dependent Ero1 and sulfhydryl oxidases participate in the oxidative folding (formation of disulfide bonds) of secretory proteins in the endoplasmic reticulum.28–31 Riboflavin deficiency impairs the oxidative folding and subsequent secretion of proteins in human cell cultures.30,31
However, there has been no documentation concerning the protection of riboflavin against diabetes-induced cardiac dysfunction to date. In the current study, we investigated the effect of riboflavin on diabetic cardiomyopathy in the STZ-induced diabetic rats. Recent studies show that HO-1 is a stress-induced protein. It protects against tissue injury through multiple mechanisms including anti-oxidation, anti-inflammation, and anti-apoptosis.32,33
Therefore, to evaluate myocardial oxidative stress in diabetic cardiac complications, we chose to investigate the possible effect of riboflavin on circulating markers of cardiac damage (CK, LDH), oxidative stress (lipid peroxides), and antioxidant enzyme (heme oxygenase-1, superoxide dismutase) in relation to left ventricular (LV) hemodynamic function during type I diabetic cardiomyopathy using the well-characterized rat streptozotocin (STZ) model of type I diabetes. To determine the cardioprotective effects against diabetic cardiovascular disease, we orally administered STZ-diabetic rats with riboflavin. We also investigated the effects of riboflavin on heart levels of CTGF.
Materials and Methods
Experimental animals
Sprague-Dawley rats weighing 200±20 g were provided by the Experimental Animal Center in Wannan Medical College and housed in a standard animal facility under controlled environmental conditions at room temperature 22±2°C and 12-h light-dark cycle. All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the Chinese National Institutes of Health.
Induction of diabetes
Diabetes was induced by a single i.p. injection of STZ (70 mg/kg) dissolved in 0.1M citrate buffer (pH 4.5) in male Sprague-Dawley rats (180–220 g). The control group rats were treated with the same volume citrate buffer. Diabetes was confirmed at 72 h after STZ injection by measuring the glucose concentrations of peripheral blood obtained from the tail vein (One Touch SureStep Meter, LifeScan, CA, USA) and weekly thereafter. Insulin was not administered, and all animals had free access to food and water. Diabetes was diagnosed by a sustained glucose concentration of more than 15 mmol/l.
Experimental protocols and riboflavin treatment
Control and diabetic rats were randomly assigned to three main experimental groups (8 animals per group) immediately after confirmation of STZ-induced diabetes: control rats received the control diet; diabetic rats, received the control diet; riboflavin rats, received orally administered riboflavin (20 mg kg−1 per day) and the control diet.
Cardiac function
After eight weeks of drug dosing, rats were anesthetized with sodium pentobarbital (50 mg/kg i.p.). The right carotid artery was cannulated with a Millar mininature catheter and advanced into the aorta to record arterial pressure. The aortic catheter was then advanced into the left ventricle to record left ventricular systolic pressure (LVSP), left ventricular developed pressure (LVDP), left ventricular end diastolic pressure (LVEDP), maximal rate of rise/fall left ventricle pressure development and decline (±dP/dtmax). All pressure data were recorded on MedLab data acquisition system (Nanjing MedEase Co., Nanjing, China). Fasting blood samples and hearts were then collected from all the groups of rats for further studies.
Biochemical analyses of blood samples
The levels of serum triacylglycerol (lipoprotein lipase method), cholesterol (cholesterol oxidase method), HDL (polyanion polymer/detergent method), LDL (catalase method), creatine kinase (CK; peroxydase method) and lactate dehydrogenase (LDH; lactic acid method) were measured as previously described.34
Measurement of myocardial oxidative stress
Left ventricular homogenate (10%, w/v) was prepared with 0.1 M PBS and centrifuged at 12,000 g for 10 min. The supernatant was used to determine SOD activity and MDA levels with commercially available kits (Nanjing Jiancheng Bioengineering Institute).
Western blotting
Left ventricles (0.2 g) were lysed and homogenized in 2 mL of lysis buffer (10 mM Tris-buffered saline, 1 mM EDTA, 1 mM EGTA, 2 mM sodium orthovanadate, 0.2 mM PMSF, 2 µg/mL leupeptin, 2 µg/mL aprotinin, and 1% Triton X-100) for 30 min on ice and cleared by centrifugation at 12,000 g for 15 min at 4°C. Total protein concentration was determined in the supernatant using the Bradford assay (Bio-Rad Laboratories, Hercules, CA, USA). For each lane, equal amounts of protein were mixed with sodium dodecyl sulfate (SDS) sample buffer and boiled for 5 min. Samples were separated on a 10% sodium dodecyl sulfatepolyacrylamide gel and then transferred to 0.2-µm nitrocellulose membrane. Nitrocellulose blots were blocked by incubation in TBST (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.1% Tween 20) containing 5% non-fat milk for 1 h at room temperature and then incubated with a rabbit polyclonal anti-HO-1, CTGF, β-actin antibody (1:500 dilution; Wuhan Boster Biotechnologies, Wuhan, China) overnight at 4°C. After 3 washing steps, a secondary anti-rabbit antibody (1:10,000 dilution; Sigma Chemical Co, St. Louis, MO, USA) was added and incubated for 1 h. After being rinsed three times with wash buffer, the reaction was visualized by DAB (Bio Basic Inc., Canada). The relative amounts of the bands were quantified by densitometry using image software.
Statistical analysis
Data are expressed as the mean ± SEM. Group means were compared by one-way analysis of variance (ANOVA). Comparison between two groups was performed by Student's t-test. Values of P<0.05 were considered significant.
Results
Change in body weight and heart weight/body weight ratio after riboflavin treatment
After STZ administration, animals showed similar hyperglycemia in all experimental groups. There was no change in blood glucose levels in diabetic rats treated with riboflavin who also showed a significant decrease in body weight compared with controls (Table 1). However, the results in Table 1 also show an increase heart weight/body weight ratio in the STZ-diabetic animals. Treatment of riboflavin prevented body weight loss in diabetic rats as compared with the untreated rats. In addition, diabetic rats had increased heart weight/body weight ratio, a marker for the development of diabetic cardiomyopathy, and this ratio was significantly decreased by treatment with riboflavin (Table 1).
Table 1. General characteristics of control and diabetic rats.
| Control rats | Diabetic rats | Riboflavin rats | |
|---|---|---|---|
| Body weight (g) | 398.6±18.7 | 169.5±12.6** | 230.4±14.7° |
| Heart weight (g) | 1.33±0.23 | 1.11±0.20* | 0.97±0.17 |
| Heart weight/body weight (103) | 3.35±0.49 | 6.57±1.37** | 4.22±0.76° |
| Blood glucose (mmol/l) | 4.91±0.46 | 24.54±2.73** | 22.27±1.50 |
Values are expressed as mean ± SD of 8 samples from each group.
P<0.05,
P<0.01, significant difference to control rats.
P<0.01, significant difference to diabetic rats.
Effects of riboflavin on cardiac function
Cardiovascular function was evaluated by LV hemodynamic analysis before rats were sacrificed (Table 2). Left ventricular hemodynamic parameters were measured in control and diabetic rats treated with and without riboflavin for assessment of ventricular performance. Table 2 shows left ventricular pressure and left ventricular dP/dt recordings obtained from control and diabetic rats treated with and without riboflavin. The untreated diabetic rats show a lower left ventricular systolic pressure (LVSP), left ventricular developed pressure (LVDP), and a higher left ventricular end diastolic pressure (LVEDP) compared to the control rats (Table 2). In addition, compared with the control rats, the untreated diabetic rats also show a depressed left ventricular ±dP/dtmax. The diabetic rats treated with riboflavin show an increased LVSP, LVDP, and a reduced LVEDP concomitant with a higher left ventricular ±dP/dtmax. Heart rate (HR) in untreated diabetic rats was significantly decreased. Riboflavin treatment increased HR (Table 2).
Table 2. Effect of riboflavin treatment on left ventricular hemodynamic parameters in control and diabetic groups of animals.
| Control rats | Diabetic rats | Riboflavin rats | |
|---|---|---|---|
| HR(bpm) | 416±8.6 | 309±19.2** | 338±13.1° |
| LVSP(mmHg) | 136.1±10.1 | 76.4±7.9** | 126.5±10.2° |
| LVEDP(mmHg) | 1.70±0.50 | 7.73±1.01** | 3.82±0.65° |
| LVDP(mmHg) | 114.0±9.7 | 62.8±7.9** | 83.7±8.4° |
| + dP/dtmax | 4229±118 | 2586±92** | 3401±103° |
| − dP/dtmax | 4135±143 | 2463±101** | 3310±82° |
Values are expressed as mean ± SD of 8 samples from each group.
P<0.05,
P<0.01, significant difference to control rats.
P<0.01, significant difference to diabetic rats.
Effects of riboflavin on CK and LDH
Compared to the controls, the circulating markers of cardiac damage in STZ diabetic rats showed a significant increase in the levels of serum CK and LDH. However, the riboflavin treatment to the diabetic rats markedly reduced the levels of LDH and CK when compared with the untreated diabetic rats (Figure 1).
Figure 1.
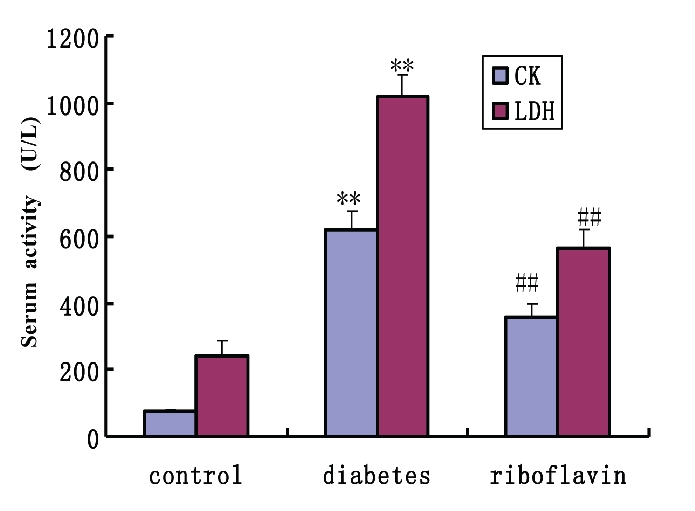
Effect of riboflavin on the level of CK and LDH in serum after treatment with riboflavin in diabetic rats. All values represent mean±SEM (n=8). **P<0.01, significant difference to control rats; ##P<0.01, significant difference to diabetic rats. CK, creatine kinase; LDH, lactate dehydrogenase.
Effects of riboflavin on serum lipid profile
Riboflavin had a significant effect on lowering triglyceride, cholesterol and LDL levels and increasing HDL levels in diabetic rats (Table 3). All these levels were completely normalized after riboflavin treatment which suggests that riboflavin is far more effective in maintaining the lipid profile near to that of the control in this animal model of diabetes.
Table 3. Effects of riboflavin on serum lipid profile in control and diabetic groups of animals.
| Group | TC(mmol/L) | TG(mmol/L) | LDL(mmol/L) | HDL(mmol/L) |
|---|---|---|---|---|
| Control rats | 1.38±0.26 | 0.67±0.13 | 1.75±0.36 | 1.12±0.15 |
| Diabetic rats | 5.31±0.77** | 2.23±0.28** | 8.89±0.94** | 0.34±0.06** |
| Riboflavin rats | 2.63±0.43° | 1.17±0.20° | 3.81±0.88° | 0.57±0.09° |
Values are expressed as mean ± SD of 8 samples from each group.
P<0.05,
P<0.01, significant difference to control rats.
P<0.01, significant difference to diabetic rats.
Antioxidant effects of riboflavin
Lipid peroxide formation (MDA), which is a direct marker for oxidative stress, significantly increased the cardiac tissue of the STZ group compared with the control (Figure 2). Riboflavin treatment significantly reduced the levels of lipid peroxidation which were very close to those of the control group (Figure 2). The level of SOD was significantly depressed in the diabetic rats compared with controls (Figure 2). Treatment with riboflavin significantly increased SOD heart activity in diabetic rats.
Figure 2.
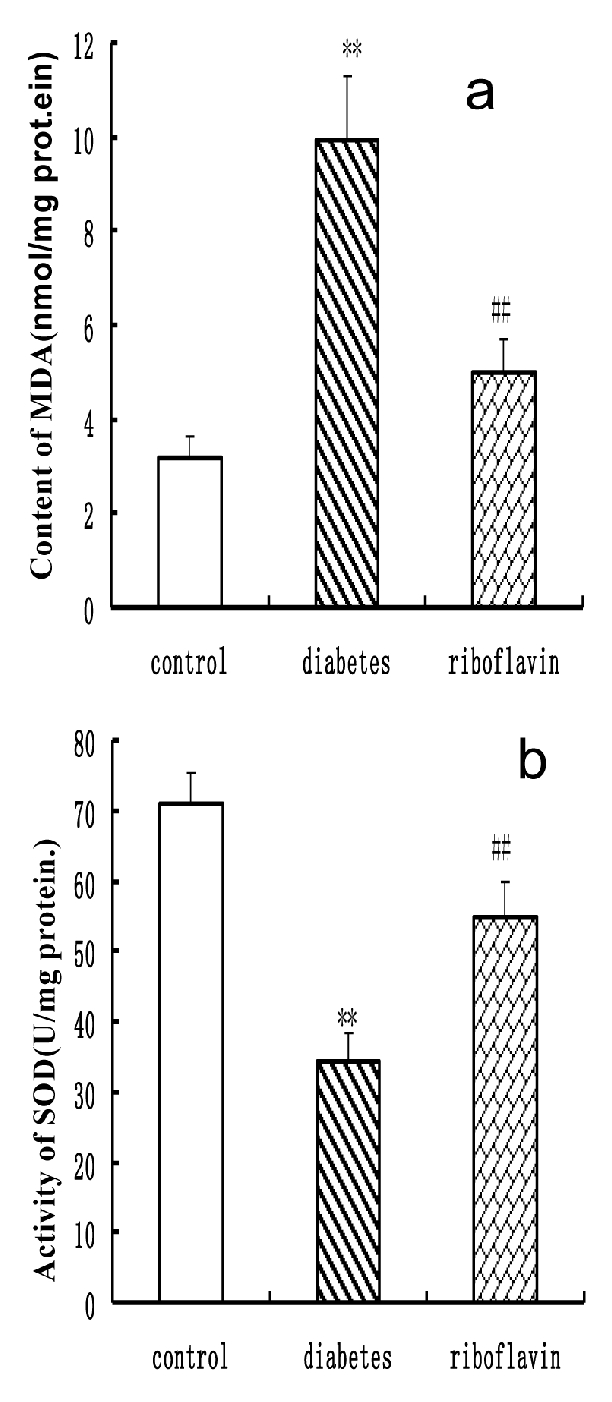
Effect of riboflavin on the level of MDA (a) and SOD (b) in diabetic rats. All the values represent mean±SED (n=8). **P<0.01, significant difference to control rats; ## P<0.01, significant difference to diabetic rats. SOD, superoxide dismutase; MDA, malondialdehyde.
Changes in HO-1, and CTGF protein by riboflavin treatment in diabetic rats
Western blot analyses showed an accumulation of HO-1 protein in diabetic rats compared with normal control rats (Figure. 3). Riboflavin treatment led to a strong upregulation of HO-1 protein compared with the untreated rats (Figure 3). To determine the effects of riboflavin on cardiac fibrotic response to diabetes, expression of cardiac CTGF was examined by Western blot. Results showed that CTGF was significantly increased in cardiac tissue from diabetic rats (Figure 3). Riboflavin treatment significantly prevented diabetes-caused upregulation of CTGF.
Figure 3.
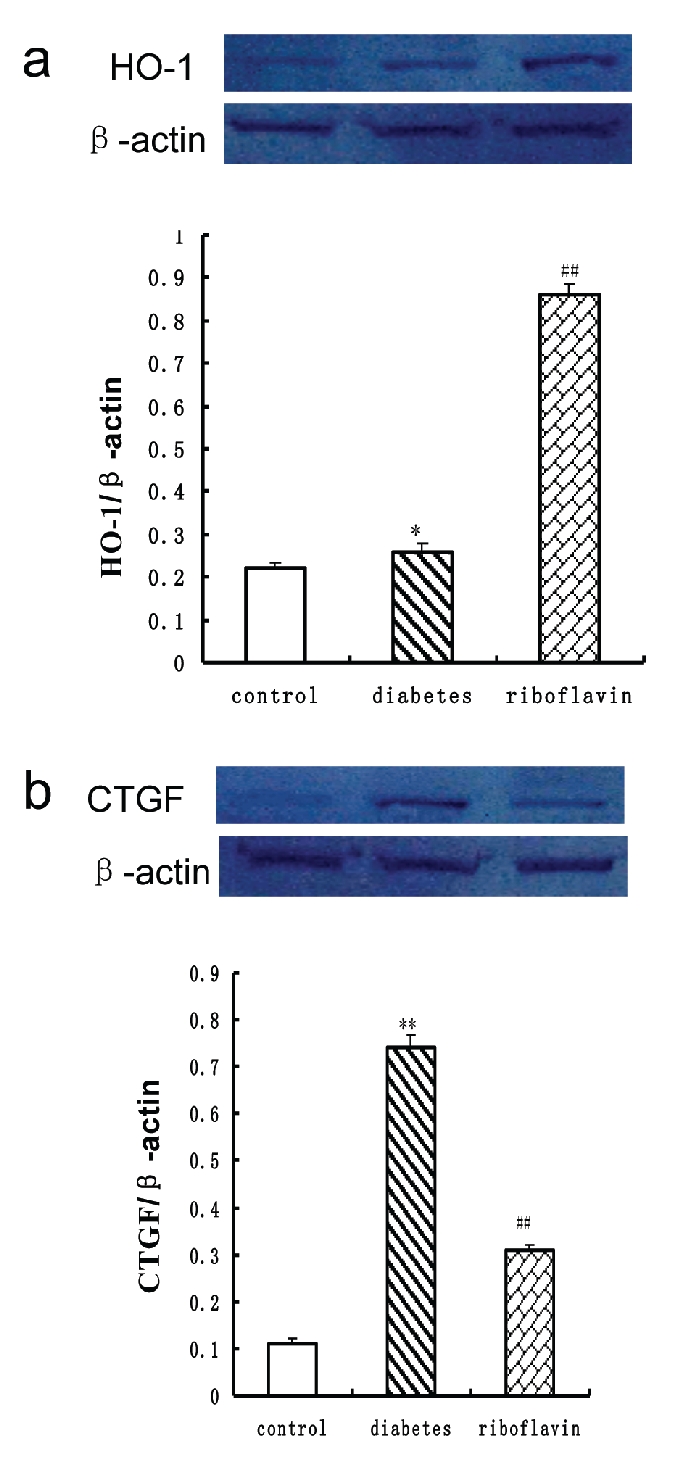
Western blot of HO-1 (a) and CTGF (b) in heart after treatment with riboflavin in diabetic rats. HO-1and CTGF were normalized with respect to actin in respective controls. Similar results were obtained in four independent sets of experiments. All the values represent mean±SEM (n=4). **P<0.01, significant difference to control rats; ##P<0.01, significant difference to diabetic rats. HO-1, heme oxygenase-1; CTGF, connective tissue growth factor.
Discussion
Diabetes is a metabolic syndrome with a cluster of common clinical disorders that is related with an increased risk for cardiovascular disease.38 Cardiovascular complications remain the leading cause of diabetes-related mortality and morbidity.36 A specific disease termed as diabetic cardiomyopathy increases the risk for cardiac dysfunction and heart failure independently of other risk factors such as coronary artery disease and hypertension, as evidenced by compelling epidemiological and clinical data.37,38 Despite the potential importance of this disease entity, the underlying mechanisms are still not well understood. Dyslipidemia, oxidative stress and inflammatory injury are important interrelated factors responsible for the development of cardiomyopathy as they are known to promote the progression of premature atherosclerosis, coronary insufficiency and myocardial infarction.39 Riboflavin, a precursor of flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD), prevents cardiac damage and enhances the oxidative folding and subsequent secretion of proteins.40–42 However, the effect of riboflavin on the antioxidant properties and altered lipid profile in experimental models of diabetes has not yet been studied. In the present study, we extensively evaluated the effect of riboflavin on cardiac damage.
Diabetic cardiomyopathy characterized by diastolic dysfunction and left ventricular hypertrophy is usually the terminal condition of heart in diabetes.43 Our results indicate that 8-week treatment of riboflavin in diabetic rats proved to be beneficial as it restrained the progression of the metabolic disorders of diabetes. The STZ diabetic rats showed elevated blood glucose levels. Also, there was a notable increase in the HW/BW ratio which signifies cardiac hypertrophy. Metabolically, the diabetic heart is characterized by diminished glucose utilization and increased fatty acid oxidation resulting in lipid accumulation in the myocardium.10,11 Riboflavin remarkably improved lipid profile. Riboflavin treatment showed a distinct positive effect on HW/BW ratio indicating that riboflavin was able to prevent cardiac hypertrophy which usually sets in as a result of diastolic dysfunction secondary to diabetes. The untreated STZ-diabetic animals showed significant depressions in LVSP, +dP/dt, and −dP/ dt and concomitant increases in LVEDP. These hemodynamic alterations in the untreated STZ-diabetic rats demonstrate abnormal left ventricular systolic and diastolic function that is the hallmark of diabetic cardiomyopathy. Riboflavin treatment attenuated these hemodynamic changes.
Increases in levels of circulating cardiac damage markers, such as CK and LDH, represent a powerful and sensitive predictor of increased cardiac complications.44,45 In the present study, riboflavin-treated diabetic rats showed a significant improvement in levels of these circulating cardiac damage markers. This indicates that riboflavin prevents cardiac damage and has beneficial properties. The decrease in the cardiac damage markers in the diabetic rats upon riboflavin treatment suggested that the riboflavin treatment reduces the risk of metabolic disorders associated with diabetics.
Increased oxidative stress and an altered antioxidant pool have been implicated in both clinical and experimental type 1 diabetes.14 The results from our study showed that an STZ induced diabetic condition resulted in an increase in the lipid peroxidation and protein carbonylation which are direct indicators of systemic oxidative stress. This was in conjunction with depletion of superoxide scavenger SOD and an increase in lipid peroxidation product MDA. Riboflavin treatment in the STZ-diabetic rats reduced the formation of lipid peroxides and carbonyl content and also restored the levels of SOD. Heme oxygenase-1 (HO-1), the inducible isoform of the HO system, is a rate-limiting enzyme which converts heme into equimolar amounts of iron, carbon monoxide and biliverdin. HO-1 is thought to have antioxidant and cytoprotective roles.31 Riboflavin treatment significantly increased the level of myocardial HO-1 in the STZ-diabetic rats. This positive beneficial effect of riboflavin could be directly attributable to its antioxidative nature which is in agreement with previous findings.40–42
Apart from the altered metabolic condition, diabetes is also an inflammation-prone condition. Hyperglycemia-induced ROS stimulates signal transduction to instigate inflammatory cytokines, e.g. TNF-α, IFN-γ and TGF-β.46 This leads to systemic inflammation, cardiac dysfunction and exacerbates the severity of diabetes.47 CTGF, a recently discovered cytokine, has been demonstrated to play an important role in fibrotic response through a TGF-β1-dependent or independent pathway.25,26 CTGF acts as a cofactor with TGF-β to induce fibroblasts to become myofibroblasts that deposit collagen, ultimately resulting in organ scarring and dysfunction, and in the most severe forms, organ failure and death. Indeed, CTGF levels in tissue, blood or vitreal fluid have been shown to correlate with the degree and severity of fibrosis in many diseases.48 Other studies showed that CTGF also exhibited prohypertrophic properties on cardiomyocytes.49 Furthermore, CTGF has been proposed as a heart failure biomarker.43–44 Stress induced CTGF in cultured cardiomyocytes.50,51 In the present study, riboflavin-treated diabetic rats showed effective suppression of CTGF. The results strongly indicate that riboflavin is a potential agent against diabetes-associated systemic fibrosis.
In our study, the altered lipid profile observed in the diabetic condition was also abrogated by riboflavin treatment. Riboflavin improved the serum cholesterol, triglycerides, LDL and HDL. These findings suggest that riboflavin possesses lipid lowering activities due to its unique anti-atherogenic properties which contribute also to its cardiovascular protective actions. This is the first report to evaluate the positive effect of riboflavin on lipid profile in STZ-diabetic rats.
Limitations
This study has several limitations. The sample size was relatively small. This preliminary result must be verified in a more numerous population, and on a long-term basis. Although myocardial oxidative stress was measured, we did not provide more detailed mechanisms.
Conclusions
In summary, the present findings show that treatment with riboflavin may attenuate the progressive cardiac dysfunction and myocardial oxidative stress in a murine model of diabetic cardiomyopathy. The beneficial effect of riboflavin is a result of its inhibition of myocardial oxidative stress processes through its potential antioxidant properties. Our results show that riboflavin administration may exert beneficial effects in diabetic cardiomyopathy by increasing antioxidant and decreasing CTGF levels. We suggest that it might be useful to re-explore the role of antioxidant therapy given immediately after the diagnosis of type I diabetes mellitus to reduce the risk of future cardiovascular complications.
References
- 1.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–23. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 2.Nishiya D, Enomoto S, Omura T, et al. The long-acting Ca2+-channel blocker azelnidipine prevents left ventricular remodeling after myocardial infarction. J Pharmacol Sci. 2007;103:391–7. doi: 10.1254/jphs.fp0061139. [DOI] [PubMed] [Google Scholar]
- 3.Hussain MJ, Peakman M, Gallati H, et al. Elevated serum levels of macrophage-derived cytokines precede and accompany the onset of IDDM. Diabetologia. 1996;39:60–9. doi: 10.1007/BF00400414. [DOI] [PubMed] [Google Scholar]
- 4.Kini AS, Kim MC, Moreno PR, et al. Comparison of coronary flow reserve and fractional flow reserve in patients with versus without diabetes mellitus and having elective percutaneous coronary intervention and abciximab therapy (from the PREDICT Trial) Am J Cardiol. 2008;101:796–800. doi: 10.1016/j.amjcard.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 5.Geddes J, Deans KA, Cormack A, et al. Cardiac troponin I concentrations in people presenting with diabetic ketoacidosis. Ann Clin Biochem. 2007;44:391–3. doi: 10.1258/000456307780945750. [DOI] [PubMed] [Google Scholar]
- 6.Mandosi E, Fallarino M, Gatti A, et al. Atorvastatin downregulates monocyte CD36 expression, nuclear NFkappaB and TNFalpha levels in type 2 diabetes. J Atheroscler Thromb. 2010;17:539–45. doi: 10.5551/jat.2956. [DOI] [PubMed] [Google Scholar]
- 7.Motomura T, Okamoto M, Kitamura T, et al. Effects of pitavastatin on serum lipids and high sensitivity C-reactive protein in type 2 diabetic patients. J Atheroscler Thromb. 2009;16:546–52. doi: 10.5551/jat.992. [DOI] [PubMed] [Google Scholar]
- 8.Guha A, Harmancey R, Taegtmeyer H. Nonischemic heart failure in diabetes mellitus. Current Opinion in Cardiology. 2008;23:241–8. doi: 10.1097/HCO.0b013e3282fcc2fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliveira PJ, Seica R, Coxito PM, et al. Enhanced permeability transition explains the reduced calcium uptake in cardiac mitochondria from streptozotocin-induced diabetic rats. FEBS Lett. 2003;554:511–4. doi: 10.1016/s0014-5793(03)01233-x. [DOI] [PubMed] [Google Scholar]
- 10.Ye G, Metreveli NS, Donthi RV, et al. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes. 2004;53:1336–43. doi: 10.2337/diabetes.53.5.1336. [DOI] [PubMed] [Google Scholar]
- 11.Zhou G, Li X, Hein DW, et al. Metallothionein suppresses angiotensin II-induced nicotinamide adenine dinucleotide phosphate oxidase activation, nitrosative stress, apoptosis, and pathological remodeling in the diabetic heart. J Am Coll Cardiol. 2008;52:655–66. doi: 10.1016/j.jacc.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Ceriello A. New insights on oxidative stress and diabetic complications may lead to a “causal” antioxidant therapy. Diabetes Care. 2003;26:1589–96. doi: 10.2337/diacare.26.5.1589. [DOI] [PubMed] [Google Scholar]
- 13.Da Ros R, Assaloni R, Ceriello A. Antioxidant therapy in diabetic complications: what is new? Curr Vasc Pharmacol. 2004;2:335–41. doi: 10.2174/1570161043385538. [DOI] [PubMed] [Google Scholar]
- 14.Vincent AM, Brownlee M, Russell JW. Oxidative stress and programmed cell death in diabetic neuropathy. Ann NY Acad Sci. 2002;959:368–83. doi: 10.1111/j.1749-6632.2002.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 15.Molavi B, Mehta JL. Oxidative stress in cardiovascular disease: molecular basis of its deleterious effects, its detection, and therapeutic considerations. Curr Opin Cardiol. 2004;19:488–93. doi: 10.1097/01.hco.0000133657.77024.bd. [DOI] [PubMed] [Google Scholar]
- 16.Marra G, Cotroneo P, Pitocco D, et al. Early increase of oxidative stress and reduced antioxidant defenses in patients with uncomplicated type 1 diabetes: a case for gender difference. Diabetes Care. 2002;25:370–5. doi: 10.2337/diacare.25.2.370. [DOI] [PubMed] [Google Scholar]
- 17.Mann DL. Stress-activated cytokines and the heart: from adaptation to maladaptation. Annu Rev Physiol. 2003;65:81–101. doi: 10.1146/annurev.physiol.65.092101.142249. [DOI] [PubMed] [Google Scholar]
- 18.Ryter SW, Alam J, Choi AM. Heme oxygenase/carbon monoxide: From basic science to therapeutic application. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 19.Stocker RY, Yamamoto Y, McDonagh AF, et al. Bilirubin is an antioxidant of possible physiological significance. Science. 1987;235:1043–6. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 20.Otterbein LE, Bach FH, Alam J, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–8. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 21.Bursell SE, Clermont AC, Aiello LP, et al. High-dose vitamin E supplementation normalizes retinal blood flow and creatinine clearance in patients with type 1 diabetes. Diabetes Care. 1999;22:1245–51. doi: 10.2337/diacare.22.8.1245. [DOI] [PubMed] [Google Scholar]
- 22.Leask A, Abraham DJ. The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem Cell Biol. 2003;81:355–63. doi: 10.1139/o03-069. [DOI] [PubMed] [Google Scholar]
- 23.Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–10. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 24.Gressner OA, Lahme B, Siluschek M, et al. Connective tissue growth factor is a Smad2 regulated amplifier of transforming growth factor beta actions in hepatocytes--but without modulating bone mor-phogenetic protein 7 signaling. Hepatology. 2009;49:2021–30. doi: 10.1002/hep.22850. [DOI] [PubMed] [Google Scholar]
- 25.Way KJ, Isshiki K, Suzuma K, et al. Expression of connective tissue growth factor is increased in injured myocardium associated with protein kinase C beta2 activation and diabetes. Diabetes. 2002;51:2709–18. doi: 10.2337/diabetes.51.9.2709. [DOI] [PubMed] [Google Scholar]
- 26.Zhou G, Li C, Cai L. Advanced glycation end-products induce connective tissue growth factor-mediated renal fibrosis predominantly through transforming growth factor beta-independent pathway. Am J Pathol. 2004;165:2033–43. doi: 10.1016/s0002-9440(10)63254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivlin RS. Riboflavin. In: Bowman BA, Russell RM, Bowman BA, Russell RM, editors. Present Knowledge in Nutrition. Washington, DC: ILSI Press; 2001. pp. 191–8. [Google Scholar]
- 28.Tu BP, Ho-Schleyer SC, Travers KL, et al. Biochemical basis of oxidative protein folding in the endoplasmic reticulum. Science. 2000;290:1571–4. doi: 10.1126/science.290.5496.1571. [DOI] [PubMed] [Google Scholar]
- 29.Thorpe C, Hoober K, Raje S, et al. Sulfhydryl oxidases: emerging catalysts of protein disulfide bond formation in eukaryotes. Arch Bioch Biophys. 2002;405:1–12. doi: 10.1016/s0003-9861(02)00337-5. [DOI] [PubMed] [Google Scholar]
- 30.Camporeale G, Zempleni J. Oxidative folding of interleukin-2 is impaired in flavin-deficient Jurkat cells, causing intracellular accumulation of interleukin-2 and increased expression of stress response genes. J Nutr. 2003;133:668–72. doi: 10.1093/jn/133.3.668. [DOI] [PubMed] [Google Scholar]
- 31.Manthey KC, Chew YC, Zempleni J. Riboflavin deficiency impairs oxidative folding and secretion of apolipoprotein B-100 in HepG2 cells, triggering stress-response systems. J Nutr. 2005;135:978–82. doi: 10.1093/jn/135.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryter SW, Choi AM. Heme oxygenase-1: redox regulation of a stress protein in lung and cell culture models. Antioxid Redox Signal. 2005;7:80–91. doi: 10.1089/ars.2005.7.80. [DOI] [PubMed] [Google Scholar]
- 33.Wunder C, Potter RF. The heme oxygenase system: its role in liver inflammation. Curr Drug Targets Cardiovasc Haematol Disord. 2003;3:199–208. doi: 10.2174/1568006033481410. [DOI] [PubMed] [Google Scholar]
- 34.Yahagi N, Shimano H, Hasty AH, et al. Absence of sterol regulatory element-binding protein-1 (SREBP-1) ameliorates fatty livers but not obesity or insulin resistance in Lep(ob)/Lep(ob) mice. J Biol Chem. 2002;277:19353–7. doi: 10.1074/jbc.M201584200. [DOI] [PubMed] [Google Scholar]
- 35.Grundy SM, Benjamin U, Burke GL, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–46. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 36.Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008;371:1800–9. doi: 10.1016/S0140-6736(08)60768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord. 2010;11:31–9. doi: 10.1007/s11154-010-9131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haim TE, Wang W, Flagg TP, et al. Palmitate Attenuates Myocardial Contractility through Augmentation of Repolarizing Kv Currents. J Mol Cell Cardiol. 2010;48:395–416. doi: 10.1016/j.yjmcc.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reasner CA. Reducing cardiovascular complications of type 2 diabetes by targeting multiple risk factors. J Cardiovasc Pharmacol. 2008;52:136–44. doi: 10.1097/FJC.0b013e31817ffe5a. [DOI] [PubMed] [Google Scholar]
- 40.Iwanaga K, Hasegawa T, Hultquist DE, et al. Riboflavin-mediated reduction of oxidant injury , rejection , and vasculopathy after cardiac allotransplantation. Transplantation. 2007;83:747–53. doi: 10.1097/01.tp.0000256283.06469.d4. [DOI] [PubMed] [Google Scholar]
- 41.Premkumar VG, Yuvaraj S, Shanthi P, et al. Co-enzyme Q10, riboflavin and niacin supplementation on alteration of DNA repair enzyme and DNA methylation in breast cancer patients undergoing tamoxifen therapy. Br J Nutr. 2008;1:1–4. doi: 10.1017/S0007114508968276. [DOI] [PubMed] [Google Scholar]
- 42.Perumal SS, Shanthi P, Sachdanadam P. Augmented efficacy of tamoxifen in rat breast tumorigenesis when gavaged along with riboflavin, niacin, and CoQ10: effects on lipid peroxidation and antioxidants in mitochondria. Chem Biol Interact. 2005;152:49–58. doi: 10.1016/j.cbi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Raev DC. Which left ventricular function is impaired earlier in the evolution of diabetic cardiomyopathy? An echocardiographic study of young type I diabetic patients. Diabetes Care. 1994;17:633–39. doi: 10.2337/diacare.17.7.633. [DOI] [PubMed] [Google Scholar]
- 44.Howard-Alpe GM, Sear JW, Foex P. Methods of detecting atherosclerosis in non-cardiac surgical patients; the role of biochemical markers. Br J Anaesth. 2006;97:758–69. doi: 10.1093/bja/ael303. [DOI] [PubMed] [Google Scholar]
- 45.Hagar HH. Folic acid and vitamin B12 supplementation attenuates isoprenaline-induced myocardial infarction in experimental hyperhomocysteinemic rats, Pharmacological Research. 2002;46:213–9. doi: 10.1016/s1043-6618(02)00095-6. [DOI] [PubMed] [Google Scholar]
- 46.Hori M, Nishida K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc Res. 2009;81:457–64. doi: 10.1093/cvr/cvn335. [DOI] [PubMed] [Google Scholar]
- 47.Mohamed-Ali V, Armstrong L, Clarke D, et al. Evidence for the regulation of levels of plasma adhesion molecules by proinflammatory cytokines and their soluble receptors in type 1 diabetes. J Intern Med. 2001;250:415–21. doi: 10.1046/j.1365-2796.2001.00900.x. [DOI] [PubMed] [Google Scholar]
- 48.Brigstock DR. Strategies for blocking the fibrogenic actions of connective tissue growth factor (CCN2): From pharmacological inhibition in vitro to targeted siRNA therapy in vivo. J Cell Commun Signal. 2009;3:5–18. doi: 10.1007/s12079-009-0043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayata N, Fujio Y, Yamamoto Y, et al. Connective tissue growth factor induces cardiac hypertrophy through Akt signaling. Biochem Biophys Res Commun. 2008;370:274–8. doi: 10.1016/j.bbrc.2008.03.100. [DOI] [PubMed] [Google Scholar]
- 50.Matsui Y, Sadoshima J. Rapid upregulation of CTGF in cardiac myocytes by hypertrophic stimuli: implication for cardiac fibrosis and hypertrophy. J Mol Cell Cardiol. 2004;37:477–81. doi: 10.1016/j.yjmcc.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 51.He Z, Way KJ, Arikawa E, et al. Differential regulation of angiotensin II-induced expression of connective tissue growth factor by protein kinase C isoforms in the myocardium. J Biol Chem. 2005;280:15719–26. doi: 10.1074/jbc.M413493200. [DOI] [PubMed] [Google Scholar]


