Abstract
Primary neuroendocrine tumors of the female genital tract have been described in the cervix, ovaries and uterus. Large cell neuroendocrine carcinoma (LCNC) of the uterine corpus is the least common and appears to behave the most aggressively. We report a rare case of a large cell neuroendocrine tumor of the endometrium. These tumors are not well characterized, unlike neuroendocrine tumors of the uterine cervix. Consequently, the optimal management remains still unclear. The treatment of our case consisted of surgery, radiotherapy, chemotherapy, and octreotide. Despite the aggressive treatment, the patient died of disease progression 12 months after the initial diagnosis. We discuss the diagnosis, prognosis, and treatment options for LCNC of the genital tract, and potential future therapeutics.
Key words: Large cell neuroendocrine carcinoma, uterine corpus, chromogranin A, octerotide.
Introduction
Neuroendocrine genital tract tumors are more common in the female than the male. Large cell neuroendocrine carcinoma of the uterine corpus is a rare variant of uterine carcinoma with features of biologic aggressiveness.
According to the WHO, large cell neuroendocrine tumor is synonymous with undifferentiated carcinoma of non-small cell neuroendocrine type and defined as a malignant tumor composed of large cells that show neuroendocrine differentiation. The criterion for neuroendocrine tumor differentiation relies on a combination of typical structural, immunohistochemical and ultrastructural findings.1
Primary neuroendocrine tumors of the female genital tract have been described in the cervix, ovaries and endometrium. Of these, large cell neuroendocrine carcinoma of the endometrium is the least common, with only one previously reported case in the medical literature. We present the second reported case of pure large cell neuroendocrine carcinoma of the endometrium.
Case Report
A 59-year-old, gravida 6 para 4, post-menopausal Hispanic woman presented with a six-month history of postmenopausal bleeding. Her past medical history was significant for hypertension, diabetes and goiter. Her mother had breast cancer. Gynecologic examination revealed an enlarged 10 cm uterus with irregular contour; the cervix and adnexa were normal. Poorly differentiated endometrial carcinoma in sheets and clusters was diagnosed on endometrial biopsy. The patient's chest X-ray, colonoscopy and mammogram were within normal limits. CA125 prior to surgery was within normal limit. Computed Tomography (CT) of the chest, abdomen and pelvis demonstrated an enlarged uterus, with a central hypodense mass measuring 7×6 cm, pelvic lymph nodes measuring up to 4 cm, para-aortic lymphadenopathy measuring up to 3 cm. Exploratory laparotomy revealed a 12 cm size uterus; the entire uterine, and bladder serosa, including peritoneum was involved with superficial tumor implants. Ovaries and upper abdomen were unremarkable. Pelvic and para-aortic nodes were enlarged. Surgical staging consisted in washings for cytology, total abdominal hysterectomy with bilateral salpingo-oophoerectomy, bilateral pelvic and para-aortic lymphadenectomy, omentectomy, appendectomy and tumor debulking was performed. Pathology examination demonstrated a 312 gram uterus. There was a polypoid, tan-white, fleshy tumor covering the entire anterior endometrium and myometrium. Micro - scopically, the tumor consisted of a poorly differentiated LCNC infiltrating through the full thickness of myometrium to the uterine serosa extending to the cervical stroma (Figure 1). Extensive lymphovascular invasion was present. Pelvic and para-aortic lymph nodes were positive for metastatic carcinoma. On immunohistochemical evaluation, the tumor cells were diffusely positive for synaptophysin (Figure 2A), Chromogranin A (CgA) (Figure 2B), Neuron Specific Enolase (NSE) (Figure 2C). Additionally, they were patchy positive for CD56, and focally positive for low molecular weight cytokeratin (CK 8/18). The tumor was negative for pan-cytokeratin (AE1/AE3), CD45 (LCA), TTF-1, estrogen, progesterone, CD10, p63, p53, s100, c-kit, vimentin, smooth muscle actin, desmin and HMB-5. The final pathology diagnosis was consistent with invasive LCNC.
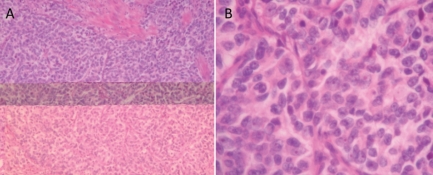
Figure 1.
A) Immunohistochemical analysis of large cell neuroendocrine carcinoma showing a composition of sheets and organoid large cells with abundant eosinophilic cytoplasm and small eosinophilic cytoplasmic granules. B) Larger magnification of large cell neuroendocrine carcinoma tissue showing cells with vesicular high grade nuclei with prominent nucleoli and many mitoses.
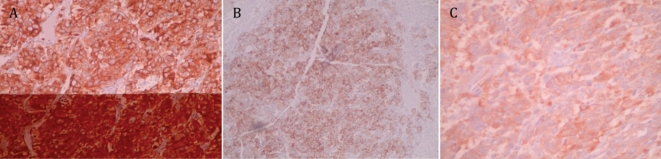
Figure 2.
Immunohistochemical staining showing expression of specific neuroendocrine markers: Synaptophysin (A), Chromagranin (B) and Neuron Specific Enolase (C).
Postoperatively, the patient was started on an institutional ‘sandwiched’ chemoradiotherapy protocol used in our division for the post-operative treatment of patients with high risk histologic types of endometrial cancer. The treatment regimen consists of six cycles of carboplatin and paclitaxel with radiation therapy administered following the third cycle of chemotherapy. The CT scan of chest, abdomen and pelvis prior to radiation was unremarkable. The radiation therapy consists of standard whole pelvic therapy with 45 Gy (1.8 Gy per fraction daily), and vaginal brachytherapy, 15Gy (5Gy per fraction weekly). After completion of sixth cycle of chemotherapy, the CT scan demonstrated soft tissue thickening along the anus and extensive retroperitoneal lymphadenopathy. The patient then received three cycles of pegylated doxorubicin. A follow-up CT revealed disease progression, with increased retroperitoneal lymphadenopathy. An octreotide scan was performed which was positive for pelvic lymphadenopathy. For the first time the serum levels of NSE and CgA were evaluated and measured at 188 units/L and 72 ng/mL respectively. The patient wished to continue with aggressive therapy and began a three cycle combination chemotherapy regimen of etoposide (100 mg/m2 IV), cisplatin (75 mg/m2 IV) and long acting depot octreotide 20 mg IM every four weeks. Oral etoposide 200 mg/m2 was also given on days 2–4. Patient did not respond to three cycle of this regimen. During use of this combination chemotherapy the NSE level decreased to 55 ng/mL, while the level of CgA increased to 249 U/L. She died 12 months following her initial diagnosis and surgery from progressive disease. During these 12 months, the patient's course was complicated by multiple admissions for DVT, neutropenic fever, and hyperglycemia.
Discussion
The College of American Pathologists and the National Cancer Institute workshop recognized four general categories of endocrine tumors. These are typical (classical), carcinoid tumor, large cell neuroendocrine carcinoma and small (oat) cell carcinoma. LCNC are poorly differentiated tumors that typically have the neuroendocrine morphologic appearance under light microscopy (organelle nesting, palisading, rosettes, and trabeculae), high mitotic rate (>10 mitoses/10 high-power fields), the presence of necrosis (centrilobular or in form of a large area), the presence of large cell size, low nuclear to cytoplasmic ratio, vesicular chromatin, and prominent nuclei, and positive immunohistochemical staining for one or more neuroendocrine markers NSE, CgA and synaptophysin.2 Among these general tumor markers CgA, although its precise function has not been established, has been shown to be a very sensitive and specific serum marker for various types of neuroendocrine tumors. It is elevated in 50–100% of patients with neuroendocrine tumors. CgA serum or plasma levels may reflect tumor load.3 LCNCs of the lung and the cervix are characterized by early tumor recurrence despite radical surgery, radiotherapy and chemotherapy. These tumors are highly aggressive neoplasms. In clinico-pathologic study of 16 cases of LCNC of the cervix, the progression of disease was within 2 years of diagnosis.4, 5 LCNC of the endometrium is a rare, aggressive tumor with an assumed unfavorable outcome similar to LCNC of the cervix. In the one reported case of LCNC of the endometrium, the patient developed cerebral and pulmonary metastases after surgery and adjuvant radiotherapy and died 4 months after recurrence.6 Our case is the second report of a LCNC of the endometrium and the first case who had the trial of octreotide treatment. Our patient reported increasing serum values of CgA, which was consistent with progressive disease and non-response to treatment.
Adjuvant chemotherapy (cisplatin, carboplatin, etoposide or cyclophosphamide based, 5-fluorouracil, streptozocin, paciltaxel) has been used in the management of LCNC of the lung and cervix4,5 Since the biological activity and reason for aggressive behavior for neuroendocrine tumors is not well delineated at present, targeted therapy is limited to octreotide. Octreotide is a synthetic octapep-tide with a structure and activity similar to somatostatin.7 Somatostatin receptors are essential for transmitting the response from somatostatin or its analogs, and they are highly represented in neuroendocrine tumors. The role of somatostatin analogs in inhibiting tumor growth has been demonstrated in a number of animal models and human tumor cell lines. The potential mechanisms include inhibition of the secretion of hormones involved in the regulation of tumor growth, direct or indirect inhibition of IGF-1, and/or other growth factors with a stimulatory effect on tumor growth, inhibition of angiogenesis, and a direct inhibitory effect on the tumor via specific somatostatin receptors.7
In the single case report of the use of octreotide for small cell neuroendocrine tumor of the endometrium, a partial response was reported.8 In our case the patient had progression of the disease with combination of chemotherapy and octreotide treatment regardless of having an octreotide positive scan. Octreotide in general, is well tolerated. However, we observed a well-known side effect of octreotide, namely poor glycemic control especially in patients with diabetes. The patient described here reported repeated episodes of hypo- and hyperglycemia.
Due to the rarity of these tumors the optimal therapy has not yet been delineated. Although a treatment recommendation cannot be established from our report, a review of the literature on LCNC of the lung and cervix suggests that primary multimodality therapy may improve prognosis in these patients.
References
- 1.Roth LM, Tsubura A, Dietel M, Senzaki H. Miscellaneous tumours and tumour-like conditions of the ovary. Tavassoli FA, Devilee P, editors. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. World Health Organization Classification of Tumours. 2004 [Google Scholar]
- 2.Abores Saavedin J, Gersell D, Gilks CB, et al. Terminology of endocrine tumors of the uterine cervix: results of a workshop sponsored by the college of American Pathologists and the National Cancer Institute. Arch Pathol Lab Med. 1997;121:34–9. [PubMed] [Google Scholar]
- 3.Chaudhry A, Kvols L. Advances in the use of somatostatins in the management of endocrine tumors. Curr Opin Oncol. 1996;8:44–8. doi: 10.1097/00001622-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Gilks CB, Young RH, Gersell DJ, et al. Large cell neuroendocrine carcinoma of the uterine cervix: a clinicopathologic study of 12 cases. Am J Surg Pathol. 1997;21:905–14. doi: 10.1097/00000478-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Wang KL, Yang YC, Wang TY, et al. Neuroendocrine carcinoma of the uterine cervix: A clinicopathologic retrospective study of 31 cases with prognostic implications. J Chemother. 2006;18:209–16. doi: 10.1179/joc.2006.18.2.209. [DOI] [PubMed] [Google Scholar]
- 6.Erhan Y, Dikmen Y, Yucebilgin MS, et al. Large cell neuroendocrine carcinoma of the uterine corpus metastatic to brain and lung: case report and review of the literature. Eur J Gynaecol Oncol. 2004;25:109–12. [PubMed] [Google Scholar]
- 7.Scully RE, Aguirre P, Delellis RA. Argyrophilia, serotonin and peptide hormones in the female genital tract and its tumors. Int J Gynecol Pathol. 1984;3:51–70. doi: 10.1097/00004347-198403010-00005. [DOI] [PubMed] [Google Scholar]
- 8.Verschraegen CF, Matei C, Loyer E, et al. Octreotide induced remission of a refractory small cell carcinoma of the endometrium Int J Gynecol Cancer. 1999;9:80–5. doi: 10.1046/j.1525-1438.1999.09886.x. [DOI] [PubMed] [Google Scholar]