Abstract
The rhizome of Curcuma longa (turmeric) is often used in Asia as a spice and as a medicine. Its most well-studied component, curcumin, has been shown to exhibit poor bioavailability in animal studies and clinical trials. We hypothesized that the presence of lipophilic components (e.g., turmerones) in turmeric extract would affect the absorption of curcumin. The effects of turmerones on curcumin transport were evaluated in human intestinal epithelial Caco-2 cells. The roles of turmerones on P-glycoprotein (P-gp) activities and mRNA expression were also evaluated. Results showed that in the presence of α- and aromatic turmerones, the amount of curcumin transported into the Caco-2 cells in 2 hours was significantly increased. α-Turmerone and verapamil (a P-gp inhibitor) significantly inhibited the efflux of rhodamine-123 and digoxin (i.e., inhibited the activity of P-gp). It is interesting that aromatic turmerone significantly increased the rhodamine-123 efflux and P-gp (MDR1 gene) mRNA expression levels. The effects of α- and aromatic turmerones on curcumin transport as well as P-gp activities were shown here for the first time. The presence of turmerones did affect the absorption of curcumin in vitro. These findings suggest the potential use of turmeric extract (including curcumin and turmerones), rather than curcumin alone, for treating diseases.
Key Words: Caco-2 cells, Curcuma longa, curcumin, P-glycoprotein, turmerone
Introduction
Turmeric is derived from the rhizome of Curcuma longa L., which has been used in India and China for centuries as both a spice and a medicine. According to the Ayurvedic Pharmacopoeia of India, essential oil from rhizome of Curcuma longa was used as a carminative, stomachic, and tonic.1 In ancient Chinese medicine, the use of Curcuma was formally recorded in the Compendium of Materia Medica (Ben Cao Gang Mu) of the Ming dynasty (A.D. 1590); according to the Chinese Pharmacopoeia, C. longa eliminates blood stasis, promotes the flow of “qi,” stimulates menstrual discharge, and relieves pain.2
Previous epidemiological studies suggested that turmeric used in Indian cooking contributes to the lower incidence of cancers, especially large-bowel cancers, in Indians.3–5 Turmeric extract consists of 3–5% essential oil and 0.02–2.0% curcuminoids.6,7 Many pharmacological studies have demonstrated anti-inflammatory, antitumor, and antioxidant activities of the essential oil8–10 and curcuminoids, especially curcumin.11–16 In our previous study, two sesquiterpenoids (α-turmerone [AL] and aromatic turmerone [AR]) as well as three curcuminoids (curcumin, demethoxycurcumin, and bisdemethoxycurcumin) were isolated from C. longa extract.16 The antiproliferative activities of these compounds have been demonstrated in human hepatoma and breast cancer cells. In human peripheral blood mononuclear cells, turmerones were shown to have immunostimulating activities.16
Although curcumin is thought to be the most active component of turmeric extract, its pharmacological potential may be limited by its extremely low water solubility and poor bioavailability. A previous report suggested that approximately 75% of ingested curcumin (1 g/kg of body weight) was excreted in the feces and that curcumin was poorly absorbed from the intestine of rats.17 Other animal studies showed that metabolites of curcumin (e.g., curcumin glucuronide and sulfates) were found in bile and urine.18,19 A recent animal study demonstrated that the oral bioavailability of curcumin was about 1% in the rat.20 Clinical trials demonstrated that curcumin was well tolerated (without any reported treatment-related toxicity), and a trace amount of curcumin was detected in plasma,21 or metabolites of curcumin were detected in feces.22 Several studies suggested ways to enhance the bioavailability of curcumin, such as combination of curcumin with piperine,23 formulation with phosphatidylcholine,24 preparing curcumin nanoparticles,25 and synthesis of analogs.26 However, it remains to be confirmed whether the presence of other compounds improves the bioavailability of curcumin. As curcumin is relatively lipid soluble,27 we hypothesized that the presence of lipophilic components (e.g., turmerones) in turmeric extract would facilitate the absorption of curcumin.
In the present study, the transport of curcumin was investigated in Caco-2 cell monolayers, which are widely used as an in vitro method for drug permeability determination because these cells have characteristics similar to those of the absorptive cells in the human intestine.28 Previous studies have demonstrated the effects of Curcuma extracts and curcumin on the activities of P-glycoprotein (P-gp) and cytochrome P450 3A4 in Caco-2 cells29–32 or LS180 cells.33 Nonetheless, the transport of curcumin in Caco-2 cells is seldom reported. Furthermore, the role of AL and AR, which were shown to have antiproliferative properties in cancer cells and immunostimulating activities,16 on the activities of P-gp has never been reported. The present study aimed to examine whether the presence of turmerones would affect the transport of curcumin and to evaluate the effects of turmerones on the activities of transporters in Caco-2 cells. The transports of two P-gp substrates—rhodamine-123 and digoxin—were used to evaluate the activities of P-gp as reported in other studies.29,30,32 On the other hand, components isolated from natural products (e.g., flavonoids) were demonstrated to alter activities of ATP-binding cassette transporters other than P-gp.34,35 Therefore, the effects of turmerones on P-gp (MDR1 gene), multidrug resistance protein (MRP2 gene), and breast cancer resistance protein (BCRP gene) expressions have also been investigated in the present study.
Materials And Methods
Materials
Purified curcumin, AL, and AR were isolated from C. longa by column chromatography as previously reported.16 Dried rhizome of India-sourced C. longa L. was purchased from a herbal supplier in Hong Kong. Organoleptic, microscopic, and chemical authentications were accomplished in accordance with the Chinese Pharmacopoeia.2 An authenticated voucher specimen (number HK 40400) was deposited in the Hong Kong Herbarium of the Agriculture, Fisheries and Conservation Department of the Hong Kong Special Administration Region, China. The identification of the purified compounds was based on the 1H and 13C nuclear magnetic resonance spectral analysis and mass spectrometry.16 All organic solvents for purification of compounds mentioned above were purchased from Lab-Scan (Bangkok, Thailand) and were of high-performance liquid chromatography (HPLC) grade.
The human colonic adenocarcinoma cell line Caco-2 was purchased from American Type Culture Collection (Rockville, MD, USA). Dulbecco's modified Eagle's medium, fetal bovine serum, nonessential amino acids, penicillin–streptomycin, trypsin–EDTA, Hanks' balanced salt solution (HBSS), TRIzol®, SuperScript® III reverse transcriptase, and deoxynucleotide triphosphates were obtained from Invitrogen GIBCO (Grand Island, NY, USA). Rhodamine-123, digoxin, verapamil, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), collagen, and HEPES were from Sigma (St. Louis, MO, USA). The real-time polymerase chain reaction (PCR) reagent iTaq™ Fast SYBR® Green Supermix was from Bio-Rad (Hong Kong). Transwell® polycarbonate cell culture inserts (24 mm in diameter, 0.4 μm in pore size, 4.71 cm2 of growth area) were from Costar (Corning, Inc., Life Sciences, Lowell, MA, USA).
Cell culture
The Caco-2 cells were maintained in Dulbecco's modified Eagle's medium containing 10% heat-inactivated fetal bovine serum, 1% nonessential amino acids, 100 units/mL penicillin, and 100 μg/mL streptomycin. The cells were incubated at 37°C in a humidified atmosphere of 5% CO2. The cells at passages 35–46 were used for all experiments. When the cells reached 80% confluence in the culture flasks, trypsin–EDTA was used to remove the cells, and the cells were used in experiments or reseeded in flasks. Turmerones and curcumin were prepared at 100 mg/mL in absolute ethanol and 100 mM in dimethyl sulfoxide (DMSO), respectively. The compounds were stored at –20°C and reconstituted in appropriate medium prior to the experiments.
Cell viability assay
Caco-2 cells (5×103 per well) were seeded in 24-well flat-bottom culture plates (Iwaki Scitech Div, Tokyo, Japan) with 1 mL of culture medium and incubated for 21 days. Subsequently, 1 mL of culture medium containing various concentrations (12.5, 25, 50, or 100 μg/mL) of AL, AR, or curcumin was added into the wells. The content of vehicle solvent (ethanol or DMSO) was 0.5% (vol/vol) in culture medium. Then the plates were incubated at 37°C for 4, 8, or 24 hours. Plain medium containing vehicle solvent (0.5% [vol/vol] ethanol or DMSO) was added to the control wells.
Following the incubation of cells with the compounds, 30 μL of 5 mg/mL MTT in phosphate-buffered saline was added to each well, and the plates were further incubated for 3 hours at 37°C. The supernatant was then removed, and 200 μL of DMSO was added to each well to dissolve the purple formazan crystals. The absorbance at 540 nm was measured with a microplate reader (FLUOstarOptima, BMG LabTech GmbH, Ortenberg, Germany). Results were expressed as the percentage of MTT absorbance with respect to vehicle-treated control cells.
Solubility of curcumin with turmerone
To evaluate the solubility of curcumin in transport buffer, curcumin was added to test tubes containing warmed HBSS buffer (pH 6.8, 37°C). In a previous Caco-2 transport study of curcumin, 40 μM (14.7 μg/mL) was used.36 Hence, three concentrations (14.7, 29.4, and 44.1 μg/mL) of curcumin were tested for solubility. Aliquots were centrifuged at 14,000 g at 4°C to remove insoluble particles. The supernatants were subjected to HPLC analysis. In another set of experiment, 14.7 μg/mL curcumin was added to transport buffer containing 5% (vol/vol) DMSO or AL+AR (each at 50 μg/mL). The sample solution was centrifuged and analyzed. The stability test of curcumin in transport buffer was also performed, in which transport buffer containing curcumin plus DMSO or AL+AR was incubated at 37°C in 5% CO2/95% air for 120 minutes. Aliquots of buffer were collected at 0, 60, and 120 minutes and analyzed by HPLC.
Transport studies of curcumin
Harvested Caco-2 cells were seeded at 3×105 cells per well onto six-well plates with Transwell inserts and cultured for 21 days prior to transport experiments. The culture medium was changed 24 hours before the transport experiment. The integrity of the monolayer was monitored by measuring the transepithelial electrical resistance (TEER) at 37°C with an epithelial volt-ohm meter (World Precision Instruments, Inc., Sarasota, FL, USA). Transport experiments were conducted on monolayers with TEER above 600 Ωcm2. Monolayer TEER was measured before and after transport experiments.
To carry out the transport experiment, Transwell inserts were washed twice and equilibrated with warmed HBSS transport buffer (pH 6.8) at 37°C for 15 minutes before the transport experiment. The HBSS on both sides of the monolayers was replaced with HBSS containing 5% (vol/vol) DMSO. Then, 14.7 μg/mL (40 μM)36 curcumin was added on the apical side of monolayers and incubated at 37°C in 5% CO2/95% air for 120 minutes in the presence or absence of AL+AR (each at 50 μg/mL) at the apical side. At the end of the experiment, HBSS buffer on both apical (1.5 mL) and basolateral (2.6 mL) sides was collected, freeze-dried and reconstituted in methanol for HPLC analysis. The Caco-2 cells on the Transwell membranes were washed twice with cold phosphate-buffered saline and then collected. The curcumin inside the cells were extracted with 400 μL of methanol for HPLC analysis.
HPLC analysis of curcumin
The samples for curcumin transport study were reconstituted in 600 μL of methanol and analyzed using an HPLC system (System Gold®, Beckman Coulter, Brea, CA, USA) equipped with a model 125 solvent pump, a model 168 diode array detector, a model 508 autosampler, and Karat™ 32 software (Beckman Coulter). An analytical column (4.6×250 [i.d.] mm) packed with hydrophobic-bonded (film thickness, 5 μm) C18 phase (Eclipse XDB-C18, Agilent, Palo Alto, CA, USA) was used, accompanied with an Agilent Eclipse XDB-C18 guard column (4.6×12.5 [i.d.] mm; film thickness, 5 μm). The mobile phase was composed of A (water; pH 3, adjusted with H3PO4) and B (methanol:acetonitrile:water:20% H3PO4, 450:450:100:1.5 by volume). The flow rate was 1.5 mL/minute. The gradient was as follows: zero-time, 30% A, 70% B; 5 minutes, 0% A, 100% B. The injection volume was 50 μL. Curcumin was detected at 425 nm, and the total run time was 17 minutes. Concentrations were obtained by extrapolation of the peak areas from a standard curve.
Transport studies of rhodamine-123
Caco-2 cells grown on Transwell inserts for 21 days were used for the transport studies of rhodamine-123. After the inserts were washed twice and equilibrated with warmed HBSS buffer, turmerones (50 or 100 μg/mL) or verapamil (a P-gp inhibitor) (100 μM) was added to the apical side of the Caco-2 monolayers and incubated at 37°C for 30 minutes. Then 10 μM rhodamine-123 was added to the basolateral side. At 30, 45, 60, 75, 90, 105, and 120 minutes, 100-μL aliquots were withdrawn from the apical side of the monolayer. Withdrawn samples were replaced with equal volumes of prewarmed HBSS transport buffer. The fluorescence of rhodamine-123 was determined on a BMG FLUOstarOptima microplate at wavelengths of 485 nm (excitation) and 530 nm (emission). The changes in rhodamine-123 amount were expressed as a percentage of the control.
At the end of 120-minute experiment, the cells on the Transwell membrane were washed twice with ice-cold phosphate-buffered saline, and the membranes were collected. The cells were then lysed with 400 μL of 0.1% Triton X-100 for 20 minutes. The lysates were subjected to total protein (using a BCA protein assay kit, Sigma) and rhodamine-123 fluorescence measurements.
Bidirectional transport studies of digoxin
Caco-2 cells grown on Transwell inserts for 21 days were used for digoxin bidirectional transport studies. After the inserts were washed twice and equilibrated with warmed HBSS buffer, turmerones (50 μg/mL) or verapamil (100 μM) was added to the apical side of the Caco-2 monolayers and incubated at 37°C for 30 minutes. Then 10 μM digoxin was added to the apical side (for apical to basolateral transport, A→B) or basolateral side (for basolateral to apical transport, B→A), the so-called donor side. At 30, 60, 90, and 120 minutes, 100 μL aliquots were withdrawn from the receiver side of the monolayer. Withdrawn samples were replaced with equal volumes of prewarmed HBSS transport buffer. The samples were frozen at −20°C until HPLC analysis. The apparent permeability coefficient (Papp) was calculated as described previously:28
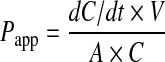 |
where dC/dt is the change of the drug concentration in the receiver chambers over time, V is the volume of the solution in the receiver chambers (in cm3), A represents the membrane surface area (in cm2), and C is the initial concentration in the donor chambers.
Development of an HPLC method for digoxin quantification
Digoxin in HBSS buffer was quantified with an HPLC system composed of an Agilent HP1100 apparatus. An aliquot of 20 μL of sample was loaded on a Waters (Milford, MA, USA) C18 column (250×4.6 [i.d.] mm; film thickness, 5 μm) protected with a Waters C18 guard column (12.5×4.6 [i.d.] mm; film thickness, 5 μm) maintained at 20°C. The isocratic elution with a mobile phase of water–acetonitrile (70:30, vol/vol) was used at a flow rate of 1.0 mL/minute for the separation of analytes. The analytes were monitored at an ultraviolet wavelength of 230 nm. The limit of detection and limit of quantification of the assay method were 0.1 μg/mL and 0.25 μg/mL, respectively. Calibration range of the compound was from 0.25 to 8 μg/mL. A simple, accurate, and specific HPLC analytical method for quantification of digoxin was established and successfully applied to determine the buffer obtained from the transport experiments with good reproducibility in the range from 0.25 to 8.0 μg/mL.
Real-time PCR analysis of MDR1, MRP2, and BCRP mRNA
Caco-2 cells were seeded in six-well plates at 3×105 cells per well, and the medium was changed after 24 hours. The cells were allowed to grow for 20 days, and medium was changed every 2 days. On day 21, testing compounds (turmerones and curcumin) were added into the wells for 24 hours. Then, the cells were washed with cold phosphate-buffered saline twice and collected by scraping. Total RNA was extracted from cells using TRIzol reagent according to the manufacturer's protocols. The RNA concentration was spectrophotometrically determined using a BioPhotometer (Eppendorf, Hauppage, NY, USA). Reverse transcription of 3 μg of total RNA was performed in a Bio-Rad iCycler using SuperScript III reverse transcriptase reagents according to the manufacturer's protocols. To quantify the amount of mRNA of MDR1, MRP2, and BCRP, real-time semiquantitative PCR of cDNA samples was performed in a Bio-Rad CFX96™ Real-Time System C1000 thermal cycler using the iTaq Fast SYBR Green Supermix. Each 20 μL of PCR sample contained 80 ng of cDNA, 10 μL of Supermix, RNase-free water, and 1.25 μL of both the specific forward and reverse primers (10 mM), which were synthesized by Invitrogen. The sequences of the primers are listed in Table 1. Reactions were performed in triplicate using the following protocol: preincubation for 3 minutes at 95°C, followed by 40 PCR cycles at 95°C for 3 seconds, 60°C for 30 seconds, and 72°C for 5 seconds. Relative quantification was obtained by the comparative threshold cycle (ΔΔCt) method (CFX Manager software, version 1.6, Bio-Rad). The specific gene mRNA levels were normalized relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA level in each sample.
Table 1.
Gene-Specific Polymerase Chain Reaction Primers
| Gene | Forward primer | Reverse primer |
|---|---|---|
| MDR1 | 5′-CAGACAGCAGGAAATGAAGTTGAA-3′ | 5′-TGAAGACATTTCCAAGGCATCA-3′ |
| MRP2 | 5′-TGCAGCCTCCATAACCATGAG-3′ | 5′-GATGCCTGCCATTGGACCTA-3′ |
| BCRP | 5′-CAGGTCTGTTGGTCAATCTCACA-3′ | 5′-TCCATATCGTGGAATGCTGAAG-3′ |
| GAPDH | 5′ CGAGATCCCTCCAAAATCAA 3′ | 5′-TTCACACCCATGGACGAACAT 3′ |
BCRP, breast cancer resistance protein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MDR1, P-glycoprotein; MRP2, multidrug resistance protein.
Statistical analysis
Data were expressed as mean±SD values. Statistical analyses and significance, as measured by Student's t test for unpaired samples were performed using GraphPad PRISM software version 5.0 (GraphPad Software, La Jolla, CA, USA). In all comparisons, P<.05 was considered statistically significant.
Results
Effects of curcumin and turmerones on cell viability of Caco-2 cells
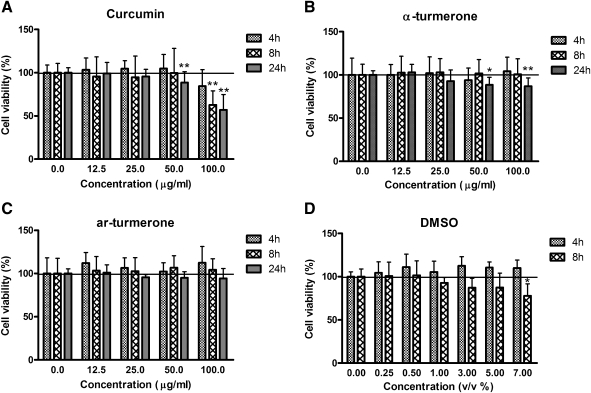
Curcumin at 50 and 100 μg/mL showed significant cytotoxicity in Caco-2 cells after a 24-hour incubation (Fig. 1); however, a 4–8-hour treatment with curcumin (12.5–50 μg/mL) did not affect viability of Caco-2 cells. AL showed mild cytotoxic effects at 50 and 100 μg/mL after a 24-hour incubation, whereas AR (12.5–100 μg/mL) did not show cytotoxic effects after a 24-hour incubation at all concentrations tested. Content of DMSO (0.25–7% vol/vol) did not affect cell viability after a 4-hour incubation (n=3) (P>.05). However, addition of 3–7% (vol/vol) DMSO in culture medium for 8 hours caused decreases in cell viability, whereas the difference between the 7% (vol/vol) DMSO treatment group and the control group was statistically significant (n=3) (P<.05). In a previous Caco-2 transport study,36 40 μM (14.7 μg/mL) curcumin did not show cytotoxicity after an 8-hour incubation. Hence, this was the concentration of curcumin adopted in our following experiments.
FIG. 1.
Effects of curcumin, turmerones, and dimethyl sulfoxide (DMSO) on cell viabilities of Caco-2 cells. Cells were treated with increasing concentrations of compounds for 4, 8, or 24 hours, and cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Results are expressed as percentages of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide absorbance with respect to the untreated vehicle control wells and are mean+SD values of three or four independent experiments with four wells each. *P<.05, **P<.01, compared with the vehicle control group. ar, aromatic.
Solubility of curcumin with turmerone
Curcumin solubility in transport buffer was determined as 0.57 μg/mL. Increased curcumin concentrations (14.7–44.1 μg/mL) did not alter the solubility (data not shown). Our experimental value of curcumin solubility was comparable to data reported in the literature (i.e., 0.6 μg/mL37). The stability test of curcumin in transport buffer revealed no significant change in concentration collected at different time points (data not shown).
In order to increase the solubility of curcumin, 5% (vol/vol) DMSO was added to transport buffer, which was not cytotoxic after a 4-hour incubation (Fig. 1). The presence of turmerones significantly decreased the solubility of curcumin as shown in Table 2.
Table 2.
Comparison of Curcumin Solubility Under Different Conditions
| |
Measured concentration (μg/mL) at given DMSO (%) in transport buffer |
||
|---|---|---|---|
| Curcumin | 1 | 5 | 5 |
| 14.7 μg/mL | 0.59±0.03 | 3.38±0.52* | — |
| 14.7 μg/mL+α-turmerone/aromatic turmerone (50 μg/mL each) | — | — | 2.17±0.38† |
Data are mean±SD values of three independent experiments.
P<.05, compared with curcumin in 1% DMSO.
P<.05, compared with curcumin in 5% DMSO alone.
Transport studies of curcumin
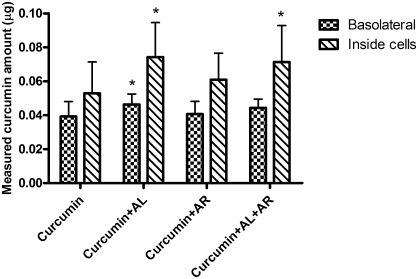
Based on data summarized from the cytotoxicity and solubility tests of curcumin, 14.7 μg/mL curcumin in transport buffer containing 5% (vol/vol) DMSO was used in the transport study. The presence of testing compounds and DMSO did not alter the TEER values (data not shown) after a 2-hour incubation, showing the intact integrity of the Caco-2 monolayers. After the 2-hour transport study, there were trace amounts of curcumin (∼0.03 μg) detected on the basolateral side of the Caco-2 cell monolayer. In the presence of turmerones (AL and AR, each at 50 μg/mL), more curcumin was detected inside the cells (P<0.05) (Table 3, Experiment A). Because the presence of turmerones decreased the solubility of curcumin in transport buffer, the actual amount of 14.7 μg/mL curcumin available for transport on the apical side was different as shown in Table 3 (1.90 μg vs. 0.67 μg). In order to ensure the same amount of curcumin at the beginning of experiment, 14.7 μg/mL curcumin was added to the transport buffer with turmerones. Then the actual amount of curcumin in the solution was quantified by HPLC, and the value was 0.60 μg. Meanwhile, a solubility curve (actual detected amount vs. added amount of curcumin) was generated by adding different amounts of curcumin (without turmerones) to the transport buffer. From the curve, 11.4 μg/mL curcumin was needed to produce a solution with the actual curcumin amount at 0.6 μg. Finally, the two solutions with the same actual curcumin amount were added into the cell monolayers. In the presence of turmerones, a significantly higher amount of curcumin was detected inside the cells (Table 3). On the basolateral side, more curcumin was detected when turmerones were present; however, the differences were not statistically significant. In another set of experiments, the effects of individual turmerone (AL or AR, each at 50 μg/mL) on the transport of curcumin were studied. As shown in Figure 2, the presence of AL significantly increased the amount of curcumin detected on the basolateral side of the monolayer and inside the cells (P<.05); in contrast, in the presence of AR, there was only a slight increase in the amount of curcumin inside the cells. When both turmerones were present in equal amounts, a significantly higher amount of curcumin was detected inside the cells. Results showed that the changes of curcumin transport into and across the Caco-2 cells were mainly contributed by AL. In fact, in the presence of AL (100 μg/mL), the measured curcumin amounts on the basolateral side of the monolayer and inside the cells were significantly increased (data not shown). However, there was no significant difference between the 50 μg/mL and 100 μg/mL AL treatment groups. Therefore, the data for minimum effective dose (i.e., 50 μg/mL) are shown in Figure 2.
Table 3.
Amount of Curcumin Measured Before and After Transport Experiments
| |
Experiment A |
Experiment B |
||
|---|---|---|---|---|
| Measured curcumin amount (μg) | Curcumin | Curcumin+turmerones | Curcumin | Curcumin+turmerones |
| Apical (before) | 1.90±0.48 | 0.67±0.076 | 0.60±0.071 | 0.60±0.12 |
| Basolateral (after) | 0.028±0.012 | 0.030±0.010 | 0.025±0.015 | 0.035±0.013 |
| Inside cells (after) | 0.032±0.0052 | 0.044±0.0080* | 0.042±0.010 | 0.068±0.024* |
In Experiment A, 40 μM curcumin with or without turmerones was added to the Transwell inserts. In Experiment B, 31 μM curcumin alone or 40 μM curcumin with turmerones were added to the Transwell inserts. After the 2-hour transport study, sample solution was collected in apical and basolateral chambers of Transwell inserts, and the cell lysate was also collected for curcumin quantification. Data are mean±SD values of three independent experiments with three Transwell inserts each.
P<.05, significantly different from curcumin-alone group.
FIG. 2.
Effects of α-turmerone (AL) and ar-turmerone (AR) on the amount of curcumin measured after transport experiments. Caco-2 monolayers were added with 40 μM curcumin with or without AL or AR (50 μg/mL) or AL+AR (each at 50 μg/mL). After 2 hours sample solutions were collected in basolateral chambers of Transwell inserts, and the cell lysates were also collected for curcumin quantification. Results are mean+SD values of four independent experiments with three Transwell inserts each. *P<.05, significantly different from curcumin-alone group.
Transport studies of rhodamine-123
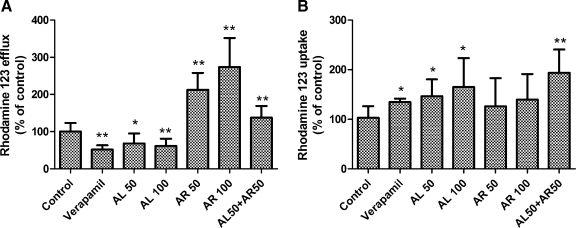
To examine the effect of turmerones on the efflux activity of P-gp, rhodamine-123 accumulation and efflux experiments were performed.30,32 After the cells were treated with 50 or 100 μg/mL AL for 30 minutes, rhodamine-123 efflux was decreased by 32% and 40%, respectively (Fig. 3). The inhibition by AL was comparable to the effect of the P-gp inhibitor verapamil (100 μM), which inhibited 48% of efflux. However, AR treatment (50 or 100 μg/mL) raised rhodamine-123 efflux by 2.12–2.73-fold. Furthermore, the efflux was increased by 37% in monolayers treated with both AL and AR (each at 50 μg/mL). On the other hand, AL (50 and 100 μg/mL) and verapamil significantly increased rhodamine-123 uptake by Caco-2 cells, whereas AR did not significantly affect the uptake. It is interesting that when both AL and AR (each at 50 μg/mL) were added to the monolayers, the rhodamine-123 uptake was significantly increased by 1.94-fold.
FIG. 3.
Effects of AL, AR, and verapamil on rhodamine-123 efflux and uptake. Caco-2 monolayers were treated with AL or AR (50 or 100 μg/mL), AL+AR (each at 50 μg/mL), or verapamil (positive control, 100 μM) for 30 minutes. The efflux and uptake of rhodamine-123 (10 μM) were measured after incubating the cells at 37°C for 120 minutes. Results are expressed as percentages of fluorescence with respect to the untreated vehicle control cells and are mean+SD values of six independent experiments with two Transwell inserts each. *P<.05, **P<.005, compared with the vehicle control group.
Bidirectional transport studies of digoxin
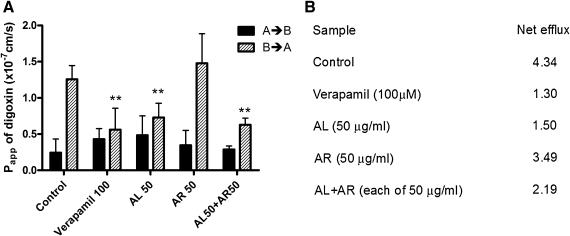
The P-gp function was also evaluated by measuring transepithelial transport of digoxin across Caco-2 cell monolayers.29,30 Digoxin transport was polarized, with basolateral to apical (B→A) permeability exceeding apical to basolateral (A→B) by an efflux ratio of 4.34 (Fig. 4B). In the presence of verapamil (100 μM) or AL (50 μg/mL), A→B digoxin transport was enhanced (but not statistically significant), whereas that in the B→A direction was significantly inhibited (P<0.05, Fig. 4A). In contrast, AR slightly increased B→A digoxin transport across the Caco-2 monolayer (P>.05, Fig. 4A). The corresponding efflux ratios are given in Figure 4B. When both AL and AR (each at 50 μg/mL) were added to the monolayers, the B→A digoxin transport was significantly decreased (P<.05).
FIG. 4.
Effects of AL, AR, and verapamil on the apparent permeability (Papp) of digoxin across Caco-2 monolayers. (A) The apical to basolateral permeability values PappA→B (black columns) and basolateral to apical permeability values PappB→A (striped columns) were determined as the slope of the linear portion of each transport–time profile. Data are mean+SD values of four independent experiments with three Transwell inserts each. **P<.005, compared with the vehicle control group (B→A). (B) Net efflux was calculated as the ratio of mean B→A Papp to mean A→B Papp.
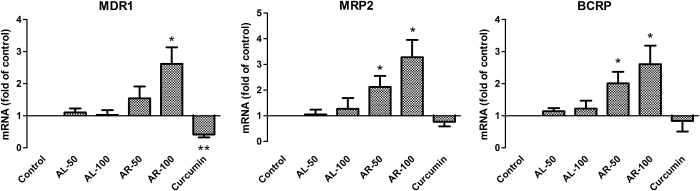
Real-time PCR analysis of MDR1, MRP2, and BCRP mRNA
The mRNA levels of MDR1, MRP2, and BCRP in Caco-2 cells treated with turmerones and curcumin for 24 hours were studied using real-time PCR. As shown in Figure 5, AR (100 μg/mL) significantly up-regulated MDR1, MRP2, and BCRP expressions. The increases of mRNA expressions seemed to be concentration-dependent. Although AL treatment (50 or 100 μg/mL) did not significantly alter the expression of these genes at this time point, the expression level of MDR1 was significantly decreased by curcumin (14.7 μg/mL, 24 hours) in Caco-2 cells. Our results agreed with previous studies, in which MDR1 mRNA expressions were decreased by curcumin at 72 hours of treatment.30,33
FIG. 5.
Quantitative real-time polymerase chain reaction analysis of MDR1, MRP2, and BCRP mRNA in AL-, AR-, or curcumin-treated Caco-2 cells. Caco-2 cells were treated with AL or AR turmerones (50 or 100 μg/mL) or curcumin (14.7 μg/mL) for 24 hours, and RNA was extracted for quantitative real-time polymerase chain reaciotn. Data (cycle threshold, Ct values) were normalized with that of GAPDH and control samples. Data are mean+SD values of three independent experiments. *P<.05, **P<.005, compared with the vehicle control group.
Discussion
The use of turmeric as a spice and a medicine is very popular in Asia. The pharmacological activities of its major compound, curcumin, have been studied intensively in laboratories all over the world.3,11,37 The poor bioavailability of curcumin also drew many scientists' attention, and enhancing bioavailability has been the objective of several studies.31,34,38 In the present study, the transport of curcumin was investigated using the in vitro Caco-2 cell monolayer model. A simple hypothesis was verified in this well-established and widely used model. We hypothesized that the lipophilic components (e.g., turmerones) in turmeric extract would affect the transport of curcumin in the intestine. Therefore the effects of turmerones (isolated from turmeric crude extract) on curcumin transport were explored in the present study.
Our results demonstrated that in the presence of turmerones, amounts of curcumin transported from the apical side to the basolateral side (absorption) of Caco-2 cell monolayers were relatively higher than those without turmerones. At the same time, the amounts of curcumin inside the Caco-2 cells were significantly higher when turmerones were present in the transport system (Table 3), especially when AL was present (Fig. 2). The in vitro transport of curcumin with turmerones was reported here for the first time. By increasing the DMSO concentration from 1% to 5% (vol/vol) in the transport buffer, more curcumin was available for the in vitro transport experiment. As a result, the curcumin amount in the sample solution collected on the basolateral side of the Caco-2 cell monolayer could be analyzed by HPLC, even though the concentration was low. Besides, because intestinal solubility may be far greater than the solubility measured in water, the use of aqueous solubility to predict drug absorption can therefore lead to very pronounced underestimates of the oral bioavailability.39 Hence, DMSO was used to increase solubility of curcumin, and this method enabled the evaluation of curcumin transport in the Caco-2 model with other substances (e.g., turmerones in this study). The increased DMSO concentration did not affect the viability of Caco-2 cells after a 4-hour incubation (Fig. 1) or the integrity (reflected by TEER) of the monolayers, and it was a more feasible method to increase the curcumin solubility than alternative heat treatment or addition of alkaline in transport buffer, which were used in previous studies.37,40 One of the limitations of the present experiment was that the amounts of curcumin in the sample solution (0.5 mL) collected on the basolateral side at 30, 60, or 90 minutess were too low to be analyzed by HPLC (data not shown). Instead, the total amount of curcumin in the basolateral solution (2.6 mL) was collected and analyzed. Therefore, the apparent permeability coefficient (Papp) of curcumin could not be calculated.
As the improvement of curcumin transport in Caco-2 cell monolayers by adding tumerones was demonstrated, the effects of turmerones on intestinal cell transporter activities were also assessed. Because the yield percentages of AL and AR from turmeric extracts were 0.03% (wt/wt) and 0.027% (wt/wt), respectively,16 equal amounts of turmerones were combined or tested separately. AL was shown to inhibit P-gp activity as it inhibited the efflux and enhanced uptake of rhodamine-123 (Fig. 3). It also inhibited the B→A transport (efflux) of another P-gp substrate, digoxin, and decreased the net efflux ratio (Fig. 4B). The effects were similar and comparable to those of the P-gp inhibitor verapamil (100 μM). As mentioned before, the presence of AL increased the amount of curcumin inside the cells, which may due to the inhibitory effects of AL toward P-gp and other transporters so that the efflux of absorbed curcumin has been decreased. In contrast, AR increased the efflux of rhodamine-123 and digoxin. When the two turmerones combined in equal amounts, the efflux of rhodamine-123 was slightly increased, whereas that of digoxin was decreased. It is possible that these two turmerones also act on other intestinal tranporters, apart from P-gp. The effect of AR dominated in rhodamine-123 transport so the combined effect was similar to that of AR alone. Conversely, AL affected digoxin transport dominantly, and AR had no significant effect; therefore, the combined effect was similar to that of AL alone. On the other hand, a simple HPLC detection method for digoxin was established in this study. In the past, [3H]digoxin was commonly used for P-gp activity evaluation.29,30 With our HPLC detection method for digoxin, no radioactive samples need to be handled in the future, and the experimental procedures can be simplified for the transport studies using digoxin.
Previous studies have demonstrated that the methanolic extract of Curcuma significantly increased the activity of P-gp by up-regulating the expressions of P-gp protein and MDR1 mRNA levels and that curcumin inhibited the activity of P-gp.30 A recent study also showed that the hydroethanolic extracts of Curcuma species decreases the rhodamine-123 efflux (i.e., P-gp activity).32 Our data presented here could explain the contradictory results of these studies. Our results also showed that AR could up-regulate the MDR1, MRP2 and BCRP expressions of Caco-2 cells. Unlike other studies in which the changes in gene expression induced by Curcuma extracts or curcumin were observed after a 72-hour exposure,30,33 the changes were observed at a much earlier time in our study because the cells were exposed to curcumin or turmerones for 24 hours only. Furthermore, the changes in P-gp activities, in terms of rhodamine-123 and digoxin transport, were observed in cell monolayers exposed to turmerones for 150 minutes (a 30-minute preincubation plus the 120-minute transport experiment). The transient effect on P-gp activities was evaluated in the present study. Nevertheless, the changes of expressions of the above-mentioned genes have also been investigated using cells exposed to turmerones for 8 and 16 hours. However, no significant change has been observed (data not shown). To further elucidate the effects of turmerones on the activities of ATP-binding cassette transporters or other transporters that may relate to the transport of curcumin, future experiments should be performed using different transporter inhibitors or receptor antagonists.
Previous epidemiological studies suggested that turmeric contributes to the lower incidence of large-bowel cancers in Indians.3–5 Previous clinical studies have also demonstrated the chemopreventive effect of curcumin during the promotion/progression stages of colon cancer.21,41 In a recent clinical trial, colorectal cancer patients ingested curcumin, and both normal and malignant colorectal tissues were found to have taken up curcumin.42 Our in vitro results demonstrated that the presence of turmerones increased the accumulation of curcumin inside intestinal epithelial cells. Our results agree with a recent clinical study in which curcumin with the noncurcuminoid components of turmeric (Biocurcumax™, Arjuna Natural Extracts, Alwaye, India) was taken by human volunteers;43 the bioavailability of Biocurcumax was sixfold higher than that of normal curcumin. Nonetheless, our study also demonstrated the roles of individual turmerones in curcumin transport. As previous studies suggested that most of the oral curcumin was excreted in the feces,11 it is possible that curcumin could reach the colon without being metabolized and that more curcumin could be absorbed into the cells with turmerones. As a result, curcumin could take advantage of its antitumor and anti-inflammatory properties toward the colorectal malignant cells. Furthermore, AR has been demonstrated to have immunostimulatory effects in human peripheral mononuclear cells in our previous study.16 Such effects might have contributed to the modulation of intestinal immunity, and the antitumor effects of curcumin might be enhanced. On the other hand, AL could have significantly inhibited the P-gp activities as shown in rhodamine-123 and digoxin transport studies (Figs. 3 and 4). The potency of inhibition by AL (50 μg/mL represents approximately 229 μM) was comparable to that of the well-known P-gp inhibitor verapamil (100 μM). The anti–drug resistance capabilities of AL will be further investigated in multidrug-resistance cancer cells, in which the P-gp expression is significantly higher. Last but not least, the bioavailability and the local tissue accumulation of curcumin should be evaluated with turmerones in animal models so that the pharmacokinetics of these compounds could be revealed. These data will certainly provide a good foundation to future clinical trials using turmeric extracts.
In conclusion, the transport of curcumin in Caco-2 cell monolayers could be enhanced in the presence of turmerones, which were isolated from turmeric crude extract. The two turmerones showed opposite effects on P-gp activities: AL inhibited the P-gp activities, whereas AR enhanced P-gp activities as well as up-regulated MDR1, MRP2 and BCRP expressions in Caco-2 cells. These findings supported the use of turmeric extract (including curcumin and turmerones), other than curcumin alone in cancer patients, especially those with colorectal cancers.
Acknowledgments
This research was supported in part by grant P50 AT002779-05 from the National Institutes of Health, the National Center for Complementary and Alternative Medicine, and the Office of Dietary Supplements. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Complementary and Alternative Medicine, the Office of Dietary Supplements, or the National Institutes of Health. The authors would like to thank Prof. Zhong Zuo for technical advice and support on Caco-2 model establishment and Dr. Erik Ko for the technical support on quantitative real-time polymerase chain reaction analysis.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ministry of Health & Family Welfare, Government of India. The Ayurvedic Pharmacopoeia of India, Part I. I. The Controller of Publications Civil Lines; New Delhi: 2001. pp. 45–46. [Google Scholar]
- 2.Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia. I. China Medical Science and Technology Press; Beijing: 2010. pp. 247–248. [Google Scholar]
- 3.Chauhan DP. Chemotherapeutic potential of curcumin for colorectal cancer. Curr Pharm Des. 2002;8:1695–1706. doi: 10.2174/1381612023394016. [DOI] [PubMed] [Google Scholar]
- 4.Mohandas KM. Desai DC. Epidemiology of digestive tract cancers in India. V. Large and small bowel. Indian J Gastroenterol. 1999;18:118–121. [PubMed] [Google Scholar]
- 5.Sinha R. Anderson DE. McDonald SS. Greenwald P. Cancer risk and diet in India. J Postgrad Med. 2003;49:222–228. [PubMed] [Google Scholar]
- 6.Gopalan B. Goto M. Kodama A. Hirose T. Supercritical carbon dioxide extraction of turmeric (Curcuma longa) J Agric Food Chem. 2000;48:2189–2192. doi: 10.1021/jf9908594. [DOI] [PubMed] [Google Scholar]
- 7.Braga MEM. Leal PF. Carvalho JE. Meireles MAA. Comparison of yield, composition and antioxidant activity of turmeric (Curcuma longa L.) extracts obtained using various techniques. J Agric Food Chem. 2003;51:6604–6611. doi: 10.1021/jf0345550. [DOI] [PubMed] [Google Scholar]
- 8.Shi XY. Ku K. Tan R. Mechanistic study of anti-tumor effect of turmeric volatile oil. Pharmacol Clin Chin Mater Med. 2003;19:15–16. [Google Scholar]
- 9.Jayaprakasha GK. Jena BS. Negi PS. Sakariah KK. Evaluation of antioxidant activities and antimutagenicity of turmeric oil: a byproduct from curcumin production. Z Naturforsch C. 2002;57:828–835. doi: 10.1515/znc-2002-9-1013. [DOI] [PubMed] [Google Scholar]
- 10.Ji M. Choi J. Lee J. Lee Y. Induction of apoptosis by ar-turmerone on various cell lines. Int J Mol Med. 2004;14:253–256. [PubMed] [Google Scholar]
- 11.Goel A. Kunnumakkara AB. Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Plummer SM. Holloway KA. Manson MM, et al. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappa B activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–6020. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal S. Ichikawa H. Takada Y. Sandur SK. Shishodia S. Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaB alpha kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 14.Anuchapreeda S. Tima S. Duangrat C. Limtrakul P. Effect of pure curcumin, demethoxycurcumin, and bisdemethoxycurcumin on WT1 gene expression in leukemic cell lines. Cancer Chemother Pharmacol. 2008;62:585–594. doi: 10.1007/s00280-007-0642-1. [DOI] [PubMed] [Google Scholar]
- 15.Cao J. Liu Y. Jia L, et al. Curcumin induces apoptosis through mitochondrial hyperpolarization and mtDNA damage in human hepatoma G2 cells. Free Radic Biol Med. 2007;43:968–975. doi: 10.1016/j.freeradbiomed.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Yue GG. Chan BC. Hon PM, et al. Evaluation of in vitro anti-proliferative and immunomodulatory activities of compounds isolated from Curcuma longa. Food Chem Toxicol. 2010;48:2011–2020. doi: 10.1016/j.fct.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wahlstrom B. Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol (Copenh) 1978;43:86–92. doi: 10.1111/j.1600-0773.1978.tb02240.x. [DOI] [PubMed] [Google Scholar]
- 18.Ravindranath V. Chandrasekhara N. Absorption and tissue distribution of curcumin in rats. Toxicology. 1980;16:259–265. doi: 10.1016/0300-483x(80)90122-5. [DOI] [PubMed] [Google Scholar]
- 19.Holder GM. Plummer JL. Ryan AJ. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica. 1978;8:761–768. doi: 10.3109/00498257809069589. [DOI] [PubMed] [Google Scholar]
- 20.Yang KY. Lin LC. Tseng TY. Wang SC. Tsai TH. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;853:183–189. doi: 10.1016/j.jchromb.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Cheng AL. Hsu CH. Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 22.Sharma RA. McLelland HR. Hill KA, et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894–1900. [PubMed] [Google Scholar]
- 23.Shoba G. Joy D. Joseph T. Majeed M. Rajendran R. Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 24.Marczylo TH. Verschoyle RD. Cooke DN. Morazzoni P. Steward WP. Gescher AJ. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol. 2007;60:171–177. doi: 10.1007/s00280-006-0355-x. [DOI] [PubMed] [Google Scholar]
- 25.Bisht S. Feldmann G. Soni S, et al. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnol. 2007;5:3. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun A. Shoji M. Lu YJ. Liotta DC. Snyder JP. Synthesis of EF24-tripeptide chloromethyl ketone: a novel curcumin-related anticancer drug delivery system. J Med Chem. 2006;49:3153–3158. doi: 10.1021/jm051141k. [DOI] [PubMed] [Google Scholar]
- 27.Tomren MA. Másson M. Loftsson T. Tønnesen HH. Studies on curcumin and curcuminoids XXXI. Symmetric and asymmetric curcuminoids: stability, activity and complexation with cyclodextrin. Int J Pharm. 2007;338:27–34. doi: 10.1016/j.ijpharm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Artursson P. Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun. 1991;175:880–885. doi: 10.1016/0006-291x(91)91647-u. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W. Lim LY. Effects of spice constituents on P-glycoprotein-mediated transport and CYP3A4-mediated metabolism in vitro. Drug Metab Dispos. 2008;36:1283–1290. doi: 10.1124/dmd.107.019737. [DOI] [PubMed] [Google Scholar]
- 30.Hou XL. Takahashi K. Tanaka K, et al. Curcuma drugs and curcumin regulate the expression and function of P-gp in Caco-2 cells in completely opposite ways. Int J Pharm. 2008;358:224–229. doi: 10.1016/j.ijpharm.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Hou XL. Takahashi K. Kinoshita N, et al. Possible inhibitory mechanism of Curcuma drugs on CYP3A4 in 1alpha,25 dihydroxyvitamin D3 treated Caco-2 cells. Int J Pharm. 2007;337:169–177. doi: 10.1016/j.ijpharm.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 32.Ampasavate C. Sotanaphun U. Phattanawasin P. Piyapolrungroj N. Effects of Curcuma spp. on P-glycoprotein function. Phytomedicine. 2010;17:506–512. doi: 10.1016/j.phymed.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Graber-Maier A. Berger BK. Aeschlimann J, et al. Effects of Curcuma extracts and curcuminoids on expression of P-glycoprotein and cytochrome P450 3A4 in the intestinal cell culture model LS180. Planta Med. 2010;76:1866–1870. doi: 10.1055/s-0030-1249980. [DOI] [PubMed] [Google Scholar]
- 34.Morris ME. Zhang S. Flavonoid-drug interactions: effects of flavonoids on ABC transporters. Life Sci. 2006;78:2116–2130. doi: 10.1016/j.lfs.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Brand W. van der Wel PA. Rein MJ, et al. Metabolism and transport of the citrus flavonoid hesperetin in Caco-2 cell monolayers. Drug Metab Dispos. 2008;36:1794–1802. doi: 10.1124/dmd.107.019943. [DOI] [PubMed] [Google Scholar]
- 36.Usta M. Wortelboer HM. Vervoort J, et al. Human glutathione S-transferase-mediated glutathione conjugation of curcumin and efflux of these conjugates in Caco-2 cells. Chem Res Toxicol. 2007;20:1895–1902. doi: 10.1021/tx7002245. [DOI] [PubMed] [Google Scholar]
- 37.Kurien BT. Singh A. Matsumoto H. Scofield RH. Improving the solubility and pharmacological efficacy of curcumin by heat treatment. Assay Drug Dev Technol. 2007;5:567–576. doi: 10.1089/adt.2007.064. [DOI] [PubMed] [Google Scholar]
- 38.Yadav VR. Prasad S. Kannappan R, et al. Cyclodextrin-complexed curcumin exhibits anti-inflammatory and antiproliferative activities superior to those of curcumin through higher cellular uptake. Biochem Pharmacol. 2010;80:1021–1032. doi: 10.1016/j.bcp.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Dressman JB. Vertzoni M. Goumas K. Reppas C. Estimating drug solubility in the gastrointestinal tract. Adv Drug Deliv Rev. 2007;59:591–602. doi: 10.1016/j.addr.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Kurien BT. Scofield RH. Curcumin/turmeric solubilized in sodium hydroxide inhibits HNE protein modification—an in vitro study. J Ethnopharmacol. 2007;110:368–373. doi: 10.1016/j.jep.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 41.Kawamori T. Lubet R. Steele VE, et al. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999;59:597–601. [PubMed] [Google Scholar]
- 42.Garcea G. Berry DP. Jones DJ, et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev. 2005;14:120–125. [PubMed] [Google Scholar]
- 43.Antony B. Merina B. Iyer VS. Judy N. Lennertz K. Joyal S. A pilot cross-over study to evaluate human oral bioavailability of BCM-95CG (Biocurcumax), a novel bioenhanced preparation of curcumin. Indian J Pharm Sci. 2008;70:445–449. doi: 10.4103/0250-474X.44591. [DOI] [PMC free article] [PubMed] [Google Scholar]