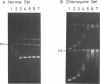
Abstract
In vitro transcription was reconstituted with HeLa cell transcription factors and RNA polymerase II, which were essentially free from DNA topoisomerase activities. DNA templates with defined negative superhelical densities were tested for transcription activity. Transcription of the Bombyx mori fibroin gene increases and plateaus from templates of increasing superhelicity, and transcription from the adenovirus 2 major late promoter rises and then falls, while transcription of the Drosophila hsp70 gene remains unchanged. Dissection of transcription into pre and post-initiation steps by the use of Sarkosyl reveals that formation of a preinitiation complex on the fibroin gene or the adenovirus 2 major late promoter is slow on relaxed DNA and accelerated by DNA superhelicity. On the contrary, the preinitiation complex assembles rapidly on the hsp70 gene irrespective of DNA topology. As is the case with the fibroin gene promoter, DNA superhelicity appears to facilitate the interaction of transcription factor IID to the adenovirus 2 major late promoter.
Full text
PDF
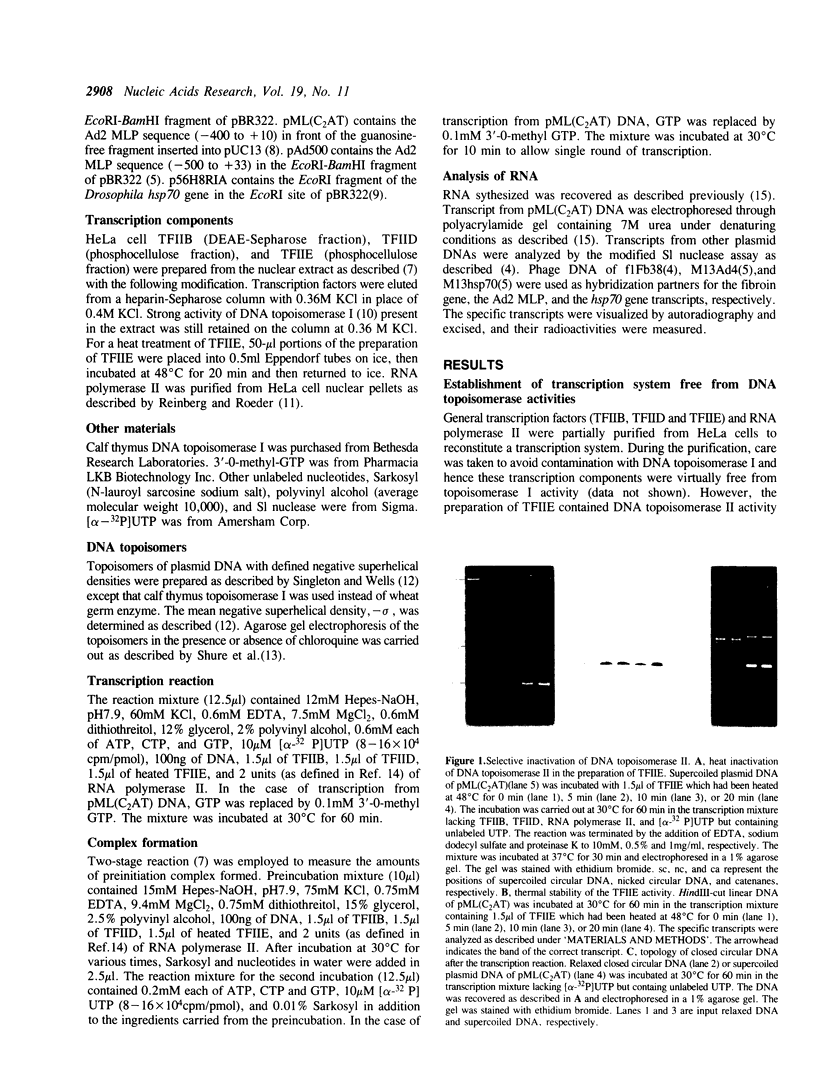
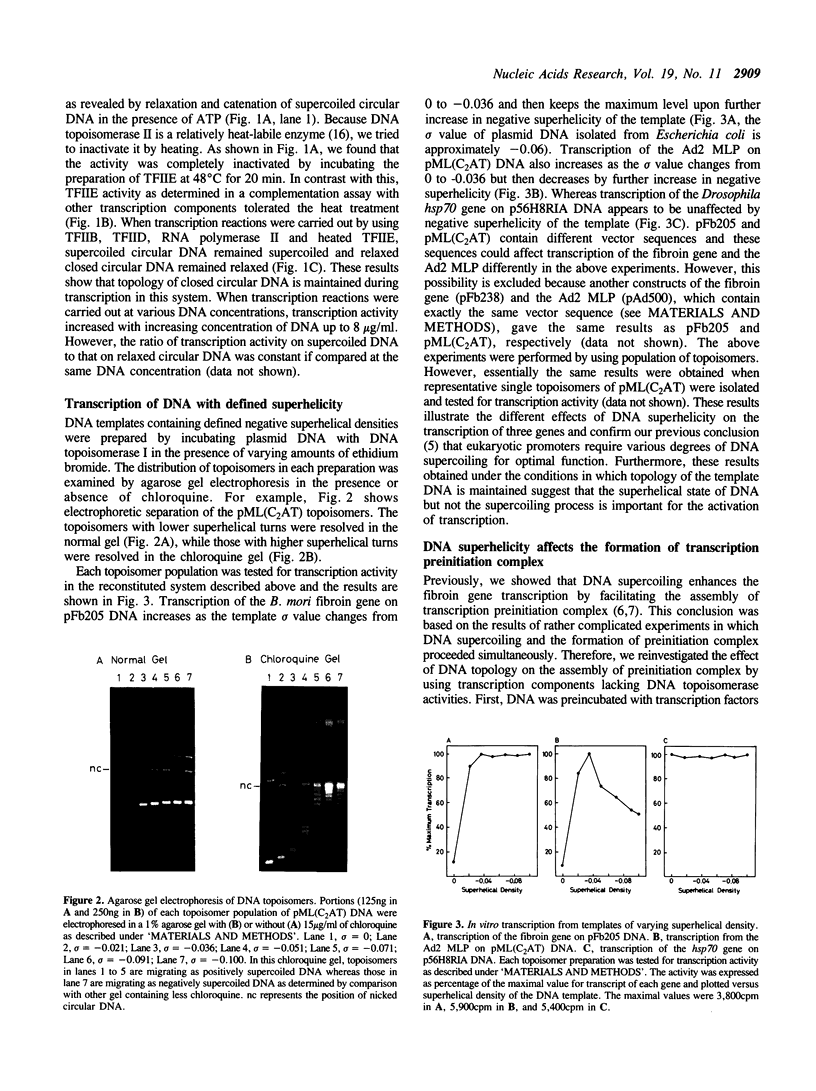
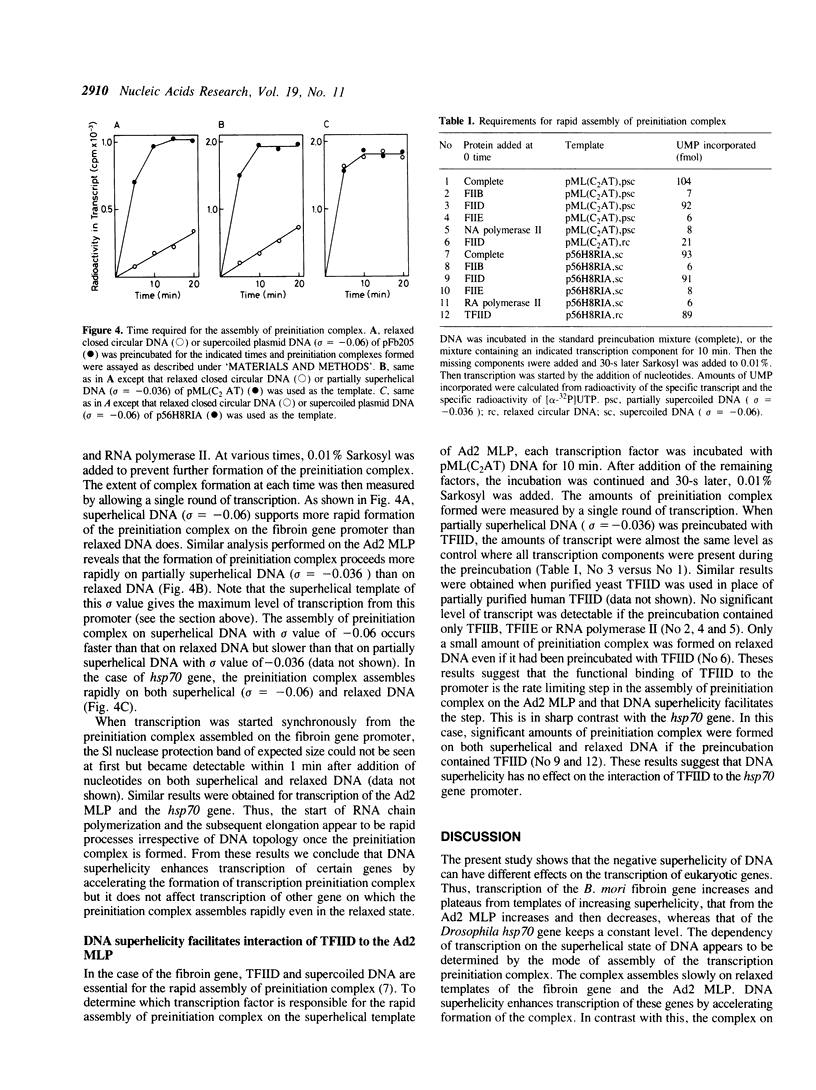

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Courey A. J., Wang J. C. Cruciform formation in a negatively supercoiled DNA may be kinetically forbidden under physiological conditions. Cell. 1983 Jul;33(3):817–829. doi: 10.1016/0092-8674(83)90024-7. [DOI] [PubMed] [Google Scholar]
- Fire A., Samuels M., Sharp P. A. Interactions between RNA polymerase II, factors, and template leading to accurate transcription. J Biol Chem. 1984 Feb 25;259(4):2509–2516. [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., Roeder R. G. Separation and partial characterization of three functional steps in transcription initiation by human RNA polymerase II. J Biol Chem. 1985 Jul 5;260(13):8163–8172. [PubMed] [Google Scholar]
- Hirose S., Suzuki Y. In vitro transcription of eukaryotic genes is affected differently by the degree of DNA supercoiling. Proc Natl Acad Sci U S A. 1988 Feb;85(3):718–722. doi: 10.1073/pnas.85.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose S., Tsuda M., Suzuki Y. Enhanced transcription of fibroin gene in vitro on covalently closed circular templates. J Biol Chem. 1985 Sep 5;260(19):10557–10562. [PubMed] [Google Scholar]
- Horikoshi M., Hai T., Lin Y. S., Green M. R., Roeder R. G. Transcription factor ATF interacts with the TATA factor to facilitate establishment of a preinitiation complex. Cell. 1988 Sep 23;54(7):1033–1042. doi: 10.1016/0092-8674(88)90118-3. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L., Tsai S. Y., Weigel N. L., Allan G. F., Riley D., Rodriguez R., Schrader W. T., Tsai M. J., O'Malley B. W. The progesterone receptor stimulates cell-free transcription by enhancing the formation of a stable preinitiation complex. Cell. 1990 Jan 26;60(2):247–257. doi: 10.1016/0092-8674(90)90740-6. [DOI] [PubMed] [Google Scholar]
- Liu L. F. HeLa toposiomerase I. Methods Enzymol. 1983;100:133–137. [PubMed] [Google Scholar]
- Mizutani M., Ohta T., Watanabe H., Handa H., Hirose S. Negative supercoiling of DNA facilitates an interaction between transcription factor IID and the fibroin gene promoter. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):718–722. doi: 10.1073/pnas.88.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osheroff N., Shelton E. R., Brutlag D. L. DNA topoisomerase II from Drosophila melanogaster. Relaxation of supercoiled DNA. J Biol Chem. 1983 Aug 10;258(15):9536–9543. [PubMed] [Google Scholar]
- Reinberg D., Roeder R. G. Factors involved in specific transcription by mammalian RNA polymerase II. Purification and functional analysis of initiation factors IIB and IIE. J Biol Chem. 1987 Mar 5;262(7):3310–3321. [PubMed] [Google Scholar]
- Rougvie A. E., Lis J. T. The RNA polymerase II molecule at the 5' end of the uninduced hsp70 gene of D. melanogaster is transcriptionally engaged. Cell. 1988 Sep 9;54(6):795–804. doi: 10.1016/s0092-8674(88)91087-2. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Factors involved in specific transcription by human RNA polymerase II: analysis by a rapid and quantitative in vitro assay. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4394–4398. doi: 10.1073/pnas.82.13.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Shure M., Pulleyblank D. E., Vinograd J. The problems of eukaryotic and prokaryotic DNA packaging and in vivo conformation posed by superhelix density heterogeneity. Nucleic Acids Res. 1977;4(5):1183–1205. doi: 10.1093/nar/4.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden R. R., Carlson J. O., Pettijohn D. E. Torsional tension in the DNA double helix measured with trimethylpsoralen in living E. coli cells: analogous measurements in insect and human cells. Cell. 1980 Oct;21(3):773–783. doi: 10.1016/0092-8674(80)90440-7. [DOI] [PubMed] [Google Scholar]
- Singleton C. K., Wells R. D. The facile generation of covalently closed, circular DNAs with defined negative superhelical densities. Anal Biochem. 1982 May 15;122(2):253–257. doi: 10.1016/0003-2697(82)90277-9. [DOI] [PubMed] [Google Scholar]
- Tabuchi H., Hirose S. DNA supercoiling facilitates formation of the transcription initiation complex on the fibroin gene promoter. J Biol Chem. 1988 Oct 25;263(30):15282–15287. [PubMed] [Google Scholar]
- Tsujimoto Y., Hirose S., Tsuda M., Suzuki Y. Promoter sequence of fibroin gene assigned by in vitro transcription system. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4838–4842. doi: 10.1073/pnas.78.8.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Török I., Karch F. Nucleotide sequences of heat shock activated genes in Drosophila melanogaster. I. Sequences in the regions of the 5' and 3' ends of the hsp 70 gene in the hybrid plasmid 56H8. Nucleic Acids Res. 1980 Jul 25;8(14):3105–3123. doi: 10.1093/nar/8.14.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Imai T., Sharp P. A., Handa H. Identification of two transcription factors that bind to specific elements in the promoter of the adenovirus early-region 4. Mol Cell Biol. 1988 Mar;8(3):1290–1300. doi: 10.1128/mcb.8.3.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. Two protein-binding sites in chromatin implicated in the activation of heat-shock genes. Nature. 1984 May 17;309(5965):229–234. doi: 10.1038/309229a0. [DOI] [PubMed] [Google Scholar]