Abstract
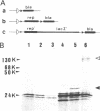
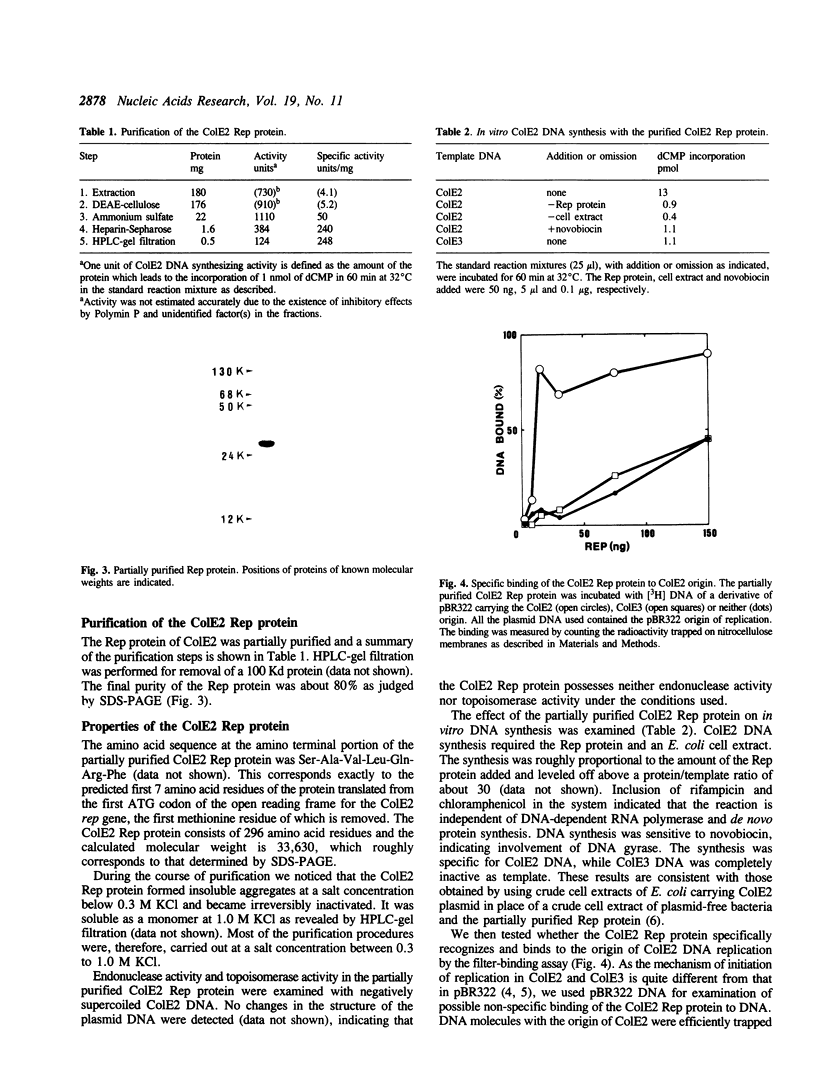
The product of the rep gene of ColE2 is required for initiation of ColE2 DNA replication. The rep gene was placed under the control of the promoters, PL and PR, and the heat-labile cl857 repressor of bacteriophage lambda. The Rep protein was identified as a 35 Kd protein by the maxicell method in combination with heat-induced expression. The protein was efficiently expressed from these promoters in unirradiated cells and accumulated up to a few per cent of the total cellular proteins. It was partially purified (about 80% pure) and its properties examined. The amino acid sequence of the amino terminal portion of the partially purified protein agreed well with that predicted from the nucleotide sequence of the rep gene. One of the characteristic features of the rep gene is frequent usage of rare codons, especially those for arginine. The protein specifically stimulated replication of ColE2 DNA but not that of ColE3 DNA in crude cell extracts of Escherichia coli. Specific binding of the protein to plasmid DNA containing the origin region of ColE2 was demonstrated by the filter binding method. Neither endonuclease activity nor topoisomerase activity was detected by using ColE2 DNA.
Full text
PDF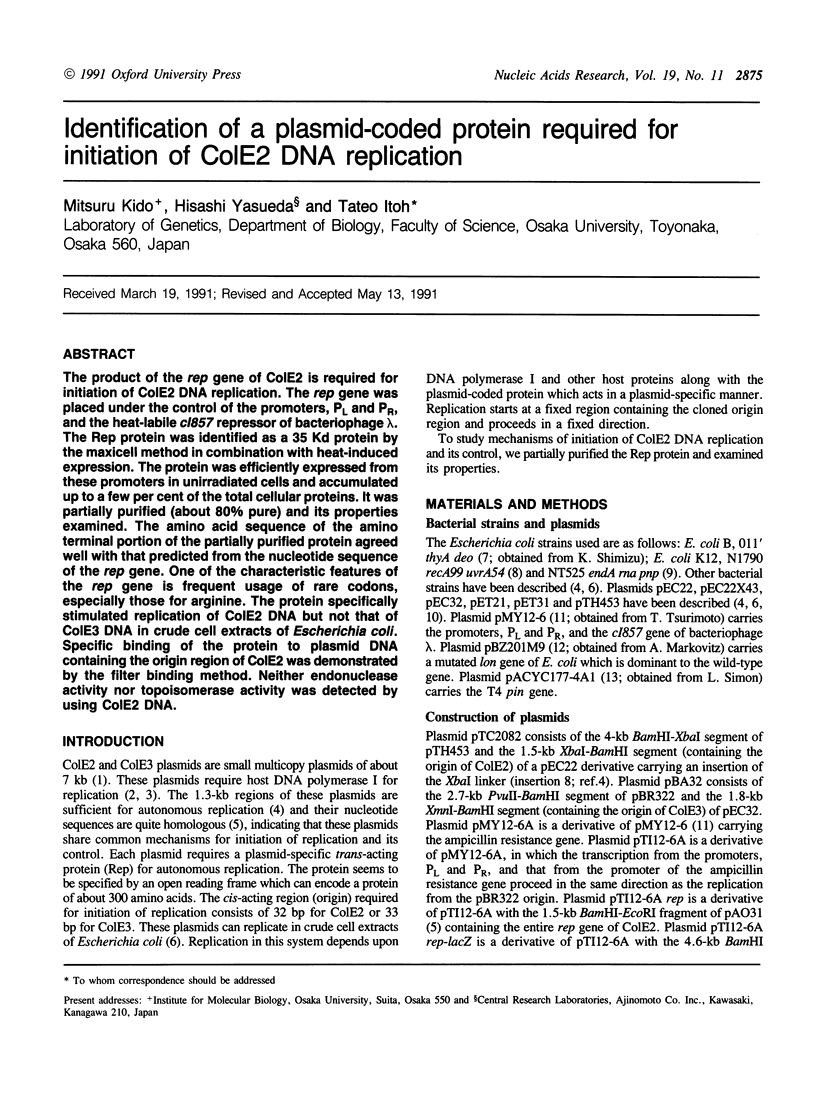



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brent R., Ptashne M. The lexA gene product represses its own promoter. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1932–1936. doi: 10.1073/pnas.77.4.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cole S. T., Saint-Joanis B., Pugsley A. P. Molecular characterisation of the colicin E2 operon and identification of its products. Mol Gen Genet. 1985;198(3):465–472. doi: 10.1007/BF00332940. [DOI] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschman H. R., Helinski D. R. Comparative study of the events associated with colicin induction. J Bacteriol. 1967 Sep;94(3):691–699. doi: 10.1128/jb.94.3.691-699.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii T., Itoh T. Replication of ColE2 and ColE3 plasmids: the regions sufficient for autonomous replication. Mol Gen Genet. 1988 May;212(2):225–231. doi: 10.1007/BF00334689. [DOI] [PubMed] [Google Scholar]
- Itoh T., Horii T. Replication of ColE2 and ColE3 plasmids: in vitro replication dependent on plasmid-coded proteins. Mol Gen Genet. 1989 Oct;219(1-2):249–255. doi: 10.1007/BF00261184. [DOI] [PubMed] [Google Scholar]
- Kameji T., Murakami Y., Fujita K., Hayashi S. Purification and some properties of ornithine decarboxylase from rat liver. Biochim Biophys Acta. 1982 Jul 16;717(1):111–117. doi: 10.1016/0304-4165(82)90387-7. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T., Helinski D. R. DNA polymerase as a requirement for the maintenance of the bacterial plasmid colicinogenic factor E1. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1538–1544. doi: 10.1016/0006-291x(70)90562-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamerichs R. M., Padilla A., Boelens R., Kaptein R., Ottleben G., Rüterjans H., Granger-Schnarr M., Oertel P., Schnarr M. The amino-terminal domain of LexA repressor is alpha-helical but differs from canonical helix-turn-helix proteins: a two-dimensional 1H NMR study. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6863–6867. doi: 10.1073/pnas.86.18.6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlon J., Lloubès R., Varenne S., Chartier M., Lazdunski C. Complete nucleotide sequence of the structural gene for colicin A, a gene translated at non-uniform rate. J Mol Biol. 1983 Oct 25;170(2):271–285. doi: 10.1016/s0022-2836(83)80148-x. [DOI] [PubMed] [Google Scholar]
- Ogawa T. Analysis of dnaB function of Escherichia coli K12 and the dnaB-like function of P1 prophage. J Mol Biol. 1975 May 25;94(3):327–340. doi: 10.1016/0022-2836(75)90206-5. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Suzuki H., Bourgeois S. Lac repressor-operator interaction. I. Equilibrium studies. J Mol Biol. 1970 Feb 28;48(1):67–83. doi: 10.1016/0022-2836(70)90219-6. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y., Tomizawa J. I. Replication of colicin E1 plasmid DNA in cell extracts. Proc Natl Acad Sci U S A. 1974 Mar;71(3):802–806. doi: 10.1073/pnas.71.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L. D., Randolph B., Irwin N., Binkowski G. Stabilization of proteins by a bacteriophage T4 gene cloned in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2059–2062. doi: 10.1073/pnas.80.7.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J. DNA sequence analysis by primed synthesis. Methods Enzymol. 1980;65(1):560–580. doi: 10.1016/s0076-6879(80)65060-5. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Tacon W., Sherratt D. ColE plasmid replication in DNA polymerase I-deficient strains of Escherichia coli. Mol Gen Genet. 1976 Sep 23;147(3):331–335. doi: 10.1007/BF00582885. [DOI] [PubMed] [Google Scholar]
- Tsurimoto T., Hase T., Matsubara H., Matsubara K. Bacteriophage lambda initiators: preparation from a strain that overproduces the O and P proteins. Mol Gen Genet. 1982;187(1):79–86. doi: 10.1007/BF00384387. [DOI] [PubMed] [Google Scholar]
- Yasueda H., Horii T., Itoh T. Structural and functional organization of ColE2 and ColE3 replicons. Mol Gen Genet. 1989 Jan;215(2):209–216. doi: 10.1007/BF00339719. [DOI] [PubMed] [Google Scholar]
- Zehnbauer B. A., Markovitz A. Cloning of gene lon (capR) of Escherichia coli K-12 and identification of polypeptides specified by the cloned deoxyribonucleic acid fragment. J Bacteriol. 1980 Aug;143(2):852–863. doi: 10.1128/jb.143.2.852-863.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Elzen P. J., Walters H. H., Veltkamp E., Nijkamp H. J. Molecular structure and function of the bacteriocin gene and bacteriocin protein of plasmid Clo DF13. Nucleic Acids Res. 1983 Apr 25;11(8):2465–2477. doi: 10.1093/nar/11.8.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]