Abstract
In contrast to the well-described various biological effects of grape wines, the potential effects of commonly consumed blackberry wine have not been studied. We examined in vitro antioxidant and vasodilatory effects of four blackberry wines and compared them with the effects of two red and two white grape wines. Although some blackberry wines had lower total phenolic content relative to the red grape wines, their antioxidant capacity was stronger, which may be related to a higher content of non-flavonoid compounds (most notably gallic acid) in blackberry wines. Although maximal vasodilation induced by blackberry wines was generally similar to that of red wines, blackberry wines were less potent vasodilators. Vasodilatory activity of all wines, in addition to their flavonoid and total phenolic content, was most significantly associated with their content of anthocyanins. No association of vasodilation with any individual polyphenolic compound was found. Our results indicate the biological potential of blackberry wines, which deserves deeper scientific attention.
Key Words: anthocyanins, antioxidant effects, blackberry, vasodilation, wine
Introduction
Different fruits and their processed products, such as wine, are rich sources of polyphenolic compounds. Epidemiological studies have demonstrated a significant negative correlation between polyphenol consumption and cardiovascular risk.1,2 It has been indicated that polyphenols may act beneficially against oxidative stress, as the main pathophysiological mechanism, and the endothelium, as the main target organ, in the development of various cardiovascular diseases. In addition to, and independently from, their antioxidant effects,3 polyphenols enhance the production of vasodilating agents (nitric oxide, endothelium-derived hyperpolarizing factor) and inhibit the production of vasoconstrictor (endothelin-1) factors in endothelial cells.4 Among polyphenol-rich food products, the phenolic composition and biological effects of red wine produced from grapes (Vitis vinifera) have received particular attention.5,6 An important and well-documented biological effect of red wine is the direct, endothelium-dependent vasodilatory activity that is mostly related to the polyphenols.7–9 In contrast to red wine, the polyphenolic content of white wine is low, and related direct vasodilatory activity is poor.8,10
Besides grape wines, there are several other commonly consumed fruit wines. Although their consumption has become increasingly popular, they have received little scientific attention. Several studies showed that fruit wines are also a potentially rich source of polyphenols, exhibiting noticeable antioxidant activity in vitro.11–13 Among them, blackberry (Rubus fructicosus) wine was indicated as a rich source of phenolics,12 with even higher in vitro antioxidant activity than grape wine.14 However, no effects of blackberry wines in biological systems have been studied so far.
In this study, we examined antioxidant and vasodilatory effects of four blackberry wines in the isolated rat aorta and compared them with the effects of two red and two white grape wines. The interrelationship between the vasodilatory activity of the tested wines with their phenolic content and composition was also examined. All tested samples were biochemically characterized with respect to their total phenolic content, high-performance liquid chromatography (HPLC) “phenolic fingerprint,” antioxidant capacity, and ethanol content.
Materials and Methods
All wines were commercially available and purchased from local pharmacy and grocery stores. Information about the source, vintage, and ethanol content of the tested grape (red and white) and blackberry wine samples are shown in Table 1.
Table 1.
Grape and Blackberry Wine Source, Vintage, and Ethanol Content Used in the Study
| Wine number | Wine sample (abbreviation)a | Name, producer, region | Year | Ethanol content (volume %) |
|---|---|---|---|---|
| 1 | Red (RW1) | Refosk, Bric, Slovenia | 2007 | 13.5 |
| 2 | Red (RW2) | Vinagra, Bric, Slovenia | 2005 | 13.0 |
| 3 | White (WW1) | Posip, Cara Korcula, Craotia | 2007 | 14.0 |
| 4 | White (WW2) | Zlatna zlahtina, Vrbnik Krk, Croatia | 2007 | 11.5 |
| 5 | Blackberry (BW1) | KupiFe, Split, Croatia | 2007 | 9.9 |
| 6 | Blackberry (BW2) | Kupinovo vino Suler, Kutina, Croatia | 2007 | 13.7 |
| 7 | Blackberry (BW3) | BIO&BIO, Orlov put, Croatia | 2008 | 10.6 |
| 8 | Blackberry (BW4) | Baranjsko kupinovo vino, Cerine, Croatia | 2006 | 13.0 |
BW, blackberry wine; RW, grape red wine; WW, grape white wine.
Animal experiments were conducted in accordance with international ethical guidelines. The study was approved by the Ethics Committee of the University of Split School of Medicine, Split, Croatia.
Preparation of aortic rings
Male Sprague–Dawley rats (n=48) weighing 330±20 g were used for this study. The animals received an intraperitoneal injection of urethane (1.2 g/kg). After becoming unresponsive to noxious stimuli, they were decapitated. The descending thoracic aorta was dissected free from the connective tissue and placed in the modified Krebs–Henseleit solution. The aorta was carefully cleaned of the adhering fat and cut into rings as previously described.15 After a washing-out and stabilization period, the rings were precontracted with a test dose of noradrenaline (10−7 M). When the contraction reached the plateau phase, functionality of endothelium was confirmed by acetylcholine (10−6 M)-induced relaxation. The relaxation was expressed as the percentage decrease of the noradrenaline-induced vasoconstriction. After triple washout and tension stabilization, the precontracted rings were randomly exposed to cumulative concentrations (0.1‰ to 8‰ final dilutions in organ baths) of each of the tested wine samples (n=15 per wine sample).
Biochemical analysis of the wine samples
Phenolic content and composition
The contents of total phenolics and their subgroup (flavonoid, non-flavonoid, and anthocyanin) were measured spectrophotometrically, whereas the individual phenolic compounds were determined by HPLC.
The total phenolic content of the samples was determined by the Folin–Ciocalteau method, and the results are expressed as gallic acid equivalents per liter. Non-flavonoid compounds were determined by the Folin–Ciocalteau method after precipitation of flavonoids with formaldehyde, and the flavonoid content was calculated as the difference between total phenolic and non-flavonoid contents.
Total anthocyanin content was determined using the bisulfite bleaching method, and the results are expressed as milligrams of malvidin-3-glucoside per liter.
Absorbances were monitored by an ultraviolet-visible spectrophotometer (Specord 200, Analytik Jena Inc., Jena, Germany), equipped with a six-cell holder and a thermostatically controlled bath. The data presented are the averages of three measurements. A more detailed description of the above-mentioned methods has been previously published.16
Individual polyphenols were identified and quantified by HPLC. The HPLC system was composed of a Varian (Palo Alto, CA, USA) ultraviolet-visible PDA 330 detector, a ternary gradient liquid Pro Star 230 pump, model 500 column heater, and Star chromatography workstation version 6.0. The polyphenolic compounds were separated on an octadecyl column (Zorbax Eclipse XDB-C18; 4.6 mm×250 mm; film thickness, 5 μm; Agilent, Palo Alto) maintained at 30°C. Wine samples were filtered through a membrane filter (pore size, 0.45 μm) and directly injected through a 20-μL fixed loop into a C18 guard column. Wine samples were diluted three times with eluent prior to injection.
A gradient consisting of solvent A (water/acetic acid, 98:2, vol/vol) and solvent B (acetonitrile/acetic acid, 99:1, vol/vol) was applied at a flow rate of 1.0 mL/minute as follows: 0 minute, 93% A/7% B; 18 minutes, 80% A/20% B; 25 minutes, 60% A/40% B; 30 minutes, 40% A/60% A; 40 minutes, 40% A/60% B; 43 minutes, 93% A/7% B; and 45 minutes, 93% A/7% B. The signal was monitored at 280 nm. Phenolic compounds were identified by their retention times and absorption spectra. Quantification was carried out by comparison with external standard calibration curves: 20–80 mg/L for gallic acid and (–)-epicatechin; 10–60 mg/L for epigallocatechin gallate, quercetin-4-glucoside, trans-resveratrol, (E)-piceid, and astringin; and 30–170 mg/L for (+)-catechin and procyanidin B2. The stock solution of cis-resveratrol isomer was prepared by ultraviolet irradiation at 254 nm of an alcoholic solution of trans-resveratrol according to Romero-Pérez et al.17 Each sample was injected twice into the chromatographic system.
Antioxidant capacity
Total antioxidant capacity of the samples was determined using the ferric reducing antioxidant power (FRAP) assay.18 In this assay, antioxidants are evaluated as reductants of Fe3+ to Fe2+, which is chelated by 2,4,6-tripyridyl-s-triazine to form a Fe2+–2,4,6-tripyridyl-s-triazine complex absorbing at 593 nm. Measurements were done in triplicate. Results were compared with a standard curve prepared with different concentrations (0.5–2 mM) of Trolox, a water-soluble analog of vitamin E, and expressed as Trolox equivalents.
Ethanol content
Ethanol concentration in the samples was measured by a Shimadzu (Kyoto, Japan) model 2010 gas chromatograph with a headspace and flame ionization detector. Ultrapure-grade helium was used as the carrier gas at a flow rate of 11.70 mL/minute. An RTX–BAC2 chromatographic column was used (fused silica; 30 m long and 0.53 mm i.d.; film thickness, 0.20 μm). The injection temperature was 200°C, and the column conditions were 3 minutes at 60°C with the flame ionization detector at 200°C.
Chemicals
All analytical-grade chemicals and reagents were obtained from Sigma Chemical Co. (St. Louis, MO, USA), Aldrich Chemical Co. (Steineheim, Germany), and Merck (Darmstadt, Germany). The resveratrol derivatives (E)-piceid (trans-3,5,4′-trihydroxystilbene-3-O-β-d-glucopyranoside), isorhapontin (trans-3,4′,5-trihydroxy-3′-methoxystilbene-3-O-β-d-glucopyranoside), and astringin (trans-3,4,3′,5′-tetrahydroxystilbene-3′-O-β-d-glucopyranoside) were obtained from Polyphenols Laboratories (Sandnes, Norway). Deionized (Milli Q®, Waters Corp., Milford, MA, USA) water was used for the preparation of all solutions and reagents.
Statistical analysis
Data were analyzed using GraphPad Prism version 4.03 for Windows (GraphPad Software, San Diego, CA, USA). For statistical analysis of vasodilatation responses, one- and two-way analysis of variance followed by Bonferroni's post hoc tests was used. All data are expressed as mean±SEM values. P<.05 was considered statistically significant. Because the wine samples differed significantly in their total phenolic and ethanol contents, we used dilution and logarithm of dilution, instead of concentration, to express 50% effective concentration (EC50) values. Nonlinear regression analysis was used to calculate EC50.
Results
Vasodilatory effects of the wines
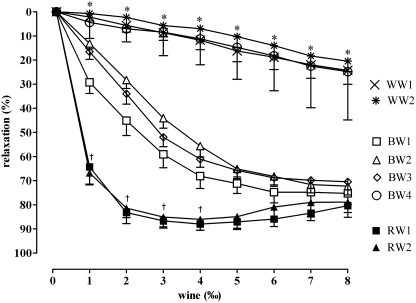
Basal tension of the rat aortic rings (n=120) following exposure to noradrenaline was 18.31±4.27 mN. Although all wines showed vasodilatory activity, they induced different concentration-dependent vasodilatory responses in the noradrenaline-precontracted rat aortic rings (Fig. 1). Generally, red grape wines were the most potent vasodilators (EC50=−3.17 and −3.24 for RW1 and RW2). Blackberry wines BW1, BW2, and BW3 showed intermediate vasodilatory potency with EC50 values of −2.85, −2.63, and −2.72, respectively, whereas blackberry wine BW4 was a significantly less potent vasodilator with an EC50 of −2.31. However, maximum vasodilation (Emax) induced by blackberry wines BW1, BW2, and BW3 was generally similar to that of red grape wines (Emax=75.27±4.67%, 72.27±3.65%, and 70.48±3.40% for BW1, BW2, and BW3, respectively, vs. 87.99±2.50% for RW1 and 86.00±4.56% for RW2). Maximal relaxation produced by white grape wines and blackberry wine BW4 was significantly smaller (24.28±5.79%, 20.53±4.17%, and 24.27±3.86% for WW1, WW2, and BW4, respectively) (P<.05) (Table 2).
FIG. 1.
Relaxation in noradrenaline-precontracted rat aortic rings following exposure to grape red (RW1 and RW2), grape white (WW1 and WW2) and blackberry (BW1, BW2, BW3, and BW4) wines. Results are shown as mean±SEM values (n=15 per wine sample). By two-way analysis of variance, Bonferroni's post hoc test: *P<.05 versus red grape (RW1 and RW2) and blackberry (BW1, BW2, and BW3) wines; †P<.05 versus blackberry wines (BW1, BW2, and BW3).
Table 2.
Vasodilating Activity of the Grape and Blackberry Wines Expressed by Maximum Vasodilation and 50% Effective Concentration
| Wine sample | Emax (%) | EC50 (CI) |
|---|---|---|
| RW1 | 87.99±2.50* | −3.17 (−3.18 to −3.03)† |
| RW2 | 86.00±4.56* | −3.24 (−3.47 to −3.01)† |
| WW1 | 24.28±5.79 | −2.44 (−2.55 to −2.32) |
| WW2 | 20.53±4.17 | −2.34 (−2.44 to −2.24) |
| BW1 | 75.27±4.67* | −2.85 (−2.93 to −2.77)* |
| BW2 | 72.27±3.65* | −2.63 (−2.64 to −2.59)* |
| BW3 | 70.48±3.40* | −2.72 (−2.77 to −2.67)* |
| BW4 | 24.27±3.86 | −2.31 (−2.38 to −2.25) |
Maximum vasodilation (Emax) values are mean±SEM values, and the 50% effective concentration (EC50) was calculated using nonlinear regression analysis. EC50 values are a log of dilution giving 50% of relaxation relative to the sample's own maximal relaxation (1‰=0.001; log 0.001=−3 and 2‰=0.002; log 0.002=−2.70), with the 95% confidence interval (CI) in parentheses. In all measurements n=15 per wine sample.
By one-way analysis of variance, Bonferroni's post hoc multiple comparison test: *P<.05 versus white grape wines (WW1 and WW2) and blackberry wine (BW4); †P<.05 versus blackberry wines (BW1, BW2, BW3, and BW4) and white grape wines (WW1 and WW2).
Biochemical analysis of the wine samples
Phenolic content and antioxidant capacity
As shown in Table 3, total phenolic content was highest in the red grape wines, followed by blackberry wines, whereas the lowest was found in the white grape wines.
Table 3.
Content of Total Phenolics, Main Phenolic Fractions, and Antioxidant Activity of the Grape and Blackberry Wines
| Total phenolics (mg of GAE/L) | Flavonoids (mg of GAE/L) | Non-flavonoids (mg of GAE/L) | Anthocyanins (mg of M-3-G/L) | FRAP (mmol of TE/L) | |
|---|---|---|---|---|---|
| RW1 | 3,313±27 | 3,002±22 | 311±4 | 212±9 | 12.6±0.6 |
| RW2 | 3,225±26 | 2,902±21 | 323±7 | 287±3 | 12.7±0.2 |
| WW1 | 482±3 | 103±2 | 379±5 | ND | 1.9±0.1 |
| WW2 | 379±3 | 58±3 | 321±5 | ND | 1.2±0.1 |
| BW1 | 2,628±29 | 1,417±14 | 1,210±11 | 135±3 | 13.9±0.7 |
| BW2 | 2,025±23 | 951±11 | 1,074±10 | 148±2 | 10.8±0.4 |
| BW3 | 2,789±27 | 1,303±14 | 1,486±12 | 164±3 | 15.8±0.6 |
| BW4 | 1,697±20 | 924±11 | 773±8 | 13±1 | 7.8±0.4 |
Data are averages of at least three independent samples and are shown as mean±SEM values.
FRAP, ferric reducing antioxidant capacity; GAE gallic acid equivalents; M-3-G, malvidin-3-glucoside; ND, not detected; TE, Trolox equivalents.
Relative to the red grape wines, blackberry wines were lower in flavonoid content but were several times richer in non-flavonoid phenolic compounds. Anthocyanins were present only in the red grape and blackberry wines. Their concentrations were higher in the red grape wines (212±9 and 287±3 mg/L malvidin-3-glucoside), relative to the blackberry wines, in which anthocyanins ranged from 13 to 164 mg/L malvidin-3-glucoside (Table 3).
The wine with the highest antioxidant capacity was blackberry wine BW3. The order of the antioxidant capacity considering FRAP was BW3>BW1>RW2>RW1>BW2>BW4>WW1>WW2. The antioxidant capacity of the white wines was fourfold lower relative to the blackberry wine with the lowest FRAP (Table 3).
Analysis of phenolic compounds by HPLC
The most abundant flavanol monomer in grape wines was catechin. In blackberry wines the flavanol content varied more than in grape wines. The best example of that is epigallocatechin gallate, which ranged in concentration from 3.5 to 148.8 mg/L in the blackberry wines. Similarly, procyanidin B2, the dimer of epicatechin, ranged in concentration from 6.1 to 77.1 mg/L in blackberry wines; thus BW1 and BW2 were richer in procyanidin B2 content than red wines.
Among non-flavonoids, stilbenes were detected in all wines. Resveratrol monomers were found in small amounts (0–2.2 mg/L), but their derivatives, (E)-piceid and astringin, were found in higher concentrations, regardless of wine type. The non-flavonoid gallic acid was also present in all wines, but its concentrations were two to three times higher in blackberry than in grape wines. Compounds identified and their concentrations are listed in Table 4.
Table 4.
Concentrations of Selected Phenolic Compounds in the Grape and Blackberry Wines
| |
Concentration (mg/L) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wine | Gallic acid | (+)-Catechin | (–)-Epicatechin | Epigallocatechin gallate | Procyanidin B2 | Quercetin-4-glucoside | cis-Resveratrol | trans-Resveratrol | (E)-Piceid | Astringin |
| RW1 | 26.4±0.1 | 13.5±0.4 | 7.8±1.4 | 12.7±0.5 | 12.3±1.6 | 1.4±0.1 | 0.6±0.1 | 1.1±0.1 | 1.5±0.2 | 5.2±0.1 |
| RW2 | 14.9±0.1 | 15.8±0.9 | 4.9±0.3 | 1.6±0.3 | 10.0±1.3 | 0.4±0.1 | 0.6±0.1 | 1±0.1 | 1.7±0.1 | ND |
| WW1 | 21.8±0.1 | 21.7±0.2 | 1.5±0.3 | 9.8±0.1 | 4.4±0.3 | ND | 0.5±0.1 | 1.1±0.1 | 2.3±0.1 | 2.4±0.2 |
| WW2 | 3.3±0.2 | 18.4±0.9 | 0.4±0.01 | 17.8±0.6 | ND | ND | 0.1±0 | 0.2±0 | 0.8±0.1 | 2.3±0.2 |
| BW1 | 59.0±1.4 | 18.7±1.5 | 34.7±3.7 | 148.8±0.8 | 77.1±3.3 | 2.6±0.1 | 1.5±0.1 | 0.7±0.1 | 5.2±0.5 | 7.9±0.8 |
| BW2 | 45.4±0.3 | 16.8±1.4 | 7.9±1.7 | 16.8±2.2 | 34.5±4.3 | 2.4±0.2 | ND | 0.5±0.1 | 2.6±0.1 | ND |
| BW3 | 50.1±0.3 | 45.2±2.9 | ND | ND | 6.1±0.2 | 2.1±0.1 | 0.2±0.1 | 0.7±0.1 | 6.5±0.4 | 12.1±0.8 |
| BW4 | 53.1±3.2 | 9.1±0.9 | 12.2±1.7 | 3.5±0 | 8.8±0.4 | ND | 0.1±0 | 0.9±0.1 | 2.0±0.4 | ND |
Data are averages of two independent samples and are shown as mean±SEM values.
Relationship between vasodilatory activity and phenolic content of the tested wines
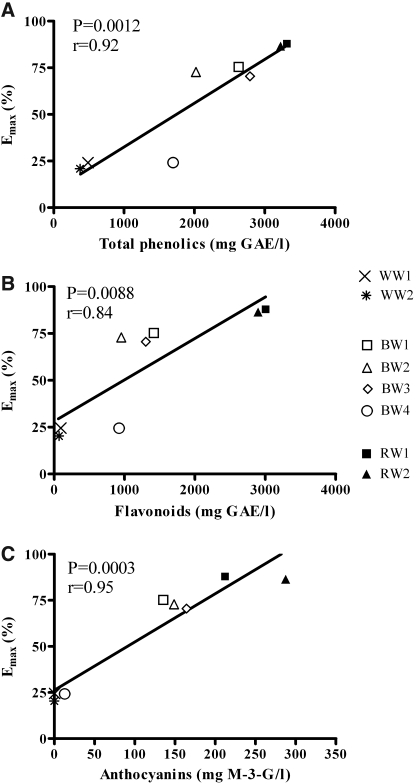
Vasodilatory activity correlated strongly with total phenolic content of the wines (r=0.92). Among phenolic fractions a strong positive correlation was found for anthocyanin content (r=0.95), followed by flavonoid content (r=0.84). No correlation was found between the levels of individual phenolic compounds and vasodilatory activity. The relationship between Emax and total phenolic, flavonoid, and anthocyanin content is shown in Figure 2.
FIG. 2.
Relationships between vasodilatory activity (Emax) and (A) total phenolic, (B) flavonoid, and (C) anthocyanin content of the tested wine samples. Total phenolic and flavonoid contents were expressed in milligrams of GAE per liter, and anthocyanin content was expressed in milligrams of M-3-G per liter.
Discussion
Results of this study indicate that blackberry wines are relatively effective direct vasodilators and even better antioxidants in vitro. The finding that some blackberry wines (BW1 and BW3) were more effective antioxidants than red grape wines is supportive of the previously published results by Pinhero and Paliyath.14 Nonetheless, this is a rather unexpected finding taking into account the lower total phenolic content of blackberry wines in comparison with red grape wines, as it has been repeatedly documented that in vitro antioxidant capacity of different beverages, as determined by FRAP, is highly related to their total phenolic content.16,19,20 A possible explanation for this discrepancy may be attributed to the higher content of non-flavonoids (most notably gallic acid) in blackberry wines relative to the red grape wines. In our previous study on antioxidant and vasodilatory effects of phenolic acids we showed that gallic acid was the most potent in vitro antioxidant but the least effective direct vasodilator.21
Another important finding of this study is that vasodilatory activity of all tested wines, in addition to their flavonoid and total phenolic contents, was most significantly associated with their anthocyanin content. In contrast to that, we found no association of vasodilation with any individual polyphenolic compound, at least not with ones that we analyzed.
The correlation between vasodilatory activity and anthocyanin level of red wines was already demonstrated by Burns et al.,7 who tested 16 different red wine samples on rabbit aortic vascular rings. However, it remains unclear as to why the anthocyanins are so strongly associated with the vascular response to wines. Results of in vitro studies with individual anthocyanidin compounds (sugar free molecules, anthocyanin aglycones) were contradictory, as only delphinidin, but not malvidin or cyanidin, caused endothelium-dependent relaxation of rat aortic rings.22 The study by Nakamura et al.23 showed that four individual anthocyanin compounds, applied either alone or in combination, caused no vasodilatory effect in the rat aorta. Similarly, Serraino et al.24 showed that cyanidine-3-O-glucoside had no effect in the contractile or in the endothelium-dependent vasodilating response of the aortic rings under basal conditions, although it provided protection against peroxynitrite-mediated vascular dysfunction. Negligible direct vasodilatory effects of anthocyanin and anthocyanidin compounds in vitro do not necessarily reflect their effectiveness under in vivo conditions when they are consumed as a part of a complex mixed solution, such as wine. The concentrations of anthocyanins in our study were in the nanomolar range (for example, 1‰ of BW3 roughly equals 310 nM malvidin-3-glucoside in the organ bath), which corresponds with the upper limits of their plasma concentrations following consumption of anthocyanin-rich foods or beverages.25 In addition, the most recent reports indicate that anthocyanins undergo substantial metabolism after being ingested and that their metabolites, formed in the small intestine and hepatic cells, as well as low-molecular-weight catabolic products of the colonic microflora, such as phenolic acids, travel around the human body in the circulatory system and may be responsible for the distinctive biological effects of “anthocyanins.”26,27 These metabolites might be the key to filling the gap between in vitro and in vivo observed biological effects of the anthocyanins.
The importance of anthocyanins in the vasodilatory activity of blackberry wine is best illustrated with the BW4 sample. It acted as a poor direct vasodilator similar to the white grape wines with Emax of 24.27±3.86% and 24.28±5.79% for BW4 and WW1, respectively, although its phenolic content is 3.5-fold higher than that of white grape wine (WW1), but its anthocyanin content was about 10-fold lower than that of other blackberry wines. Numerous studies were undertaken in order to identify polyphenolic compounds responsible for the vasodilatory effect of the red grape wine. Among wine phenolics, a significant role has been attributed to resveratrol in wine-mediated cardiovascular protection, including vasodilatory activity.28 Vasodilatory response in this study could not be associated with the resveratrol content of the wine samples examined. However, it should be noted that the resveratrol content in the wines examined was rather low (up to 2.2 mg/L), and after dilution in the organ bath its concentration was in the low nanomolar range, which is an order of magnitude lower than in the studies examining the dilatory effects of resveratrol in vitro.29 Procyanindins were also suggested as the principal vasoactive polyphenols in red grape wine.30 Although procyanidin B2 concentrations in our samples were within the range of the vasodilation threshold (from 0.5 to 4 μg/L),31 we found no significant correlation between procyanidin B2 concentration and Emax (r=0.41, P=.31). Taken together, these results support the notion that the biological effect of a complex solution, such as wine, cannot be exclusively attributed to a single phenolic compound. Rather, it is a result of the synergistic effect of different polyphenolics, as shown for various biological effects under different experimental conditions.9,32,33
In conclusion, we have confirmed potent in vitro antioxidant and vasodilatory effects of blackberry wines that are roughly comparable to those of red grape wines.
These results justify the need for examining cardiovascular effects of blackberry wines in human subjects.
Acknowledgments
This work was supported by grants 216-2160547-0537 and 011-2160547-2226 from the Ministry of Science, Education and Sports of the Republic of Croatia. The authors thank Mr. Enver Moralic for his donation of the red wines used in this study and Mrs. Shelly Pranic for technical assistance.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Scalbert A. Manach C. Morand C. Remesy C. Jimenez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 2.Chong MF. Macdonald R. Lovegrove JA. Fruit polyphenols and CVD risk: a review of human intervention studies. Br J Nutr. 2010;104(Suppl 3):S28–S39. doi: 10.1017/S0007114510003922. [DOI] [PubMed] [Google Scholar]
- 3.Rice-Evans C. Miller N. Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. [Google Scholar]
- 4.Stoclet JC. Chataigneau T. Ndiaye M, et al. Vascular protection by dietary polyphenols. Eur J Pharmacol. 2004;500:299–313. doi: 10.1016/j.ejphar.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Velez M. Martinez-Martinez F. Del Valle-Ribes C. The study of phenolic compounds as natural antioxidants in wine. Crit Rev Food Sci Nutr. 2003;43:233–244. doi: 10.1080/10408690390826509. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigo R. Miranda A. Vergara L. Modulation of endogenous antioxidant system by wine polyphenols in human disease. Clin Chim Acta. 2010;412:410–424. doi: 10.1016/j.cca.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 7.Burns J. Gardner PT. O'Neil J, et al. Relationship among antioxidant activity, vasodilation capacity, and phenolic content of red wines. J Agric Food Chem. 2000;48:220–230. doi: 10.1021/jf9909757. [DOI] [PubMed] [Google Scholar]
- 8.Fitzpatrick DF. Hirschfield SL. Coffey RG. Endothelium-dependent vasorelaxing activity of wine and other grape products. Am J Physiol. 1993;265:H774–H778. doi: 10.1152/ajpheart.1993.265.2.H774. [DOI] [PubMed] [Google Scholar]
- 9.Boban M. Modun D. Music I, et al. Red wine induced modulation of vascular function: separating the role of polyphenols, ethanol, and urates. J Cardiovasc Pharmacol. 2006;47:695–701. doi: 10.1097/01.fjc.0000211762.06271.ce. [DOI] [PubMed] [Google Scholar]
- 10.Flesch M. Schwarz A. Bohm M. Effects of red and white wine on endothelium-dependent vasorelaxation of rat aorta and human coronary arteries. Am J Physiol. 1998;275:H1183–H1190. doi: 10.1152/ajpheart.1998.275.4.H1183. [DOI] [PubMed] [Google Scholar]
- 11.Heinonen IM. Lehtonen PJ. Hopia AI. Antioxidant activity of berry and fruit wines and liquors. J Agric Food Chem. 1998;46:25–31. doi: 10.1021/jf970489o. [DOI] [PubMed] [Google Scholar]
- 12.Yildrim H. Evaluation of colour parameters and antioxidant activities of fruit wines. Int J Food Sci Nutr. 2006;57:47–63. doi: 10.1080/09637480600655993. [DOI] [PubMed] [Google Scholar]
- 13.Negi B. Dey G. Comparative analysis of total phenolic content in sea buckthorn wine and other selected fruit wines. World Acad Sci Eng Technol. 2009;54:99–102. [Google Scholar]
- 14.Pinhero RG. Paliyath G. Antioxidant and calmodulin-inhibitory activities of phenolic components in fruit wines and its biotechnological implications. Food Biotechnol. 2001;15:179–192. [Google Scholar]
- 15.Music I. Modun D. Katalinic V. Salamunic I. Kozina B. Boban M. Effects of four-weeks moderate drinking of red wine and ethanol on the rat isolated heart and aortic rings reactivity during ischemia and hypoxia. Period Biol. 2005;107:165–173. [Google Scholar]
- 16.Katalinic V. Milos M. Modun D. Music I. Boban M. Antioxidant effectiveness of selected wines in comparison with (+)-catechin. Food Chem. 2004;86:593–600. [Google Scholar]
- 17.Romero-Pérez AI. Lamuela-Raventós RM. Waterhouse AL. de la Torre-Boronat MC. Levels on cis- and trans-resveratrol and their glucosides in white and rosé Vitis vinifera wines from Spain. J Agric Food Chem. 1996;44:2124–2128. [Google Scholar]
- 18.Benzie IFF. Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;23:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 19.Piazzon A. Forte M. Nardini M. Characterization of phenolics content and antioxidant activity of different beer types. J Agric Food Chem. 2010;58:10677–10683. doi: 10.1021/jf101975q. [DOI] [PubMed] [Google Scholar]
- 20.Dudonne S. Vitrac X. Coutiere P. Woillez M. Merillon JM. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J Agric Food Chem. 2009;57:1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- 21.Mudnic I. Modun D. Rastija V, et al. Antioxidative and vasodilatory effects of phenolic acids in wine. Food Chem. 2010;119:1205–1210. [Google Scholar]
- 22.Andriambeloson E. Magnier C. Haan-Archipoff G, et al. Natural dietary polyphenolic compounds cause endothelium-dependent vasorelaxation in rat thoracic aorta. J Nutr. 1998;128:2324–2333. doi: 10.1093/jn/128.12.2324. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura Y. Matsumoto H. Todoki K. Endothelium-dependent vasorelaxation induced by black currant concentrate in rat thoracic aorta. Jpn J Pharmacol. 2002;89:29–35. doi: 10.1254/jjp.89.29. [DOI] [PubMed] [Google Scholar]
- 24.Serraino I. Dugo L. Dugo P, et al. Protective effects of cyanidin-3-O-glucoside from blackberry extract against peroxynitrite-induced endothelial dysfunction and vascular failure. Life Sci. 2003;73:1097–1114. doi: 10.1016/s0024-3205(03)00356-4. [DOI] [PubMed] [Google Scholar]
- 25.Cao G. Muccitelli H. Sanchez-Moreno C. Prior R. Anthocyanins are absorbed in glycated forms in elderly women: a pharmacokinetic study. Am J Clin Nutr. 2001;73:920–926. doi: 10.1093/ajcn/73.5.920. [DOI] [PubMed] [Google Scholar]
- 26.Vitaglione P. Donnarumma G. Napolitano A, et al. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J Nutr. 2007;137:2043–2048. doi: 10.1093/jn/137.9.2043. [DOI] [PubMed] [Google Scholar]
- 27.Forester S. Watehouse A. Metabolites are key to understanding health effects of wine polyphenolics. J Nutr. 2009;138:1824S–1831S. doi: 10.3945/jn.109.107664. [DOI] [PubMed] [Google Scholar]
- 28.Bertelli AA. Das DK. Grapes, wines, resveratrol, and heart health. J Cardiovasc Pharmacol. 2009;54:468–476. doi: 10.1097/FJC.0b013e3181bfaff3. [DOI] [PubMed] [Google Scholar]
- 29.Novakovic A. Bukarica L. Kanjuh V. Heinle H. Potassium channels-mediated vasorelaxation of rat aorta induced by resveratrol. Basic Clin Pharmacol Toxicol. 2006;99:360–364. doi: 10.1111/j.1742-7843.2006.pto_531.x. [DOI] [PubMed] [Google Scholar]
- 30.Corder R. Mullen W. Khan N, et al. Oenology red wine procyanidins and vascular health. Nature. 2006;444:566. doi: 10.1038/444566a. [DOI] [PubMed] [Google Scholar]
- 31.Dell'Agli M. Busciala A. Bosisio E. Vascular effects of wine polyphenols. Cardiovasc Res. 2004;63:593–602. doi: 10.1016/j.cardiores.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Papadopoulou C. Soulti K. Roussis IG. Potential antimicrobial activity of red and white wine phenolic extracts against strains of Staphylococcus aureus, Escherichia coli and Candida albicans. Food Technol Biotechnol. 2005;43:41–46. [Google Scholar]
- 33.Wallerath T. Li H. Godtel-Ambrust U. Schwarz PM. Forstermann U. A blend of polyphenolic compounds explains the stimulatory effect of red wine on human endothelial NO synthase. Nitric Oxide. 2005;12:97–104. doi: 10.1016/j.niox.2004.12.004. [DOI] [PubMed] [Google Scholar]