Abstract
Apolipoprotein E (APOE) genotype is the major genetic risk factor for Alzheimer disease (AD); the ε4 allele increases risk and the ε2 allele is protective. In the central nervous system (CNS), apoE is produced by glial cells, is present in high-density-like lipoproteins, interacts with several receptors that are members of the low-density lipoprotein receptor (LDLR) family, and is a protein that binds to the amyloid-β (Aβ) peptide. There are a variety of mechanisms by which apoE isoform may influence risk for AD. There is substantial evidence that differential effects of apoE isoform on AD risk are influenced by the ability of apoE to affect Aβ aggregation and clearance in the brain. Other mechanisms are also likely to play a role in the ability of apoE to influence CNS function as well as AD, including effects on synaptic plasticity, cell signaling, lipid transport and metabolism, and neuroinflammation. ApoE receptors, including LDLRs, Apoer2, very low-density lipoprotein receptors (VLDLRs), and lipoprotein receptor-related protein 1 (LRP1) appear to influence both the CNS effects of apoE as well as Aβ metabolism and toxicity. Therapeutic strategies based on apoE and apoE receptors may include influencing apoE/Aβ interactions, apoE structure, apoE lipidation, LDLR receptor family member function, and signaling. Understanding the normal and disease-related biology connecting apoE, apoE receptors, and AD is likely to provide novel insights into AD pathogenesis and treatment.
The ε4 allele of the apolipoprotein E gene is a strong genetic risk factor for late-onset Alzheimer disease. It may exert its effect by altering amyloid-β accumulation in the brain.
Alzheimer disease (AD), specifically the late-onset form of AD (LOAD), is the most common cause of dementia in individuals older than 60 years of age. Although mutations in the genes PS1, PS2, and APP cause less common forms of early-onset, autosomal dominant familial AD (FAD), these cases represent <1% of AD. In addition to the genes that cause FAD, LOAD also has a strong genetic component. Although several susceptibility genes for AD have been reported, by far the strongest genetic risk factor for LOAD is apolipoprotein E (APOE) genotype, with the ε4 allele being an AD risk factor and the ε2 allele being protective relative to the prevalent ε3 allele (Corder et al. 1993; Strittmatter et al. 1993a). Strong evidence suggests a major mechanism by which apoE influences AD and cerebral amyloid angiopathy (CAA) is via its effects on Aβ metabolism (Kim et al. 2009a; Castellano et al. 2011). Current understanding of apoE biology in the CNS and how apoE/Aβ interactions are relevant to AD will be reviewed in the first section. There are several apoE receptors that are members of the LDLR family (Fig. 1). Some of these receptors, such as LDLR and LRP1, influence apoE levels (Fryer et al. 2005a; Liu et al. 2007). Others, such as Apoer2 and VLDLR, although apoE receptors, are also receptors for other ligands such as the neuromodulatory signaling protein Reelin, which plays an important role in neurodevelopment and synpatic function. These receptors are involved in neural signaling and tau phosphorylation, and there is evidence that apoE can counteract some of the neurotoxicity caused by Aβ. Apoer2 and VLDLR will be reviewed in the second section. LDLR and LRP1 are important receptors for apoE in the brain that regulate CNS apoE levels. Although LDLR has no known ligand other than apoE in the CNS, LRP is somewhat unique in that it has multiple ligands, binds to both APP and Aβ, and influences APP and Aβ metabolism. LDLR and LRP1 will be reviewed in the third section. Although there are currently no apoE-based therapies for AD, given the effects of apoE and apoE receptors on both Aβ and CNS development and function, a variety of apoE/apoE receptor-based approaches will be discussed.
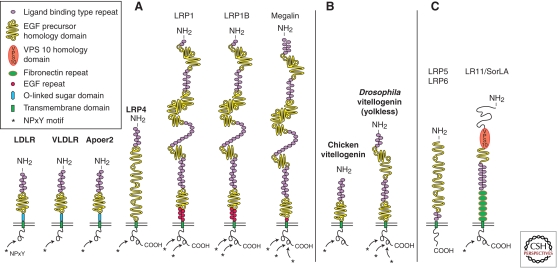
Figure 1.
The low-density lipoprotein (LDL) receptor gene family. (A) The core LDL receptor gene family as it exists in mammalian species. These family members are characterized by one or more ligand-binding domains, epidermal growth factor (EGF), homology domains consisting of EGF repeats and YWTD propeller (β-propeller) domains involved in pH-dependent release of ligands in the endosomes, a single transmembrane domain and a cytoplasmic tail containing at least one NPxY motif. The latter represents both the endocytosis signal as well as a binding site for adaptor proteins linking the receptor to intracellular signaling pathways. Furthermore, LDLR, VLDLR, and Apoer2 carry an O-linked sugar domain. (B) Equivalent receptors that are structurally and functionally distinct family members in nonmammalian species. (C) A subgroup of functionally important, but more distantly related family members that share some, but not all, of the structural requirements of the “core members.” In addition, they could also contain domains, e.g., vacuolar protein sorting (VPS) domains, which are not present in the core family. (From Dieckmann et al. 2010; reprinted, with permission, from Walter de Gruyter GmbH © 2010.)
ApoE: POTENTIAL ROLE IN AD
Neurobiology of ApoE
The human apoE protein is a 299 amino acid glycoprotein that is expressed by several cell types, but with highest expression in the liver and in the CNS (Mahley 1988). In the brain, apoE is expressed predominantly by astrocytes but also by microglia (Fig. 2) (Pitas et al. 1987; Grehan et al. 2001). Under certain conditions, such as after excitotoxic injury, some neurons appear to be able to synthesize apoE (Xu et al. 1999; Xu et al. 2006). Under physiological conditions, apoE is present in lipoprotein particles (Fig. 2). Although it is present in lipoproteins of different size classes in plasma, in the CNS, it is the most abundantly produced apoprotein and is secreted by glial cells in nascent high-density lipoprotein (HDL)-like particles (Pitas et al. 1987; DeMattos et al. 2001) that are discoidal in shape and contain phospholipids and cholesterol. ApoE is also present in cerebrospinal fluid (CSF) at a concentration of ∼5 µg/ml, in spherical particles that are similar to glial-secreted HDL, except that they also contain a cholesteryl ester core (LaDu et al. 1998). Although apoE-containing lipoproteins may play a role in reverse cholesterol transport as well as in cholesterol and lipid delivery, their role in CNS lipid and cholesterol homeostasis is not yet clearly defined. As in the periphery, apoE functions as a ligand in receptor-mediated endocytosis of lipoprotein particles in the CNS. In vitro studies have shown that cholesterol released from apoE-containing lipoprotein particles is used to support synaptogenesis (Mauch et al. 2001) and the maintenance of synaptic connections (Pfrieger 2003). Although there is some in vitro (Nathan et al. 1994; Holtzman et al. 1995) and in vivo (Masliah et al. 1995; Poirier 2003) data suggesting that apoE can play a role in neuronal sprouting after injury, whether apoE plays a major role in supporting synaptogenesis and maintenance of synaptic connections in vivo in the uninjured brain has not yet been proven. For example, several studies have shown that the brain of apoE knockout mice, for the most part, appears normal in the absence of injury (Anderson et al. 1998; Fagan et al. 1998). Moreover, no overt cognitive defects have been reported in humans with genetic ApoE deficiency. In addition to apoE, several other apolipolipoproteins are present in the CNS, the most abundant being apoAI and apoJ, also called clusterin. ApoE in the CNS is derived from the CNS; the same appears to be true for clusterin. In contrast, apoAI in CNS is derived from the periphery (Sorci-Thomas et al. 1988). Whether apoAI and clusterin play a role in CNS lipid metabolism, or in normal brain function, is not clear. Genetic deficiency of either protein in humans or mice does not result in an obvious CNS phenotype (Schaefer et al. 1982; McLaughlin et al. 2000). Interestingly, single-nucleotide polymorphisms in clusterin have been shown to be a risk factor for AD (Harold et al. 2009; Lambert et al. 2009). The mechanism for this is unclear, although animal model data have shown that clusterin strongly influences Aβ aggregation and toxicity in vivo (DeMattos et al. 2002; DeMattos et al. 2004).
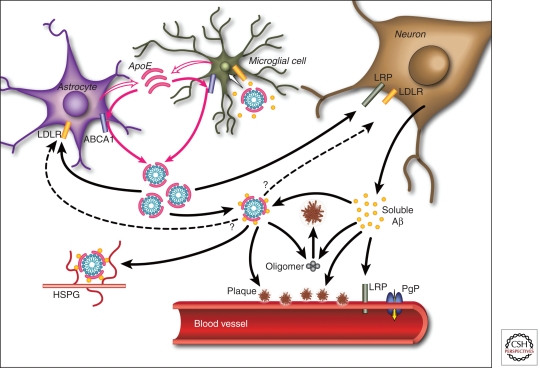
Figure 2.
Pathways by which apoE and Aβ interact in the brain. ApoE is primarily produced by both astrocytes and microglia and is subsequently lipidated by ABCA1 to form lipoprotein particles. In the extracellular space, lipidated apoE binds to soluble Aβ in an isoform-dependent pattern (E2 > E3 > E4) and influences the formation of parenchymal amyloid plaques and transport of Aβ within the CNS. ApoE is endocytosed into various cell types within the brain by different members of the LDL receptor family, including LDLR and LRP1. ApoE may also facilitate the cellular uptake of Aβ through the endocytosis of a complex of apoE-containing lipoprotein particles bound to Aβ in a manner that likely depends on the isoforms and its level of lipidation. Furthermore, apoE has been shown to directly enhance both the degradation of Aβ within microglial cells and the ability of astrocytes to clear diffuse Aβ deposits (Koistinaho et al. 2004; Jiang et al. 2008). Aβ associated with apoE-containing lipoprotein particles may also be retained within the CNS through their binding to heparin sulfate proteoglycan (HSPG) moieties present in the extracellular space (Mahley and Rall 2000). At the blood–brain barrier (BBB), soluble Aβ is predominantly transported from the interstitial fluid into the bloodstream via LRP1 and P-glycoprotein (Cirrito et al. 2005; Zlokovic 2008). ApoE has been shown to slow the transport of Aβ across the BBB in an isoform-dependent manner (E4 > E3 > E2) (Bell et al. 2007; Ito et al. 2007; Deane et al. 2008). In addition, apoE can influence the pathogenesis of CAA in an amyloid protein precursor (APP)-transgenic mouse model, with apoE4 increasing the amount of vascular plaques in comparison to apoE3 (Fryer et al. 2005b). (From Kim et al. 2009; reprinted, with permission, from Elsevier © 2009.)
Genetic, Clinical, and Biomarker Observations on Relationship of ApoE and AD
The human apoE gene contains several single-nucleotide polymorphisms (SNPs) distributed across the gene (Nickerson et al. 2000). The most common three SNPs lead to changes in the coding sequence and result in the three common isoforms of apoE: apoE2 (cys112, cys158), apoE3 (cys112, arg158), and apoE4 (arg112, arg158). Although the three common isoforms differ by only one or two amino acids at residues 112 or 158, these differences alter apoE structure and function (Mahley et al. 2006). In regard to the connection between apoE and AD, apoE was found to colocalize with amyloid plaques in the early 1990s (Namba et al. 1991; Wisniewski and Frangione 1992). After that, the ε4 allele of the APOE gene was discovered to be a strong genetic risk factor for AD (Corder et al. 1993; Strittmatter et al. 1993a). Since then, numerous studies have confirmed that the ε4 allele is the strongest genetic risk factor for both AD and CAA, or a combination of both disorders (Schmechel et al. 1993; Greenberg et al. 1995; Bertram et al. 2007). As compared to individuals with no ε4 alleles, the increased risk for AD is approximately threefold in people with one ε4 allele and ∼ 12-fold in those with two ε4 alleles. The odds ratio for ε4 versus ε3 alleles by meta-analysis of multiple studies is 3.68 as of June 2011 (www.alzgene.org). Importantly, the ε2 allele of apoE is associated with a lower risk for AD (Corder et al. 1994; Farrer et al. 1997) with an odds ratio of ε2 versus ε3 of 0.62 as of June 2011 (www.alzgene.org).
Evidence of a Key Role for ApoE on Aβ Metabolism in AD Pathogenesis
In vitro and in vivo data including data in humans and animal models suggests that the physical interaction of apoE with Aβ plays an important role in AD and CAA pathogenesis (Fig. 1). It was first proposed that apoE was an Aβ-binding protein in the brain that induces a pathological β-sheet conformational change in Aβ (Wisniewski and Frangione 1992). Pathological studies showed a positive correlation between plaque density and ε4 allele dose in AD patients at autopsy (Rebeck et al. 1993; Schmechel et al. 1993). Although some studies reported conflicting findings (Benjamin et al. 1995; Heinonen et al. 1995), a large autopsy study strongly suggested that ε4 dosage is associated with increased neuritic plaques in AD (Tiraboschi et al. 2004). If the effect of apoE4 is to accelerate the average onset of Aβ deposition in the brain, an expectation would be that middle-aged individuals at risk to develop AD in the future who are still cogntively normal would have larger amounts of Aβ deposition in the brain. This has now been shown to be the case as evidenced by amyloid-imaging studies with Pittsburgh compound B as well as CSF studies using CSF Aβ42 in which a decrease has been shown to indicate brain amyloid deposition. Cognitively normal apoE4-positive middle-aged and elderly individuals are much more likely to have brain amyloid (Reiman et al. 2009; Morris et al. 2010) and low CSF Aβ42 (Sunderland et al. 2004; Morris et al. 2010) than apoE4-negative individuals. Further, apoE2-positive individuals rarely develop fibrillar Aβ as defined by a positive amyloid-imaging scan (Morris et al. 2010).
Whereas human data supports the idea that apoE isoforms result in differential susceptibilty to Aβ aggregation in the brain, animal studies utilizing genetically modified mice that develop Aβ deposition and express human apoE isoforms show more directly that human apoE isoforms have a strong effect on the time of onset of Aβ aggregation as well as the amount, location, and conformation of Aβ in the brain. Early studies with APP-transgenic (Tg) mice that develop Aβ deposition in the brain (PDAPP and Tg2576 models) showed that when these mice were crossed with apoE−/− mice, there was less Aβ deposition and a virtual abolishment of true amyloid plaques, plaque-associated neuritic dystrophy, CAA, and CAA-associated microhemorrhage in the absence of apoE (Bales et al. 1997; Bales et al. 1999; Holtzman et al. 2000b; Fryer et al. 2003). In addition, the anatomical pattern of Aβ deposition differs in the absence of apoE (Holtzman et al. 2000a; Irizarry et al. 2000). The expression of human apoE isoforms in either PDAPP or Tg2576 Tg mice resulted in a marked delay in the deposition of Aβ and formation of neuritic plaques, compared with APP Tg mice expressing no apoE or mouse apoE (Fagan et al. 2002; Fryer et al. 2005b). Importantly, expression of human apoE isoforms in APP Tg mice results in an isoform-specific effect on the amount of Aβ accumulation as well as true amyloid deposits (E4 > E3 > E2) (Holtzman et al. 2000a; Fagan et al. 2002; Fryer et al. 2005b). In addition to the isoform-specific effects of human apoE on parenchymal Aβ pathology, crossing human apoE knockin mice to Tg2576 mice resulted in a relative shift of Aβ deposition from the brain parenchyma to arterioles in the form of CAA in apoE4 expressing mice relative to apoE3 or mouse apoE (Fryer et al. 2005b). A similar effect of apoE4 predisposing to CAA is seen in humans. These data strongly suggest that understanding the in vivo mechanisms underlying the apoE isoform-mediated difference in Aβ accumulation is critical for relating these findings to the pathogenesis of AD.
The underlying mechanism underlying how apoE influences Aβ aggregation and accumulation in the brain is on its way to being elucidated. In vitro and in vivo studies suggest that apoE may influence Aβ seeding and fibrillogenesis, as well as soluble Aβ clearance. Lipid-free and lipidated (physiological) forms of apoE can interact with Aβ in vitro (Strittmatter et al. 1993b; LaDu et al. 1994; Sanan et al. 1994; Aleshkov et al. 1997; Yang et al. 1997; Tokuda et al. 2000). Most studies show that the efficiency of complex formation between lipidated apoE and Aβ follows the order of apoE2 > apoE3 >> apoE4. The effect of apoE isoforms on Aβ aggregation has also been investigated extensively in vitro. Some studies show apoE causing greater fibrillization (E4 > E3 > E2) (Ma et al. 1994; Wisniewski et al. 1994; Castano et al. 1995), whereas others show that apoE inhibits fibrillization (Evans et al. 1994; Wood et al. 1996). Conflicting results between in vitro studies may be owing to the differences in apoE and Aβ preparations or other factors. Altering the lipidation state of apoE in the brain is associated with strong effects on Aβ fibrillization in vivo. ATP-binding cassette A1 (ABCA1) normally lipidates apoE in the brain. When APP Tg mice are crossed onto an Abca1−/− background, this decreases apoE lipidation and increases amyloid deposition (Hirsch-Reinshagen et al. 2005; Koldamova et al. 2005a; Wahrle et al. 2005), whereas increasing ABCA1 increases apoE lipidation and decreases amyloid deposition (Wahrle et al. 2008).
In addition to the effects of apoE on fibrillogenesis, there is evidence that apoE alters both the transport and clearance of soluble Aβ in the brain (Fig. 1). A recent study shows that apoE isoforms do not differentially influence Aβ production in vivo; however, apoE isoforms differentially affect Aβ clearance before Aβ deposition with E4 resulting in clearance that is slower than E3 and E2 (Castellano et al. 2011). These results suggest the difference in Aβ accumulation between apoE isoforms is likely because of isoform-specific differences in Aβ clearance. ApoE seems to play an important role in the clearance of Aβ through several possible mechanisms. ApoE-containing lipoprotein particles may sequester Aβ and modulate the cellular uptake of an apoE-Aβ complex by receptor-mediated endocytosis. Alternatively, apoE may modulate Aβ removal from the brain to the systemic circulation by transport across the blood–brain barrier. Data from in vitro studies support the idea that apoE facilitates the binding and internalization of soluble Aβ by cells or its clearance via enzymes such as neprilysn (Beffert et al. 1998; Yang et al. 1999; Cole and Ard 2000; Koistinaho et al. 2004; Jiang et al. 2008). Although in vitro studies suggest that apoE enhances cellular Aβ uptake and degradation (Kim et al. 2009a), there is in vivo evidence that apoE retards Aβ clearance from the brain (DeMattos et al. 2004; Bell et al. 2007; Deane et al. 2008), possibly via an effect at the blood–brain barrier (BBB) (Fig. 1) (Zlokovic 2008). More work is clearly needed to determine the exact role that apoE has in modifying brain Aβ clearance, the role of the BBB in the process, and whether isoform-specific effects exist.
Several key questions remain to be further addressed regarding the effect of apoE on Aβ. Whether it is better to increase or decrease human apoE levels (regardless of isoform) to reduce Aβ levels is still unanswered. Analyzing whether, and to what extent, altering human apoE level affects Aβ pathology will help determine whether targeting apoE levels may be a viable therapeutic option for influencing Aβ levels and toxicity, and ultimately treating AD.
ApoE RECEPTORS AND SYNAPTIC PLASTICITY
The strong association of ApoE4 with late-onset AD raised the possibility that ApoE is mediating its powerful effect on the average age of disease onset at least in part through the receptors to which it binds. These ApoE receptors include the core, as well as potentially several more distantly related members of the LDLR gene family (Fig. 1). LRP1 has been repeatedly, albeit weakly, associated with AD risk (Beffert et al. 1999; Vazquez-Higuera et al. 2009), and a coding polymorphism in the distantly related Wnt coreceptor LRP6 has also been implicated (De Ferrari et al. 2007). None of the other family members have so far been convincingly associated with AD by human genetic data. The absence of genetic association, however, does not preclude important roles for these multifunctional receptors in the molecular mechanisms that underlie the disease process. The very nature of their essential functions during the development of the embryo in general (Herz et al. 1992; Johnson et al. 2005; Dietrich et al. 2010; Karner et al. 2010), and the brain, in particular (Willnow et al. 1996; Trommsdorff et al. 1999; May et al. 2004; Boycott et al. 2005; Boycott et al. 2009), may occlude their participation in AD pathogenesis, which would manifest itself much later in life.
Mechanisms by which ApoE receptors may contribute to AD development and progression may include roles in the control of inflammation (Lillis et al. 2008; Zurhove et al. 2008), cholesterol metabolism (reviewed in Herz et al. 2009), neurogenesis (Gajera et al. 2010), or the generation and trafficking of APP and Aβ (reviewed in the third section). Other potential mechanisms by which ApoE receptors may promote neuronal survival (Beffert et al. 2006b) during aging involve signaling pathways that control microtubule and actin dynamics (Beffert et al. 2002; Assadi et al. 2003; Brich et al. 2003; Ohkubo et al. 2003; Chai et al. 2009; Forster et al. 2010; Rust et al. 2010), dendritogenesis (Niu et al. 2004), spine formation (Niu et al. 2008), glutamate receptor function and synaptic plasticity (Zhuo et al. 2000; Weeber et al. 2002; Beffert et al. 2005; Chen et al. 2005; D’Arcangelo 2005; Sinagra et al. 2005; Groc et al. 2007; Durakoglugil et al. 2009; Korwek et al. 2009; Chen et al. 2010), as well as learning and memory (reviewed in Herz and Beffert 2000; Herz and Chen 2006; Bu 2009; Herz 2009). In this section we will mainly focus on the role of the ApoE receptors Apoer2 and Vldlr and their ligand Reelin in these processes.
Molecular Basis of Signal Transduction by Neuronal ApoE Receptors
ApoE receptors contain only short cytoplasmic tails, which lack functional enzymatic domains through which many cell-surface receptors transmit extracellular signals into the cell. However, they harbor a variety of short conserved sequence stretches, such as the tetra-amino acid NPxY motif, which serve as docking sites for a wide array of cytoplasmic adaptor and scaffolding proteins (Trommsdorff et al. 1998; Gotthardt et al. 2000; Beffert et al. 2005; Hoe et al. 2006a; Hoe et al. 2006b). The receptors can also interact as coreceptors through their extracellular domains with other types of signaling proteins and modules, and thereby modulate their intrinsic activity (Boucher et al. 2002; Loukinova et al. 2002; Huang et al. 2003; Lillis et al. 2008; Zurhove et al. 2008), including that of the N-methyl-d-aspartate (NMDA) receptor (May et al. 2004; Beffert et al. 2005; Hoe et al. 2006b).
Apoer2 and Vldlr are a notable exception, inasmuch as they do not need to associate with another protein with intrinsic signal transduction activity to elicit an intracellular signal. Both receptors bind the large homo-oligomeric signaling protein Reelin with high affinity (D’Arcangelo et al. 1999; Hiesberger et al. 1999), resulting in their clustering at the plasma membrane (Strasser et al. 2004). The simultaneous interaction of the adapter protein Disabled 1 (Dab1) with NPxY motifs in their intracellular domains (ICDs) (Trommsdorff et al. 1998; Stolt et al. 2005) results in the progressive recruitment and transphosphorylation of Src family tyrosine kinases (SFKs) (Howell et al. 1997; Arnaud et al. 2003; Bock and Herz 2003). This in turn initiates a kinase casade inside the neuron, starting with the activation of phosphoinositide-3-kinase (PI3K), which subsequently activates protein kinase B (also known as Akt), and ending with the inhibition of glycogen synthase 3β (GSK3β) (Beffert et al. 2002), one of the primary kinases that phosphorylate the microtubule stabilizing protein tau on the same sites that are typically abnormally phosphorylated in the neurofibrillary tangles in the AD-afflicted brain.
Reelin signaling is essential for normal brain development by regulating a pathway that controls the migration and positioning of the neuronal cell bodies in their appropriate cortical layers of the neocortex and the cerebellum (Tissir and Goffinet 2003), as well as neuronal connectivity (Del Rio et al. 1997). Activation of SFKs is the “master switch” that is required for the initiation of all subsequent downstream signaling events, which are not limited to the control of GSK3β activity but also involve the regulation of Lis1-dependent nuclear translocation (Shu et al. 2004), n-cofilin-mediated actin reorganization (Chai et al. 2009; Frotscher 2010), and tyrosine phosphorylation of NMDA receptor subunits (Beffert et al. 2005; Chen et al. 2005).
Regulation of Tau Phosphorylation
Genetic disruption of any component of this Reelin-Apoer2/Vldlr-Dab1 signaling pathway in the mouse, i.e., loss-of-function mutations in the ligand, the receptors, or the adaptor protein, results in reduced phosphorylation of GSK3β on an inhibitory serine residue, which leads to disinhibition of the enzyme and hyperphosphorylation of tau (Hiesberger et al. 1999; Beffert et al. 2002; Brich et al. 2003; Ohkubo et al. 2003). High levels of tau phosphorylation disrupt neuronal vesicle transport by compromising microtubule stability (Mudher et al. 2004), and consequently lead to variable degrees of neuronal dysfunction and premature death of signaling defective mutant mice (Sheldon et al. 1997; Trommsdorff et al. 1999; Brich et al. 2003). Intriguingly, genetic deficiency of tau prevents APP/Aβ-induced cognitive defects as well as excitotoxicity in mice (Roberson et al. 2007), indicating that the presence of abnormally phosphorylated tau, rather than its functional loss, is the likely reason for the severe motor defects that cause the premature death in the Reelin pathway mutants. This is further supported by a series of recent studies that showed a broad effect of mislocalized, phosphorylated tau on the spinodendritic targeting of Fyn (Ittner et al. 2010) and of the scaffolding protein JIP1 (Ittner et al. 2010), as well as on the disruption of glutamate receptor trafficking and recycling (Hoover et al. 2010). Intriguingly, the tau-induced synaptic defects are prevented or reversed by reducing the tau levels (Roberson et al. 2011; Sydow et al. 2011).
Loss of LRP1 also results in increased GSK3β activity, at least in fibroblasts and adipocytes, as a result of a loss of autocrine Wnt5a expression (Terrand et al. 2009). Conditional LRP1 knockout mice, lacking LRP1 expression exclusively in postmitotic neurons, also display severe locomotor abnormalities (May et al. 2004), raising the possibility that these dysfunctions could also be caused in part by defective regulation of tau phosphorylation, although this has not been explored at the time of this writing.
Tau hyperphosphorylation in Reelin signaling defective animals on a mixed strain background is highly variable (Hiesberger et al. 1999) and strongly dependent on the background strains. This observation was exploited in an unbiased approach to map genetic modifiers of ApoE receptor/Dab1-dependent tau phosphorylation in the mouse (Brich et al. 2003). Surprisingly, the strongest modifier mapped to a narrow genomic region centered around APP on mouse chromosome 16, in addition to a suggestive quantitative trait in the vicinity of Presenilin 1 on chromosome 12. Together these findings add further support to a model in which ApoE receptors functionally interact with APP, Aβ, and tau to control the molecular mechanisms that underlie the pathogenesis of AD.
Regulation of Dendritic Spines, Glutamatergic Neurotransmission, and Synaptic Plasticity
Numerous independent studies and observations point toward a role for Reelin and ApoE receptors in the formation of neuronal connections (Del Rio et al. 1997; Borrell et al. 2007) and the generation of dendritic complexity (Trommsdorff et al. 1999; Costa et al. 2001; Niu et al. 2004; Matsuki et al. 2008; Hoe et al. 2009). The latter may, however, not be entirely dependent on Apoer2 and Vldlr (Chameau et al. 2009) and may also involve interactions with APP (Hoe et al. 2009). Reelin signaling also regulates dendritic spine morphology (Costa et al. 2001; Niu et al. 2008; Pujadas et al. 2010), which likely involves regulation of actin dynamics and the participation of n-cofilin (Chai et al. 2009; Rust et al. 2010). It activates LIM kinase (LIMK), which inhibits the actin-depolymerizing activity of n-cofilin. The dynamic remodeling of synaptic connections requires constant reorganization of actin filaments (Dillon and Goda 2005). Consequently, postnatal disruption of n-cofilin in mice leads to increased synapse density and enlargement of axospinous synapses (Rust et al. 2010) with defects in long-term potentiation (LTP) and long-term depression (LTD). Although synaptic AMPA receptor mobility is not affected, diffusion of extrasynaptic AMPA receptors is reduced owing to F-actin stabilization, preventing efficient egress of AMPA receptors from the synaptic into the extrasynaptic domain, and thus LTD, in the n-cofilin mutants. Similarly, Reelin has been shown to regulate surface mobility and synaptic residency of NMDA receptor NR2B subunits (Groc et al. 2007). It is thus required for NMDA receptor maturation (Sinagra et al. 2005) and for the maintenance of normal NR2A/B ratios (Campo et al. 2009).
These findings explain the profound effect of Reelin on glutamatergic neurotransmission and synaptic plasticity ex corpore (Weeber et al. 2002; Beffert et al. 2005; Beffert et al. 2006b; Qiu et al. 2006; Campo et al. 2009) and in vivo (Pujadas et al. 2010) (E Weeber, pers. comm.). Reelin potently increases LTP, which requires the presence of both receptors, Apoer2 and Vldlr (Weeber et al. 2002). This increase of synaptic plasticity is mediated by the effect Reelin has on NMDA and AMPA receptor trafficking and conductance, which determine the synaptic acitivity of these glutamate receptors (Qiu et al. 2006). It further requires the presence of a 59 amino acid insert encoded by an alternatively spliced exon in the cytoplasmic domain of Apoer2 (Beffert et al. 2005). Only when the insert is present can Apoer2 functionally couple with NMDA receptors and induce tyrosine phosphorylation of NR2 subunits in response to Reelin. Increased tyrosine phosphorylation of the NMDA receptor increases ion gating and reduces its endocytosis, thereby increasing NMDA receptor activity overall (Salter and Kalia 2004; Snyder et al. 2005). Intriguingly, differential splicing of this exon is regulated in a circadian, activity-driven manner (Beffert et al. 2005), suggesting that periodic variation of NMDA receptor activity by Reelin is physiologically significant for synapse function, learning, and memory (Beffert et al. 2002; Weeber et al. 2002; Beffert et al. 2005; D’Arcangelo 2005; Beffert et al. 2006b; Pujadas et al. 2010).
ApoE Receptors as Antagonists of Aβ-Induced Synaptic Suppression
Aβ1-42 oligomers are strong inducers of synaptic suppression, and several mechanisms have been proposed to explain this effect (Lambert et al. 1998; Walsh et al. 2002; Lacor et al. 2004; Snyder et al. 2005; Lesne et al. 2006; Townsend et al. 2006; Haass and Selkoe 2007; Shankar et al. 2007; Berman et al. 2008; Puzzo et al. 2008; Shankar et al. 2008; Nygaard and Strittmatter 2009; Gimbel et al. 2010; Palop and Mucke 2010; Renner et al. 2010; Ronicke et al. 2010) (see Mucke and Selkoe 2011). Synaptic suppression by the oligomers correlates with reduced NMDA receptor activity (Snyder et al. 2005; Shankar et al. 2007), which is caused by NMDA receptor dephosphorylation and accelerated endocytosis (Snyder et al. 2005). Snyder, Greengard, and colleagues (Snyder et al. 2005) proposed that this is mediated by the Aβ-mediated activation of phosphatases (STEP and calcineurin). The concomitant reduction of NMDA receptor activity induces dendritic spine loss and, intriguingly, this requires calcineurin, as well as the active, i.e., Ser3-unphosphorylated form of n-cofilin (Shankar et al. 2007). A dominant negative form of n-cofilin, in which Ser3 is replaced with a phosphomimetic amino acid (Ser3Asp), prevents oligomer-induced spine loss (Shankar et al. 2007).
Reelin induces the phosphorylation of n-cofilin at the inhibitory Ser3 residue, thereby promoting spine stability (Chai et al. 2009; Frotscher 2010). Moreover, Reelin signaling activates SFKs (Chen et al. 2005), which would directly oppose the activity of the phosphatases on the NMDA receptor (Snyder et al. 2005). This hypothesis was tested by measuring the effect of different Aβ preparations, including Aβ-containing extracts from human AD brain, on hippocampal synaptic plasticity, NMDA receptor tyrosine phosphorylation, and activity in response to Reelin (Durakoglugil et al. 2009). At low to intermediate, but not at unphysiologically high (>400 nM) Aβ concentrations, activation of Reelin signaling can completely prevent the synaptic suppression induced by the oligomers, suggesting that Reelin is a physiological mediator of neuroprotection. Importantly, ApoE4 strongly interferes with these synapse-enhancing functions of Reelin by sequestering ApoE receptors in intracellular compartments (Chen et al. 2010) (Fig. 3), thus providing a novel mechanism by which accelerated spine loss, increased tau phosphorylation (Kocherhans et al. 2010), loss of network homeostasis (Palop et al. 2007; Palop and Mucke 2010), and earlier disease onset through loss of neuroprotective compensatory bandwidth (Korwek et al. 2009) can be readily explained. This mechanism is also consistent with the finding that ApoE4 reduces spine density and dendritic complexity in cortical neurons in vivo (Dumanis et al. 2009). Moreover, Reelin expression levels are reduced in the brains of AD patients and in the entorhinal cortex of APP overexpressing mice (Chin et al. 2007), suggesting that Aβ can reduce Reelin expression in a subset of entorhinal pyramidal neurons, thereby adding further support to a role of diminished Reelin signaling in AD progression (Herz and Chen 2006).
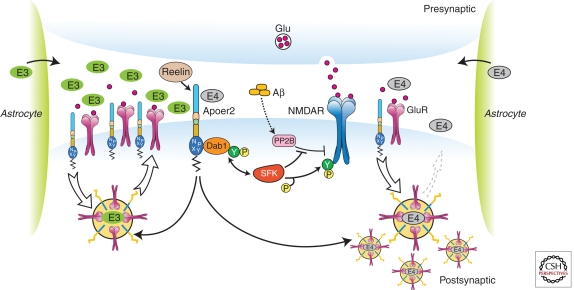
Figure 3.
ApoE isoforms differentially impair ApoE receptor and glutamate receptor recycling at the synapse. Apoer2 induces N-methyl-d-aspartate receptor (NMDAR) tyrosine phosphorylation by activating Src family tyrosine kinases (SFKs) in response to Reelin in the postsynaptic neuron. Astrocyte-derived ApoE3 (green ovals) or ApoE4 (gray ovals) bind to Apoer2 and are constitutively but slowly internalized. Apoer2 undergoes accelerated endocytosis in response to Reelin signaling. ApoE4 sequesters Apoer2 in intracellular compartments along with glutamate receptors (NMDAR and GluR), thereby reducing the ability of the postsynaptic neuron to recycle these proteins with normal kinetics, whereas ApoE2 or ApoE3 efficiently recycle back to the cell surface and thus deplete surface Apoer2 and glutamate receptor levels to a lesser extent (illustrated on the left for ApoE3). Aβ oligomers interfere with NMDAR tyrosine phosphorylation by activating tyrosine phosphatases (Snyder et al. 2005). (Modified from Chen et al. 2010; reprinted, with permission, from the National Academy of Sciences © 2010.)
ApoE Receptors Protect against Neurodegeneration
Recent evidence shows that ApoE receptors, specifically Apoer2 and LRP1, directly protect against the loss of neurons and dendrites in vivo (Beffert et al. 2006b). Apoer2 was found to protect against the loss of corticospinal neurons during the normal aging process (Beffert et al. 2006b). This protection requires the presence of the alternatively spliced cytoplasmic insert, which functionally couples Apoer2 to NMDA receptors, presumably through a PSD95-mediated interaction (Beffert et al. 2005; Hoe et al. 2006b), but also serves to recruit a c-jun amino-terminal kinase (JNK) signaling complex to the cytoplasmic tail of Apoer2 (Gotthardt et al. 2000; Stockinger et al. 2000). Intriguingly, JNK3 knockout mice and Apoer2 mutants lacking the alternatively spliced insert are resistant to lesion-induced neuronal death, suggesting that JNK recruitment to the Apoer2 cytoplasmic domain can promote both, neuronal loss or survival, depending on context (Beffert et al. 2006b).
Apoer2 also protects against neurodegeneration through another, mechanistically distinct mechanism, which involves its role in the uptake of essential selenoproteins into the brain (Burk et al. 2007). Loss of Apoer2 sensitizes the animals to low dietary selenium levels resulting in death from rapid and severe neurodegeneration. Selenoprotein 1 (Sepp1)-deficient animals show a pronounced LTP defect owing to selenium deficiency in the CNS when fed a selenium-reduced diet (Peters et al. 2006). However, the synaptic defects of Apoer2 knockin mutants (Beffert et al. 2005; Beffert et al. 2006a; Beffert et al. 2006b) are not caused by selenium depletion, but by the loss of NMDA and AMPA receptor regulation (Masiulis et al. 2009). LRP2 also mediates transport of this essential micronutrient (Chiu-Ugalde et al. 2010).
Neuroprotection by Apoer2 is further likely to involve its potent ability to mediate phosphorylation of cyclic AMP response element binding protein (CREB), a neuroprotective transcription factor that is also involved in long-term memory formation. Ca2+ influx through synaptic NMDA receptors stimulates CREB phosphorylation and promotes neuronal survival, whereas excitotoxic stimulation of extrasynaptic NMDA receptors shuts off CREB phosphorylation and promotes neuronal death (Hardingham et al. 2002). Reelin signaling strongly promotes CREB phosphorylation in cultured primary cortical neurons and this requires NMDA receptor activity (Chen et al. 2005). However, this is almost completely prevented by ApoE4 (Chen et al. 2010), suggesting another potential mechanism by which this apolipoprotein may contribute to the premature onset of neurodegeneration in ApoE4 carriers.
LRP1: EFFECTS ON APP, Aβ, AND ApoE METABOLISM
Regulation of APP Trafficking and Processing by LRP1 and Other ApoE Receptors
The β-secretase BACE1 is abundantly present and active in acidic endosomes (Cole and Vassar 2007). Consequently, increased APP endocytosis and distribution in endosomes lead to increased amyloidogenic processing and Aβ production. Conversely, if APP is retained at the cell surface, it has a greater availability for the cell-surface-localized α-secretase and is cleaved to sAPPα and a minimally toxic peptide p3 (see Haass et al. 2011 for details). Therefore, APP-interacting proteins or cellular conditions that alter APP trafficking and/or distribution are expected to impact APP processing to Aβ. Indeed, several apoE receptors interact with APP and modulate its trafficking and processing. LRP1 interacts with APP extracellularly by binding to the Kunitz-type protease inhibitor (KPI) domain that is present in the longer forms of APP (APP751 and APP770) (Kounnas et al. 1995). Further studies showed that LRP1 also interacts with the neuronal isoform of APP lacking the KPI domain (APP695). Two different phosphotyrosine-binding (PTB) domains within the adaptor protein FE65 bind to the NPxY motifs within APP and LRP1, thus bridging an interaction between these two membrane proteins intracellularly (Trommsdorff et al. 1998; Kinoshita et al. 2001; Pietrzik et al. 2004). Because of the rapid endocytosis rate of LRP1 compared with that of APP (Cam et al. 2005), the consequence of APP and LRP1 interaction is accelerated APP endocytic trafficking and processing to Aβ (Ulery et al. 2000; Zerbinatti et al. 2004; Cam et al. 2005; Zerbinatti et al. 2006). The in vivo role of LRP1 in APP trafficking and processing requires further investigation.
Several other apoE receptors also interact with APP and regulate its trafficking and processing to Aβ. LRP1B, which shares high sequence homology with LRP1 but has a significantly slower rate of endocytosis retains APP at the cell surface and reduces its processing to Aβ (Cam et al. 2004). Apoer2 either decreases or increases Aβ production depending on the experimental conditions. In the presence of F-spondin, a common ligand that bridges APP and Apoer2 extracellular interaction, the slow endocytosis rate of Apoer2 inhibits APP endocytic trafficking and reduces Aβ production (Hoe et al. 2005). However, in the absence of common ligands, Apoer2 increases the distribution of APP into lipid rafts and APP processing to Aβ (Fuentealba et al. 2007). A third apoE receptor that modulates APP trafficking and processing to Aβ is sorLA/LR11 whose expression is significantly reduced in AD brains (Scherzer et al. 2004). Cell biological studies show that sorLA in neurons shifts APP distribution to the Golgi compartment and decreases its processing to Aβ (Andersen et al. 2005). Importantly, a deletion of the Sorla gene in mice increases concentration of Aβ in the brain (Andersen et al. 2005). Supporting a role for sorLA in AD pathogenesis, a genetic study showed that inherited variants in the SORL1 gene (which encodes sorLA) are associated with LOAD (Rogaeva et al. 2007). Collectively, these studies show that apoE receptors are intimately associated with APP in neurons and regulate APP trafficking and processing.
LRP1 and LDLR in Cellular and Brain Aβ Metabolism
Impaired Aβ clearance is likely a major pathogenic event for LOAD. There are two major pathways by which Aβ is cleared from the brain: (1) receptor-mediated clearance by cells in brain parenchyma (microglia, astrocytes, neurons), along the interstitial fluid (ISF) drainage pathway or through the BBB and (2) through endopeptidase-mediated proteolytic degradation (see Saido and Leissring 2011 for details on proteolytic degradation of Aβ). Receptor-mediated clearance of Aβ in the brain is at least partially mediated by the apoE receptors LRP1, LDLR, and VLDLR, which are widely expressed in neurons, astrocytes, and microglia of brain parenchyma, as well as in endothelial cells, astrocytes, and smooth muscle cells at the BBB and cerebral arteries.
The best-characterized Aβ clearance receptor in the brain is LRP1, which along with several of its ligands are present in amyloid plaques (Rebeck et al. 1995). The important function of LRP1 in brain Aβ clearance was shown in amyloid mouse model with decreased LRP1 expression owing to a deletion of their chaperone RAP (Van Uden et al. 2002). Recombinant RAP and LRP1 antibody also reduce Aβ efflux from mouse brain (Shibata et al. 2000). In humans, a decreased LRP1 expression in the brain capillaries in AD brains may contribute to impaired Aβ clearance (Deane et al. 2004), whereas circulating soluble LRP1 (sLRP) might provide peripheral “sink” activity for Aβ clearance through the BBB (Sagare et al. 2007).
LRP1 binds Aβ directly (Deane et al. 2004) or indirectly via its ligands, which include α2-macroglobulin (Narita et al. 1997), RAP (Kanekiyo and Bu 2009), and apoE (Bu 2009; Kim et al. 2009a). ApoE is the best-characterized Aβ chaperone. Because apoE immunoreactivity is commonly found in amyloid plaques (Namba et al. 1991; Wisniewski and Frangione 1992), it is likely that apoE interacts with Aβ directly in the human brain. The region of apoE that is responsible for Aβ binding is in the carboxy-terminal domain overlapping with the lipid-binding region (Strittmatter et al. 1993b; Tamamizu-Kato et al. 2008), suggesting that the lipophilic Aβ peptide associates with apoE in a process that is analogous to lipid binding. Indeed, Aβ binding to apoE compromises its lipid-binding function (Tamamizu-Kato et al. 2008). Furthermore, Aβ peptides modulate the binding of apoE isoforms differently to apoE receptors (Beffert et al. 1998; Hone et al. 2005). These results show that Aβ peptides can interfere with the normal function of apoE under in vitro conditions. It is unclear if in vivo this is the case. A fraction of apoE3/lipoprotein binds to Aβ with higher affinity than apoE4/lipoprotein (LaDu et al. 1994). However, whether Aβ binding to apoE/lipoprotein leads to enhanced or reduced Aβ clearance, has not yet been clarifed (Kim et al. 2009a). If Aβ is cleared more efficiently through apoE/lipoprotein-independent pathway, one would expect that Aβ binding to apoE/lipoprotein will impede its clearance. On the other hand, if apoE/lipoprotein/Aβ complexes enter cells via apoE receptor (e.g., LRP1 and LDLR) pathways more efficiently than Aβ alone, apoE/lipoprotein likely promotes Aβ clearance. The in vivo role of apoE/lipoprotein in Aβ clearance is likely influenced by apoE lipidation state, receptor expression, and local Aβ concentration (Bu 2009; Kim et al. 2009a). Interestingly, a recent study shows that Aβ binding to apoE4 redirects its clearance from LRP1 to VLDLR, which internalizes Aβ-apoE4 complexes at the BBB more slowly than LRP1 (Deane et al. 2008). In contrast, Aβ-apoE2 and Aβ-apoE3 complexes are cleared at the BBB via both VLDLR and LRP1 at a substantially faster rate than Aβ-apoE4 complexes.
LDLR is another receptor that is implicated in brain Aβ clearance, although the effects of LDLR loss-of-function on Aβ clearance are still being worked out. One study showed that LDLR deficiency was associated with increased amyloid deposition in Tg2576 APP-transgenic mice (Cao et al. 2006). In contrast, another study using PDAPP-transgenic mice did not find a significant effect of Ldlr deletion on Aβ level or deposition although there was a trend for increased Aβ in the absence of LDLR (Fryer et al. 2005a). Using a gain-of-function approach, a more recent study showed that overexpression of the LDLR in the brain of transgenic mice enhanced Aβ clearance and decreased Aβ deposition (Kim et al. 2009b). It is not clear whether the effect of LDLR overexpression on Aβ clearance is because of the reduced level of apoE in the LDLR transgenic mice, or a direct effect on Aβ, or both. Nonetheless, these findings indicate that increasing LDLR expression may represent a novel therapeutic strategy to treat AD.
The receptor-mediated clearance is, in principle, an efficient way of reducing brain Aβ because most Aβ that is internalized by apoE receptors is delivered to lysosomes for degradation (see Fig. 4) or transcytosed into the plasma via BBB. However, it is possible that receptor-mediated clearance of Aβ into neurons can lead to intraneuronal accumulation of Aβ (Fig. 3), which under certain conditions may be toxic (Billings et al. 2005). A portion of Aβ that is internalized by neurons, in particular oligomeric Aβ42, accumulates in multivesicular bodies (MVBs)/late endosomes and lysosomes, and contributes to lysosomal dysfunction and neuronal toxicity. In contrast, receptor-mediated internalization of Aβ by astrocytes (Koistinaho et al. 2004) and microglia (Mandrekar et al. 2009) is likely to represent a more functional pathway to clear and eventually degrade Aβ.
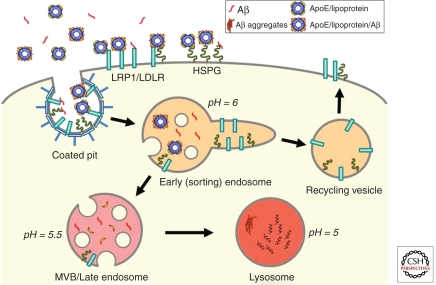
Figure 4.
Schematic model of LRP1/LDLR-mediated cellular transport of apoE/lipoprotein and Aβ. Three cell-surface receptors, LRP1, LDLR, and HSPG, are capable of binding to apoE/lipoprotein, Aβ, and apoE/lipoprotein/Aβ complexes. On clathrin-mediated endocytosis, ligands are mostly dissociated from the receptors within the early/sorting endosomes owing to lower pH. Whereas receptors are typically recycled back to the cell surface, ligands are delivered to multivesicular bodies (MVBs)/late endosomes and eventually to lysosomes for degradation. Lipid components are transported out of the lysosomes for storage or reutilization. Depending on the concentrations and cellular conditions, some Aβ molecules might aggregate within the lysosomes as intracellular Aβ, which could eventually serve to seed amyloid plaques (Hu et al. 2009).
Evidence that LRP1 and LDLR Are Key Metabolic Receptors for ApoE/Lipoprotein in the Brain
Although several LDLR family members are expressed in the brain, accumulating evidence indicates that the LDLR and LRP1 are the two primary metabolic receptors for apoE/lipoprotein. Deletion of the Ldlr gene in mice increases apoE levels in brain parenchyma and CSF (Fryer et al. 2005a), suggesting impaired metabolism of apoE. In contrast, overexpression of the LDLR in the brain decreases apoE levels, reflecting an increased metabolism of apoE (Kim et al. 2009b). Similarly, conditional deletion of the Lrp1 gene in mouse forebrain neurons increases apoE levels (Liu et al. 2007) and overexpression of a functional LRP1 minireceptor in mouse brain decreases brain apoE levels (Zerbinatti et al. 2006). Although both LDLR and LRP1 play roles in brain apoE/lipoprotein metabolism, there are important differences between them. First, whereas LRP1 is highly expressed in neurons and to a lesser degree in glia, LDLR is more prominently expressed in glia than neurons (Rebeck et al. 1993; Rapp et al. 2006). Second, deletion of the Lrp1 gene in mouse forebrain neurons reduces brain cholesterol levels (Liu et al. 2007), whereas cholesterol levels in Ldlr knockout mice are unchanged (Fryer et al. 2005a). Third, apoE/lipoprotein particles secreted by astrocytes have higher affinity for LDLR than LRP1 (Fryer et al. 2005a), whereas recombinant apoE (Narita et al. 2002), apoE-enriched lipoprotein particles (Kowal et al. 1990), and CSF-isolated HDL particles (Fagan et al. 1996) bind more avidly to LRP1. The receptor-binding specificity of apoE is likely influenced by its conformation and lipidation state. It is possible that apoE/lipoprotein particles secreted by astrocytes recruit additional apoE molecules, perhaps bound to heparan sulfate proteoglycan (HSPG), before being transported to the CSF or binding to LRP1 at the neuronal cell surface.
There is some evidence that apoE isoforms may differ in their function in regard to cholesterol transport and efflux (Michikawa et al. 2000; Gong et al. 2002; Rapp et al. 2006), although not all studies show this (Hirsch-Reinshagen et al. 2004). Thus the roles of apoE isoforms in brain cholesterol metabolism require further investigation. The structural differences among apoE isoforms that determine their lipid- and receptor-binding specificities in the brain environment could account for their differences in modulating brain cholesterol metabolism. It is important to note that there are likely LRP1-mediated cholesterol transport mechanisms in the brain that are independent of apoE because LRP1 deficiency in the brain, but not apoE deficiency, leads to decreased brain cholesterol levels. Other LRP1 ligands such as lipoprotein lipase might also play a role. Alternatively, LRP1 may serve as a cholesterol sensor that influences cholesterol synthesis and/or intracellular transport.
In addition to cholesterol, apoE also mediates the transport of other brain lipids, some of which are not produced in astrocytes. For example, sulfatide, an oligodendrocyte-synthesized lipid that is crucial for neuronal spine and myelin sheath integrity, is actively transported by an apoE- and LRP1-dependent mechanism (Han 2007). It is possible that apoE/lipoprotein particles are modified by myelin-associated lipids before being transported into neurons. Interestingly, sulfatide is a potential biomarker for AD diagnosis as its levels are decreased in AD brains (Han 2007). Whether LRP1/LDLR-mediated apoE/cholesterol transport is impaired in AD brains and how this in turn contributes to AD pathogenesis is currently not clear. However, because of the important role of apoE/cholesterol in injury repair, the cholesterol transport pathway should be considered when apoE/LRP1/LDLR pathways are explored as therapeutic targets for AD.
SUMMARY
APOE genotype is the strongest genetic risk factor for AD, and understanding the mechanism underlying this relationship has the potential to lead to new therapeutic approaches. A major reason that appears to underlie this relationship is the fact that apoE isoforms result in differential onset of Aβ accumulation in the brain with the onset E4 earlier than E3, which is earlier than E2. There is direct in vivo evidence that differences in Aβ clearance is one factor that accounts for this (Castellano et al. 2011), although effects of apoE on Aβ aggregation independent of clearance may also be important. From a therapeutic standpoint, pathways that stimulate Aβ clearance via apoE-dependent mechanisms are one possible approach to decrease Aβ accumulation and its toxic effects. Targeting the liver X receptor (LXR) pathway (Koldamova et al. 2005b), apoE lipidation state via ABCA1, as well as LDLR, LRP1, and other apoE receptors are potential ways to stimulate apoE-dependent Aβ clearance. In terms of apoE-dependent Aβ aggregation, interrupting the apoE-Aβ interaction may also have therapeutic potential (Sadowski et al. 2006). In addition to effects on the apoE/Aβ pathway, apoE receptors such as Apoer2 and Vldlr play important roles in synaptic plasticity, tau phosphorylation, and neuroprotection. Determining ways to activate these receptors may be another strategy to delay or halt the progressive neurodegenerative process that occurs in AD.
Footnotes
Editors: Dennis J. Selkoe, Eckhard Mandelkow, and David M. Holtzman
Additional Perspectives on The Biology of Alzheimer Disease available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Aleshkov S, Abraham CR, Zannis VI 1997. Interaction of nascent apoE2, apoE3, and apoE4 isoforms expressed in mammalian cells with amyloid peptide β(1-40). Relevance to Alzheimer’s disease. Biochemistry 36: 10571–10580 [DOI] [PubMed] [Google Scholar]
- Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, Behlke J, von Arnim CA, Breiderhoff T, Jansen P, Wu X, et al. 2005. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. In Proc Natl Acad Sci 102: 13461–13466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R, Barnes JC, Bliss TV, Cain DP, Cambon K, Davies HA, Errington ML, Fellows LA, Gray RA, Hoh T, et al. 1998. Behavioural, physiological and morphological analysis of a line of apolipoprotein E knockout mouse. Neuroscience 85: 93–110 [DOI] [PubMed] [Google Scholar]
- Arnaud L, Ballif BA, Forster E, Cooper JA 2003. Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr Biol 13: 9–17 [DOI] [PubMed] [Google Scholar]
- Assadi AH, Zhang G, Beffert U, McNeil RS, Renfro AL, Niu S, Quattrocchi CC, Antalffy BA, Sheldon M, Armstrong DD, et al. 2003. Interaction of reelin signaling and Lis1 in brain development. Nat Genet 35: 270–276 [DOI] [PubMed] [Google Scholar]
- Bales KR, Verina T, Dodel RC, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, et al. 1997. Lack of apolipoprotein E dramatically reduces amyloid β-peptide deposition. Nat Genet 17: 263–264 [DOI] [PubMed] [Google Scholar]
- Bales KR, Verina T, Cummins DJ, Du Y, Dodel JC, Saura J, Fishman CE, DeLong CA, Piccardo P, Petegnief V, et al. 1999. Apolipoprotein E is essential for amyloid deposition in the APPV717F transgenic mouse model of Alzheimer’s disease. Proc Natl Acad Sci 96: 15233–15238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffert U, Arguin C, Poirier J 1999. The polymorphism in exon 3 of the low density lipoprotein receptor-related protein gene is weakly associated with Alzheimer’s disease. Neurosci Lett 259: 29–32 [DOI] [PubMed] [Google Scholar]
- Beffert U, Aumont N, Dea D, Lussier-Cacan S, Davignon J, Poirier J 1998. β-amyloid peptides increase the binding and internalization of apolipoprotein E to hippocampal neurons. J Neurochem 70: 1458–1466 [DOI] [PubMed] [Google Scholar]
- Beffert U, Morfini G, Bock HH, Reyna H, Brady ST, Herz J 2002. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3β. J Biol Chem 277: 49958–49964 [DOI] [PubMed] [Google Scholar]
- Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, Sweatt JD, Li WP, Adelmann G, Frotscher M, Hammer RE, et al. 2005. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron 47: 567–579 [DOI] [PubMed] [Google Scholar]
- Beffert U, Durudas A, Weeber EJ, Stolt PC, Giehl KM, Sweatt JD, Hammer RE, Herz J 2006a. Functional dissection of Reelin signaling by site-directed disruption of Disabled-1 adaptor binding to apolipoprotein E receptor 2: Distinct roles in development and synaptic plasticity. J Neurosci 26: 2041–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffert U, Nematollah Farsian F, Masiulis I, Hammer RE, Yoon SO, Giehl KM, Herz J 2006b. ApoE receptor 2 controls neuronal survival in the adult brain. Curr Biol 16: 2446–2452 [DOI] [PubMed] [Google Scholar]
- Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, Zlokovic BV 2007. Transport pathways for clearance of human Alzheimer’s amyloid β-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab 27: 909–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin R, Leake A, Ince PG, Perry RH, McKeith IG, Edwardson JA, Morris CM 1995. Effects of apolipoprotein E genotype on cortical neuropathology in senile dementia of the Lewy body and Alzheimer’s disease. Neurodegeneration 4: 443–448 [DOI] [PubMed] [Google Scholar]
- Berman DE, Dall’Armi C, Voronov SV, McIntire LB, Zhang H, Moore AZ, Staniszewski A, Arancio O, Kim TW, Di Paolo G 2008. Oligomeric amyloid-β peptide disrupts phosphatidylinositol-4,5-bisphosphate metabolism. Nat Neurosci 11: 547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE 2007. Systematic meta-analyses of Alzheimer disease genetic association studies: The AlzGene database. Nat Genet 39: 17–23 [DOI] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, Laferla FM 2005. Intraneuronal Aβ causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron 45: 675–688 [DOI] [PubMed] [Google Scholar]
- Bock HH, Herz J 2003. Reelin activates SRC family tyrosine kinases in neurons. Curr Biol 13: 18–26 [DOI] [PubMed] [Google Scholar]
- Borrell V, Pujadas L, Simo S, Dura D, Sole M, Cooper JA, Del Rio JA, Soriano E 2007. Reelin and mDab1 regulate the development of hippocampal connections. Mol Cell Neurosci 36: 158–173 [DOI] [PubMed] [Google Scholar]
- Boucher P, Liu P, Gotthardt M, Hiesberger T, Anderson RG, Herz J 2002. Platelet-derived growth factor mediates tyrosine phosphorylation of the cytoplasmic domain of the low density lipoprotein receptor-related protein in caveolae. J Biol Chem 277: 15507–15513 [DOI] [PubMed] [Google Scholar]
- Boycott KM, Flavelle S, Bureau A, Glass HC, Fujiwara TM, Wirrell E, Davey K, Chudley AE, Scott JN, McLeod DR, et al. 2005. Homozygous deletion of the very low density lipoprotein receptor gene causes autosomal recessive cerebellar hypoplasia with cerebral gyral simplification. Am J Human Genet 77: 477–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott KM, Bonnemann C, Herz J, Neuert S, Beaulieu C, Scott JN, Venkatasubramanian A, Parboosingh JS 2009. Mutations in VLDLR as a cause for autosomal recessive cerebellar ataxia with mental retardation (dysequilibrium syndrome). J Child Neurol 24: 1310–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brich J, Shie FS, Howell BW, Li R, Tus K, Wakeland EK, Jin LW, Mumby M, Churchill G, Herz J, et al. 2003. Genetic modulation of tau phosphorylation in the mouse. J Neurosci 23: 187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu G 2009. Apolipoprotein E and its receptors in Alzheimer’s disease: Pathways, pathogenesis and therapy. Nat Rev Neurosci 10: 333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk RF, Hill KE, Olson GE, Weeber EJ, Motley AK, Winfrey VP, Austin LM 2007. Deletion of apolipoprotein E receptor-2 in mice lowers brain selenium and causes severe neurological dysfunction and death when a low-selenium diet is fed. J Neurosci 27: 6207–6211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cam JA, Zerbinatti CV, Knisely JM, Hecimovic S, Li Y, Bu G 2004. The low density lipoprotein receptor-related protein 1B retains β-amyloid precursor protein at the cell surface and reduces amyloid-β peptide production. J Biol Chem 279: 29639–29646 [DOI] [PubMed] [Google Scholar]
- Cam JA, Zerbinatti CV, Li Y, Bu G 2005. Rapid endocytosis of the low density lipoprotein receptor-related protein modulates cell surface distribution and processing of the β-amyloid precursor protein. J Biol Chem 280: 15464–15470 [DOI] [PubMed] [Google Scholar]
- Campo CG, Sinagra M, Verrier D, Manzoni OJ, Chavis P 2009. Reelin secreted by GABAergic neurons regulates glutamate receptor homeostasis. PLoS One 4: e5505 10.1371/journal.pone.0005505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Fukuchi K, Wan H, Kim H, Li L 2006. Lack of LDL receptor aggravates learning deficits and amyloid deposits in Alzheimer transgenic mice. Neurobiol Aging 27: 1632–1643 [DOI] [PubMed] [Google Scholar]
- Castano EM, Prelli F, Wisniewski T, Golabek A, Kumar RA, Soto C, Frangione B 1995. Fibrillogenesis in Alzheimer’s disease of amyloid β peptides and apolipoprotein E. Biochem J 306: 599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Kim J, Stewart FR, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Paul SM, Bateman RJ, et al. 2011. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med 3: 89ra57.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X, Forster E, Zhao S, Bock HH, Frotscher M 2009. Reelin acts as a stop signal for radially migrating neurons by inducing phosphorylation of n-cofilin at the leading edge. Commun Integr Biol 2: 375–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chameau P, Inta D, Vitalis T, Monyer H, Wadman WJ, van Hooft JA 2009. The N-terminal region of reelin regulates postnatal dendritic maturation of cortical pyramidal neurons. Proc Natl Acad Sci 106: 7227–7232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Beffert U, Ertunc M, Tang TS, Kavalali ET, Bezprozvanny I, Herz J 2005. Reelin modulates NMDA receptor activity in cortical neurons. J Neurosci 25: 8209–8216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Durakoglugil MS, Xian X, Herz J 2010. ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc Natl Acad Sci 107: 12011–12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J, Massaro CM, Palop JJ, Thwin MT, Yu GQ, Bien-Ly N, Bender A, Mucke L 2007. Reelin depletion in the entorhinal cortex of human amyloid precursor protein transgenic mice and humans with Alzheimer’s disease. J Neurosci 27: 2727–2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu-Ugalde J, Theilig F, Behrends T, Drebes J, Sieland C, Subbarayal P, Kohrle J, Hammes A, Schomburg L, Schweizer U 2010. Mutation of megalin leads to urinary loss of selenoprotein P and selenium deficiency in serum, liver, kidneys and brain. Biochem J 431: 103–111 [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, et al. 2005. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-β deposition in an Alzheimer disease mouse model. J Clin Invest 115: 3285–3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole GM, Ard MD 2000. Influence of lipoproteins on microglial degradation of Alzheimer’s amyloid β-protein. Microsc Res Tech 50: 316–324 [DOI] [PubMed] [Google Scholar]
- Cole SL, Vassar R 2007. The Alzheimer’s disease β-secretase enzyme, BACE1. Mol Neurodegener 2: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA 1993. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261: 921–923 [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC Jr, Rimmler JB, Locke PA, Conneally PM, Schmader KE, et al. 1994. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet 7: 180–184 [DOI] [PubMed] [Google Scholar]
- Costa E, Davis J, Grayson DR, Guidotti A, Pappas GD, Pesold C 2001. Dendritic spine hypoplasticity and downregulation of reelin and GABAergic tone in schizophrenia vulnerability. Neurobiol Dis 8: 723–742 [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G 2005. Apoer2: A reelin receptor to remember. Neuron 47: 471–473 [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T 1999. Reelin is a ligand for lipoprotein receptors. Neuron 24: 471–479 [DOI] [PubMed] [Google Scholar]
- Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, et al. 2004. LRP/amyloid β-peptide interaction mediates differential brain efflux of Aβ isoforms. Neuron 43: 333–344 [DOI] [PubMed] [Google Scholar]
- Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV 2008. apoE isoform-specific disruption of amyloid β peptide clearance from mouse brain. J Clin Invest 118: 4002–4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ferrari GV, Papassotiropoulos A, Biechele T, Wavrant De-Vrieze F, Avila ME, Major MB, Myers A, Saez K, Henriquez JP, Zhao A, et al. 2007. Common genetic variation within the low-density lipoprotein receptor-related protein 6 and late-onset Alzheimer’s disease. Proc Natl Acad Sci 104: 9434–9439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio JA, Heimrich B, Borrell V, Forster E, Drakew A, Alcantara S, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, et al. 1997. A role for Cajal-Retzius cells and reelin in the development of hippocampal connections. Nature 385: 70–74 [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Brendza RP, Heuser JE, Kierson M, Cirrito JR, Fryer JD, Sullivan PM, Fagan AM, Han X, Holtzman DM 2001. Purification and characterization of astrocyte-secreted apolipoprotein E and J-containing lipoproteins from wild-type and human apoE transgenic mice. Neurochem Int 39: 415–425 [DOI] [PubMed] [Google Scholar]
- DeMattos RB, O’dell MA, Parsadanian M, Taylor JW, Harmony JAK, Bales KR, Paul SM, Aronow BJ, Holtzman DM 2002. Clusterin promotes amyloid plaque formation and is critical for neuritic toxicity in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci 10: 10843–10848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattos RB, Cirrito JR, Parsadanian M, May PC, O’Dell MA, Taylor JM, Harmony JAK, Aronow BJ, Bales KR, Paul SM, et al. 2004. ApoE and clusterin cooperatively suppress Ab levels and deposition: Evidence that apoE regulates extracellular Ab metabolism in vivo. Neuron 41: 193–202 [DOI] [PubMed] [Google Scholar]
- Dieckmann M, Dietrich MF, Herz J 2010. Lipoprotein receptors—An evolutionarily ancient multifunctional receptor family. Biol Chem 391: 1341–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MF, van der Weyden L, Prosser HM, Bradley A, Herz J, Adams DJ 2010. Ectodomains of the LDL receptor-related proteins LRP1b and LRP4 have anchorage independent functions in vivo. PLoS One 5: e9960 10.1371/journal.pone.0009960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon C, Goda Y 2005. The actin cytoskeleton: Integrating form and function at the synapse. Annu Rev Neurosci 28: 25–55 [DOI] [PubMed] [Google Scholar]
- Dumanis SB, Tesoriero JA, Babus LW, Nguyen MT, Trotter JH, Ladu MJ, Weeber EJ, Turner RS, Xu B, Rebeck GW, et al. 2009. ApoE4 decreases spine density and dendritic complexity in cortical neurons in vivo. J Neurosci 29: 15317–15322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durakoglugil MS, Chen Y, White CL, Kavalali ET, Herz J 2009. Reelin signaling antagonizes β-amyloid at the synapse. Proc Natl Acad Sci 106: 15938–15943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans KC, Berger EP, Cho C-G, Weisgraber KH, Lansbury PT 1994. Apolipoprotein E is a kinetic but not a thermodynamic inhibitor of amyloid formation: Implications for the pathogenesis and treatment of Alzheimer’s disease. Proc Natl Acad Sci 92: 763–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Bu G, Sun Y, Daugherty A, Holtzman DM 1996. Apolipoprotein E-containing high density lipoprotein promotes neurite outgrowth and is a ligand for the low density lipoprotein receptor-related protein. J Biol Chem 271: 30121–30125 [DOI] [PubMed] [Google Scholar]
- Fagan AM, Murphy BA, Patel SN, Kilbridge JF, Mobley WC, Bu G, Holtzman DM 1998. Evidence for normal aging of the septo-hippocampal cholinergic system in apoE−/− mice but impaired clearance of axonal degeneration products following injury. Exp Neurol 151: 314–325 [DOI] [PubMed] [Google Scholar]
- Fagan AM, Watson M, Parsadanian M, Bales KR, Paul SM, Holtzman DM 2002. Human and murine apoE markedly influence Aβ metabolism both prior and subsequent to plaque formation in a mouse model of Alzheimer’s disease. Neurobiol Dis 9: 305–318 [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM 1997. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 278: 1349–1356 [PubMed] [Google Scholar]
- Forster E, Bock HH, Herz J, Chai X, Frotscher M, Zhao S 2010. Emerging topics in Reelin function. Eur J Neurosci 31: 1511–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M 2010. Role for Reelin in stabilizing cortical architecture. Trends Neurosci 33: 407–414 [DOI] [PubMed] [Google Scholar]
- Fryer JD, Taylor JW, DeMattos RB, Bales KR, Paul SM, Parsadanian M, Holtzman DM 2003. Apolipoprotein E markedly facilitates age-dependent cerebral amyloid angiopathy and spontaneous hemorrhage in APP transgenic mice. J Neurosci 23: 7889–7896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer JD, Demattos RB, McCormick LM, O’Dell M A, Spinner ML, Bales KR, Paul SM, Sullivan PM, Parsadanian M, Bu G, et al. 2005a. The low density lipoprotein receptor regulates the level of central nervous system human and murine apolipoprotein E but does not modify amyloid plaque pathology in PDAPP mice. J Biol Chem 280: 25754–25759 [DOI] [PubMed] [Google Scholar]
- Fryer JD, Simmons K, Parsadanian M, Bales KR, Paul SM, Sullivan PM, Holtzman DM 2005b. ApoE4 alters the amyloid-β 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an APP transgenic model. J Neurosci 25: 2803–2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba RA, Barria MI, Lee J, Cam J, Araya C, Escudero CA, Inestrosa NC, Bronfman FC, Bu G, Marzolo MP 2007. ApoER2 expression increases Aβ production while decreasing Amyloid Precursor Protein (APP) endocytosis: Possible role in the partitioning of APP into lipid rafts and in the regulation of γ-secretase activity. Mol Neurodegener 2: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajera CR, Emich H, Lioubinski O, Christ A, Beckervordersandforth-Bonk R, Yoshikawa K, Bachmann S, Christensen EI, Gotz M, Kempermann G, et al. 2010. LRP2 in ependymal cells regulates BMP signaling in the adult neurogenic niche. J Cell Sci 123: 1922–1930 [DOI] [PubMed] [Google Scholar]
- Gimbel DA, Nygaard HB, Coffey EE, Gunther EC, Lauren J, Gimbel ZA, Strittmatter SM 2010. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J Neurosci 30: 6367–6374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JS, Kobayashi M, Hayashi H, Zou K, Sawamura N, Fujita SC, Yanagisawa K, Michikawa M 2002. Apolipoprotein E (ApoE) isoform-dependent lipid release from astrocytes prepared from human ApoE3 and ApoE4 knock-in mice. J Biol Chem 277: 29919–29926 [DOI] [PubMed] [Google Scholar]
- Gotthardt M, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, Stockinger W, Nimpf J, Herz J 2000. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J Biol Chem 275: 25616–25624 [DOI] [PubMed] [Google Scholar]
- Greenberg SM, Rebeck GW, Vonsattel JPG, Gomez-Isla T, Hyman BT 1995. Apolipoprotein E ε4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol 38: 254–259 [DOI] [PubMed] [Google Scholar]
- Grehan S, Tse E, Taylor JM 2001. Two distal downstream enhancers direct expression of the human apolipoprotein E gene to astrocytes in the brain. J Neurosci 21: 812–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Choquet D, Stephenson FA, Verrier D, Manzoni OJ, Chavis P 2007. NMDA receptor surface trafficking and synaptic subunit composition are developmentally regulated by the extracellular matrix protein Reelin. J Neurosci 27: 10165–10175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ 2007. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid β-peptide. Nat Rev Mol Cell Biol 8: 101–112 [DOI] [PubMed] [Google Scholar]
- *.Haass C, Kaether C, Sisodia S, Thinakaran G 2011. Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med 10.1101/cshperspect.a006270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X 2007. Potential mechanisms contributing to sulfatide depletion at the earliest clinically recognizable stage of Alzheimer’s disease: A tale of shotgun lipidomics. J Neurochem 103: 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H 2002. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci 5: 405–414 [DOI] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, et al. 2009. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet 41: 1088–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen O, Lehtovirta M, Soininen H, Helisalmi S, Mannermaa A, Sorvari H, Kosunen O, Paljarvi L, Ryynanen M, Riekkinen PJ Sr 1995. Alzheimer pathology of patients carrying apolipoprotein E ε4 allele. Neurobiol Aging 16: 505–513 [DOI] [PubMed] [Google Scholar]
- Herz J 2009. Apolipoprotein E receptors in the nervous system. Curr Opin Lipidol 20: 190–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Beffert U 2000. Apolipoprotein E receptors: Linking brain development and Alzheimer’s disease. Nature Rev 1: 51–58 [DOI] [PubMed] [Google Scholar]
- Herz J, Chen Y 2006. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci 7: 850–859 [DOI] [PubMed] [Google Scholar]
- Herz J, Clouthier DE, Hammer RE 1992. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell 71: 411–421 [DOI] [PubMed] [Google Scholar]
- Herz J, Chen Y, Masiulis I, Zhou L 2009. Expanding functions of lipoprotein receptors. J Lipid Res 50(Suppl): S287–S292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J 1999. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron 24: 481–489 [DOI] [PubMed] [Google Scholar]
- Hirsch-Reinshagen V, Zhou S, Burgess BL, Bernier L, McIsaac SA, Chan JY, Tansley GH, Cohn JS, Hayden MR, Wellington CL 2004. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J Biol Chem 279: 41197–41207 [DOI] [PubMed] [Google Scholar]
- Hirsch-Reinshagen V, Maia LF, Burgess BL, Blain JF, Naus KE, McIsaac SA, Parkinson PF, Chan JY, Tansley GH, Hayden MR, et al. 2005. The absence of ABCA1 decreases soluble ApoE levels but does not diminish amyloid deposition in two murine models of Alzheimer disease. J Biol Chem 280: 43243–43256 [DOI] [PubMed] [Google Scholar]
- Hoe HS, Wessner D, Beffert U, Becker AG, Matsuoka Y, Rebeck GW 2005. F-spondin interaction with the apolipoprotein E receptor ApoEr2 affects processing of amyloid precursor protein. Mol Cell Biol 25: 9259–9268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe HS, Freeman J, Rebeck GW 2006a. Apolipoprotein E decreases tau kinases and phospho-tau levels in primary neurons. Mol Neurodegen 1: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe HS, Pocivavsek A, Chakraborty G, Fu Z, Vicini S, Ehlers MD, Rebeck GW 2006b. Apolipoprotein E receptor 2 interactions with the N-methyl-d-aspartate receptor. J Biol Chem 281: 3425–3431 [DOI] [PubMed] [Google Scholar]
- Hoe HS, Fu Z, Makarova A, Lee JY, Lu C, Feng L, Pajoohesh-Ganji A, Matsuoka Y, Hyman BT, Ehlers MD, et al. 2009. The effects of amyloid precursor protein on postsynaptic composition and activity. J Biol Chem 284: 8495–8506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Pitas RE, Kilbridge J, Nathan B, Mahley RW, Bu G, Schwartz AL 1995. LRP mediates apolipoprotein E-dependent neurite outgrowth in a CNS-derived neuronal cell line. Proc Natl Acad Sci 92: 9480–9484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, et al. 2000a. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci 97: 2892–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Fagan AM, Mackey B, Tenkova T, Sartorius L, Paul SM, Bales K, Ashe KH, Irizzary MC, Hyman BT 2000b. ApoE facilitates neuritic and cerebrovascular plaque formation in the APPsw mouse model of Alzheimer’s disease. Ann Neurol 47: 739–747 [PubMed] [Google Scholar]
- Hone E, Martins IJ, Jeoung M, Ji TH, Gandy SE, Martins RN 2005. Alzheimer’s disease amyloid-β peptide modulates apolipoprotein E isoform specific receptor binding. J Alzheimers Dis 7: 303–314 [DOI] [PubMed] [Google Scholar]
- Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, Pitstick R, Carlson GA, Lanier LM, Yuan LL, et al. 2010. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 68: 1067–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BW, Gertler FB, Cooper JA 1997. Mouse disabled (mDab1): A Src binding protein implicated in neuronal development. EMBO J 16: 121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Crick SL, Bu G, Frieden C, Pappu RV, Lee JM 2009. Amyloid seeds formed by cellular uptake, concentration, and aggregation of the amyloid-β peptide. Proc Natl Acad Sci 106: 20324–20329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SS, Ling TY, Tseng WF, Huang YH, Tang FM, Leal SM, Huang JS 2003. Cellular growth inhibition by IGFBP-3 and TGF-β1 requires LRP-1. FASEB J 17: 2068–2081 [DOI] [PubMed] [Google Scholar]
- Irizarry MC, Cheung BS, Rebeck GW, Paul SM, Bales KR, Hyman BT 2000. Apolipoprotein E affects the amount, form, and anatomical distribution of amyloid β-peptide deposition in homozygous APP(V717F) transgenic mice. Acta Neuropathol 100: 451–458 [DOI] [PubMed] [Google Scholar]
- Ito S, Ohtsuki S, Kamiie J, Nezu Y, Terasaki T 2007. Cerebral clearance of human amyloid-β peptide (1–40) across the blood-brain barrier is reduced by self-aggregation and formation of low-density lipoprotein receptor-related protein-1 ligand complexes. J Neurochem 103: 2482–2490 [DOI] [PubMed] [Google Scholar]
- Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wolfing H, Chieng BC, Christie MJ, Napier IA, et al. 2010. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer’s disease mouse models. Cell 142: 387–397 [DOI] [PubMed] [Google Scholar]
- Jiang Q, Lee CY, Mandrekar S, Wilkinson B, Cramer P, Zelcer N, Mann K, Lamb B, Willson TM, Collins JL, et al. 2008. ApoE promotes the proteolytic degradation of Aβ. Neuron 58: 681–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EB, Hammer RE, Herz J 2005. Abnormal development of the apical ectodermal ridge and polysyndactyly in Megf7-deficient mice. Hum Mol Genet 14: 3523–3538 [DOI] [PubMed] [Google Scholar]
- Kanekiyo T, Bu G 2009. Receptor-associated protein interacts with amyloid-β peptide and promotes its cellular uptake. J Biol Chem 284: 33352–33359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karner CM, Dietrich MF, Johnson EB, Kappesser N, Tennert C, Percin F, Wollnik B, Carroll TJ, Herz J 2010. Lrp4 regulates initiation of ureteric budding and is crucial for kidney formation—A mouse model for Cenani-Lenz syndrome. PLoS One 5: e10418 10.1371/journal.pone.0010418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM 2009a. The role of apolipoprotein E in Alzheimer’s disease. Neuron 63: 287–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Castellano JM, Jiang H, Basak JM, Parsadanian M, Pham V, Mason SM, Paul SM, Holtzman DM 2009b. Overexpression of low-density lipoprotein receptor in the brain markedly inhibits amyloid deposition and increases extracellular Aβ clearance. Neuron 64: 632–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A, Whelan CM, Smith CJ, Mikhailenko I, Rebeck GW, Strickland DK, Hyman BT 2001. Demonstration by fluorescence resonance energy transfer of two sites of interaction between the low-density lipoprotein receptor-related protein and the amyloid precursor protein: Role of the intracellular adapter protein Fe65. J Neurosci 21: 8354–8361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocherhans S, Madhusudan A, Doehner J, Breu KS, Nitsch RM, Fritschy JM, Knuesel I 2010. Reduced Reelin expression accelerates amyloid-beta plaque formation and tau pathology in transgenic Alzheimer’s disease mice. J Neurosci 30: 9228–9240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J, Higgs R, Liu F, Malkani S, Bales KR, et al. 2004. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-β peptides. Nat Med 10: 719–726 [DOI] [PubMed] [Google Scholar]
- Koldamova R, Staufenbiel M, Lefterov I 2005a. Lack of ABCA1 considerably decreases brain ApoE level and increases amyloid deposition in APP23 mice. J Biol Chem 280: 43224–43235 [DOI] [PubMed] [Google Scholar]
- Koldamova RP, Lefterov IM, Staufenbiel M, Wolfe D, Huang S, Glorioso JC, Walter M, Roth MG, Lazo JS 2005b. The liver X receptor ligand T0901317 decreases amyloid β production in vitro and in a mouse model of Alzheimer’s disease. J Biol Chem 280: 4079–4088 [DOI] [PubMed] [Google Scholar]
- Korwek KM, Trotter JH, Ladu MJ, Sullivan PM, Weeber EJ 2009. ApoE isoform-dependent changes in hippocampal synaptic function. Molec Neurodegen 4: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounnas MZ, Moir RD, Rebeck GW, Bush AI, Argraves WS, Tanzi RE, Hyman BT, Strickland DK 1995. LDL-receptor-related protein, a multifunctional apoE receptor, binds secreted β-amyloid precursor protein and mediates its degradation. Cell 82: 331–340 [DOI] [PubMed] [Google Scholar]
- Kowal RC, Herz J, Weisgraber KH, Mahley RW, Brown MS, Goldstein JL 1990. Opposing effects of apolipoproteins E and C on lipoprotein binding to low density lipoprotein receptor-related protein. J Biol Chem 265: 10771–10779 [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, et al. 2004. Synaptic targeting by Alzheimer’s-related amyloid β oligomers. J Neurosci 24: 10191–10200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDu MJ, Falduto MT, Manelli AM, Reardon CA, Getz GS, Frail DE 1994. Isoform-specific binding of apolipoprotein E to β-amyloid. J Biol Chem 269: 23404–23406 [PubMed] [Google Scholar]
- LaDu MJ, Gilligan SM, Lukens SR, Cabana VG, Reardon CA, Van Eldik LJ, Holtzman DM 1998. Nascent astrocyte particles differ from lipoproteins in CSF. J Neurochem 70: 2070–2081 [DOI] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, et al. 1998. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci 95: 6448–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, et al. 2009. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet 41: 1094–1099 [DOI] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH 2006. A specific amyloid-β protein assembly in the brain impairs memory. Nature 440: 352–357 [DOI] [PubMed] [Google Scholar]
- Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK 2008. LDL receptor-related protein 1: Unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev 88: 887–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Zerbinatti CV, Zhang J, Hoe HS, Wang B, Cole SL, Herz J, Muglia L, Bu G 2007. Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron 56: 66–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukinova E, Ranganathan S, Kuznetsov S, Gorlatova N, Migliorini MM, Loukinov D, Ulery PG, Mikhailenko I, Lawrence DA, Strickland DK 2002. Platelet-derived growth factor (PDGF)-induced tyrosine phosphorylation of the low density lipoprotein receptor-related protein (LRP). Evidence for integrated co-receptor function between LRP and the PDGF. J Biol Chem 277: 15499–15506 [DOI] [PubMed] [Google Scholar]
- Ma J, Yee A, Brewer HB, Das S, Potter H 1994. Amyloid-associated proteins α-1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer beta-protein into filaments. Nature 372: 92–94 [DOI] [PubMed] [Google Scholar]
- Mahley RW 1988. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science 240: 622–630 [DOI] [PubMed] [Google Scholar]
- Mahley RW, Rall SC Jr 2000. Apolipoprotein E: Far more than a lipid transport protein. Annu Rev Genomics Hum Genet 1: 507–537 [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y, Weisgraber KH 2006. Putting cholesterol in its place: ApoE and reverse cholesterol transport. J Clin Invest 116: 1226–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar S, Jiang Q, Lee CY, Koenigsknecht-Talboo J, Holtzman DM, Landreth GE 2009. Microglia mediate the clearance of soluble Aβ through fluid phase macropinocytosis. J Neurosci 29: 4252–4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiulis I, Quill TA, Burk RF, Herz J 2009. Differential functions of the Apoer2 intracellular domain in selenium uptake and cell signaling. Biol Chem 390: 67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Ge N, Alford M, Veinbergs I, Roses AD 1995. Neurodegeneration in the central nervous system of apoE-deficient mice. Exp Neurol 136: 107–122 [DOI] [PubMed] [Google Scholar]
- Matsuki T, Pramatarova A, Howell BW 2008. Reduction of Crk and CrkL expression blocks reelin-induced dendritogenesis. J Cell Sci 121: 1869–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW 2001. CNS synaptogenesis promoted by glia-derived cholesterol. Science 294: 1354–1357 [DOI] [PubMed] [Google Scholar]
- May P, Rohlmann A, Bock HH, Zurhove K, Marth JD, Schomburg ED, Noebels JL, Beffert U, Sweatt JD, Weeber EJ, et al. 2004. Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. Mol Cell Biol 24: 8872–8883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin L, Zhu G, Mistry M, Ley-Ebert C, Stuart WD, Florio CJ, Groen PA, Witt SA, Kimball TR, Witte DP, et al. 2000. Apolipoprotein J/clusterin limits the severity of murine autoimmune myocarditis. J Clin Invest 106: 1105–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michikawa M, Fan QW, Isobe I, Yanagisawa K 2000. Apolipoprotein E exhibits isoform-specific promotion of lipid efflux from astrocytes and neurons in culture. J Neurochem 74: 1008–1016 [DOI] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA 2010. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 67: 122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Mucke L, Selkoe DJ 2011. Neurotoxicity of amyloid β-protein: Synaptic and network dysfunction. Cold Spring Harb Perspect Med 10.1101/cshperspect.a006338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudher A, Shepherd D, Newman TA, Mildren P, Jukes JP, Squire A, Mears A, Drummond JA, Berg S, MacKay D, et al. 2004. GSK-3β inhibition reverses axonal transport defects and behavioural phenotypes in Drosophila. Mol Psychiatry 9: 522–530 [DOI] [PubMed] [Google Scholar]
- Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K 1991. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer’s disease kuru plaque amyloid in Creutzfeldt-Jacob disease. Brain Res 541: 163–166 [DOI] [PubMed] [Google Scholar]
- Narita N, Holtzman DM, Schwartz AL, Bu G 1997. α2-Macroglobulin complexes with and mediates the endocytosis of β-amyloid peptide via cell surface low-density lipoprotein receptor-related protein. J Neurochem 69: 1904–1911 [DOI] [PubMed] [Google Scholar]
- Narita M, Holtzman DM, Fagan AM, LaDu MJ, Yu L, Han X, Gross RW, Bu G, Schwartz AL 2002. Cellular catabolism of lipid poor apolipoprotein E via cell surface LDL receptor-related protein. J Biochem 132: 743–749 [DOI] [PubMed] [Google Scholar]
- Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE 1994. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science 264: 850–852 [DOI] [PubMed] [Google Scholar]
- Nickerson DA, Taylor SL, Fullerton SM, Weiss KM, Clark AG, Stengard JH, Salomaa V, Boerwinkle E, Sing CF 2000. Sequence diversity and large-scale typing of SNPs in the human apolipoprotein E gene. Genome Res 10: 1532–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu S, Renfro A, Quattrocchi CC, Sheldon M, D’Arcangelo G 2004. Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron 41: 71–84 [DOI] [PubMed] [Google Scholar]
- Niu S, Yabut O, D’Arcangelo G 2008. The Reelin signaling pathway promotes dendritic spine development in hippocampal neurons. J Neurosci 28: 10339–10348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard HB, Strittmatter SM 2009. Cellular prion protein mediates the toxicity of β-amyloid oligomers: Implications for Alzheimer disease. Arch Neurol 66: 1325–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo N, Lee YD, Morishima A, Terashima T, Kikkawa S, Tohyama M, Sakanaka M, Tanaka J, Maeda N, Vitek MP, et al. 2003. Apolipoprotein E and Reelin ligands modulate tau phosphorylation through an apolipoprotein E receptor/disabled-1/glycogen synthase kinase-3β cascade. FASEB J 17: 295–297 [DOI] [PubMed] [Google Scholar]
- Palop JJ, Mucke L 2010. Amyloid-β-induced neuronal dysfunction in Alzheimer’s disease: From synapses toward neural networks. Nat Neurosci 13: 812–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, et al. 2007. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55: 697–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters MM, Hill KE, Burk RF, Weeber EJ 2006. Altered hippocampus synaptic function in selenoprotein P deficient mice. Mol Neurodegener 1: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfrieger FW 2003. Cholesterol homeostasis and function in neurons of the central nervous system. Cell Mol Life Sci 60: 1158–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzik CU, Yoon IS, Jaeger S, Busse T, Weggen S, Koo EH 2004. FE65 constitutes the functional link between the low-density lipoprotein receptor-related protein and the amyloid precursor protein. J Neurosci 24: 4259–4265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitas RE, Boyles JK, Lee SH, Foss D, Mahley RW 1987. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim Biophys Acta 917: 148–161 [DOI] [PubMed] [Google Scholar]
- Poirier J 2003. Apolipoprotein E and cholesterol metabolism in the pathogenesis and treatment of Alzheimer’s disease. Trends Mol Med 9: 94–101 [DOI] [PubMed] [Google Scholar]
- Pujadas L, Gruart A, Bosch C, Delgado L, Teixeira CM, Rossi D, de Lecea L, Martinez A, Delgado-Garcia JM, Soriano E 2010. Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J Neurosci 30: 4636–4649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzzo D, Privitera L, Leznik E, Fa M, Staniszewski A, Palmeri A, Arancio O 2008. Picomolar amyloid-β positively modulates synaptic plasticity and memory in hippocampus. J Neurosci 28: 14537–14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Zhao LF, Korwek KM, Weeber EJ 2006. Differential reelin-induced enhancement of NMDA and AMPA receptor activity in the adult hippocampus. J Neurosci 26: 12943–12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp A, Gmeiner B, Huttinger M 2006. Implication of apoE isoforms in cholesterol metabolism by primary rat hippocampal neurons and astrocytes. Biochimie 88: 473–483 [DOI] [PubMed] [Google Scholar]
- Rebeck GW, Reiter JS, Strickland DK, Hyman BT 1993. Apolipoprotein E in sporadic Alzheimer’s disease: Allelic variation and receptor interactions. Neuron 11: 575–580 [DOI] [PubMed] [Google Scholar]
- Rebeck GW, Harr SD, Strickland DK, Hyman BT 1995. Multiple, diverse senile plaque-associated proteins are ligands of an apolipoprotein receptor, the α2-macroglobulin receptor/low-density-lipoprotein receptor-related protein. Ann Neurol 37: 211–217 [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J, Reeder SA, Langbaum JB, et al. 2009. Fibrillar amyloid-β burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci 106: 6820–6825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner M, Lacor PN, Velasco PT, Xu J, Contractor A, Klein WL, Triller A 2010. Deleterious effects of amyloid β oligomers acting as an extracellular scaffold for mGluR5. Neuron 66: 739–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L 2007. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer’s disease mouse model. Science 316: 750–754 [DOI] [PubMed] [Google Scholar]
- Roberson ED, Halabisky B, Yoo JW, Yao J, Chin J, Yan F, Wu T, Hamto P, Devidze N, Yu GQ, et al. 2011. Amyloid-β/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. J Neurosci 31: 700–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, Katayama T, Baldwin CT, Cheng R, Hasegawa H, et al. 2007. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet 39: 168–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronicke R, Mikhaylova M, Ronicke S, Meinhardt J, Schroder UH, Fandrich M, Reiser G, Kreutz MR, Reymann KG 2010. Early neuronal dysfunction by amyloid β oligomers depends on activation of NR2B-containing NMDA receptors. Neurobiol Aging 10.1016/j.neurobiolaging.2010.01.011 [DOI] [PubMed] [Google Scholar]
- Rust MB, Gurniak CB, Renner M, Vara H, Morando L, Gorlich A, Sassoe-Pognetto M, Banchaabouchi MA, Giustetto M, Triller A, et al. 2010. Learning, AMPA receptor mobility and synaptic plasticity depend on n-cofilin-mediated actin dynamics. EMBO J 29: 1889–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski MJ, Pankiewicz J, Scholtzova H, Mehta PD, Prelli F, Quartermain D, Wisniewski T 2006. Blocking the apolipoprotein E/amyloid-β interaction as a potential therapeutic approach for Alzheimer’s disease. Proc Natl Acad Sci 103: 18787–18792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, Marky A, Lenting PJ, Wu Z, Zarcone T, et al. 2007. Clearance of amyloid-β by circulating lipoprotein receptors. Nat Med 13: 1029–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Saido T, Leissring MA 2011. Proteolytic degradation of amyloid β-protein. Cold Spring Harb Perspect Med 10.1011/cshperspect.a006379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, Kalia LV 2004. Src kinases: A hub for NMDA receptor regulation. Nat Rev Neurosci 5: 317–328 [DOI] [PubMed] [Google Scholar]
- Sanan DA, Weisgraber KH, Russel SJ, Mahley RW, Huang D, Saunders A, Schmechel D, Wisniewksi T, Frangione B, Roses B, et al. 1994. Apolipoprotein E associates with β amyloid peptide of Alzheimer’s disease to form novel monofibrils. J Clin Invest 94: 860–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer EJ, Heaton WH, Wetzel MG, Brewer HB Jr 1982. Plasma apolipoprotein A-1 absence associated with a marked reduction of high density lipoproteins and premature coronary artery disease. Arteriosclerosis 2: 16–26 [DOI] [PubMed] [Google Scholar]
- Scherzer CR, Offe K, Gearing M, Rees HD, Fang G, Heilman CJ, Schaller C, Bujo H, Levey AI, Lah JJ 2004. Loss of apolipoprotein E receptor LR11 in Alzheimer disease. Arch Neurol 61: 1200–1205 [DOI] [PubMed] [Google Scholar]
- Schmechel DE, Saunders AM, Strittmattter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD 1993. Increased amyloid β-peptide deposition in cerebral cortex as a consequence of apolipoprotein genotype in late-onset Alzheimer disease. Proc Natl Acad Sci 90: 9649–9653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL 2007. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci 27: 2866–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. 2008. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med 14: 837–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon M, Rice DS, D’Arcangelo G, Yoneshima H, Nakajima K, Mikoshiba K, Howell BW, Cooper JA, Goldowitz D, Curran T 1997. Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice. Nature 389: 730–733 [DOI] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, et al. 2000. Clearance of Alzheimer’s amyloid-β1–40 peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest 106: 1489–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T, Ayala R, Nguyen MD, Xie Z, Gleeson JG, Tsai LH 2004. Ndel1 operates in a common pathway with LIS1 and cytoplasmic dynein to regulate cortical neuronal positioning. Neuron 44: 263–277 [DOI] [PubMed] [Google Scholar]
- Sinagra M, Verrier D, Frankova D, Korwek KM, Blahos J, Weeber EJ, Manzoni OJ, Chavis P 2005. Reelin, very-low-density lipoprotein receptor, and apolipoprotein E receptor 2 control somatic NMDA receptor composition during hippocampal maturation in vitro. J Neurosci 25: 6127–6136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, et al. 2005. Regulation of NMDA receptor trafficking by amyloid-β. Nat Neurosci 8: 1051–1058 [DOI] [PubMed] [Google Scholar]
- Sorci-Thomas M, Prack MM, Dashti N, Johnson F, Rudel LL, Williams DL 1988. Apolipoprotein (apo) A-I production and mRNA abundance explain plasma apoA-I and high density lipoprotein differences between two nonhuman primate species with high and low susceptibilities to diet-induced hypercholesterolemia. J Biol Chem 263: 5183–5189 [PubMed] [Google Scholar]
- Stockinger W, Brandes C, Fasching D, Hermann M, Gotthardt M, Herz J, Schneider WJ, Nimpf J 2000. The reelin receptor ApoER2 recruits JNK-interacting proteins-1 and -2. J Biol Chem 275: 25625–25632 [DOI] [PubMed] [Google Scholar]
- Stolt PC, Chen Y, Liu P, Bock HH, Blacklow SC, Herz J 2005. Phosphoinositide binding by the disabled-1 PTB domain is necessary for membrane localization and Reelin signal transduction. J Biol Chem 280: 9671–9677 [DOI] [PubMed] [Google Scholar]
- Strasser V, Fasching D, Hauser C, Mayer H, Bock HH, Hiesberger T, Herz J, Weeber EJ, Sweatt JD, Pramatarova A, et al. 2004. Receptor clustering is involved in Reelin signaling. Mol Cell Biol 24: 1378–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD 1993a. Apolipoprotein E: High avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci 90: 1977–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Weisgraber KH, Huang DY, Dong L-Y, Salvesen GS, Pericak-Vance M, Schmechel D, Saunders AM, Goldgaber D, Roses AD 1993b. Binding of human apolipoprotein E to synthetic amyloid β peptide: Isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci 90: 8098–8102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland T, Mirza N, Putnam KT, Linker G, Bhupali D, Durham R, Soares H, Kimmel L, Friedman D, Bergeson J, et al. 2004. Cerebrospinal fluid β-amyloid1-42 and tau in control subjects at risk for Alzheimer’s disease: The effect of APOE ε4 allele. Biol Psychiatry 56: 670–676 [DOI] [PubMed] [Google Scholar]
- Sydow A, Van der Jeugd A, Zheng F, Ahmed T, Balschun D, Petrova O, Drexler D, Zhou L, Rune G, Mandelkow E, et al. 2011. Tau-induced defects in synaptic plasticity, learning, and memory are reversible in transgenic mice after switching off the toxic tau mutant. J Neurosci 31: 2511–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamizu-Kato S, Cohen JK, Drake CB, Kosaraju MG, Drury J, Narayanaswami V 2008. Interaction with amyloid β peptide compromises the lipid binding function of apolipoprotein E. Biochemistry 47: 5225–5234 [DOI] [PubMed] [Google Scholar]
- Terrand J, Bruban V, Zhou L, Gong W, El Asmar Z, May P, Zurhove K, Haffner P, Philippe C, Woldt E, et al. 2009. LRP1 controls intracellular cholesterol storage and fatty acid synthesis through modulation of Wnt signaling. J Biol Chem 284: 381–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiraboschi P, Hansen LA, Masliah E, Alford M, Thal LJ, Corey-Bloom J 2004. Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology 62: 1977–1983 [DOI] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM 2003. Reelin and brain development. Nat Rev Neurosci 4: 496–505 [DOI] [PubMed] [Google Scholar]
- Tokuda T, Calero M, Matsubara E, Vidal R, Kumar A, Permanne B, Zlokovic B, Smith JD, Ladu MJ, Rostagno A, et al. 2000. Lipidation of apolipoprotein E influences its isoform-specific interaction with Alzheimer’s amyloid β peptides. Biochem J 348: 359–365 [PMC free article] [PubMed] [Google Scholar]
- Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ 2006. Effects of secreted oligomers of amyloid β-protein on hippocampal synaptic plasticity: A potent role for trimers. J Physiol 572: 477–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommsdorff M, Borg JP, Margolis B, Herz J 1998. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J Biol Chem 273: 33556–33560 [DOI] [PubMed] [Google Scholar]
- Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J 1999. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 97: 689–701 [DOI] [PubMed] [Google Scholar]
- Ulery PG, Beers J, Mikhailenko I, Tanzi RE, Rebeck GW, Hyman BT, Strickland DK 2000. Modulation of β-amyloid precursor protein processing by the low density lipoprotein receptor-related protein (LRP). Evidence that LRP contributes to the pathogenesis of Alzheimer’s disease. J Biol Chem 275: 7410–7415 [DOI] [PubMed] [Google Scholar]
- Van Uden E, Mallory M, Ieinbergs I, Alford M, Rockenstein E, Masliah E 2002. Increased extracellular amyloid deposition and neurodegeneration in human amyloid precursor protein transgenic mice deficient in receptor-associated protein. J Neurosci 22: 9298–9304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Higuera JL, Mateo I, Sanchez-Juan P, Rodriguez-Rodriguez E, Pozueta A, Infante J, Berciano J, Combarros O 2009. Genetic interaction between tau and the apolipoprotein E receptor LRP1 Increases Alzheimer’s disease risk. Dement Geriat Cogn Disord 28: 116–120 [DOI] [PubMed] [Google Scholar]
- Wahrle SE, Jiang H, Parsadanian M, Hartman RE, Bales KR, Paul SM, Holtzman DM 2005. Deletion of Abca1 increases Aβ deposition in the PDAPP transgenic mouse model of Alzheimer disease. J Biol Chem 280: 43236–43242 [DOI] [PubMed] [Google Scholar]
- Wahrle SE, Jiang H, Parsadanian M, Kim J, Li A, Knoten A, Jain S, Hirsch-Reinshagen V, Wellington CL, Bales KR, et al. 2008. Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J Clin Invest 118: 671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ 2002. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416: 535–539 [DOI] [PubMed] [Google Scholar]
- Weeber EJ, Beffert U, Jones C, Christian JM, Forster E, Sweatt JD, Herz J 2002. Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J Biol Chem 277: 39944–39952 [DOI] [PubMed] [Google Scholar]
- Willnow TE, Hilpert J, Armstrong SA, Rohlmann A, Hammer RE, Burns DK, Herz J 1996. Defective forebrain development in mice lacking gp330/megalin. Proc Natl Acad Sci 93: 8460–8464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T, Frangione B 1992. Apolipoprotein E: A pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett 135: 235–238 [DOI] [PubMed] [Google Scholar]
- Wisniewski T, Castano EM, Golabek A, Vogel T, Frangione B 1994. Acceleration of Alzheimer’s fibril formation by apolipoprotein E in vitro. Am J Pathol 145: 1030–1035 [PMC free article] [PubMed] [Google Scholar]
- Wood SJ, Chan W, Wetzel R 1996. Seeding of Aβ fibril formation is inhibited by all three isotypes of apolipoprotein E. Biochemistry 35: 12623–12628 [DOI] [PubMed] [Google Scholar]
- Xu P-T, Schmechel D, Qiu H-L, Herbstreith M, Rothrock-Christian T, Eyster M, Roses AD, Gilbert JR 1999. Sialylated human apolipoprotein E (apoEs) is preferentially associated with neuron-enriched cultures from APOE transgenic mice. Neurobiol Dis 6: 63–75 [DOI] [PubMed] [Google Scholar]
- Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y 2006. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci 26: 4985–4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D-S, Smith JD, Zhou Z, Gandy S, Martins RN 1997. Characterization of the binding of amyloid-β peptide to cell culture-derived native apolipoprotein E2, E3, and E4 isoforms and to isoforms from human plasma. J Neurochem 68: 721–725 [DOI] [PubMed] [Google Scholar]
- Yang DS, Small DH, Seydel U, Smith JD, Hallmayer J, Gandy SE, Martins RN 1999. Apolipoprotein E promotes the binding and uptake of β-amyloid into Chinese hamster ovary cells in an isoform-specific manner. Neuroscience 90: 1217–1226 [DOI] [PubMed] [Google Scholar]
- Zerbinatti CV, Wozniak DF, Cirrito J, Cam JA, Osaka H, Bales KR, Zhuo M, Paul SM, Holtzman DM, Bu G 2004. Increased soluble amyloid-β peptide and memory deficits in amyloid model mice overexpressing the low-density lipoprotein receptor-related protein. Proc Natl Acad Sci 101: 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbinatti CV, Wahrle SE, Kim H, Cam JA, Bales K, Paul SM, Holtzman DM, Bu G 2006. Apolipoprotein E and low density lipoprotein receptor-related protein facilitate intraneuronal Aβ42 accumulation in amyloid model mice. J Biol Chem 281: 36180–36186 [DOI] [PubMed] [Google Scholar]
- Zhuo M, Holtzman DM, Li Y, Osaka H, DeMaro J, Jacquin M, Bu G 2000. Role of tissue plasminogen activator receptor LRP in hippocampal long-term potentiation. J Neurosci 20: 542–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV 2008. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57: 178–201 [DOI] [PubMed] [Google Scholar]
- Zurhove K, Nakajima C, Herz J, Bock HH, May P 2008. γ-secretase limits the inflammatory response through the processing of LRP1. Sci Signal 1: ra15. [DOI] [PMC free article] [PubMed] [Google Scholar]