Abstract
Chinese bayberry fruit is a rich source of anthocyanins, especially cyanidin-3-glucoside (C3G). The present study investigated the protective effects of C3G-rich bayberry fruit extract (CRBFE) against pancreatic β cells against oxidative stress–induced injury as well as its hypoglycemic effect in diabetic mice. Bayberry extract from “Biqi” was used for both in vitro and in vivo testing because of its high C3G content and high antioxidant capacity. Pretreatment of β cells with CRBFE (containing 0.5 μmol/L C3G) prevented cell death, increased cellular viability, and decreased mitochondrial reactive oxygen species production and cell necrosis induced by 800 or 1,200 μmol/L H2O2. CRBFE dose-dependently up-regulated pancreatic duodenal homeobox 1 gene expression, contributing to increased insulin-like growth factor II gene transcript levels and insulin protein in INS-1 cells. In addition, administration of CRBFE (150 μg of C3G/10 g of body weight twice per day) significantly reduced blood glucose in streptozotocin-induced diabetic ICR mice and increased the glucose tolerance in an oral glucose tolerance test (P<.05). Such results indicated that CRBFE might be useful in prevention and control of diabetes mellitus and diabetes-associated complications.
Key Words• anthocyanins, antioxidants, diabetes, fruit extract, oxidative stress
Introduction
Anthocyanins are the most abundant and important water-soluble pigments present in fruit and vegetables. They are glycosides and acylglycoside derivatives of six common anthocyanidins—pelargonidin, cyanidin, malvidin, delphinidin, peonidin, and petunidin—which are classified according to the number and position of hydroxyl groups on the flavan nucleus.1 These anthocyanins endow fruit and vegetables with diverse colors from blue and red to purple. Recent epidemiological studies showed various bioactivities for these colorful compounds such as antioxidant activity,2,3 anti-atherogenic activity,4 anticarcinogenesis,5 prevention of obesity,6 prevention of cardiovascular diseases,7 and vision improvement.8,9 Therefore, a diet rich in anthocyanins is highly recommended for their potential benefit to human health.
Diabetes is an epidemic disease characterized as the inability to produce or improperly use insulin in the human body. There are two types of diabetes: type 1 (insulin-dependent diabetes) and type 2 (non–insulin-dependent diabetes). The cause of diabetes continues to be a mystery, although both genetics and environmental factors appear to play roles. With the development of economic and lifestyle changes the incidence of diabetes and its multiple complications has increased significantly in the past few decades and caused increasing health problems worldwide. The global prevalence of diabetes is estimated to reach 366 million by 2030, and the regions with the greatest potential increases in diabetes are Asia and Africa.10 So far, there are more than 40 million people in China who have diabetes. Prevention and control programs are urgent to inhibit the rising incidence of diabetes and its complications.
Recently, several studies showed that a diet rich in anthocyanins may decrease the incidence of diabetes, improve glucose disposal, and inhibit the development of diabetes-associated complications. Five anthocyanins (i.e., malvidin-3-glucoside, delphinidin-3-glucoside, cyanidin-3-glucoside [C3G], petunidin-3-glucoside, and peonidin-3-glucoside) from grape skins showed various inhibitory effects on the diabetic cataract.8 C3G-rich purple-colored corn was demonstrated to prevent obesity and ameliorate hyperglycemia in mice fed with high fat diet.6 C3G and delphinidin-3-glucoside showed high-level ability to stimulate insulin secretion from rodent pancreatic β cells in the presence of 4 and 10 mmol/L glucose concentrations.11 Furthermore, C3G from purple corn was proved to ameliorate hyperglycemia and insulin sensitivity in type 2 diabetic mice through down-regulation of retinol binding protein 4 expression.12 Tart cherry, which is rich in anthocyanins, was shown to reduce blood glucose as well as hyperlipidemia, hyperinsulinemia, and metabolic syndrome in Dahl salt-sensitive rats.13
Chinese bayberry (Myrica rubra Sieb. & Zucc.), a subtropical fruit native to China, is a fruit with high nutrition and health values. The color of the fruit ranges from white to dark red depending on the cultivar and fruit maturity.14 The characteristic red color of Chinese bayberry is due to the presence of anthocyanins, especially C3G, which accounts for at least 85% of the total anthocyanins in colored fruit cultivars.3 Therefore, the red Chinese bayberry fruit is a rich source of anthocyanins. High antioxidant capacities were reported for Chinese bayberry fruit under different storage conditions.3,15–17 The present study was designed to investigate a possible hypoglycemic effect of the C3G-rich bayberry fruit extract (CRBFE) and its possible mechanisms on the health benefit for prevention of diabetic diseases.
Materials and Methods
Chemicals and reagents
Standards of nine anthocyanins (i.e., pelargonin, cyanin, malvin, C3G, delphinidin, cyanidin, pelargonidin, peonidin, and malvidin), dimethyl sulfoxide, 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye) and streptozotocin (STZ) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Methanol (high-performance liquid chromatography [HPLC] grade) was obtained from Caledon Laboratories Co. (Georgetown, ON, Canada).
Preparation of CRBFE
Mature Chinese bayberry fruit of four cultivars with different colors—“Biqi” (dark red), “Dongkui” (red), “Fenhong” (pink), and “Shuijing” (white)—were obtained from Yuyao County, Zhejiang Province, China.
Anthocyanins from Chinese bayberry fruit were extracted according to our previous method3 with slight modification. The fruit flesh sample was ground and extracted five times (1:5, wt/vol) with solvent consisting of ethanol and 1.0% formic acid. The extract was filtered and centrifuged at 5,000 g for 5 minutes. Five milliliters of upper clear solution was evaporated to dryness at 30°C in a rotary evaporator and dissolved in methanol, H2O, and formic acid (50:45.5:4.5 by volume). After filtration and centrifugation to remove impurities such as protein and sugar, the solution was evaporated to dryness again, dissolved in 1 mL of double distilled water, and prepared for HPLC analysis. CRBFEs used for experiments with pancreatic β cells and diabetic mice were extracted by the same method from “Biqi” with increased volume.
HPLC analysis of anthocyanins in CRBFE
The composition and concentration of anthocyanins from Chinese bayberry fruit were determined using an HPLC apparatus equipped with a model 2695 pump and a model 2996 diode array detector (Waters Corp., Milford, MA, USA). Separation was achieved on a reverse-phase C30 column (250 mm×4.6 mm i.d.; film thickness, 5 μm), and the detection wavelength was 520 nm. Chromatography was carried out at 30°C with 4.5% formic acid in water as solvent A and 100% HPLC-grade methanol as solvent B. The gradient elution program (% solvent B) with a flow rate of 1 mL/minute was as follows: 1–5 minutes, 15–35%; 5–25 minutes, 35–50%; 25–40 minutes, 50–80%; 40–42 minutes, 80%; 42–45 minutes, 80–15%; and 45–50 minutes, 15% to finish a cycle. The retention time and peak area were used to identify different anthocyanins and calculate their concentrations, respectively.
Antioxidant capacity of CRBFE
The antioxidant capacity of CRBFE of the four cultivars was determined using the peroxyl radical scavenging capacity (PSC) assay as described by Adom and Liu.18 In brief, for preparations of dichlorofluorescin (DCFH) solution, 900 μL of 1.0 mmol/L KOH was used to hydrolyze 80 μL of 2.48 mmol/L DCFH diacetate for 3–5 minutes to remove the diacetate moiety, and then 75 mmol/L sodium phosphate buffer (pH 7.4) was used to dilute to a final volume of 6 mL. A Varioskan® fluorescent spectrophotometer (Thermo Electron Corp., Vantaa, Finland) was used to monitor the assay as follows: 100 μL of standards or fruit extract appropriately diluted in 75 mmol/L sodium phosphate buffer (pH 7.4) was transferred into reaction cells on a 96-well plate, and 100 μL of DCFH was added. The solution in each cell was mixed in the Varioskan spectrophotometer by shaking at 480 rpm for 20 seconds. The reaction was then initiated by adding 50 μL of freshly prepared 2,2′-azobis(amidinopropane) (400 mmol/L) in 75 mmol/L sodium phosphate buffer (pH 7.4). For control reaction, 75 mmol/L sodium phosphate buffer (pH 7.4) alone was used. The temperature for reaction was 37°C, and the wavelengths of excitation and emission for monitoring fluorescence were 485 nm and 538 nm, respectively. Data were acquired with Skanlt software version 2.2 (Thermo Electron Corp.). The area under the average fluorescence–reaction time kinetic curve (AUC) values for both control and samples were integrated and used as the basis for calculating antioxidant activity according to the following equation: PSC unit = 1 – (SA/CA), where SA is AUC for the sample or standards and CA is AUC for the control. The median effective concentration was defined as the dose required to cause 50% inhibition (PSC unit=0.5) for each fruit extract and was used as the basis for comparing different samples. AUCs of samples, which were close to 0.5 PSC units, were compared with a standard curve of Trolox concentrations, and the antioxidant capacity was expressed as millimoles of Trolox equivalents per gram.
Cell culture and reagents
The rat insulinoma cell line INS-1 was purchased from the China Center for Type Culture Collection (Wuhan University, Wuhan, China) and grown in monolayer cultures in regular RPMI 1640 medium supplemented with 10 mmol/L HEPES, 10% heat-inactivated fetal calf serum, 2 mmol/L l-glutamine, 1 mmol/L sodium pyruvate, 50 μmol/L β-mercaptoethanol, 100 IU/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere (5% CO2, 95% air). Cells at passages 40–60 were used in the following experiments.
Cell viability assay
The protective effect of CRBFE on cell viability was assessed by using MTT dye. In brief, INS-1 cells were seeded in 96-well plates at a density of 8–10×103 per well, and 12–18 hours later cells were incubated with CRBFE (containing 0.5 μmol/L C3G) for 24 hours. After careful washing, cells were further treated with 800 or 1,200 μmol/L H2O2, the concentrations of which were based on preliminary experiments. Three hours later, the medium was replaced by fresh medium without H2O2, and 20 μL of MTT (dissolved in phosphate-buffered saline, 5 mg/mL) was added to each well and incubated for 4 hours at 37°C to form formazan crystals. The medium was gently removed, and the crystals were dissolved in 150 μL of dimethyl sulfoxide. Six wells were used for each treatment. Formazan crystals were quantified at 570 nm using a plate reader (model ELx800, Bio-Tek, Winooski, VT, USA). Cellular viability was expressed as the ratio compared with control cells.
Flow cytometry analysis
To assess the effect of CRBFE on oxidative stress–induced injury of pancreatic β cells, flow cytometry was used to quantitatively evaluate necrosis. In brief, cells were incubated with or without CRBFE (containing 0.5 μmol/L C3G) for 24 hours, and after the medium was discarded, cells were further stimulated with 800 or 1,200 μmol/L H2O2 for another 3 hours. Thereafter, cells (including the detached cells) were harvested, washed twice with prechilled phosphate-buffered saline (pH 7.2), and resuspended in 500 μL of binding buffer. A volume of 9.8 μL of propidium iodide was then added to the cell suspension and incubated for 15 minutes in the dark at room temperature. Triplicate wells were used for each treatment. Cell necrosis was determined with a flow cytometry system with CXP software (model FC500, Beckman Coulter, Fullerton, CA, USA).
Mitochondrial reactive oxygen species
The intensity of mitochondrial reactive oxygen species (ROS) was analyzed by fluorescence microscopy and flow cytometry. After various treatments, INS-1 cells were washed with Hanks' balanced salt solution containing Ca2+ and Mg2+ and then incubated with dimethyl sulfoxide–soluble MitoSOX™ Red Superoxide Indicator (catalog number M36008, Invitrogen, Madison, WI, USA) (5 μmol/L) for 10 minutes at 37°C, protected from light. The cells were washed three times, mounted, and observed under fluorescence microscopy. The intensity of fluorescence was measured by flow cytometry.
Real-time quantitative polymerase chain reaction
Total RNA from the cells was isolated with TRIzol® reagent (Invitrogen), and the concentration was detected using a spectrophotometer. Total RNA (2.0 μg) was reverse-transcribed to cDNA in a reaction mixture in a final volume of 20 μL using a Fermentas (Vilnius, Lithuania) RNA polymerase chain reaction kit (Moloney murine leukemia virus) according to the manufacturer's directions. Gene expression was quantified using the real-time polymerase chain reaction system (Roche [Basel, Switzerland] Lightcycler 2.0) using SYBR® Green dye (TAKARA Bio, Shiga, Japan). The housekeeping gene glyceraldehyde 3-phospahte dehydrogenase (gapdh) served as the internal control. The primers were as follows: pancreatic duodenal homeobox 1 (pdx-1), forward 5′-AAACGCCACACACAAGGAGAA-3′, reverse 5′-AGACCTGGCGGTTCACATG-3′; insulin-like growth factor II (Ins2), forward 5′-ACCCACAAGTGGCACAACTGGAG-3′, reverse 5′-TTCATTGCAGAGGGGTGGACAGG-3′; and gapdh, forward 5′-GGTGGACCTCATGGCCTACAT-3′, reverse 5′-GCCTCTCTCTTGCTCTCAGTATCCT-3′. Data analysis was performed using the 2–ΔΔCT method normalized to GAPDH mRNA and expressed relative to the control subjects.
Western blot
Total protein levels were measured (BCA method, Pierce Biotech, Rockford, IL, USA), and 20–40 μg of protein per lane was separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels and transferred to a polyvinylidine difluoride membrane. Membranes were incubated with insulin antibody (1:500 dilution) (Santa Cruz Biotech, Santa Cruz, CA, USA) overnight at 4°C. Equal loading of protein between lanes was confirmed by subsequent actin immunoblots (1:5,000 dilution) (Sigma-Aldrich). After incubation with horseradish peroxidase–conjugated secondary antibody for 1 hour at room temperature, immunodetection was performed by enhanced chemiluminescence. The protein density was quantified using Bandscan version 5.0 (Glyko, Hayward, CA, USA). The relative ratios of insulin protein to actin were calculated for both control and CRBFE-treated cells, the former of which was set at 1.
Animals and diets
Male ICR mice (weighing 22–25 g) were used and maintained at 23–25°C and 50–60% humidity in the Laboratory Animal Center of Zhejiang University (Hangzhou, China). The mice were fed with standard laboratory diet of the following composition: 20% protein, 4% fat, 57% carbohydrate, 5% crude fiber, 4% minerals, and 10% water. After 3 days of adaptation, STZ dissolved in citrate buffer (10 mmol/L, pH 4.5) was injected intraperitoneally at a dose of 200 mg/kg of body weight after 6 hours of fasting. Five days after the injection, the glucose level of serum sampled from the tail vein was determined, and the mice with a serum glucose level higher than 16.7 mmol/L were selected as diabetic mice. The mice were then randomized into four groups (I, II, III, and IV) of seven mice each: Group I, control normal mice; Group II, normal mice treated with CRBFE; Group III, control diabetic mice; and Group IV, diabetic mice treated with CRBFE. Control mice (both Groups I and III) received saline (0.9% NaCl) via oral gavage only, whereas Group II and IV were treated with 0.1 mL of CRBFE (containing 150 μg of C3G)/10 g of body weight twice per day. Glucose level was monitored for the four groups on the initial day and days 7, 15, and 30 thereafter. All experiments were carried out in accordance with the ethical guidelines of the Animal Experimentation Committee in College of Medicine, Zhejiang University.
Oral glucose tolerance test
Animals were fasted for 6 hours before the oral glucose tolerance test. Mice were given an oral gavage of 0.1 mL of CRBFE (containing 150 μg of C3G)/10 g of body weight or equivalent saline, and blood glucose was measured 1 hour later as the baseline level. Then, mice in four groups (five mice each) were orally administered glucose (2.5 g/kg). Blood samples were obtained from the tail vein at 0.5-, 1-, and 2-hour intervals, and blood glucose levels were determined using a blood glucose meter (Roche Molecular Diagnostics, Pleasanton, CA, USA).
Statistical analysis
A completely randomized design was used in the present study. All data are mean±SD values. Numbers of mice or cell replicates are indicated in individual tables and figures. Data were subjected to analysis of variance using SAS software (release 8.01, SAS Institute, Inc., Cary, NC, USA). Mean separations for anthocyanin content and antioxidant capacity of the four bayberry cultivars were done by Duncan's new multiple range test, and Pearson's correlation coefficient was calculated among them. Student's t test was used to compare the effect of CRBFE treatment on the viability and necrosis of β cells treated with different concentrations of H2O2 and on the blood glucose level and oral glucose tolerance of diabetic mice. Differences were considered significant at P<.05.
Results
HPLC analysis of anthocyanins in Chinese bayberry fruit
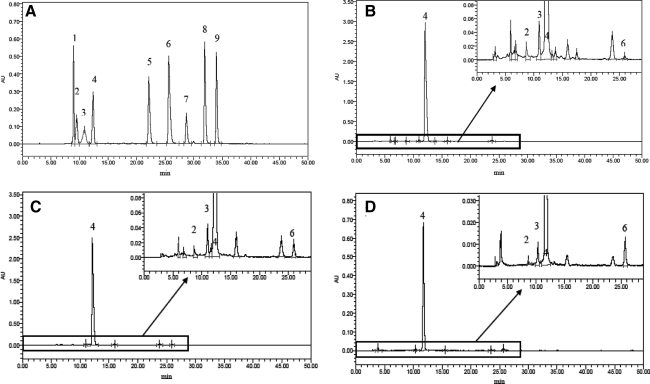
Nine anthocyanin standards were readily detected at 520 nm by diode array detection and had fine separations in the HPLC profile (Fig. 1A). The anthocyanin profile of “Biqi,” “Dongkui,” and “Fenhong” fruit extracts showed several major peaks in the 3–30-minute region of the chromatogram, and four of them were identified as cyanin, malvin, C3G, and cyanidin, respectively, by comparing their retention times with those of the authentic standards (Fig. 1B– D). Under the same HPLC condition, only the peak for C3G could be detected in “Shuijing” fruit extract (Fig. 1E). C3G was the major anthocyanin (>95% of total area of all peaks) of all four bayberry fruit extracts, although its content differed among cultivars (P<.05) (Table 1).
FIG. 1.
High-performance liquid chromatography profile of (A) anthocyanin standards and Chinese bayberry anthocyanin extract from the cultivars (B) “Biqi,” (C) “Dongkui,” (D) “Fenhong,” and (E) “Shuijing” (λ=520 nm). Nine anthocyanin standards are indicated: peak 1, pelargonin; peak 2, cyanin; peak 3, malvin; peak 4, cyanidin-3-glucoside; peak 5, delphinidin; peak 6, cyanidin; peak 7, pelargonidin; peak 8, peonidin; and peak 9, malvidin. AU, arbitrary units.
Table 1.
Content of Cyanin, Malvin, Cyanidin-3-Glucoside, and Cyanidin in Chinese Bayberry of Four Cultivars
| |
Anthocyanin content (μg/g fresh weight) |
|||
|---|---|---|---|---|
| Cultivar | Cyanin | Malvin | C3G | Cyanidin |
| “Biqi” | 3.4±0.3a | 11.3±1.1a | 467.9±21.3a | 0.4±0.1c |
| “Dongkui” | 1.7±0.2b | 8.6±0.6b | 344.6±13.3b | 1.4±0.1a |
| “Fenhong” | ND | 1.9±0.2c | 78.1±5.5c | 0.9±0.1b |
| “Shuijing” | ND | ND | 3.5±0.3d | ND |
Data are mean±SD values (n=3).
Means within a column without a common letter differ, P<.05.
C3G, cyanidin-3-glucoside; ND, not detected.
Antioxidant capacity of CRBFE
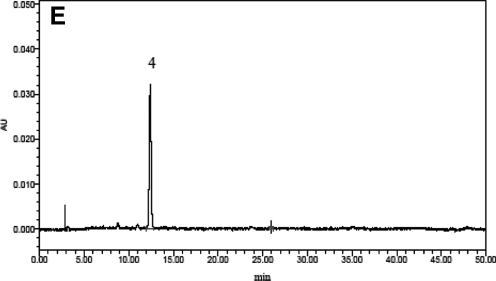
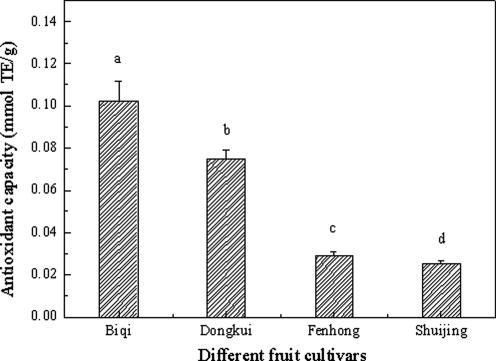
The PSC assay showed different antioxidant capacities for CRBFE from the four bayberry cultivars (P<.05), with the highest antioxidant capacity detected from the dark-red “Biqi” and the lowest from the white “Shuijing” (Fig. 2). Correlation analysis showed that antioxidant capacity was significantly correlated (r=0.995, P<.0001) with the C3G content in the four cultivars and was directly related to fruit color (Fig. 3). Therefore, CRBFE from “Biqi” was used for subsequent in vitro and in vivo tests.
FIG. 2.
Antioxidant capacity of C3G-rich extract from four bayberry cultivars. Data are mean±SD values (n=3). abcdMeans without a common letter differ, P<.05. TE, Trolox equivalents.
FIG. 3.
Correlation analysis of antioxidant capacity and C3G content from four bayberry cultivars.
Effect of CRBFE on viability of INS-1 cells under H2O2-induced oxidative stress
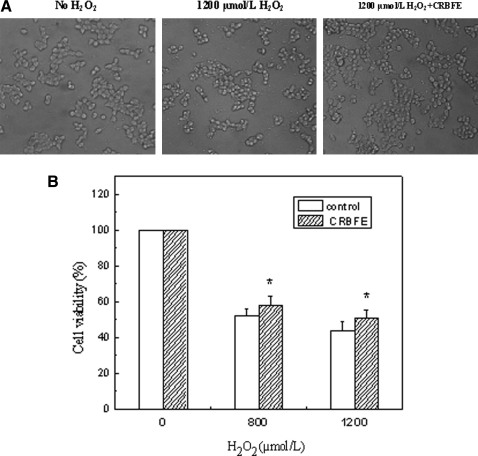
We investigated whether CRBFE could protect INS-1 cells against oxidative injury. H2O2 was added to the cell culture medium to establish the oxidative stress model, and MTT assays were further carried out for quantitatively evaluating cell viability. The 800 or 1,200 μmol/L H2O2 treatment induced obvious cell death, where cells appeared to be round and detached (Fig. 4A), and the cell viability was reduced (Fig. 4B). Preincubation of the cells with CRBFE protected INS-1 cells from H2O2-induced oxidative injury (Fig. 4A) and increased the cell viability after a 3-hour treatment with 800 or 1,200 μmol/L H2O2 (P<.05) (Fig. 4B).
FIG. 4.
Effects of C3G-rich bayberry fruit extract (CRBFE) on viability of INS-1 cells under H2O2-induced oxidative stress. (A) Representative cell images were obtained with an inverted microscope 3 hours after H2O2 treatment. (B) Formazan crystals formed were quantified with a plate reader at 570 nm, and cellular viability was expressed as the ratio of the value for experimental cells versus control cells. Data are mean±SD values (n=6). *Different from respective control for 800 or 1,200 μmol/L H2O2 treatment, P<.05.
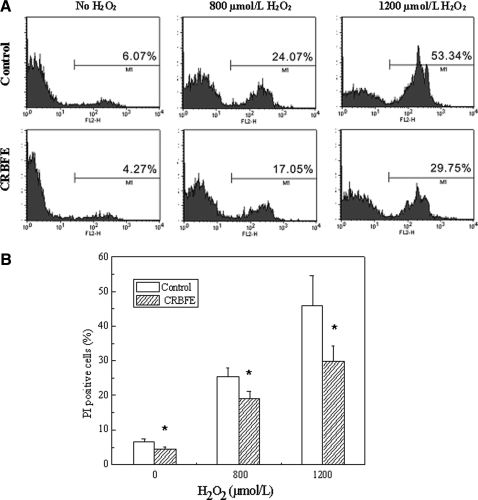
Effect of CRBFE on oxidative stress–induced cell necrosis of INS-1 cells
Treatment of INS-1 cells with 800 or 1,200 μmol/L H2O2 caused a significant increase in cell necrosis (Fig. 5A). However, pretreatment of INS-1 cells with CRBFE resulted in decreased cell necrosis in both the 800 and 1,200 μmol/L H2O2-treated cells (P<.05) (Fig. 5B). Also, CRBFE decreased the cell necrosis in β cells without H2O2 treatment (P<.05).
FIG. 5.
Effects of CRBFE on oxidative stress–induced cell necrosis of INS-1 cells. (A) Flow cytometry quantifying cells with propidium iodide (PI) staining. (B) Cell necrosis presented as the percentage of PI-positive cells with the different treatments. Data are mean±SD values (n=3). *Different from respective control for 0, 800, or 1200 μmol/L H2O2 treatment, P<.05.
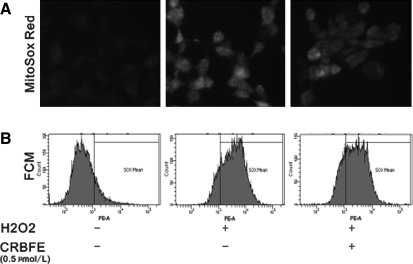
Effect of CRBFE on mitochondrial ROS production
MitoSOX Red is a new redox-sensitive dye that is targeted to mitochondria and was used in the present study to evaluate mitochondrial ROS production. Our data revealed that H2O2 stimulation significantly enhanced mitochondrial ROS production as evidenced by the increased intensity of red fluorescence in H2O2-treated INS-1 cells, whereas CRBFE decreased MitoSOX staining intensity (Fig. 6A). Such results were further confirmed by flow cytometry analysis. CRBFE pretreatment reduced the proportion of cells strongly stained by MitoSOX probe, compared with cells treated with H2O2 alone (Fig. 6B).
FIG. 6.
Effect of CRBFE on mitochondrial reactive oxygen species production detected with the MitoSOX probe. (A) Fluorescence microscopy images of INS-1 cells stained with MitoSOX (5 μmol/L): (left) control group, (middle) H2O2 group, and (right) H2O2+CRBEF group. Changes in the intensity of red fluorescence emitted by MitoSOX in INS-1 cells indicated changes in the mitochondrial reactive oxygen species levels. (B) Flow cytometry (FCM) analysis of intracellular MitoSOX Red fluorescence in INS-1 cells.
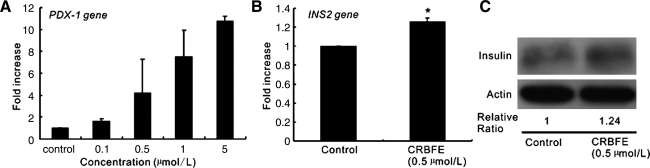
Effect of CRBFE on pdx-1 and Ins2 gene expressions and insulin protein in INS-1 cells
PDX-1 is an important transcription factor for insulin gene transcription, which binds to the A element motif of the insulin genes and contributes to directing their β-cell-specific gene expression. By using different concentrations of CRBFE, we observed that CRBFE could increase the expression of pdx-1 gene in INS-1 cells in a dose-dependent manner (Fig. 7A). Also, after 12-hour CRBFE pretreatment of INS-1 cells, increased Ins2 gene expression (P<.01) and increased insulin protein content were observed, compared with those in control cells, as verified by quantitative polymerase chain reaction (Fig. 7B) and western blot (Fig. 7C), respectively.
FIG. 7.
Effect of CRBFE on pancreatic duodenal homeobox 1 (pdx-1) and insulin-like growth factor II (Ins2) gene expressions and insulin protein in INS-1 cells. (A) pdx-1 gene expression assessed by quantitative polymerase chain reaction in INS-1 cells treated with different concentrations of CRBFE. (B) Ins2 gene expression in INS-1 cells treated with or without CRBFE. (C) Western blot analysis of insulin protein in INS-1 cells treated with or without CRBFE. Protein density was quantified using Bandscan version 5.0 software, and the relative ratios of insulin protein to actin were calculated. In all cases, the control was set at 1.
Effect of CRBFE on body weight, feeding behaviors, and blood glucose levels of mice
Apparent polydipsia and polyphagia symptoms in diabetic mice were observed in our study, and the body weight of mice in all four groups increased slightly during the experiment (30 days). CRBFE administration attenuated the polydipsia and polyphagia symptoms in diabetic mice, although no statistical significance was observed. Overall, the extent of body weight increase was lower in diabetic mice than that of normal control animals (P<.05), regardless of whether CRBFE was administered (data not shown). There was no significant difference in body weight between normal Group I and Group II. Also, there was no significant difference in body weight between the two diabetes groups (Group III and Group IV).
Food intake and water consumption of mice in all four groups were investigated for a 24-hour period at the end of the experiment. Food intake and water consumption of the two diabetic groups (Group III and Group IV) were higher than those of control mice (P<.05) (data not shown). CRBFE administration attenuated the polydipsia and polyphagia symptoms in diabetic mice; however, no statistical significance was observed.
Compared with the initial levels, there was a significant increase in blood glucose levels in STZ-induced diabetic mice within 30 days (P<.05) (Table 2). CRBFE from “Biqi” inhibited such increase in diabetic mice, and blood glucose levels decreased 30 days after fruit extract treatment (P<.05). Therefore there was a significant difference between the blood glucose levels of the two diabetes groups (Group III and Group IV) on day 30 (P<.01). For the normal mice, blood glucose levels were stable during the experimental period, and CRBFE slightly decreased the blood glucose level on day 30 (P=.053) (Table 2).
Table 2.
Effects of Cyanidin-3-Glucoside-Rich Bayberry Fruit Extract on Blood Glucose Levels of Diabetic and Normal Mice
| |
Blood glucose (mmol/L) |
|||
|---|---|---|---|---|
| Group | Initial | Day 7 | Day 15 | Day 30 |
| Normal | 7.1±1.0 | 7.0±0.5 | 6.9±0.3 | 7.9±0.9 |
| Normal+CRBFE | 7.5±0.7 | 6.9±0.4 | 6.3±0.7 | 6.6±0.8 |
| Diabetes | 27.7±2.1 | 29.3±2.9 | 28.4±2.5 | 32.6±1.2* |
| Diabetes+CRBFE | 28.1±3.1 | 29.1±2.2 | 26.6±2.0 | 24.3±1.3*† |
Data are mean±SD values (n=7).
Different compared with the initial level for each treatment, P<.05.
Different compared with the diabetes control group (without CRBFE treatment) on day 30, P<.0001.
Effect of CRBFE on oral glucose tolerance test
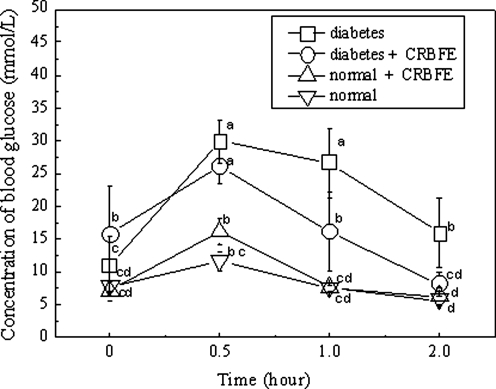
STZ-induced diabetic mice displayed a marked glucose intolerance compared with the normal mice. The blood glucose level remained high at 26.7±5.3 mmol/L at 1 hour and 15.9±5.3 mmol/L at 2 hours after the oral intake of glucose in diabetic mice, which was an apparent impairment of the glucose tolerance (P<.05) (Fig. 8). Administration of CRBFE increased the glucose tolerance in diabetic mice (P<.05), and the blood glucose levels were reduced to 16.1±6.0 mmol/L at 1 hour and 8.2±1.8 mmol/L at 2 hours following glucose intake (Fig. 8).
FIG. 8.
Effects of CRBFE on the oral glucose tolerance of diabetic and normal mice during an oral glucose tolerance test. The streptozotocin-induced diabetic mice displayed marked glucose intolerance as they maintained a high blood glucose level at 1 hour and 2 hours after the oral intake of glucose (2.5 g/kg). Administration of CRBFE increased the glucose tolerance in diabetic mice. Data are mean±SD values (n=5). abcdMeans without a common letter differ, P<.05.
Discussion
Anthocyanins are valuable nutrients because of their various positive effects on human health and marked daily intake.1,19 The protective effects of anthocyanins are attributed to their typical chemical structures, which contain many hydroxyl groups and various glycosylations and other modifications. The natural electron deficiency of anthocyanins endows these compounds with high reactivity toward ROS, and they are therefore among the most powerful natural antioxidants in dietary plants. Direct interaction of anthocyanins with ROS was demonstrated in different ROS-generating systems by Amorini et al.20
C3G alone or together with other cyanidins and their glycosides has been widely investigated for its antioxidant capacities in different experimental conditions.2,21 The antioxidant activities of C3G were 3.5 to four times higher than that of Trolox, a vitamin E analog.22,23 Also, Sarma et al.24 showed higher antioxidant ability for cyanidin-derived anthocyanins than for ascorbic acid due to their ability to chelate metal ions and form an ascorbic acid–metal–anthocyanin complex and thus to prevent oxidation of ascorbic acid. C3G and cyanidin were reported as free radical scavengers, DNA cleavage protectors, and inhibitors of xanthine oxidase.25 C3G from blackberry (Rubus species) juice acted as a peroxynitrite scavenger and prevented peroxynitrite-induced endothelial dysfunction and vascular failure in human umbilical vein endothelial cells.26 The cytoprotective effect of C3G against oxidative DNA damage caused by vitamin E deficiency was observed in human clonocytes.27
Oxidative stress is regarded as an important mediator of many cellular dysfunctions. For both type 1 and type 2 diabetes, hyperglycemia associated with diabetes causes oxidative stress due to increased production of endogenous ROS such as O2– and glucose autooxidation.28 These ROS can damage cells by directly oxidizing biological macromolecules such as DNA, proteins, and lipids. On the other hand, ROS can also indirectly attack cells by activating a variety of stress-sensitive intracellular signaling transduction pathways such as mitogen-activated protein kinase.29 In the absence of adequate compensatory response from the endogenous or exogenous antioxidant system against such oxidative stress, numerous stress-related gene products cause further cellular damage in different organs. Eventually, chronic elevations of glucose level may result in dysfunction of pancreatic β cells and development of large-scale diabetes complications.30 Therefore, up-regulation of antioxidant levels and suppression of oxidative stress are important strategies to ameliorate diabetes and its complications.
Recently, the antidiabetic properties of natural products have attracted much attention. The potential therapeutic implications of dietary C3G for prevention and amelioration of diabetic diseases were investigated in C3G-rich dietary food such as colored rice,8 purple corn,6,12 and tart cherry.13 In our study, high antioxidant activity of CRBFE was confirmed by the PSC method. Administration of CRBFE protected pancreatic β cells in vitro and attenuated oxidative stress–induced cell necrosis. In addition, we observed that the CRBFE had a hypoglycemic effect in STZ-induced type 1 diabetic mice.
Based on the in vitro and in vivo data in the present study, we hypothesized that bayberry extract might lower the blood glucose level through two possible mechanisms. On one hand, CRBFE may protect the pancreatic β cells from oxidative stress, allowing more β cells to survive diabetes-induced oxidative damage. During the onset of STZ-induced diabetes, a significant portion of β cells underwent cellular death (including necrosis, apoptosis, and autophagy), and ROS played a pivotal role.31–33 The present results showed that early administration of CRBFE may reduce the cellular ROS level, improve the antioxidant status, and provide a protective microenvironment for pancreatic β cells, thereby faciliating β cell survival under chemical and subsequent hyperglucose-mediated oxidative injury. Such an assumption was consistent with that proposed by Nizamutdinova et al.,34 where the rate of apoptotic β cells in diabetic rats was decreased after administration of anthocyanins. On the other hand, the CRBFE may protect the insulin secretion ability of the surviving β cells and therefore prevented the insulin insufficiency that caused the STZ-induced diabetes. Our results showed that CRBFE up-regulated the expression of the insulin transcription factor PDX-1 and increased insulin gene (Ins2) expression and insulin protein in β cells. Overall, these effects may lead to enhanced insulin secretion, contributing to the hypoglycemic effect. Studies on Cornus fruits extract showed that anthocyanins from this fruit could increase insulin release of INS-1 cells and that C3G was the most effective insulin secretagogue among the nine anthocyanins tested.11 Recently, research showed that administration of black soybean-derived anthocyanins significantly increased serum insulin levels in STZ-induced diabetic rats.34 Also, the insulin-enhancing property of tea polyphenols was demonstrated by Anderson and Polansky.35 The possible role of phenolic compounds other than anthocyanins in CRBFE in protecting β cells remains unknown. Therefore, the mechanisms of the protective effect of CRBFE on ameliorating hyperglycemia in STZ-induced diabetic mice deserve further investigation.
Although plant treatment for diabetes dates back to about 1550 BC as conventional therapy and more than 400 plant treatments for diabetes have been recorded, only a small number of these have been received scientifically evaluated for their mechanism.36 In the late 1980s, plant extracts from cerasee,37 xiaoke tea,38 agrimony, alfalfa, coriander, eucalyptus, and juniper39 were all reported to have antihyperglycemic effects during the development of diabetes in mice. Recently, various hypoglycemic compounds have been identified, and different possible mechanisms were proposed for different plant extracts that could be preventive and/or curative in diabetic animal models. In the present study, CRBFE showed high antioxidant capacity and a notable protective effect for pancreatic β cells against oxidative stress. In addition, CRBFE significantly reduced the blood glucose level and restored glucose tolerance in STZ-induced diabetic mice. Based on these observations, conclusive studies on the mechanisms and in vivo metabolism, absorption, and bioavailability of the CRBFE for preventing and healing of diabetes and its complications are warranted.
Acknowledgments
We thank Lin Xu and Qiuping Xie from the Department of Surgery at Zhejiang University for their invaluable help during this work. The work was supported by the National Science Foundation of China (grants 30800766 and 30872531), the Special Scientific Research Fund of Agricultural Public Welfare Profession of China (grant 200903044), and the 111 project (grant B06014).
Author Disclosure Statement
No competing financial interest exists.
References
- 1.Kong JM. Chia LS. Goh NK. Chia TF. Brouillard R. Analysis and biological activities of anthocyanins. Phytochemistry. 2003;64:923–933. doi: 10.1016/s0031-9422(03)00438-2. [DOI] [PubMed] [Google Scholar]
- 2.Galvano F. La Fauci L. Vitaglione P. Fogliano V. Vanella L. Felgines C. Bioavailability, antioxidant and biological properties of the natural free-radical scavengers cyanidin and related glycosides. Ann Ist Super Sanita. 2007;43:382–393. [PubMed] [Google Scholar]
- 3.Zhang WS. Li X. Wang XX, et al. Bioactive components and antioxidant capacity of Chinese bayberry (Myrica rubra Sieb. & Zucc.) fruit in relation to fruit maturity and postharvest storage. Eur Food Res Technol. 2008;227:1091–1097. [Google Scholar]
- 4.Xia XD. Ling WH. Ma J, et al. An anthocyanin-rich extract from black rice enhances atherosclerotic plaque stabilization in apolipoprotein E-deficient mice. J Nutr. 2006;136:2220–2225. doi: 10.1093/jn/136.8.2220. [DOI] [PubMed] [Google Scholar]
- 5.Nichenametla SN. Taruscio TG. Barney DL. Exon JH. A review of the effects and mechanisms of polyphenolics in cancer. Crit Rev Food Sci Nutr. 2006;46:161–183. doi: 10.1080/10408390591000541. [DOI] [PubMed] [Google Scholar]
- 6.Tsuda T. Horio F. Uchida K. Aoki H. Osawa T. Dietary cyanidin 3-O-beta-d-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J Nutr. 2003;133:2125–2130. doi: 10.1093/jn/133.7.2125. [DOI] [PubMed] [Google Scholar]
- 7.Dell'Agli M. Buscialà A. Bosisio E. Vascular effects of wine polyphenols. Cardiovasc Res. 2004;63:593–602. doi: 10.1016/j.cardiores.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Morimitsu Y. Kubota K. Tashiro T. Hashizume E. Kamiya T. Osawa T. Inhibitory effect of anthocyanins and colored rice on diabetic cataract formation in the rat lenses. Int Congr Ser. 2002;1245:503–508. [Google Scholar]
- 9.Lee J. Lee HK. Kim CY, et al. Purified high-dose anthocyanoside oligomer administration improves nocturnal vision, clinical symptoms in myopia subjects. Br J Nutr. 2005;93:895–899. doi: 10.1079/bjn20051438. [DOI] [PubMed] [Google Scholar]
- 10.Amos AF. McCarty DJ. Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;145(Suppl 5):1s–85s. [PubMed] [Google Scholar]
- 11.Jayaprakasam B. Vareed SK. Olson LK. Nair MG. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J Agric Food Chem. 2005;53:28–31. doi: 10.1021/jf049018+. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki R. Nishimura N. Hoshino H, et al. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochem Pharmacol. 2007;74:1619–1627. doi: 10.1016/j.bcp.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Seymour EM. Singer AA. Kirakosyan A. Urcuyo-Llanes DE. Kaufman PB. Bolling SF. Altered hyperlipidemia, hepatic steatosis, and hepatic peroxisome proliferator-activated receptors in rats with intake of tart cherry. J Med Food. 2008;11:252–259. doi: 10.1089/jmf.2007.658. [DOI] [PubMed] [Google Scholar]
- 14.Zhang WS. Chen KS. Zhang B, et al. Postharvest responses of Chinese bayberry fruit. Postharvest Biol Technol. 2005;37:241–251. [Google Scholar]
- 15.Bao JS. Cai YZ. Sun M. Wang GY. Corke H. Anthocyanins, flavonols, and free radical scavenging activity of Chinese bayberry (Myrica rubra) extracts and their color properties and stability. J Agric Food Chem. 2005;53:2327–2332. doi: 10.1021/jf048312z. [DOI] [PubMed] [Google Scholar]
- 16.Wang K. Jin P. Cao S. Shang H. Yang Z. Zheng Y. Methyl jasmonate reduces decay and enhances antioxidant capacity in Chinese bayberries. J Agric Food Chem. 2009;57:5809–5815. doi: 10.1021/jf900914a. [DOI] [PubMed] [Google Scholar]
- 17.Yang Z. Zheng Y. Cao S. Effect of high oxygen atmosphere storage on quality, antioxidant enzymes, and DPPH-radical scavenging activity of Chinese bayberry fruit. J Agric Food Chem. 2009;57:176–181. doi: 10.1021/jf803007j. [DOI] [PubMed] [Google Scholar]
- 18.Adom KK. Liu RH. Rapid peroxyl radical scavenging capacity (PSC) assay for assessing both hydrophilic and lipophilic antioxidants. J Agric Food Chem. 2005;53:6572–6580. doi: 10.1021/jf048318o. [DOI] [PubMed] [Google Scholar]
- 19.Galvano F. La Fauci L. Lazzarino G, et al. Cyanidins: metabolism and biological properties. J Nutr Biochem. 2004;15:2–11. doi: 10.1016/j.jnutbio.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Amorini AM. Fazzina G. Lazzarino G, et al. Activity and mechanism of the antioxidant properties of cyanidin-3-O-beta-glucopyranoside. Free Radic Res. 2001;35:953–966. doi: 10.1080/10715760100301451. [DOI] [PubMed] [Google Scholar]
- 21.Kähkönen MP. Heinonen M. Antioxidant activity of anthocyanins and their aglycons. J Agric Food Chem. 2003;51:628–633. doi: 10.1021/jf025551i. [DOI] [PubMed] [Google Scholar]
- 22.Rice-Evans CA. Miller NJ. Bolwell PG. Bramley PM. Pridham JB. The relative antioxidant activities of plant-derived polyphenolic flavnonoids. Free Radic Res. 1995;22:375–383. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 23.Wang H. Cao GH. Prior RL. Oxygen radical absorbing capacity of anthocyanins. J Agric Food Chem. 1997;2:304–309. [Google Scholar]
- 24.Sarma AD. Sreelakshmi Y. Sharma R. Antioxidant ability of anthocyanins against ascorbic acid oxidation. Phytochemistry. 1997;45:671–674. [Google Scholar]
- 25.Acquaviva R. Russo A. Galvano F, et al. Cyanidin and cyanidin 3-O-beta-d-glucoside as DNA cleavage protectors and antioxidants. Cell Biol Toxicol. 2003;19:243–252. doi: 10.1023/b:cbto.0000003974.27349.4e. [DOI] [PubMed] [Google Scholar]
- 26.Serraino I. Dugo L. Dugo P, et al. Protective effects of cyanidin-3-O-glucoside from blackberry extract against peroxynitrite-induced endothelial dysfunction and vascular failure. Life Sci. 2003;73:1097–1114. doi: 10.1016/s0024-3205(03)00356-4. [DOI] [PubMed] [Google Scholar]
- 27.Duthie SJ. Gardner PT. Morrice PC, et al. DNA stability and lipid peroxidation in vitamin E-deficient rats in vivo and colon cells in vitro—modulation by the dietary anthocyanin, cyanidin-3-glycoside. Eur J Nutr. 2005;44:195–203. doi: 10.1007/s00394-004-0511-1. [DOI] [PubMed] [Google Scholar]
- 28.Robertson RP. Harmon JS. Pancreatic islet beta-cell and oxidative stress: the importance of glutathione peroxidase. FEBS Lett. 2007;581:3743–3748. doi: 10.1016/j.febslet.2007.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans JL. Goldfine ID. Maddux BA. Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type-2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 30.Evans JL. Goldfine ID. Maddux BA. Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Ohkuwa T. Sato Y. Naoi M. Hydroxyl radical formation in diabetic rats induced by streptozotocin. Life Sci. 1995;56:1789–1798. doi: 10.1016/0024-3205(95)00150-5. [DOI] [PubMed] [Google Scholar]
- 32.Chen H. Carlson EC. Pellet L. Moritz JT. Epstein PN. Overexpression of metallothionein in pancreatic beta-cells reduces streptozotocin-induced DNA damage and diabetes. Diabetes. 2001;50:2040–2046. doi: 10.2337/diabetes.50.9.2040. [DOI] [PubMed] [Google Scholar]
- 33.Manna P. Sinha M. Sil PC. Protective role of arjunolic acid in response to streptozotocin-induced type-1 diabetes via the mitochondrial dependent and independent pathways. Toxicology. 2009;257:53–63. doi: 10.1016/j.tox.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Nizamutdinova IT. Jin YC. Chung JI, et al. The anti-diabetic effect of anthocyanins in streptozotocin-induced diabetic rats through glucose transporter 4 regulation and prevention of insulin resistance and pancreatic apoptosis. Mol Nutr Food Res. 2009;53:1419–1429. doi: 10.1002/mnfr.200800526. [DOI] [PubMed] [Google Scholar]
- 35.Anderson RA. Polansky MM. Tea enhances insulin activity. J Agric Food Chem. 2002;50:7182–7186. doi: 10.1021/jf020514c. [DOI] [PubMed] [Google Scholar]
- 36.Bailey CJ. Day C. Traditional plant medicines as treatments for diabetes. Diabetes Care. 1989;12:553–564. doi: 10.2337/diacare.12.8.553. [DOI] [PubMed] [Google Scholar]
- 37.Bailey CJ. Day C. Turner SL. Leatherdale BA. Cerasee, a traditional treatment for diabetes. Studies in normal and streptozotocin diabetic mice. Diabetes Res. 1985;2:81–84. [PubMed] [Google Scholar]
- 38.Hale PJ. Horrocks PM. Wright AD. Fitzgerald MG. Nattrass M. Bailey CJ. Xiaoke tea, a Chinese herbal treatment for diabetes mellitus. Diabet Med. 1989;6:675–676. doi: 10.1111/j.1464-5491.1989.tb01255.x. [DOI] [PubMed] [Google Scholar]
- 39.Swanston-Flatt SK. Day C. Bailey CJ. Flatt PR. Traditional plant treatments for diabetes. Studies in normal and streptozotocin diabetic mice. Diabetologia. 1990;33:462–464. doi: 10.1007/BF00405106. [DOI] [PubMed] [Google Scholar]