Abstract
This study was carried out to evaluate the anticancer effects of guava leaf extracts and its fractions. The chemical compositions of the active extracts were also determined. In the present study, we set out to determine whether the anticancer effects of guava leaves are linked with their ability to suppress constitutive AKT/mammalian target of rapamycin (mTOR)/ribosomal p70 S6 kinase (S6K1) and mitogen-activated protein kinase (MAPK) activation pathways in human prostate cancer cells. We found that guava leaf hexane fraction (GHF) was the most potent inducer of cytotoxic and apoptotic effects in PC-3 cells. The molecular mechanism or mechanisms of GHF apoptotic potential were correlated with the suppression of AKT/mTOR/S6K1 and MAPK signaling pathways. This effect of GHF correlated with down-regulation of various proteins that mediate cell proliferation, cell survival, metastasis, and angiogenesis. Analysis of GHF by gas chromatography and gas chromatography–mass spectrometry tentatively identified 60 compounds, including β-eudesmol (11.98%), α-copaene (7.97%), phytol (7.95%), α-patchoulene (3.76%), β-caryophyllene oxide (CPO) (3.63%), caryophylla-3(15),7(14)-dien-6-ol (2.68%), (E)-methyl isoeugenol (1.90%), α-terpineol (1.76%), and octadecane (1.23%). Besides GHF, CPO, but not phytol, also inhibited the AKT/mTOR/S6K1 signaling pathway and induced apoptosis in prostate cancer cells. Overall, these findings suggest that guava leaves can interfere with multiple signaling cascades linked with tumorigenesis and provide a source of potential therapeutic compounds for both the prevention and treatment of cancer.
Key Words: AKT/mammalian target of rapamycin/ribosomal p70 S6 kinase, apoptosis, guava leaves, prostate cancer
Introduction
Guava (Psidium Guajava Linn.) is a member of the Myrtaceae family, which contains at least 133 genera and more than 3800 plant species. African folk medicine uses guava leaf to treat many diseases, including diabetes mellitus, diarrhea, cough, painful menstruation, and hypertension. It is also used to treat acne, painful menses, tooth decay, gum infection, and sore throat, as a disinfectant for wounds, and as an antiseptic (see www.boyikporowealth.com). Other investigations have already examined antibacterial,1 antidiarrheic,2 antihyperglycemic,3 anti-acne,4 and sedative5 effects, as well as anticough6 and narcotic-like7 activities of the plant species. Many reports have shown that the leaves of guava are rich in triterpenoids,8 flavonoids,9 essential oil,10 and tannins.11 Recently, guava leaf extracts have been reported to exhibit anti-inflammatory,12 spermatoprotective,13 and chemopreventive14–17 effects. For instance, they have been shown to suppress the proliferation of various tumor cells, including prostate adenocarcinoma, mouth epidermal carcinoma, colon carcinoma, and murine leukemia. In contrast, it also protects human umbilical vein endothelial cells from loss of viability caused by hyperglycemia.18
Prostate cancer is an androgen-dependent disease and is usually treated with androgen deprivation therapy, which is usually effective in the first stages of the disease.19 However, androgen deprivation therapy ultimately fails in many men, and continuous androgen deprivation usually leads to recurrent androgen-independent prostate cancer.20,21 The phosphatidylinositol 3-kinase (PI3K)/AKT signal transduction pathway plays a key role in cell survival and the protection of cells from apoptosis in human prostate cancer development and progression.22,23 Activation of PI3K leads to the phosphorylation or activation of several downstream targets such as AKT. The activation of AKT is markedly increased in androgen-independent cells compared with androgen-dependent cells.24 PI3K/AKT signaling has been implicated in the regulation of the mammalian target of rapamycin (mTOR) pathway, which regulates cell growth.24 The overexpression of AKT is reportedly involved in the formation of a prostate intraepithelial neoplasia lesion, which is reversed through mTOR inhibition in a transgenic mouse model.25 Hence, mTOR has become a key player in metastatic prostate cancer because of its effects on growth by regulating of hypoxia-inducible factor-1α26 and inhibiting of transforming growth factor-β1.27 The mTOR signaling pathway has also been identified as an important drug target in cancer therapy recently.28 In addition, several studies have demonstrated that ribosomal p70 S6 kinase (S6K1) activity is controlled by mTOR by direct29,30 and indirect31,32 mechanisms. S6K1 is a mitogen-activated serine/threonine kinase that has a critical role in control of cell cycle, growth, and survival. Recently, it has been reported to inactivate the pro-apoptotic molecule BAD by phosphorylation, thereby also promoting cell survival.33
Because overactivation in the AKT/mTOR/S6K1 signaling pathway is closely linked with tumorigenesis, angiogenesis, and metastasis34,35 in prostate cancer, we hypothesized that the guava leaf hexane fraction (GHF) mediates its effects, in part, through the inhibition of the AKT/mTOR/S6K1 pathway. We tested this hypothesis in human prostate cancer PC-3 cells. In our experiments, GHF indeed suppressed constitutive AKT/mTOR/S6K1 activation. This inhibition decreased cell survival and down-regulated expression of proliferative, metastatic, and angiogenic proteins, ultimately leading to the induction of apoptosis via caspase-3 activation in human prostate cancer cells.
Materials and Methods
Cell culture
PC-3 and LNCaP cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin (100 units/mL), and streptomycin (100 μg/mL) at 37°C in a humidified atmosphere with 5% CO2. All experiments were performed 1 day after seeding the cells.
Preparation of various fractions from guava leaves
Air-dried Jeju guava leaves were pulverized and extracted with 80% methanol and occasional sonication for 3 days at room temperature. After being filtered, the extract was concentrated with a vacuum rotary evaporator under reduced pressure and lyophilized. The methanol extract was suspended in water and further fractionated with four different solvents in a stepwise manner. The resulting fractions were the n-hexane fraction, chloroform fraction, ethyl acetate fraction, n-butanol fraction, and water fraction. The extract powder was dissolved in dimethyl sulfoxide and diluted with phosphate-buffered saline (PBS) (pH 7.4) to give the final concentrations.
Chemicals
Anti-phospho (p)-AKT (Ser473), anti-AKT, anti-p-mTOR (Ser2448), anti-mTOR, anti-p-S6K1 (Thr421/Ser424), anti-S6K1, anti-p-extracellular signal-regulated kinase (ERK) (Thr202/Tyr204), anti-ERK, anti-p-c-Jun N-terminal kinase (JNK) (Thr183/Tyr185), anti-JNK, anti-p-p38 (Thr180/Tyr182), anti-p38, anti-caspase-3, and anti-cleaved caspase-3 antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Anti-Bcl-2, anti-Bcl-xL, anti-survivin, anti-inhibitor of apoptotic proteins (IAP)-1, anti-IAP-2, anti-cyclin D1, anti-cyclooxygenase-2 (COX-2), anti-vascular endothelial growth factor (VEGF) anti-caspase-8, anti-caspase-9, anti-poly(ADP-ribose) polymerase (PARP), anti-β-actin antibodies, and horseradish peroxidase–conjugated secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Extracts of guava leaves (methanol, n-butanol, CHCl3, hexane, water, and ethyl acetate) were kindly provided by Dr. Somi Cho (Cheju National University, Jeju, Korea). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 4′,6-diamidino-2-phenylindole (DAPI), and propidium iodide (PI) were purchased from Sigma (St. Louis, MO, USA). Rhodamine-123 was from Molecular Probes (Eugene, OR, USA). Annexin V was from BD Biosciences (Palo Alto, CA, USA). The terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) assay kit was from Roche Diagnostics GmbH (Mannheim, Germany). AKT inhibitor (SH-5) was from Calbiochem (Nottingham, United Kingdom). Rapamycin and NVP-BEZ235 were from Cayman Chemical Co. (Ann Arbor, MI, USA).
MTT assay
Cell viability was measured by an MTT assay to detect NADH-dependent dehydrogenase activity. Fifty microliters of MTT solution (5 mg/mL) in 1× PBS was directly added to the cells, which were then incubated for 4 h to allow MTT to metabolize to formazan. Absorbance was measured with an automated spectrophotometric plate reader at a wavelength of 570 nm. Cell viability was normalized as relative percentages in comparison with untreated controls.
Western blot analysis
After PC-3 cells were treated with the indicated concentration of GHF, the cells were lysed, and the total protein concentration was determined with the Bradford reagent (Bio-Rad, Hercules, CA). Equal amounts of lysates were resolved on sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (Bio-Rad), and the membrane was blocked with 1× Tris-buffered saline containing 0.1% Tween 20 and 5% skim milk or 5% bovine serum albumin for 1 h at room temperature. After blocking, the membranes were incubated overnight at 4°C with the respective primary antibodies. The membranes were washed three times and incubated with diluted (1:10,000) horseradish peroxidase–conjugated secondary antibodies for 1 h at room temperature. After three washes, the membranes were detected using the enhanced chemiluminescence kit (Millipore, Bedford, MA, USA).
Cell cycle analysis
PC-3 cells were seeded onto six-well plates at a density of 5×105 cells per well and incubated for 1 day. After treatment with the indicated concentration of GHF for 24 h, the cells were collected and washed with 1× PBS. Cell pellets were fixed in 70% cold ethanol overnight at −20°C. The fixed cells were resuspended in 1× PBS containing 1 mg/mL RNase A and incubated for 1 h at 37°C, and the cells were stained by adding 50 μg/mL PI for 30 min at room temperature in the dark. The DNA contents of the stained cells were analyzed using CellQuest™ software with a FACSVantage™ SE (Becton Dickinson, Heidelberg, Germany) flow cytometer.
TUNEL analysis
Individual apoptotic cell death was observed using a TUNEL assay kit. For a flow cytometer assay, PC-3 cells were seeded onto six-well plates at a density of 1×106 cells per well, incubated for 1 day, and then treated with GHF for 24 h. The cells were collected and washed with 1× PBS. Cell pellets were fixed with 4% paraformaldehyde for 30 min at room temperature. The fixed cells were permeabilized by 0.2% Triton X-100 in 1× PBS for 15 min, washed, resuspended in 1× PBS containing TUNEL reaction solution, and incubated for 1 h at 37°C in the dark. For the fluorescence microscopy assay, the cells were seeded onto a poly-L-lysine-coated slide, fixed with 4% paraformaldehyde for 30 min at room temperature, and washed three times with 1× PBS. At 15 min after permeabilization with 0.2% Triton X-100, the cells were washed three times with 1× PBS, and TUNEL reaction solution was added. The cells were covered with aluminum foil and incubated for 1 h at 37°C in a humidified CO2 incubator. Then, the cells were then stained with 1 μg/mL DAPI solution for 5 min at room temperature in the dark.
Annexin V assay
Cells (1×106) were treated with GHF for 24 h, fixed with 4% paraformaldehyde, and stained by annexin V conjugated to fluorescein isothiocyanate or with 1 μg/mL DAPI solution. The cells were washed and analyzed with a flow cytometer or a confocal microscope.
Measurement of mitochondrial membrane potential
Rhodamine-123 was used as the mitochondrial membrane potential (Δψm)–sensitive dye. At 24 h after treatment with GHF, the cells washed with 1× PBS and incubated with 10 μM rhodamine-123 for 30 min at room temperature in the dark. The cells were washed with 1× PBS and fixed with 4% paraformaldehyde. Next, the cells were stained with 1 μg/mL DAPI solution, mounted, and analyzed using a fluorescence microscope.
Identification of active constituents from GHF (Table 1)
Table 1.
Compounds Tentatively Identified in the Hexane Fraction of Guava Leaf Extract
| Number | Compound | RTa | Homology (%)b | RPA (%)c |
|---|---|---|---|---|
| 1 | 6-Methyl-5-hepten-2-one | 11.27 | 86 | 0.03 |
| 2 | m-Cymene | 12.73 | 90 | 0.02 |
| 3 | Nonanal | 15.89 | 90 | 0.06 |
| 4 | Phenylethyl alcohol | 16.07 | 95 | 0.29 |
| 5 | Terpinene-4-ol | 18.62 | 95 | 0.06 |
| 6 | α-Terpineol | 19.18 | 91 | 1.76 |
| 7 | Dodecane | 19.42 | 91 | 0.03 |
| 8 | Decanal | 19.62 | 91 | 0.05 |
| 9 | 4-Methoxybenzaldehyde | 21.28 | 91 | 0.06 |
| 10 | Tridecane | 22.93 | 83 | 0.04 |
| 11 | exo-2-Hydroxycineole acetate | 24.79 | 64 | 0.04 |
| 12 | Butyl butyrate | 25.21 | 72 | 0.03 |
| 13 | (E)-5-Octadecene | 25.96 | 90 | 0.05 |
| 14 | 1,6-Dimethyl-2-pyridthione | 26.09 | 59 | 0.11 |
| 15 | Tetradecane | 26.24 | 97 | 0.31 |
| 16 | Cyclotetradecane | 26.38 | 86 | 0.03 |
| 17 | (E)-Caryophyllene | 26.72 | 99 | 0.39 |
| 18 | (Z)-Methyl isoeugenol | 27.86 | 97 | 0.30 |
| 19 | 1-Tetradecanol | 28.58 | 60 | 0.14 |
| 20 | 1-Cyclohexyl-1-butyne | 28.78 | 56 | 0.77 |
| 21 | Germacrene D | 28.65 | 96 | 0.07 |
| 22 | (E)-Methyl isoeugenol | 29.15 | 98 | 1.90 |
| 23 | δ-Cadinene | 29.81 | 96 | 0.08 |
| 24 | 2,4,6-Trimethyl-1,3,6-heptatriene | 29.98 | 53 | 0.16 |
| 25 | α-Calacorene | 30.48 | 91 | 0.07 |
| 26 | Nerolidol | 31.12 | 95 | 1.28 |
| 27 | α-Caryophyllenol | 31.48 | 64 | 0.51 |
| 28 | Caryophyllene oxide | 31.67 | 99 | 3.63 |
| 29 | Guaiol | 32.10 | 91 | 0.84 |
| 30 | Hexadecane | 32.33 | 98 | 1.33 |
| 31 | Humulene oxide | 32.47 | 90 | 0.82 |
| 32 | γ-Cadinene | 32.65 | 53 | 0.65 |
| 33 | Chrysomelidial | 32.75 | 78 | 0.40 |
| 34 | Caryophylla-3(15),7(14)-dien-6-ol | 33.28 | 98 | 2.68 |
| 35 | T-Cardinol | 33.43 | 90 | 1.16 |
| 36 | T-Muurolol | 33.49 | 91 | 1.12 |
| 37 | α-Copaene | 33.57 | 97 | 7.97 |
| 38 | β-Eudesmol | 33.81 | 95 | 11.98 |
| 39 | α-Patchoulene | 33.89 | 87 | 3.76 |
| 40 | Caryophylla-3,8(13)-dien-5β-ol | 34.21 | 97 | 0.86 |
| 41 | α-Bisabolol | 34.71 | 91 | 0.42 |
| 42 | 2,6-Dimethoxy-4-(2-propenyl)-phenol | 34.95 | 86 | 0.39 |
| 43 | Heptadecane | 35.13 | 96 | 0.32 |
| 44 | Loliolide | 36.78 | 56 | 0.29 |
| 45 | β-Oplopenone | 36.85 | 70 | 0.22 |
| 46 | Germacrene B | 37.72 | 56 | 0.26 |
| 47 | Octadecane | 37.81 | 96 | 1.23 |
| 48 | Longifolenaldehyde | 38.35 | 78 | 0.25 |
| 49 | Nonadecane | 40.35 | 96 | 0.10 |
| 50 | Hexadecanoic acid, methyl ester | 40.95 | 99 | 0.69 |
| 51 | Dehydroaromadendrene | 42.20 | 70 | 0.07 |
| 52 | Eicosane | 42.78 | 98 | 0.44 |
| 53 | 9,12-Octadecadienoic acid, methyl ester | 44.86 | 99 | 0.30 |
| 54 | 9,12,15-Octadecatrienoic acid, methyl ester | 44.99 | 95 | 0.22 |
| 55 | (E)-8-Octadecenoic acid, methyl ester | 45.04 | 99 | 0.69 |
| 56 | Phytol | 45.26 | 95 | 7.95 |
| 57 | Neophytadiene | 46.14 | 78 | 0.12 |
| 58 | Docosane | 47.35 | 98 | 0.42 |
| 59 | (Z)-9-Octadecenamide | 50.56 | 95 | 0.59 |
| 60 | Tetracosane | 51.53 | 98 | 0.22 |
Retention time (RT) (in min).
Homology: MS/RI, mass spectrum was identical with that of Wiley 275 mass spectrum database and retention index was consistent with those of the literature (Kondjoyan and Berdague36 and Acree and Ahn37); MS, mass spectrum was consistent with that of the Wiley 275 mass spectrum database.
Relative peak area (RPA) percentage (peak area relative to the total peak area percentage).
The dried extracts were dissolved in 1 mL of hexane, and 1 μL of the solution was injected for gas chromatography–mass spectrometry analysis, which was performed using an Agilent 6890N gas chromatograph with a model 5975 mass-selective detector (Hewlett-Packard, Palo Alto, CA, USA) equipped with a DB-5MS fused silica column (30 m length×0.25 mm i.d.×0.25 μm film thickness, J&W Scientific, Folsom, CA, USA). The carrier gas was helium with a constant flow rate of 1.0 mL/min. One aliquot (1 μL) was injected into the capillary column in a splitless mode. The oven temperature was programmed as follows: 40°C (2 min); 5°C/min to 150°C; 2°C/min to 200°C; 5°C/min to 250°C (3 min). The injector and transfer-line temperatures were 230°C and 250°C, respectively. The ionization energy was 70 eV, and the mass scan range was m/z=35–550. Compounds were identified by comparing their retention times and mass spectra with those of the Wiley 275 library (available with the Agilent device). The retention index value of compounds was calculated with n-paraffins from C7 to C22 as external standards.36,37
Statistical analysis
All numerical values are represented as the mean±SD. Statistical significance of the data compared with the untreated control was determined using Student's unpaired t test. Significance was set at P<.05.
Results and Discussion
Although guava leaves have been shown to suppress the proliferation of a wide variety of cell types and induce apoptosis, the exact mechanism of action of guava leaves has not been elucidated. The goal of this study was to investigate the effect of guava leaves on the AKT/mTOR/S6K1 signaling, various proteins, and cellular responses. Here, we observed that GHF not only exerted significant apoptotic effects, but it also suppressed constitutive activation of PI3K/AKT/mTOR/S6K1 and mitogen-activated protein kinases (MAPKs) in human prostate PC-3 cancer cells. Consequently, GHF down-regulated various proteins involved in cell proliferation, anti-apoptosis, and metastasis. We also clearly demonstrated that GHF can modulate both PI3K/AKT/mTOR/S6K1 and MAPK signaling pathways and induce apoptosis in PC-3 cells.
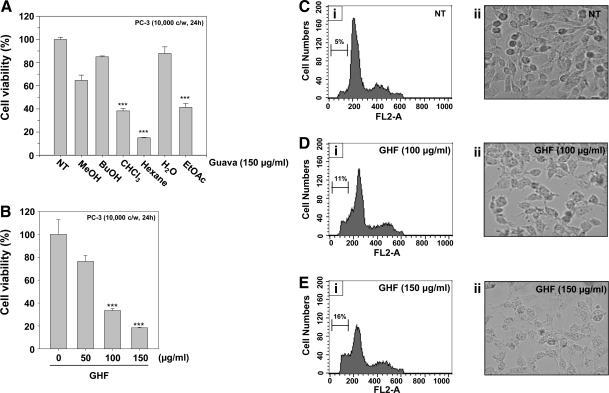
Compared with the other fractions, GHF exerted the strongest cytotoxicity in PC-3 cells (15.02±0.36, P<0.001, n=4; Fig. 1A) and induced cell death even at 50 μg/mL, although the induction was significant from 100 μg/mL (27.37±1.46, P<0.001, n=4; Fig. 1B). GHF induced morphological changes and cell loss (Fig. 1C-ii, 1D-ii; 1E-ii), and PI staining revealed the peaks that represent the apoptotic cells (sub-G1): they remarkably increased from 5% to 16% in a dose-dependent manner (Fig. 1C-i, 1D-i, 1E-i). These data suggest that GHF decreases cell viability by arresting the cell cycle at the sub-G1 phase in PC-3 cells. Previous studies have shown that guava leaves can suppress the growth only in two human prostate cancer cells, Du145 and LNCaP cells; Chen et al.15 showed that guava leaves contain a novel rhamnoallosan, which exhibited potent anticancer activity in Du145 cells through an unknown mechanism.
FIG. 1.
Effects of fractions of guava leaves on cell viability in human prostate cancer PC-3 cells. (A) After PC-3 cells (1×104 cells per well) were seeded on 96-well plates, fractions of guava leaves (methanol [MeOH], n-butanol [BuOH], CHCl3, hexane, water [H2O], and ethyl acetate [EtOAc]) were treated at 150 μg/mL for 24 h. (B) The cells were treated with various indicated concentrations of guava leaf hexane fraction (GHF) for 24 h. The representative cell viability results shown in (A) and (B) were accessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. (C–E) After 5×105 cells were seeded onto six-well plates, indicated concentrations of GHF; 0 (C), 100 (D) or 150 (E) μg/mL were used for 24 h. Then, the cells were fixed and analyzed using a flow cytometer (C-i, D-i, E-i). or observed using a microscope (C-ii, D-ii, E-ii). ***P<.001 compared with nontreated (0 μg/mL GHF) (NT) cells.
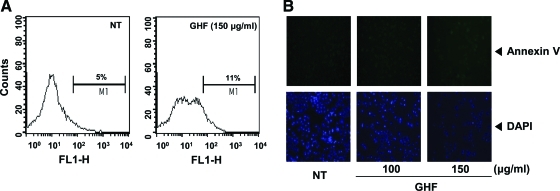
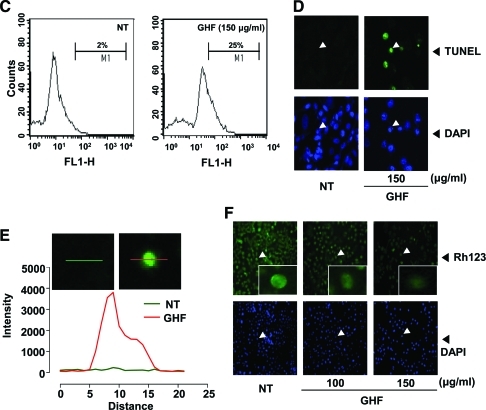
Although the sub-G1 peaks on PI staining represent an apoptotic feature, it can also represent mechanically damaged cells, cells with different chromatin structure, or normal cells with lower DNA content. Therefore, we further demonstrated the effects of GHF on cell death by examining early apoptosis by membrane phosphatidylesterase exposure using the annexin V antibody. The annexin V–positive cells (regarded as early apoptotic cells) were weakly increased compared with the nontreated cells when evaluated by flow cytometry (Fig. 2A) or fluorescence microscopy (Fig. 2B). On the other hand, late apoptotic cells were drastically increased by GHF when we examined late apoptosis by DNA strand breaks through the TUNEL assay (Fig. 2C–E). The collapse of Δψm results in mitochondria dysfunction leading to mitochondrial swelling, cytochrome c releases, and apoptosis.38 Therefore, we examined whether GHF induces changes in the Δψm. As shown in Figure 2F, the accumulation of rhodamine-123 in the mitochondria was notably decreased by GHF compared with the nontreated cells. These results indicate that GHF induces both early and late apoptosis via mitochondrial dysfunction in PC-3 cells.
FIG. 2.
GHF induced apoptosis via loss of mitochondrial membrane potential. PC-3 cells were treated with 100 or 150 μg/mL GHF for 24 h and the cells were incubated with a fluorescein isothiocyanate–conjugated annexin V antibody and then analyzed by (A) flow cytometry and (B) fluorescence microscopy as described in Materials and Methods. DAPI, 4′,6-diamidino-2-phenylindole. Color images available online at www.liebertonline.com/jmf. After treatment with GHF for 24 h, the cells were fixed, incubated using terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) reaction solution, and then analyzed by (C) flow cytometry and (D) under a fluorescence microscope as described in Materials and Methods. (E) The graph shows fluorescent intensity measured along the line drawn across cells of magnified images from indicated cells marked with an arrowhead in (D). (F) PC-3 cells were treated with 100 or 150 μg/mL GHF for 24 h, and the cells were incubated with rhodamine-123 (Rh123). The representative results shown here were analyzed using a confocal microscope. Color images available online at www.liebertonline.com/jmf
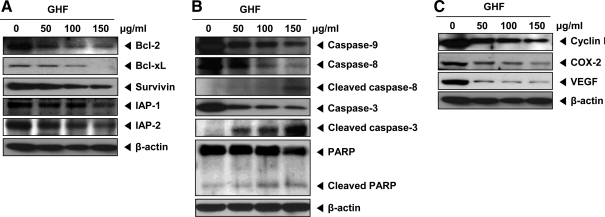
Because the constitutive expression of the proteins Bcl-2, Bcl-xL, survivin, IAP-1, and IAP-2 has been implicated in cell survival and anti-apoptosis,39–41 we examined the effect of GHF on the constitutive expressions of these genes. GHF suppressed the expression of these proteins in a concentration-dependent manner (Fig. 3A). Because procaspase-9, procaspase-8, procaspase-3, and PARP are associated with the apoptotic cell death pathway,42,43 we next investigated their levels in GHF-treated PC-3 cells. Here, GHF slightly induced the cleavage of procaspase-9, whereas procaspase-8 and procaspase-3 were clearly induced by GHF at 100–150 μg/mL concentrations as seen by the disappearance of the procaspase band and appearance of its cleavage forms. In addition, GHF induced PARP cleavage in a concentration-dependent manner (Fig. 3B). Overall, these results demonstrate that GHF mainly induced the activation of caspase-8 and caspase-3 that led to the cleavage of PARP. Also, GHF repressed cell cycle protein (cyclin D1) and proteins linked with metastasis and angiogenesis (COX-2 and VEGF) (Fig. 3C).
FIG. 3.
GHF down-regulated anti-apoptotic, proliferative, metastatic, and angiogenetic proteins and induced apoptosis by activating caspases. PC-3 cells were treated with various indicated concentrations of GHF for 24 h. (A, B) Then, equal amounts of lysates were analyzed by western blot analysis using antibodies against Bcl-2, Bcl-xL, survivin, inhibitor of apoptotic protein (IAP)-1, IAP-2, caspase-3, -8, and -9, and poly(ADP-ribose) polymerase (PARP). An anti-β-actin antibody was used as a loading control. (C) Equal amounts of lysates were analyzed by western blot analysis using antibodies against cyclin D1, cyclooxygenase-2 (COX-2), and vascular endothelial growth factor (VEGF). An anti-β-actin antibody was used as a loading control. The experiments shown here were repeated with similar results.
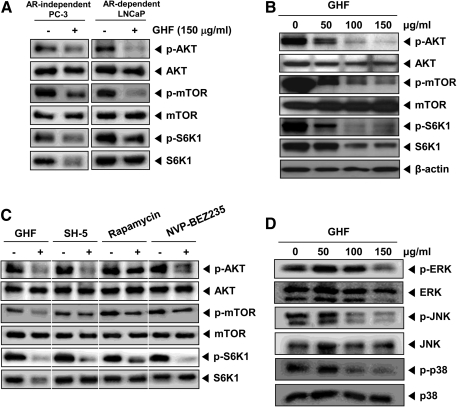
We next investigated whether GHF modulates the activity of AKT, which is representative of proteins involved in cell survival and proliferation. As shown in Figure 4A, GHF suppressed constitutive AKT/mTOR/S6K1 signaling in both PC-3 and LNCaP cells. In particular, GHF suppressed constitutive AKT activation in a concentration-dependent manner (Fig. 4B). The downstream proteins of AKT—constitutive mTOR and S6K1 activation—were also inhibited by GHF. In addition, total expression level of S6K1 distinctly reduced by GHF may be due to various terpenes such as β-eudesmol, α-copaene, phytol, or it might be a combination of various phytochemicals present in the fraction. Our data indicate that suppression of the AKT/mTOR/S6K1 signaling by GHF could partially affect its cytotoxicity in PC-3 cells. Recently, Chen et al.16 have demonstrated that guava leaves induce apoptosis through the inactivation of AKT and activation of p38 and ERK1/2 in LNCaP cells. Because the growth of PC-3 and DU-145 cells is androgen-independent, it would be quite different from cell growth signaling compared with the LNCaP cells, which are androgen-dependent.44 It has been shown that the level of AKT activation was drastically enhanced in androgen-independent cells compared with androgen-dependent cells.24 AKT is also reported to be linked to nuclear factor-κB (NF-κB) activation through the phosphorylation of p65 to enhance the transcriptional activity of NF-κB.45 Furthermore, Akt is positively involved in COX-2 expression in the Toll-like receptor-4 pathway.46 Choi et al.12 showed that guava leaves inhibit lipopolysaccharide-stimulated inducible nitric oxide synthase and COX-2 production via the down-regulation of NF-κB activation. NF-κB activation is known to regulate the expression of cell survival, proliferative, metastatic, and angiogenic proteins.47
FIG. 4.
GHF suppressed the AKT-mammalian target of rapamycin (mTOR)-ribosomal p70 S6 kinase (S6K1) signaling pathway in both PC-3 and LNCaP cells. (A) PC-3 and LNCaP cells were treated with 150 μg/mL GHF for 6 h. Then, equal amounts of lysates were analyzed by western blot analysis using antibodies against phospho (p)-AKT (Ser473) and AKT, p-mTOR (Ser2448) and mTOR, and p-S6K1 (Thr 421/Ser424) and S6K1. AR, androgen receptor. (B) PC-3 cells were treated with different indicated concentrations of GHF for 6 h, after which equal amounts of lysates were analyzed by western blot analysis using antibodies against p-AKT (Ser473) and AKT, p-mTOR (Ser2448) and mTOR, p-S6K1 (Thr 421/Ser424) and S6K1, and β-actin. The results shown are representative of three independent experiments. (C) PC-3 cells were treated with GHF (150 μg/mL), AKT inhibitor (SH-5, 3 μM), rapamycin (20 nM), or NVP-BEZ235 (50 nM) for 6 h and then analyzed by western blot analysis using antibodies against p-AKT (Ser473) and AKT, p-mTOR (Ser2448) and mTOR, and p-S6K1 (Thr 421/Ser424) and S6K1. (D) Cells were treated with various indicated concentrations of GHF for 6 h. Then equal amounts of lysates were analyzed by western blot analysis using antibodies against p-extracellular signal-regulated kinase (ERK) (Thr202/Tyr204) and ERK, p-c-Jun N-terminal kinase (JNK) (Thr183/Tyr185) and JNK, and p-p38 (Thr180/Tyr182) and p38. The results shown here are representative of three independent experiments.
GHF inhibited constitutive mTOR (Ser2448) activation in both PC-3 and LNCaP cells. Many reports have shown that overactivation of mTOR is closely associated with tumorigenesis.48–50 In recent years, specific mTOR inhibitors have shown promise in clinical trials of treatments for malignant tumors.51 It is implied that agents targeting the mTOR signaling can be very powerful and potential candidates for the prevention and treatment of cancer. We also found that GHF reduced constitutive S6K1 (Thr421/Ser424) activation, which is a direct downstream target of the mTORC1 (mTOR, Gβ1, and raptor). Several reports have described the role of p70S6K1 in inducing actin filament remodeling, forming lamellipodia and filopodia structures, and decreasing active stress fibers by regulating cell migration.52–54 Thus, the inhibition of the AKT/mTOR/S6K pathways may also contribute to the anticancer effect of GHF. To understand how GHF modulates various cell signaling pathways, the potential effects of GHF on the AKT/mTOR/S6K1 signaling cascade were compared with those of an AKT inhibitor (SH-5), an mTOR inhibitor (rapamycin), and dual PI3K/mTOR inhibitor (NVP-BEZ235). We found that SH-5 and rapamycin were the most potent in inhibiting AKT and mTOR activation, respectively (Fig. 4C). However, GHF and NVP-BEZ235 could broadly block all three kinases in AKT/mTOR/S6K1 signaling axis compared with SH-5 and rapamycin, thereby indicating that GHF can regulate multiple molecular targets and signaling pathways in PC-3 cells.
Akt signaling through mTOR is important for oncogenesis; it protects cancer cells from apoptosis and drug resistance in vivo.55 Thus, this implies that suppression of AKT/mTOR/S6K activation by GHF could facilitate apoptosis. Activated AKT exerts anti-apoptotic effects, positively regulates NF-κB transcription, modulates angiogenesis, promotes tumor invasion/metastasis, and antagonizes cell cycle arrest.56 The down-regulation of cyclin D1 expression by GHF correlated with suppression of the accumulation of cells in the G1 phase of the cell cycle, which is consistent with the requirement of cells for cyclin D1. p70S6K has been also shown to be required for cell growth and G1 cell cycle progression.57 Because Bcl-2 and Bcl-xL differentially protect human prostate cancer cells from induction of apoptosis,58 its down-regulation could contribute to the ability of GHF to induce apoptosis in the cells. Zhang et al.59 demonstrated that survivin mediates resistance to anti-androgen therapy in prostate cancer. We further observed that GHF dramatically suppressed constitutive survivin expression, suggesting that GHF is a novel strategy for enhancing sensitivity to androgen ablation therapy. Consistent with this evidence, we found that GHF could clearly suppress VEGF expression at a lower concentration of 50 μg/mL. Although we do not know whether GHF inhibits endothelial cell migration and invasion, it is possible that the suppression of VEGF is the potential link for inhibition of angiogenesis by GHF.
To prove the inhibition of signaling with MAPKs is responsible for the anticancer activity of GHF, we determined whether GHF suppresses the phosphorylation of ERK, JNK, and p38 in PC-3 cells. We found that GHF suppressed the constitutive activation of ERK, JNK, and p38 without affecting the expression of the kinase protein (Fig. 4D). These results suggest that the inhibition of signaling with MAPKs also plays a critical role in mediating the anticancer activities of GHF in PC-3 cells.
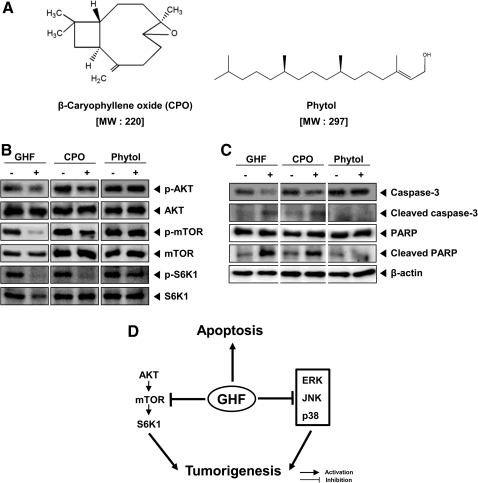
Gas chromatography–mass spectrometry analysis revealed the presence of terpenes, including β-eudesmol (11.98%), α-copaene (7.97%), phytol (7.95%), α-patchoulene (3.76%), β-caryophyllene oxide (CPO) (3.63%), caryophylla-3(15),7(14)-dien-6-ol (2.68%), (E)-methyl isoeugenol (1.90%), and α-terpineol (1.76%), and some hydrocarbons, including hexadecane (1.33%) and octadecane (1.23%). The major compound, β-eudesmol, was previously isolated from several plants60,61 and was shown to have potent anti-inflammatory60 and antitumor activities.61 In addition, there are a few more terpene compounds such as α-copaene and phytol that were also shown to have anti-inflammatory activity62 and an anti-scratching behavioral effect,63 respectively. Therefore, the significant anti-inflammatory activity exhibited by GHF may be due to each of these compounds—β-eudesmol, α-copaene, and phytol—or it might be a combination of others or all phytochemicals present in the fraction. It is interesting that we also observed that CPO clearly suppressed the constitutive activation of AKT/mTOR/S6K1 and induced apoptosis through caspase-3 activation, but not phytol (Fig. 5A–C). These results suggest that CPO is potentially involved in regulating the pathway.
FIG. 5.
GHF and β-caryophyllene oxide (CPO) suppressed the constitutive activation of AKT/mTOR/S6K1 and induced apoptosis. (A) Chemical structures of CPO and phytol. MW, molecular weight. (B) PC-3 cells were treated with GHF (150 μg/mL), CPO (50 μM), or phytol (50 μM) for 6 h and then analyzed by western blot analysis using antibodies against p-AKT (Ser473) and AKT, p-mTOR (Ser2448) and mTOR, and p-S6K1 (Thr 421/Ser424) and S6K1. (C) PC-3 cells were treated with GHF (150 μg/mL), CPO (50 μM), or phytol (50 μM) for 6 h and then analyzed by western blot analysis using antibodies against caspase-3 and PARP. An anti-β-actin antibody was used as a loading control. (D) Schematic diagram showing the effects of GHF on phosphatidylinositol 3-kinase/AKT/mTOR/S6K1 and mitogen-activated protein kinase signaling pathways and apoptosis.
When these results are taken together, GHF specifically suppresses AKT/mTOR/S6K kinase signaling and leads to the induction of apoptosis through the down-modulation of proteins that mediate tumor cell survival, proliferation, metastasis, and angiogenesis in human prostate cancer cells. Because of the lack of any known toxicity, guava leaves should be further explored for indentifying its active constituents and anticancer potential in a wide variety of tumor cells. Also, studies in animals are required to validate these findings.
Acknowledgments
We thank Prof. Gautam Sethi (National University of Singapore) and Mr. Keith Grainger for their review of and feedback on the article. This work was supported by a Korea Science and Engineering Foundation grant (2011-0006220) funded by the Korean Ministry of Education, Science and Technology.
Author Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Goncalves FA. Andrade Neto M. Bezerra JN, et al. Antibacterial activity of guava, Psidium guajava Linnaeus, leaf extracts on diarrhea-causing enteric bacteria isolated from Seabob shrimp, Xiphopenaeus kroyeri (Heller) Rev Inst Med Trop Sao Paulo. 2008;50:11–15. doi: 10.1590/s0036-46652008000100003. [DOI] [PubMed] [Google Scholar]
- 2.Lozoya X. Reyes-Morales H. Chavez-Soto MA. Martinez-Garcia Mdel C. Soto-Gonzalez Y. Doubova SV. Intestinal anti-spasmodic effect of a phytodrug of Psidium guajava folia in the treatment of acute diarrheic disease. J Ethnopharmacol. 2002;83:19–24. doi: 10.1016/s0378-8741(02)00185-x. [DOI] [PubMed] [Google Scholar]
- 3.Deguchi Y. Miyazaki K. Anti-hyperglycemic and anti-hyperlipidemic effects of guava leaf extract. Nutr Metab (Lond) 2010;7:9. doi: 10.1186/1743-7075-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qadan F. Thewaini AJ. Ali DA. Afifi R. Elkhawad A. Matalka KZ. The antimicrobial activities of Psidium guajava and Juglans regia leaf extracts to acne-developing organisms. Am J Chin Med. 2005;33:197–204. doi: 10.1142/S0192415X05002783. [DOI] [PubMed] [Google Scholar]
- 5.Steinhaus M. Sinuco D. Polster J. Osorio C. Schieberle P. Characterization of the key aroma compounds in pink guava (Psidium guajava L.) by means of aroma re-engineering experiments and omission tests. J Agric Food Chem. 2009;57:2882–2888. doi: 10.1021/jf803728n. [DOI] [PubMed] [Google Scholar]
- 6.Jaiarj P. Khoohaswan P. Wongkrajang Y, et al. Anticough and antimicrobial activities of Psidium guajava Linn. leaf extract. J Ethnopharmacol. 1999;67:203–212. doi: 10.1016/s0378-8741(99)00022-7. [DOI] [PubMed] [Google Scholar]
- 7.Lutterodt GD. Maleque A. Effects on mice locomotor activity of a narcotic-like principle from Psidium guajava leaves. J Ethnopharmacol. 1988;24:219–231. doi: 10.1016/0378-8741(88)90155-9. [DOI] [PubMed] [Google Scholar]
- 8.Osman AM. Younes MG. Sheta AE. Triterpenoids of the leaves of Psidium guaijava. Phytochemistry. 1974;13:2015–2016. [Google Scholar]
- 9.Prabu GR. Gnanamani A. Sadulla S. Guaijaverin—plant flavonoid as potential antiplaque agent against Streptococcus mutans. J Appl Microbiol. 2006;101:487–495. doi: 10.1111/j.1365-2672.2006.02912.x. [DOI] [PubMed] [Google Scholar]
- 10.Li J. Chen F. Luo J. [GC-MS analysis of essential oil from the leaves of Psidium guajava] Zhong Yao Cai. 1999;22:78–80. [PubMed] [Google Scholar]
- 11.Tanaka T. Ishida N. Ishimatsu M. Nonaka G. Nishioka I. Tannins and related compounds. CXVI. Six new complex tannins, guajavins, psidinins and psiguavin from the bark of Psidium guajava L. Chem Pharm Bull. 1992;40:2092–2098. [Google Scholar]
- 12.Choi SY. Hwang JH. Park SY, et al. Fermented guava leaf extract inhibits LPS-induced COX-2 and iNOS expression in mouse macrophage cells by inhibition of transcription factor NF-kappaB. Phytother Res. 2008;22:1030–1034. doi: 10.1002/ptr.2419. [DOI] [PubMed] [Google Scholar]
- 13.Akinola OB. Oladosu OS. Dosumu OO. Spermatoprotective activity of the leaf extract of Psidium guajava Linn. Niger Postgrad Med J. 2007;14:273–276. [PubMed] [Google Scholar]
- 14.Manosroi J. Dhumtanom P. Manosroi A. Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett. 2006;235:114–120. doi: 10.1016/j.canlet.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Chen KC. Hsieh CL. Huang KD. Ker YB. Chyau CC. Peng RY. Anticancer activity of rhamnoallosan against DU-145 cells is kinetically complementary to coexisting polyphenolics in Psidium guajava budding leaves. J Agric Food Chem. 2009;57:6114–6122. doi: 10.1021/jf901268w. [DOI] [PubMed] [Google Scholar]
- 16.Chen KC. Peng CC. Chiu WT, et al. Action mechanism and signal pathways of Psidium guajava L. aqueous extract in killing prostate cancer LNCaP cells. Nutr Cancer. 2010;62:260–270. doi: 10.1080/01635580903407130. [DOI] [PubMed] [Google Scholar]
- 17.Kawakami Y. Nakamura T. Hosokawa T, et al. Antiproliferative activity of guava leaf extract via inhibition of prostaglandin endoperoxide H synthase isoforms. Prostaglandins Leukot Essent Fatty Acids. 2009;80:239–245. doi: 10.1016/j.plefa.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh CL. Huang CN. Lin YC. Peng RY. Molecular action mechanism against apoptosis by aqueous extract from guava budding leaves elucidated with human umbilical vein endothelial cell (HUVEC) model. J Agric Food Chem. 2007;55:8523–8533. doi: 10.1021/jf071858b. [DOI] [PubMed] [Google Scholar]
- 19.Edwards J. Bartlett JM. The androgen receptor and signal-transduction pathways in hormone-refractory prostate cancer. Part 1: Modifications to the androgen receptor. BJU Int. 2005;95:1320–1326. doi: 10.1111/j.1464-410X.2005.05526.x. [DOI] [PubMed] [Google Scholar]
- 20.Petrylak D. Therapeutic options in androgen-independent prostate cancer: building on docetaxel. BJU Int. 2005;96(Suppl 2):41–46. doi: 10.1111/j.1464-410X.2005.05946.x. [DOI] [PubMed] [Google Scholar]
- 21.Shaw GL. Wilson P. Cuzick J, et al. International study into the use of intermittent hormone therapy in the treatment of carcinoma of the prostate: a meta-analysis of 1446 patients. BJU Int. 2007;99:1056–1065. doi: 10.1111/j.1464-410X.2007.06770.x. [DOI] [PubMed] [Google Scholar]
- 22.Martin GS. Cell signaling and cancer. Cancer Cell. 2003;4:167–174. doi: 10.1016/s1535-6108(03)00216-2. [DOI] [PubMed] [Google Scholar]
- 23.Yang L. Xie S. Jamaluddin MS, et al. Induction of androgen receptor expression by phosphatidylinositol 3-kinase/Akt downstream substrate, FOXO3a, and their roles in apoptosis of LNCaP prostate cancer cells. J Biol Chem. 2005;280:33558–33565. doi: 10.1074/jbc.M504461200. [DOI] [PubMed] [Google Scholar]
- 24.Murillo H. Huang H. Schmidt LJ. Smith DI. Tindall DJ. Role of PI3K signaling in survival and progression of LNCaP prostate cancer cells to the androgen refractory state. Endocrinology. 2001;142:4795–4805. doi: 10.1210/endo.142.11.8467. [DOI] [PubMed] [Google Scholar]
- 25.Majumder PK. Febbo PG. Bikoff R, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 26.Hudson CC. Liu M. Chiang GG, et al. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Poel HG. Mammalian target of rapamycin and 3-phosphatidylinositol 3-kinase pathway inhibition enhances growth inhibition of transforming growth factor-beta1 in prostate cancer cells. J Urol. 2004;172:1333–1337. doi: 10.1097/01.ju.0000138829.97838.19. [DOI] [PubMed] [Google Scholar]
- 28.Baldo P. Cecco S. Giacomin E. Lazzarini R. Ros B. Marastoni S. mTOR pathway and mTOR inhibitors as agents for cancer therapy. Curr Cancer Drug Targets. 2008;8:647–665. doi: 10.2174/156800908786733513. [DOI] [PubMed] [Google Scholar]
- 29.Burnett PE. Barrow RK. Cohen NA. Snyder SH. Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA. 1998;95:1432–1437. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isotani S. Hara K. Tokunaga C. Inoue H. Avruch J. Yonezawa K. Immunopurified mammalian target of rapamycin phosphorylates and activates p70 S6 kinase alpha in vitro. J Biol Chem. 1999;274:34493–34498. doi: 10.1074/jbc.274.48.34493. [DOI] [PubMed] [Google Scholar]
- 31.Westphal RS. Coffee RL., Jr Marotta A. Pelech SL. Wadzinski BE. Identification of kinase-phosphatase signaling modules composed of p70 S6 kinase-protein phosphatase 2A (PP2A) and p21-activated kinase-PP2A. J Biol Chem. 1999;274:687–692. doi: 10.1074/jbc.274.2.687. [DOI] [PubMed] [Google Scholar]
- 32.Brown EJ. Beal PA. Keith CT. Chen J. Shin TB. Schreiber SL. Control of p70 s6 kinase by kinase activity of FRAP in vivo. Nature. 1995;377:441–446. doi: 10.1038/377441a0. [DOI] [PubMed] [Google Scholar]
- 33.Harada H. Andersen JS. Mann M. Terada N. Korsmeyer SJ. p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc Natl Acad Sci USA. 2001;98:9666–9670. doi: 10.1073/pnas.171301998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao N. Zhang Z. Jiang BH. Shi X. Role of PI3K/AKT/mTOR signaling in the cell cycle progression of human prostate cancer. Biochem Biophys Res Commun. 2003;310:1124–1132. doi: 10.1016/j.bbrc.2003.09.132. [DOI] [PubMed] [Google Scholar]
- 35.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Kondjoyan N. Berdague JL. A Compilation of Relative Retention Indices for the Analysis of Aromatic Compounds. Laboratoire Flaveur Ed., INRA; Clermont-Ferrand, France: 1996. [Google Scholar]
- 37.Acree TE. Ahn H. Flavornet. Geneva: Cornell University; 1997. [Google Scholar]
- 38.Kluck RM. Bossy-Wetzel E. Green DR. Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 39.Hockenbery D. Nunez G. Milliman C. Schreiber RD. Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 40.Huang H. Joazeiro CA. Bonfoco E. Kamada S. Leverson JD. Hunter T. The inhibitor of apoptosis, cIAP2, functions as a ubiquitin-protein ligase and promotes in vitro monoubiquitination of caspases 3 and 7. J Biol Chem. 2000;275:26661–26664. doi: 10.1074/jbc.C000199200. [DOI] [PubMed] [Google Scholar]
- 41.Hoffman WH. Biade S. Zilfou JT. Chen J. Murphy M. Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. J Biol Chem. 2002;277:3247–3257. doi: 10.1074/jbc.M106643200. [DOI] [PubMed] [Google Scholar]
- 42.Earnshaw WC. Martins LM. Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 43.Slee EA. Harte MT. Kluck RM, et al. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9–dependent manner. J Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Steenbrugge GJ. van Uffelen CJ. Bolt J. Schroder FH. The human prostatic cancer cell line LNCaP and its derived sublines: an in vitro model for the study of androgen sensitivity. J Steroid Biochem Mol Biol. 1991;40:207–214. doi: 10.1016/0960-0760(91)90184-7. [DOI] [PubMed] [Google Scholar]
- 45.Madrid LV. Mayo MW. Reuther JY. Baldwin AS., Jr Akt stimulates the transactivation potential of the RelA/p65 subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 2001;276:18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 46.Lee JY. Ye J. Gao Z, et al. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- 47.Ahn KS. Sethi G. Aggarwal BB. Nuclear factor-kappa B: from clone to clinic. Curr Mol Med. 2007;7:619–637. doi: 10.2174/156652407782564363. [DOI] [PubMed] [Google Scholar]
- 48.Strimpakos AS. Karapanagiotou EM. Saif MW. Syrigos KN. The role of mTOR in the management of solid tumors: an overview. Cancer Treat Rev. 2009;35:148–159. doi: 10.1016/j.ctrv.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 49.Inoki K. Corradetti MN. Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 50.DeBerardinis RJ. Lum JJ. Hatzivassiliou G. Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Campone M. Levy V. Bourbouloux E, et al. Safety and pharmacokinetics of paclitaxel and the oral mTOR inhibitor everolimus in advanced solid tumours. Br J Cancer. 2009;100:315–321. doi: 10.1038/sj.bjc.6604851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qian Y. Corum L. Meng Q, et al. PI3K induced actin filament remodeling through Akt and p70S6K1: implication of essential role in cell migration. Am J Physiol Cell Physiol. 2004;286:C153–C163. doi: 10.1152/ajpcell.00142.2003. [DOI] [PubMed] [Google Scholar]
- 53.Qiu Q. Yang M. Tsang BK. Gruslin A. Both mitogen-activated protein kinase and phosphatidylinositol 3-kinase signalling are required in epidermal growth factor-induced human trophoblast migration. Mol Hum Reprod. 2004;10:677–684. doi: 10.1093/molehr/gah088. [DOI] [PubMed] [Google Scholar]
- 54.Berven LA. Willard FS. Crouch MF. Role of the p70(S6K) pathway in regulating the actin cytoskeleton and cell migration. Exp Cell Res. 2004;296:183–195. doi: 10.1016/j.yexcr.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 55.Wendel HG. De Stanchina E. Fridman JS, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 56.Testa JR. Bellacosa A. AKT plays a central role in tumorigenesis. Proc Natl Acad Sci USA. 2001;98:10983–10985. doi: 10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pullen N. Thomas G. The modular phosphorylation and activation of p70s6k. FEBS Lett. 1997;410:78–82. doi: 10.1016/s0014-5793(97)00323-2. [DOI] [PubMed] [Google Scholar]
- 58.Lebedeva IV. Sarkar D. Su ZZ, et al. Bcl-2 and Bcl-xL differentially protect human prostate cancer cells from induction of apoptosis by melanoma differentiation associated gene-7, mda-7/IL-24. Oncogene. 2003;22:8758–8773. doi: 10.1038/sj.onc.1206891. [DOI] [PubMed] [Google Scholar]
- 59.Zhang M. Latham DE. Delaney MA. Chakravarti A. Survivin mediates resistance to antiandrogen therapy in prostate cancer. Oncogene. 2005;24:2474–2482. doi: 10.1038/sj.onc.1208490. [DOI] [PubMed] [Google Scholar]
- 60.Ho CL. Wang EI. Tseng YH, et al. Composition and antimicrobial activity of the leaf and twig oils of Litsea mushaensis and L. linii from Taiwan. Nat Prod Commun. 2010;5:1823–1828. [PubMed] [Google Scholar]
- 61.Ali NA. Marongiu B. Piras A, et al. Essential oil composition of leaves of Stachys yemenensis obtained by supercritical CO. Nat Prod Res. 2010;24:1823–1829. doi: 10.1080/14786411003754272. [DOI] [PubMed] [Google Scholar]
- 62.Jordan MJ. Margaria CA. Shaw PE. Goodner KL. Volatile components and aroma active compounds in aqueous essence and fresh pink guava fruit puree (Psidium guajava L.) by GC-MS and multidimensional GC/GC-O. J Agric Food Chem. 2003;51:1421–1426. doi: 10.1021/jf020765l. [DOI] [PubMed] [Google Scholar]
- 63.Paterson WG. Barkun AN. Hopman WM, et al. Wait times for gastroenterology consultation in Canada: the patients' perspective. Can J Gastroenterol. 2010;24:28–32. doi: 10.1155/2010/912970. [DOI] [PMC free article] [PubMed] [Google Scholar]