Abstract
Gastrointestinal stromal tumors (GISTs) are uncommon mesenchymal tumors of the gastrointestinal tract. Up to one-third of GISTs are malignant with a high rate of metastasis. Surgical resection is the mainstay of care for patients with resectable disease. Imatinib mesylate, a selective tyrosine kinase inhibitor, is the current standard of care for GISTs that cannot be completely resected or in cases of metastatic GIST. Although often overlooked, radiation therapy is a viable option for select patients with GIST. We report the case of a patient with unresectable GIST who was treated with local radiotherapy and achieved long-term response. We also present a review of the literature regarding the use of radiotherapy in the treatment of GIST. GIST has been shown to be a radiosensitive tumor. Radiotherapy can offer long-term local control and should be considered in the adjuvant or palliative setting. The role of radiotherapy delivered concurrently with imatinib in the treatment of GIST may warrant further investigation.
Key words: gastrointestinal stromal tumor, GIST, radiotherapy, radiation therapy.
Introduction
Gastrointestinal stromal tumors (GISTs) are uncommon mesenchymal tumors that arise from the precursors to the interstitial cells of Cajal in the wall of the gastrointestinal tract. In the United States, approximately 4500 to 6000 cases of GIST are diagnosed each year. Up to one-third of cases of GIST are malignant with a high rate of metastasis, often to the retroperitoneum and liver.1 Prior to 1983, GISTs were usually classified as leiomyomas or leiomyosarcomas.2 Immunohistochemical studies have demonstrated that GISTs do not show a differentiation to smooth muscle but instead are a heterogeneous group of tumors with varied differentiation and with mutation in the c-KIT receptor tyrosine kinase or, less often, platelet-derived growth factor receptor-alpha (PDGFRA).3,4 GIST has three main histologic patterns: spindle cell type (70%), epitheliod cell type (20%) or a mixture of both (Figure 1). Over 95% of GISTs show immunopositivity for CD117 (KIT) (Figure 2). Other common markers for GIST include CD34 and smooth muscle actin (SMA), which are expressed in approximately 70% and 40% of GISTs respectively (Figure 3).5
Figure 1.
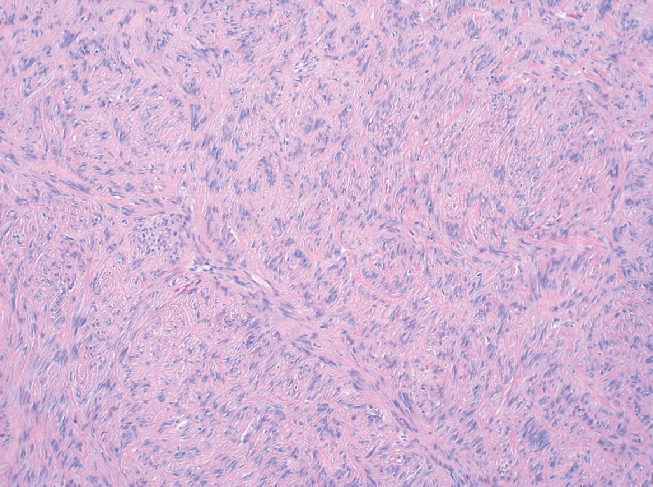
Gastrointestinal stromal tumor (GIST) of the stomach with spindle cells in a fasicular pattern (H&E stain; magnification 100×).
Figure 2.
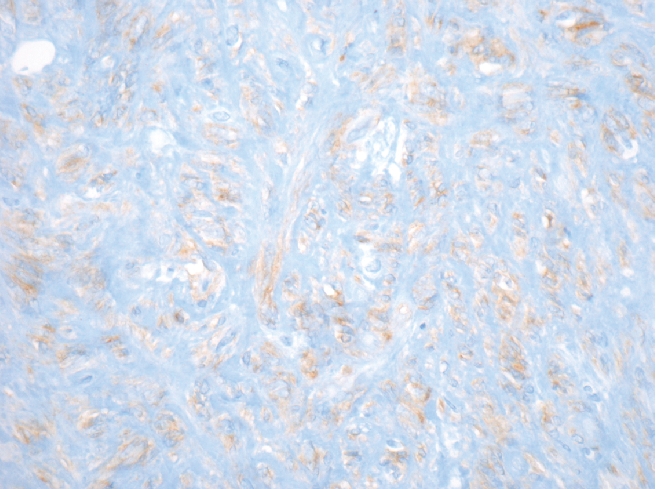
Spindle cell GIST showing diffuse immunopositivity for CD117 (KIT) (magnification 400×).
Figure 3.
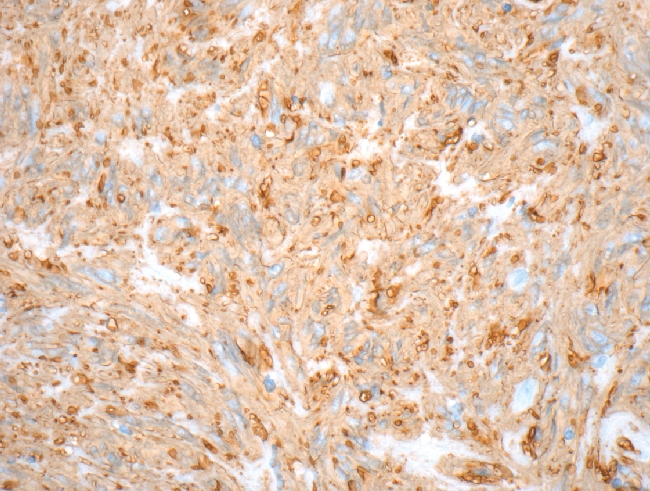
Spindle cell GIST with strong immunostaining for CD34 (magnification 400×).
For patients with GIST, complete surgical resection offers the greatest chance for increased disease-free survival. Imatinib mesylate, a selective tyrosine kinase inhibitor of c-KIT and PDGRA (as well as BCR-ABL in chronic myelogenous leukemia), is the current standard of care for GIST that cannot be completely resected. Eighty-five to ninety percent of patients experience meaningful tumor control and prolonged overall survival with imatinib. Tumors may demonstrate primary resistance or develop secondary resistance to imatinib. Sunitinib, a multi-targeted inhibitor of tyrosine kinase, has recently been approved for use in patients with imatinib-refractory GIST.3 Both imatinib and sunitinib are classified as tyrosine kinase inhibitors (TKI). In addition, some patients are not able to tolerate imatinib or sunitinib, which are oral agents, due to sequelae from their disease or side effects from the medication.
While often overlooked, radiation therapy may also be a useful treatment modality for select patients with GIST. The benefit of adjuvant or primary radiation therapy in the treatment of GIST remains unclear. This is likely due to several factors, including the rarity of the disease and the limited dose tolerance of the gastrointestinal organs. We present the case of a patient with unresectable GIST who was diagnosed prior to the widespread use of imatinib for treatment of GIST. The patient was treated with external beam radiation therapy at our institution. We also review the literature addressing the role of radiotherapy in the treatment of GIST.
Case Report
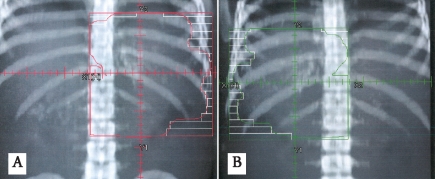
At our institution, a 37-year-old man underwent surgery for acute intestinal obstruction. Abnormal tissue was seen in the wall of the antrum and small intestine. Several local lymph nodes were found to be involved. Although the frozen section was read as consistent with Hodgkin's disease, the final pathologic diagnosis was eosinophilic gastroenteritis. The area of disease was deemed unresectable; debulking surgery was performed. The patient was symptom-free for four years, at which time he developed recurrent symptoms of obstruction plus a palpable abdominal mass. Excisional biopsy of a mass in the antrum was performed, with similar histologic findings. Additional review of the pathology concluded that the initial diagnosis was incorrect. The tumor was determined to be a GIST. The area of disease, including most of the stomach, was treated with a 1.5 cm margin to 36 Gy delivered in 24 fractions of 1.5 Gy each with anteroposterior/posteroanterior (AP/PA) fields (Figure 4A and 4B). Radiotherapy was well tolerated. The patient's gastroenterological symptoms resolved and he did not experience any late term toxicity from treatment. He did well and was clinically disease-free on imaging and physical examination until he was diagnosed with rectal adenocarcinoma almost 20 years later. He died later that year from multiple medical comorbidities without recurrence of the GIST.
Figure 4.
Digitally reconstructed radiographs demonstrating a re-creation of the antero-posterior (A) and posteroanterior (B) radiation treatment fields.
Discussion
Only a small number of published studies regarding the treatment of GIST address radiation therapy. Several have shown that adjuvant radiotherapy may be a useful modality in the treatment of GISTs that cannot be completely resected. At Princess Margaret Hospital (Toronto, ON), 10 of 50 patients with GIST treated between 1989 and 1998 received radiotherapy.6 They were not treated with chemotherapy. Two received preoperative radiation treatment for a fixed mass. Eight patients were treated postoperatively due to residual, fixed tumor. Median postoperative radiation dose was 45 Gy in 1.8 fractions. Long-term local control within the radiation field was achieved in six of nine evaluable patients who received radiation therapy. This case series provides evidence that GIST is a radiosensitive tumor.
A case study published by Pollack et al.7 demonstrates that radiotherapy may benefit patients with GIST that have microscopic residual disease. The patient had a 7 cm rectal GIST removed in piecemeal fashion with microscopically positive margins. She refused further surgery and was therefore treated with radiotherapy, including 36 Gy to the whole pelvis, followed by an additional 9 Gy to a reduced pelvic field plus a 5.4 Gy conedown to the tumor bed with a 2 cm margin, for a total of 50.4 Gy. At follow-up of two years, the patient was disease-free. An additional case study published in Italy in 2002 describes a case of GIST of the rectum treated with abdominoperineal resection with a microscopically positive margin.8 The patient received adjuvant radiotherapy of 50 Gy. At 32 months following surgery, the patient was without disease. These studies suggest that postoperative radiation therapy for residual microscopic disease may enhance local control.
In contrast, a retrospective study from Massachusetts General Hospital does not support the use of radiotherapy in cases of incomplete resection of GIST.9 The study includes 70 patients with GIST treated between 1973 and 1998. Twenty of these patients received radiation therapy, largely for intermediate- to high-grade tumors following incomplete resection and no evidence of metastasis. Radiation therapy provided no advantage in disease-specific survival. Furthermore, patients who received radiotherapy showed a significantly increased rate of recurrence (hazard ratio=5.40; P=.002). However, the authors note that those who received radiotherapy likely had more aggressive disease. Also, the radiation treatment was not standardized and no information is provided regarding the dose and radiation technique.
A case study from Japan published in 2001 indicates that radiotherapy may also be useful as a component of treatment for large metastatic lesions.10 The patient underwent total gastrectomy, distal pancreatectomy, and splenectomy in 1990 for GIST. In 1993, she presented with an 11 cm retroperitoneal metastasis, which was treated with radiotherapy and concomitant chemotherapy (carboplatin and epirubicin). A total of 51 Gy in 1.5 Gy fractions was delivered to the tumor. The patient's elevated serum lactate dehydrogenase level (2142 IU/L) began to decrease following the initiation of radiotherapy and was within normal range at the end of the radiation treatment course. CT scan immediately after completion of radiotherapy indicated decreased density of the tumor. In addition, the patient received four injections of OK432, an immunotherapeutic agent, following the conclusion of radiotherapy. Six years after treatment, the tumor had markedly decreased density and measured 20 mm, demonstrating excellent results. This case supports the use of radiotherapy as a component of palliative treatment of an isolated metastatic lesion.
A more recent abstract by Hurwitz et al. from Royal Marsden Hospital presented in 2008 described their experience with palliative radiotherapy for locally progressive and/or symptomatic metastatic GISTs.11 Most patients received 30 Gy in 10 fractions. Eleven of 12 patients experienced improvement in symptoms. Treatment is described as well-tolerated, with most toxicities being grade 1 or 2.
Two additional published case studies address the use of radiotherapy delivered concomitantly with imatinib. In one case study from Turkey,12 the patient presented with a 15×10 cm mass at the area of the sacrum and coccyx with invasion into the rectum. Multiple liver lesions were also seen. The pelvic mass was incompletely resected; histopathological and immunohistochemical examination demonstrat ed GIST with expression of c-KIT. The residual tumor was irradiated to 54 Gy in 2 Gy fractions with concomitant imatinib. Dramatic regression of the mass and alleviation of pain occurred following radiotherapy. Treatment with imatinib was continued. CT scans at two and four months post-radiotherapy showed continued regression of the pelvic tumor; the liver lesions remained stable. The pelvic tumor regressed completely at 27 months after the completion of radiotherapy. At 37 months, the liver lesions began to progress. The dose of imatinib was increased; however, the liver lesions continued to increase in size. There was no evidence of recurrence in the radiation field. We can conclude that the long-term local control of the pelvic mass was due to the radiotherapy.
A second case study by Ciresa et al. illustrates the use of concomitant pre-operative radiotherapy and imatinib.13 The patient presented with a 4.7cm rectal mass with extension to the anal canal. Biopsy revealed a GIST, positive for c-KIT. The entire mesorectum was included in the treatment field. Treatment was stopped at 37.8 Gy due to grade 3 hematologic toxicity and grade 2 proctitis. There was a marked clinical response on physical examination and imaging. The patient underwent a sphincter-sparing low anterior resection with a pathologic complete response. In these two cases, concomitant radiotherapy and imatinib were delivered safely.
Our institutional experience demonstrates that external beam radiotherapy may be a viable option for local control of unresectable GIST. Our patient's excellent long-term local control indicates that GIST is a radiosensitive tumor. This is also seen in the small number of published studies that demonstrate the effectiveness of radiotherapy in the treatment of residual and metastatic GIST. Radiation therapy, therefore, may be a useful tool in the management of GIST. Radiation therapy may be especially valuable for patients who cannot tolerate imatinib or sunitinib or who have TKI-resistant disease. Radiotherapy should be considered for patients with unresectable disease, with gross or microscopic residual disease following surgery or with recurrent or metastatic disease requiring palliation. For symptomatic patients, palliative radiotherapy may offer relief with minimal acute side effects. There is currently an open trial at Helsinki University looking at the role of radiotherapy for palliation in patients with progressive metastatic disease during or after treatment with a TKI (http://clinicaltrials.gov/ct2/show/NCT005159). In addition, the use of radiation therapy concurrently with imatinib holds promise for patients with unresectable tumor. Further investigation is warranted.
References
- 1.American Cancer Society. Detailed guide: gastrointestinal stromal tumors. Atlanta, GA: American Cancer Society; 2009. [Google Scholar]
- 2.Mazur MT, Clark HB. Gastric stromal tumors: reappraisal of histogenesis. Am J Surg Pathol. 1983;7:507–19. doi: 10.1097/00000478-198309000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Stein E, Aranha O, Agulink M. New therapeutic options in gastrointestinal stromal tumors. Oncology. 2008;22:206–11. [PubMed] [Google Scholar]
- 4.Fenton RG, Longo DL. Cancer cell biology and angiogenesis. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. Harrison's principles of internal medicine. 16th ed. New York; McGraw-Hill: 2005. pp. 453–464. [Google Scholar]
- 5.Fletcher CDM, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459–65. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 6.Crosby JA, Catton CN, Davis A, et al. Malignant gastrointestinal stromal tumors of the small intestine: a review of 50 cases from a prospective database. Ann Surg Oncol. 2001;8:50–9. doi: 10.1007/s10434-001-0050-4. [DOI] [PubMed] [Google Scholar]
- 7.Pollack J, Morgan D, Denobile J, Williams J. Adjuvant radiotherapy for gastrointestinal stromal tumor of the rectum. Dig Dis Sci. 2001;46:268–72. doi: 10.1023/a:1005581000712. [DOI] [PubMed] [Google Scholar]
- 8.Ricca L, Ferri M, De Siena T, et al. Tumori stromali gastrointestinali (GIST) a localiz-zazione rettale. Un nuovo caso e revisione della letteratura. Chir Ital. 2002;54:709–16. [Italian] [PubMed] [Google Scholar]
- 9.Pierie JEN, Choudry U, Muzikansky A, et al. The effect of surgery and grade on outcome of gastrointestinal stromal tumors. Arch Surg. 2001;136:383–9. doi: 10.1001/archsurg.136.4.383. [DOI] [PubMed] [Google Scholar]
- 10.Shioyama Y, Yakishi Y, Watanabe T, et al. Long-term control for a retroperitoneal metastasis of malignant gastrointestinal stromal tumor after chemoradiotherapy and immunotherapy. Acta Oncol. 2001;40:102–4. doi: 10.1080/028418601750071154. [DOI] [PubMed] [Google Scholar]
- 11.Hurwitz J, Constantinidou A, Saran F, et al. The role of radiotherapy in metastatic gastrointestinal stromal tumour (GIST). Proceedings of the Connective Tissue Oncology Society; 2008. Abstract 35023, available from: http://www.ctos.org/meeting/2008/program_abstracts.pdf. [Google Scholar]
- 12.Boruban C, Sencan O, Akmansu M, et al. Metastatic gastrointestinal stromal tumor with long-term response after treatment with concomitant radiotherapy and imatinib mesylate. Anticancer Drugs. 2007;18:969–72. doi: 10.1097/CAD.0b013e3280e94982. [DOI] [PubMed] [Google Scholar]
- 13.Ciresa M, D'Angelillo RM, Ramella S, et al. Molecularly targeted therapy and radiotherapy in the management of localized gastrointestinal stromal tumor (GIST) of the rectum: a case report. Tumori. 2009;95:236–9. doi: 10.1177/030089160909500217. [DOI] [PubMed] [Google Scholar]