Abstract
Background
Peripheral irritation-induced sensory plasticity may involve catecholaminergic innervation of sensory neurons in the dorsal root ganglia (DRG).
Methods
Catecholaminergic fiber outgrowth in the thoracolumbar DRG (T13-L2) was examined by tyrosine hydroxylase (TH) immunostaining, or by sucrosepotassium phosphate-glyoxylic acid histofluorescence method. TH level was examined by Western blot. Colonic afferent neurons were labeled by retrograde neuronal tracing. Colitis was induced by intracolonic instillation of tri-nitrobenzene sulfonic acid (TNBS).
Key Results
The catecholaminergic fibers formed ‘basket-like’ structures around the DRG cells. At 7 days following TNBS treatment, the number of DRG neurons surrounded by TH-immunoreactive fibers and the protein levels of TH were significantly increased in T13, L1, and L2 DRGs (two- to threefold, P < 0.05). The DRG neurons that were surrounded by TH immunoreactivity were 200 kDa neurofilament-positive, but not isolectin IB4-positve or calcitonin gene-related peptide-positive. The TH-immunoreactive fibers did not surround but adjoin the specifically labeled colonic afferent neurons, and was co-localized with glial marker S-100. Comparison of the level of TH and the severity of colonic inflammation showed that following TNBS treatment, the degree of colonic inflammation was most severe at day 3, subsided at day 7, and significantly recovered by day 21. However, the levels of TH in T13-L2 DRGs were increased at both 3 days and 7 days post TNBS treatment and persisted up to 21 days (two- to fivefold increase, P < 0.05) as examined.
Conclusions & Inferences
Colonic inflammation induced prolonged catecholaminergic innervation of sensory neurons, which may have relevance to colitis-induced chronic visceral hypersensitivity and/or referred pain.
Keywords: colitis, dorsal root ganglia, rat, sympathetic sprouting, tyrosine hydroxylase
INTRODUCTION
The catecholamine norepinephrine is a neuromodulator of the peripheral sympathetic nervous system. Tyrosine hydroxylase (TH), a rate-limiting enzyme responsible for catalyzing the conversion of L-tyrosine to the precursor of dopamine and then norepinephrine and epinephrine,1 has been widely used as a marker to detect sympathetic nerve endings. Postganglionic sympathetic innervation of sensory neurons in the dorsal root ganglia (DRG) was discovered a century ago in both humans and animals, and has been reviewed by Garcia-Poblete et al.2 Sympathetic fibers sprout into the DRG where they form ring-like structures surrounding the sensory neurons, resembling synapse and affecting the function of sensory neurons.3–5 This type of sympathetic outflow and the coupled sensory inflow may play a significant role in the generation and maintenance of neuropathic pain and/or inflammation-induced hyperalgesia.
The involvement of sympathetic nervous system in sensory activity and neuropathic pain has been suggested from several experimental animal models showing that chemical or surgical sympathectomy alleviated nerve injury-induced pain behaviors including mechanical allodynia and thermal hyperalgesia6–9; in turn, in vitro stimulation of sympathetic fibers increased the excitability and spontaneous activity of rat sensory neurons.10 The role of sympathetic nervous system in peripheral nerve injury-induced pain was also demonstrated in some patients showing that interruption of the sympathetic supply or manipulation of the sympathetic activity modified pain sensation. 11 The mechanism underlying the excess sympathetic input on the sensory activity in neuropathic pain state has been proposed showing that the extent of sympathetic sprouting to the sensory neurons in the DRG were well correlated to the degree of pain behaviors caused by peripheral nerve injury.4,12 The increases in sympathetic sprouting in the lumbar DRG were also observed in a model of inflammation-induced neuropathic pain when the DRG itself was inflamed.13,14
Inflammatory irritation to the peripheral organ can also affect the activity of sympathetic system and sensory neurons. This is particularly true for colonic inflammation (colitis). In patients with ulcerative colitis or irritable bowel syndrome (IBS), an increase in sympathetic activity has been noted as assessed by spectral analysis of heart rate variability and skin conductance.15,16 These pathophysiologic states also correspond with visceral hypersensitivity consistent with increased levels of neurotrophins, neuropeptides, and electrophysiologic activities in the DRG in rodent models of colonic inflammation.17–21 In this form of visceral pain, it was not known if there were changes in sympathetic innervation to the afferent neurons, and was examined in the present study.
Postinflammatory visceral pain and sensory hypersensitivity have been observed in animal models of colonic inflammation,22,23 and have been considered as one of the possible mechanisms underlying IBS in patients.24 This also suggests a prolonged neuroplasticity in the primary sensory pathways post colonic inflammation. The present study aimed to examine if colonic inflammation induced catecholaminergic sprouting to the DRG neurons. We examined the immunoreactive profile of TH in the DRG during (at 3 days and 7 days) and post (at 21 days) colonic inflammation induced by tri-nitrobenzene sulfonic acid (TNBS) in rat. Studies with neural retrograde tracing demonstrated the segmental distribution pattern of colonic primary afferents in thoracolumbar and lumbosacral DRGs that were at the spinal levels T13-L2 and L6-S119,25,26; the two peaks of labeling are likely to be due to innervation from the splanchnic and pelvic afferents 27 respectively. Thus, in the present study, we examined the thoracolumbar segment T13, L1, and L2 DRGs for the catecholaminergic innervation with sacral S1 segment as control in visceral pain state during and post colonic inflammation.
EXPERIMENTAL PROCEDURE
Induction of colonic inflammation
Adult male Sprague–Dawley rats (150–200 g) from Harlan Sprague Dawley, Inc., (Indianapolis, IN, USA) were used for this study. To induce inflammation in the distal colon, fasted rats were anesthetized (2% isoflurane, SurgiVet, Smiths Medical PM, Inc., Waukesha, WI, USA) and TNBS was instilled into the lumen of the colon 6 cm proximal to the anus at a dose of 90 mg kg−1 (1.5 mL kg−1 of 60 mg mL−1 solution in 50% EtOH) through a polyethylene catheter. Control animals received a similar volume of 50% EtOH. All protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee, and animal care was in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care and National Institutes of Health guidelines. All efforts were made to minimize the potential for animal pain, stress or distress. Animals were divided into control (n = 14) and colitis groups for different time course (3 days, n = 4; 7 days, n = 14; and 21 days, n = 8). Among these animals, 11 animals (four for controls, four for TNBS 7 days, and three for TNBS 21 days) were used for Western blot, 6 animals (three for controls and three for TNBS 7 days) for retrograde neuronal dye labeling of colonic afferent neurons, and the rest for immunohistochemistry and histofluorescence staining (see below).
Retrograde labeling
Under anesthesia (2.5% isoflurane, SurgiVet, Smiths Medical PM, Inc., Waukesha, WI, USA), the rat distal colon was exposed under a sterile environment with a lower abdominal incision. A neuronal tracing agent, Fast Blue (FB, 4% weight per volume; Polysciences, Inc., Warrington, PA, USA), was injected at 4 μL per site for 10 sites into the muscle wall of the descending colon (5–7 cm proximal to the external anal sphincter) to label colonic afferent neurons innervating this area. A sterilized Hamilton syringe (10 μL size) was used for injection. To prevent labeling of adjacent tissues, the needle was left in place for 30 s after each injection and a cotton swab was held close to the injection site to wipe off any excess dye that might leak from the needle tip during the needle withdrawal. After injection, the incision was closed with 4-0 sutures. The distribution of labeled DRG neurons was examined and was consistent to our previous findings.19
Perfusion and tissue harvesting
Animals were deeply anesthetized with isoflurane (2–3%) and then underwent euthanasia via intracardiac perfusion with oxy-genated Krebs buffer (pH 7.4; 95% O2, 5% CO2) followed by 4% paraformaldehyde at the designated time point after TNBS treatment. The thoracolumbar (T13-L2) and sacral (S1) DRG were identified and isolated. Following post-fixation and dehydration, the DRG underwent cryosectioning parasagitally at a thickness of 20 μm. Tissues from control and inflamed animals were handled in a similar manner.
Immunohistochemistry
An on-slide technique was used for immunostaining of the DRG sections. DRG sections were incubated with blocking solution containing 3% normal donkey serum in PBST (0.3% Triton X-100 in 0.1 mol L−1 PBS, pH 7.4) for 30 min, followed by rabbit anti-tyrosine hydroxylase (TH, 1 : 1000; Chemicon International Inc., Temecula, CA, USA) primary antibody overnight at room temperature. Following rinse (3 × 10 min with 0.1 mol L−1 PBS), sections were incubated with fluorescence-conjugated species-specific secondary antibody Alexa 594 [1 : 500; Molecular Probes (Invitrogen), Carlsbad, CA, USA] for 2 h. The slides were coverslipped with Citifluor (Citifluor Ltd, London, UK). Some of the slides underwent double immunostaining for the study of distribution of TH immunoreactivity associated with different subtypes of DRG population. The markers used for differentiation of DRG neuron subtypes were 200 kDa neurofilament recognized by RT97 mouse monoclonal antibody (1 : 400; Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA), mouse anticalcitonin gene-related peptide (CGRP, 1 : 2000, Abcam Inc., MA, USA), and isolectin IB4 [1 : 500; Molecular Probes (Invitrogen)]. Mouse anti-S100 (1 : 400, Millipore, Billerica, MA, USA) was used to identify glial cells and nerve fibers. Alexa 488-conjugated secondary antibody was used to visualize the markers where applicable. DAPI [1 : 500; Molecular Probes (Invitrogen)] was used to visualize the nucleus of the DRG cells (both neurons and satellite glial cells). Immunostaining in the absence of primary or secondary antibody was processed for control conditions. The specificity of TH antibody was examined by Western blot.
Dorsal root ganglia cells were counted under a Zeiss fluorescent photomicroscope (Carl Zeiss, Inc., Thornwood, NY, USA). Dorsal root ganglia neurons surrounded by TH-positive fibers were counted in four to six sections of each selected DRG (T13-L2 and S1), and expressed as mean ± SE for n animals. Within the specific segment of DRG (such as L1 DRG), similar sized sections were chosen and all the positive cells were counted in the sections using the microscope measurement program. The results were expressed as number of cells per section. Only differences between TNBS-treated and untreated animals were compared for a specific segment of DRG. No comparison was made between different segments of the DRG due to size discrepancy. We have chosen every third section for TH staining to avoid double counting.
Histofluorescence staining
Dorsal root ganglia sections were subject to histofluorescence staining with a solution composed of sucrose, potassium phosphate, and glyoxylic acid (SPG) for the visualization of catecholamines. 28 The SPG solution (pH 7.4) contains 10.2 g sucrose, 4.8 g monobasic KH2PO4 and 1.5 g glyoxylic acid monohydride prepared in 150 mL distilled water. Following sectioning, the DRG slides were immediately dipped three times (about one dip per second) in SPG solution at room temperature, and was dried with cold air from a hairblower. The slides were mounted with light mineral oil and heated for 2.5 min at 95 ° C. The slides were then coverslipped with an additional drop of mineral oil. The staining was visualized under a fluorescent microscope with a filter set with wavelength: ex 485 ± 20 nm/em 515–565. Some of the slides were pre-stained with DAPI to identify the cellular profiles.
Western blot
Proteins extracted from freshly isolated DRG were separated on a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane. The membrane was blocked with 5% milk in Tris-buffered saline for 1 h and then incubated with rabbit anti-TH (1 : 1000) antibody overnight in a cold room. After rinse, the membrane was then incubated with an HRP-conjugated secondary antibody for 1 h at room temperature. For internal loading control, the same membrane was striped and re-probed with anti-β-actin. The bands were visualized with enhanced chemiluminescence technique. Densitometric quantitation of immunoreactive bands was performed using the software FluorChem 8800 (Alpha Innotech, San Leabdro, CA, USA). The density of the specific band for TH was normalized with the density of the band for β-actin, and expressed as a fold change. A single band at a molecular weight of 62 kDa was detected for TH immunoblotting, indicating the specificity of the antibody used (Fig. S1).
Hematoxylin and eosin (H&E) stain to assess the severity of colonic inflammation
The distal colons from all animals were sectioned transversely at a thickness of 7 μm and were stained with an H&E staining kit according to the protocol provided by the manufacture (Richard-Allan-Scientific, Kalamazoo, MI, USA). The sections were examined with a Zeiss brightfield microscope. The histology score was graded to reflect the severity of the colonic inflammation (1, no inflammation; 2, very low inflammation; 3, low level of infiltration; 4, high level of infiltration and vascular density; 5, transmural infiltrations, loss of goblet cells, and high vascular density). The thickness of the muscular wall, the width of the submucosal space, the depth of the mucosal layer and the average width of the crypts were also measured independently with the built-in measurement software.
Statistical analysis
The results from each study were presented as mean ± SE for n animals. Comparison between control and experimental groups was made by using one-way ANOVA followed by Dunnett’s test. A Student’s t-test was used for comparison of two groups. Differences between means at a level of P ≤ 0.05 were considered to be significant.
RESULTS
Increase in tyrosine hydroxylase immunoreactivity in thoracolumbar but not sacral DRG during colonic inflammation
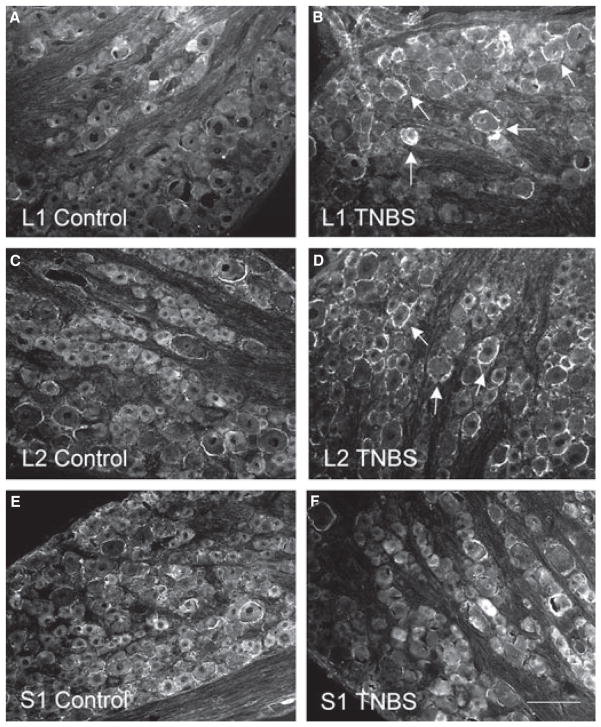
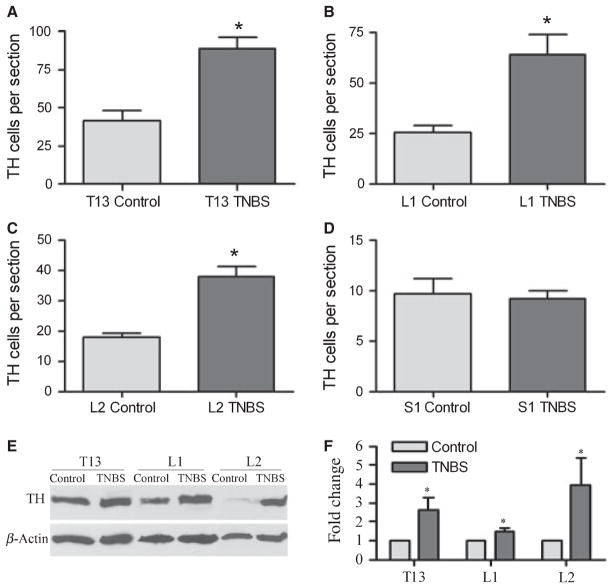
Tyrosine hydroxylase immunoreactivity was examined in the thoracolumbar T13, L1, and L2 DRGs and the sacral S1 DRG from control animals and animals treated with TNBS (Fig. 1, arrows). In control animals, there was baseline TH immunoreactivity surrounding the DRG neurons (Fig. 1A, C, E). At 7 days post colitis induction, the number of DRG neurons surrounded by TH immunoreactivity was significantly (P < 0.05) increased (Figs 1B, D, and 2A–C) in T13, L1, and L2 DRGs by 2- to 2.5-fold (T13: control, 41.6 ± 6.2; TNBS: 89.1 ± 7.0 cells per DRG section. L1: control, 25.3 ± 3.7; TNBS: 63.8 ± 10.2 cells per DRG section. L2: control, 17.7 ± 1.6; TNBS: 37.9 ± 3.3 cells per DRG section). In comparison, we examined the TH cell profiles in the sacral S1 DRG (Fig. 1E, F), and found that there were no changes (Fig. 2D) in the number of cells associated with TH immunoreactivity during colitis (9.2 ± 0.8 cells per DRG section) when compared with control (9.6 ± 1.5 cells per DRG section). The increases in TH levels in the thoracolumbar DRGs at 7 days of colitis were further confirmed with Western blot analysis (Fig. 2E, F).
Figure 1.
Immuno-profile of tyrosine hydroxylase (TH) immunoreactivity in thoracolumbosacral dorsal root ganglia (DRG). Photomicrographs showed that TH immunoreactivity was at very low baseline levels in all DRGs from control animals (A, C, E), and there were more cells immunoreactive to TH (arrows in B and D) in the thoracolumbar levels of DRG (examples showed L1 and L2 DRGs) but not in the sacral level of DRG (F) from animals sacrificed at day 7 post tri-nitrobenzene sulfonic acid treatment. Bar = 100 μm.
Figure 2.
Increases in tyrosine hydroxylase (TH) expression in thoracolumbar dorsal root ganglias (DRGs) but not in sacral DRG following tri-nitrobenzene sulfonic acid (TNBS) treatment. At day 7 post TNBS treatment, animals demonstrated significant increases in the levels of TH immunoreactivity in T13 (A), L1 (B), and L2 (C) DRGs (2- to 3.5-fold) when compared to their matched controls. In sacral S1 DRG, there were no significant differences in the level of TH immunoreactivity detected between the control and TNBS-treated groups (D). Western blot analysis (E) confirmed the increase in TH levels in T13, L1, and L2 DRGs at day 7 of colitis (F). *P < 0.05.
Formation of ‘basket-like’ structure surrounding the DRG neurons by thread-like catecholaminergic fibers during colitis
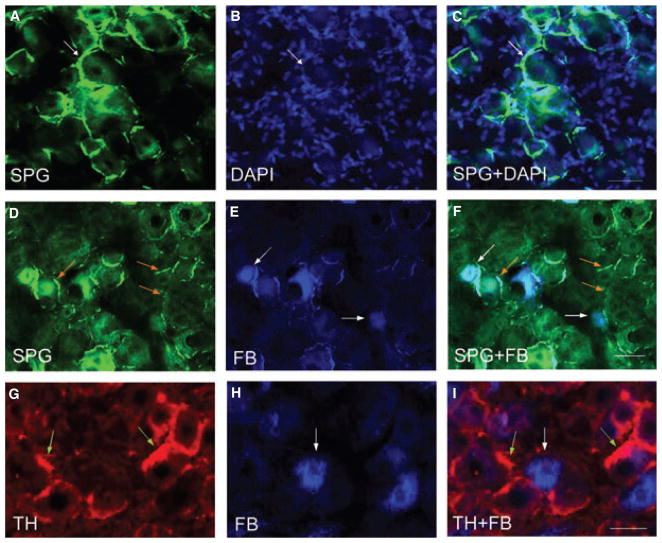
Increases in the TH immunoreactive structures in the thoracolumbar but not sacral DRG during colitis indicate that colonic inflammation induced an increase in sympathetic input into these DRGs. To better visualize the fine catecholaminergic fibers in the DRG, we utilized a histofluorescence staining (the SPG staining) that detects catecholamines (Fig. 3A). In the thoracolumbar DRG, thin axons sprouted around the DRG neurons (Fig. 3B, DAPI staining showed the DRG cells) forming basket-like skeins (Fig. 3A–C, arrows).
Figure 3.
Sympathetic sprouting surrounding the dorsal root ganglia (DRG) neurons. Histofluorescence staining for catecholamines in the thoracolumbar DRGs demonstrated thread-like sympathetic fibers (A, arrows) formed ‘basket’ structure surrounding the DRG neurons identified by DAPI staining (B). The sympathetic fibers extended between cells forming a cluster (C). The photomicrographs D–F demonstrated that SPG-stained fibers (green fibers indicated by orange arrows) were not surrounding but adjacent to FB-labeled colonic afferent neurons (blue cells indicated by white arrows). Tyrosine hydroxylase immunostaining of FB-labeled DRGs (G–I) also showed that sympathetic fibers sprouted not around but next to colonic afferent neurons. The photomicrographs showed examples from L1 DRG treated for tri-nitrobenzene sulfonic acid treatment for 7 days. Bars = 50 μm.
To examine if a subpopulation of thoracolumbar DRG neurons surrounded by catecholaminergic fibers were colonic afferent neurons, we specifically labeled the DRG neurons with retrograde neuronal tracing dye FB injected into the muscular wall of the distal colon (Fig. 3E, and H, blue cells indicated by white arrows). SPG staining (Fig. 3D) and TH immunostaining (Fig. 3G) of these DRG sections showed that the FB-labeled colonic afferent neurons were not surrounded by but adjacent to the catecholaminergic fibers in the DRGs (Fig. 3F, I).
Distribution of TH immunoreactivity in thoracolumbar DRG
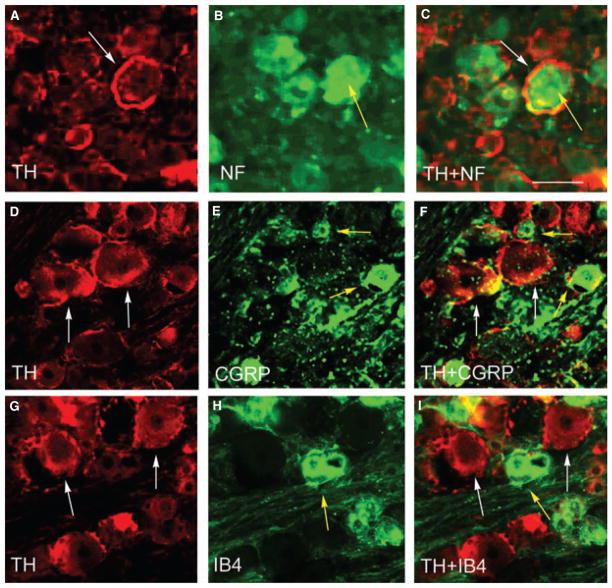
Tyrosine hydroxylase immunoreactivity was present in medium to large diameter (53 ± 9 μm) DRG neurons in thoracolumbar segment of the DRGs (Fig. 1, arrows). To determine the cell profiles of these DRG neurons, DRG sections were double immunostained with TH and CGRP, TH and IB4, or TH and RT97 antibodies. Results showed that TH immunoreactivity (Fig. 4, white arrows) was present around RT97-positive cells (Fig. 4A–C, yellow arrow), but not CGRP- (Fig. 4D–F, yellow arrows) or IB4- (Fig. 4G–I, yellow arrow) positive cells. Although TH immunoreactivity was present around RT97-positive DRG neurons, it was not part of the cell membrane because there was scarce co-localization of TH immunoreactivity with RT97 immunoreactivity in the DRG (Fig. 4C).
Figure 4.
Sprouting of sympathetic fibers around RT97-positive neurons in dorsal root ganglia (DRG). Double immunostaining showed that in thoracolumbar DRGs, tyrosine hydroxylase (TH) immunoreactivity (white arrows) was present around RT97-positive cells (A–C, yellow arrow), but not calcitonin gene-related peptide- (D–F, yellow arrow) or IB4- (G–I, yellow arrow) positive cells. Note that TH immunoreactivity was not co-localized with RT97 immunoreactivity in the DRG (C). Bar = 40 μm.
Co-localization of TH immunoreactivity with glial marker
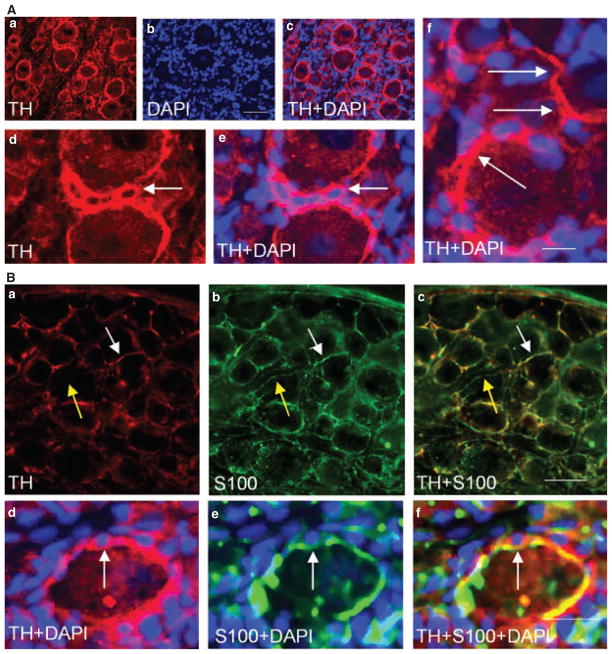
We observed that the TH immunoreactive structure was present surrounding the satellite cells around the medium to large diameter DRG neurons (Fig. 5Aa–c); these cells were identified with nucleus staining with DAPI (Fig. 5Ab,e,f, blue staining, arrows). Tyrosine hydroxylase immunoreactivity appeared as fiber-like extending from one cell to another (Fig. 5Ad, arrows), or sometimes wrapped a cluster of satellite cells (Fig. 5Ae,f). Further examination showed that TH immunoreactivity was largely co-localized with the glial marker S-100 (Fig. 5Ba–c, white arrow indicated co-localization; yellow arrow showed S-100 fiber had no TH). When these cells were stained with DAPI, the fiber-like structures surrounding the small satellite cells were both TH and S-100 positive (Fig. 5Bd–f, arrow).
Figure 5.
Sprouting of sympathetic fibers around glial cells. Some of the tyrosine hydroxylase (TH) immunoreactive fibers (Aa) wrapped the satellite cells that were identified by nucleus staining with DAPI (Ab). In some cases, TH fibers extended from one cell to another (Ad, arrows), and sometimes a cluster of glial cells was wrapped together by TH fibers (Ae and Af, arrow). Double immunostaining showed that TH immunoreactivity was largely co-localized with the glial marker S-100 (Ba–Bc, white arrow indicated co-localization; yellow arrow showed S-100 fiber had no TH), and together these fibers surrounded the small satellite cells (Bd–Bf, arrow). The photomicrograph represented L2 dorsal root ganglia from animals sacrificed at day 7 post tri-nitrobenzene sulfonic acid treatment. Bar = 50 μm in Aa–Ac; 20 μm in Ad–Af; 60 μm in Ba–Bc; and 30 μm in Bd–Bf.
Association of colonic inflammation and catecholaminergic sprouting
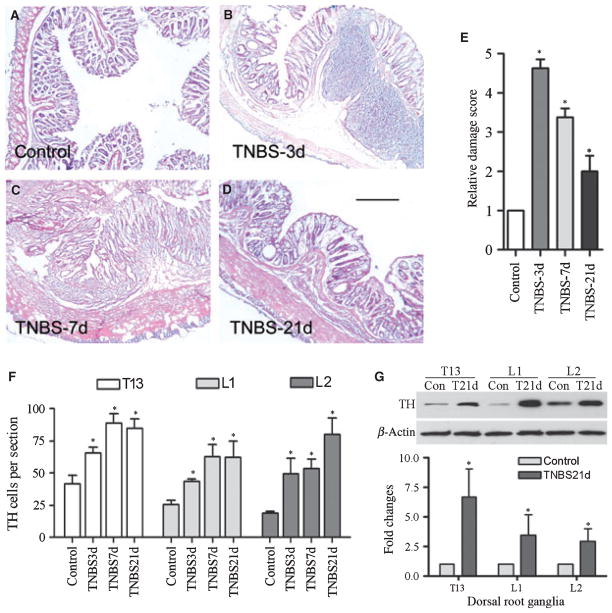
To examine if there was an association between the extent of catecholaminergic sprouting and the severity of colonic inflammation, we stained the distal colon with H&E (Fig. 6A–D), and the T13-L2 DRGs with anti-TH at 3, 7 and 21 days post colitis induction. Following TNBS instillation, the animals started signs of inflammation including diarrhea that was most severe at day 3 to day 4 and started to recover at day 7 and beyond. Microscopic examination showed that at day 3 there were severe inflammatory infiltration, edema, loss of the mucosal architecture, and high level of vascular density (Fig. 6B). The thickness of the muscle layer was significantly increased, and the submuscosal spaces also expanded dramatically (Table S1). At day 7 post TNBS treatment, there were some infiltration, and the mucosal structure started to recover (Fig. 6C). At day 21, the distal colon appeared normal except for smooth muscle layer hypertrophy (Fig. 6D, Table S1). The time-dependent changes in the severity of the colonic inflammation (Fig. 6E) did not parallel the changes in the density of TH immunoreactivity in the DRG at these time points (Fig. 6F). In the thoracolumbar DRG, the number of neurons surrounded by TH-fibers was significantly increased (P < 0.05) not only at day 3 and day 7 but also persisted at a high level till day 21 as examined (Fig. 6F). The increases in TH levels in the DRGs at day 21 following colitis inductions were also confirmed by Western blot analysis (Fig. 6G).
Figure 6.
Sympathetic sprouting during and post colonic inflammation. Intracolonic instillation of vehicle (50% EtOH) did not induce colonic inflammation (A), while intracolonic instillation of tri-nitrobenzene sulfonic acid (TNBS) induced colonic inflammation with the most severe case at 3 days (B) and gradually recovered at 7 days (C) and beyond (D). The relative damage score (E) showed a robust colonic inflammation at 3 days and then back toward normal level at 21 days post TNBS treatment. The extent of the sympathetic sprouting in thoracolumbar DRGs was persistently increased from 3 days to 21 days post colitis induction (F). The increases in TH levels in the DRG at 21 days of colitis were confirmed with Western blot analysis (G). Bar = 250 μm. *P < 0.05.
DISCUSSION
The ‘fight-or-flight’ response to stress is associated with sympathetic signal spreading to the periphery. Sympathetic neurotransmission involves the accumulation of epinephrine from the circulation by sympathetic nerve endings and the release of norepinephrine to the target tissue by postganglionic sympathetic neurons. The present study demonstrated increases in the catecholaminergic innervation of the thoracolumbar DRG during TNBS-induced colitis. The immunoreactivity of TH, the rate-limiting enzyme that converts tyrosine to catecholamines, was significantly enhanced in the DRG post colitis induction. The TH immunoreactivity was not only increased at the acute state (3 days) of colonic irritation, but it was also sustained till 21 days when the colitis was almost resolved. These results suggest that acute colonic inflammation induced a continuous sympathetic response and signal output into the sensory pathways, where it may chronically regulate the sensory sensitivity innervating the distal colon or other organs.
Neuronal tracing dye back-labeling has demonstrated that colonic primary afferent neurons in rodents were located in thoracolumbar including T13, L1, and L2, and lumbosacral L6 and S1 DRGs..19,25,26 The two peaks of labeling are likely to be due to innervation from the splanchnic afferents of the sympathetic origin and pelvic afferents of the parasympathetic origin respectively.27 The present study examined the sympathetic sprouting in the thoracolumbar segments of DRG (T13, L1, and L2) and the sacral segment of the S1 DRG during colitis. Not surprisingly, we found that the TH immunoreactivity was only increased in the thoracolumbar DRGs by colitis; this is consistent with the anatomic localization of the sympathetic pathway.
The increases in sympathetic sprouting in the DRG have been observed in several nerve injury models. 4,14,29–33 The current study showed that the profile of catecholaminergic sprouting in the DRG following colonic inflammation was similar to that seen following nerve injury, i.e., the TH-immunoreactive fibers sprouted around the medium to large diameter DRG neurons. Further examination by the present study showed that these neurons were positive for RT97 antibody with neurofilament-rich soma and A-fibers conduction velocities.34,35 The C-fiber-related DRG small cells positive to either CGRP or IB4 were not surrounded by TH immunoreactivity. Consistently, the TH-immunoreactive fibers did not sprout around FB-labeled colonic afferent neurons, of which in mice 81% expressed CGRP and 20% expressed IB4.36 Electron microscopy 37 and the double immunostaining showed that the TH-immunoreactive fibers did not come into direct contact with neuronal membrane surface, but sprouted along the path of the glial cells in the DRG. It is unclear whether the glial cells release an ‘attractant’ guiding the TH-immunoreactive fibers during colonic inflammation. It can be speculated from earlier studies in nerve injury models showing that satellite cells around the large diameter DRG neurons expressed elevated levels of nerve growth factor (NGF) and neurotrophin-3 (NT-3), and their low affinity receptor p75NTR,38 and that glial overexpression of NGF promoted the sympathetic sprouting into the DRG.39
The effects of peripheral neurotrophins in sympathetic innervation of the sensory neurons have also been suggested. When NGF was overexpressed in the skin, it caused increases in sprouting of sympathetic fibers within the trigeminal ganglia.40 During colonic inflammation, the level of NGF was increased in the distal colon, and NGF receptor TrkA was increasingly expressed in small diameter colonic afferent neurons. 41 Although these colonic afferent neurons were not surrounded by catecholaminergic fibers, they were spatially close to the catecholaminergic fiber clusters that sprouted along the path of the glial cells around the large diameter neurons. One explanation could be that activation of TrkA-mediated signaling in the colonic afferent neurons by local or retrograde NGF may lead to activation of nearby glial cells in the DRG, which then cause release of inflammatory factors and induce sympathetic sprouting. The activation of glial cells in DRG has been observed following localized DRG inflammation, which was accompanied by release of cytokines and robust sprouting of sympathetic fibers.13 Direct injection of the proinflammatory cytokine, leukemia inhibitory factor, intrathecally resulted in increased TH immunoreactivity in the DRG.42 Taken together it shows that both neurotrophins and inflammatory factors play a role in sympathetic sprouting during pathophysiological states.
The physiological significance of sympathetic sprouting in the DRG has been implicated in both animal models and humans. In rodents, the extent of sympathetic sprouting was associated with the degree of the nerve injury,12 and blockade of nerve activity by resiniferatoxin or by anesthetic drug lidocaine reduced sympathetic sprouting near sensory neurons.33,43 In patients with inflammatory bowel disease or IBS exhibited increased sympathetic activity.15,16 In the present study, we found extended catecholaminergic sprouting surrounding the non-colonic afferent neurons that were next to the colonic afferent neurons. Both of these neurons, if expressing receptors to catecholamines, would be able to increase or decrease activities modulated by the catecholaminergic sprouting, thus affecting the sensitivity of the colon or other organs. It was also demonstrated in the present study that the TH immunoreactivity persisted in the thoracolumbar DRG at both the early and later phases of colonic inflammation. The correlation of this persistent up-regulation of catecholaminergic activity to visceral inflammation-induced chronic hypersensitivity and referred pain suggests a possible role of sympathetic nervous system in modification of sensory plasticity.
Supplementary Material
Figure S1. Western blot analysis of TH immunoreactivity shows a single band indicating the specificity of the antibody used.
Table S1. Time-dependent changes in the degree of colonic inflammation induced by TNBS.
Acknowledgments
This work was supported by Grants DK077917 (LYQ) and DK046367 (HIA).
Footnotes
COMPETING INTERESTS
The authors have no competing interests.
AUTHOR CONTRIBUTIONS
CMX designed and conducted most of the experiments and wrote part of the manuscript; DGC and HIA designed and conducted some of the experiments and wrote part of the manuscript; LYQ designed the project, conducted some of the experiments, wrote and finalized the manuscript.
References
- 1.Kaufman S. Tyrosine hydroxylase. Adv Enzymol Relat Areas Mol Biol. 1995;70:103–220. doi: 10.1002/9780470123164.ch3. [DOI] [PubMed] [Google Scholar]
- 2.García-Poblete E, Fernández-García H, Moro-Rodríguez E, et al. Sympathetic sprouting in dorsal root ganglia (DRG): a recent histological finding? Histol Histopathol. 2003;18:575–86. doi: 10.14670/HH-18.575. [DOI] [PubMed] [Google Scholar]
- 3.McLachlan EM, Janig W, Devor M, Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature. 1993;363:543–6. doi: 10.1038/363543a0. [DOI] [PubMed] [Google Scholar]
- 4.Chung K, Lee BH, Yoon YW, Chung JM. Sympathetic sprouting in the dorsal root ganglia of the injured peripheral nerve in a rat neuropathic pain model. J Comp Neurol. 1996;376:241–52. doi: 10.1002/(SICI)1096-9861(19961209)376:2<241::AID-CNE6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Chung K, Chung JM. Sympathetic sprouting in the dorsal root ganglion after spinal nerve ligation: evidence of regenerative collateral sprouting. Brain Res. 2001;895:204–12. doi: 10.1016/s0006-8993(01)02092-3. [DOI] [PubMed] [Google Scholar]
- 6.Kim SH, Na HS, Sheen K, Chung JM. Effects of sympathectomy on a rat model of peripheral neuropathy. Pain. 1993;55:85–92. doi: 10.1016/0304-3959(93)90187-T. [DOI] [PubMed] [Google Scholar]
- 7.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–76. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 8.Desmeules JA, Kayser V, Weil-Fuggaza J, Bertrand A, Guilbaud G. Influence of the sympathetic nervous system in the development of abnormal pain-related behaviours in a rat model of neuropathic pain. Neuroscience. 1995;67:941–51. doi: 10.1016/0306-4522(95)00098-4. [DOI] [PubMed] [Google Scholar]
- 9.Xie J, Park SK, Chung K, Chung JM. The effect of lumbar sympathectomy in the spinal nerve ligation model of neuropathic pain. J Pain. 2001;2:270–8. doi: 10.1054/jpai.2001.24559. [DOI] [PubMed] [Google Scholar]
- 10.Xie W, Strong JA, Zhang JM. Increased excitability and spontaneous activity of rat sensory neurons following in vitro stimulation of sympathetic fiber sprouts in the isolated dorsal root ganglion. Pain. 2010;151:447–59. doi: 10.1016/j.pain.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loh L, Nathan PW. Painful peripheral states and sympathetic blocks. J Neurol Neurosurg Psychiatry. 1978;41:664–71. doi: 10.1136/jnnp.41.7.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HJ, Na HS, Back SK, Hong SK. Sympathetic sprouting in sensory ganglia depends on the number of injured neurons. Neuroreport. 2001;12:3529–32. doi: 10.1097/00001756-200111160-00031. [DOI] [PubMed] [Google Scholar]
- 13.Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang JM. Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience. 2006;142:809–22. doi: 10.1016/j.neuroscience.2006.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Xie W, Strong JA, Zhang JM. Systemic antiinflammatory corticosteroid reduces mechanical pain behavior, sympathetic sprouting, and elevation of proinflammatory cytokines in a rat model of neuropathic pain. Anesthesiology. 2007;107:469–77. doi: 10.1097/01.anes.0000278907.37774.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tillisch K, Mayer EA, Labus JS, Stains J, Chang L, Naliboff BD. Sex specific alterations in autonomic function among patients with irritable bowel syndrome. Gut. 2005;54:1396–401. doi: 10.1136/gut.2004.058685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganguli SC, Kamath MV, Redmond K, et al. A comparison of autonomic function in patients with inflammatory bowel disease and in healthy controls. Neurogastroenterol Motil. 2007;19:961–7. doi: 10.1111/j.1365-2982.2007.00987.x. [DOI] [PubMed] [Google Scholar]
- 17.Beyak MJ, Ramji N, Krol KM, Kawaja MD, Vanner SJ. Two TTX-resistant Na+ currents in mouse colonic dorsal root ganglia neurons and their role in colitis-induced hyperexcitability. Am J Physiol Gastrointest Liver Physiol. 2004;287:845–55. doi: 10.1152/ajpgi.00154.2004. [DOI] [PubMed] [Google Scholar]
- 18.Malykhina AP, Qin C, Greenwoodvan Meerveld B, Foreman RD, Lupu F, Akbarali HI. Hyperexcitability of convergent colon and bladder dorsal root ganglion neurons after colonic inflammation: mechanism for pelvic organ cross-talk. Neurogastroenterol Motil. 2006;18:936–48. doi: 10.1111/j.1365-2982.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 19.Qiao LY, Grider JR. Up-regulation of calcitonin gene-related peptide and receptor tyrosine kinase TrkB in rat bladder afferent neurons following TNBS colitis. Exp Neurol. 2007;204:667–79. doi: 10.1016/j.expneurol.2006.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiao LY, Gulick MA, Bowers J, Kuemmerle JF, Grider JR. Differential changes in brain-derived neurotrophic factor and extracellular signalregulated kinase in rat primary afferent pathways with colitis. Neurogastroenterol Motil. 2008;20:928–38. doi: 10.1111/j.1365-2982.2008.01119.x. [DOI] [PubMed] [Google Scholar]
- 21.Qiao LY, Grider JR. Colitis induces calcitonin gene-related peptide expression and Akt activation in rat primary afferent pathways. Exp Neurol. 2009;219:93–103. doi: 10.1016/j.expneurol.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Traub RJ, Tang B, Ji Y, Pandya S, Yfantis H, Sun Y. A rat model of chronic postinflammatory visceral pain induced by deoxycholic acid. Gastroenterology. 2008;135:2075–83. doi: 10.1053/j.gastro.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes PA, Brierley SM, Martin CM, Brookes SJ, Linden DR, Blackshaw LA. Post-inflammatory colonic afferent sensitisation: different subtypes, different pathways and different time courses. Gut. 2009;58:1333–41. doi: 10.1136/gut.2008.170811. [DOI] [PubMed] [Google Scholar]
- 24.Hughes PA, Brierley SM, Blackshaw LA. Post-inflammatory modification of colonic afferentmechanosensitivity. Clin Exp Pharmacol Physiol. 2009;36:1034–40. doi: 10.1111/j.1440-1681.2009.05248.x. [DOI] [PubMed] [Google Scholar]
- 25.Keast JR, De Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol. 1992;319:615–23. doi: 10.1002/cne.903190411. [DOI] [PubMed] [Google Scholar]
- 26.Traub RJ, Hutchcroft K, Gebhart GF. The peptide content of colonic afferents decreases following colonic inflammation. Peptides. 1999;20:267–73. doi: 10.1016/s0196-9781(98)00157-0. [DOI] [PubMed] [Google Scholar]
- 27.Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil. 2004;16(Suppl 1):28–33. doi: 10.1111/j.1743-3150.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- 28.De la Torre JC. An improved approach to histofluorescence using the SPG method for tissue monoamines. J Neurosci Methods. 1980;3:1–5. doi: 10.1016/0165-0270(80)90029-1. [DOI] [PubMed] [Google Scholar]
- 29.Ramer MS, Bisby MA. Rapid sprouting of sympathetic axons in dorsal root ganglia of rats with a chronic constriction injury. Pain. 1997;70:237–44. doi: 10.1016/s0304-3959(97)03331-9. [DOI] [PubMed] [Google Scholar]
- 30.Lee BH, Yoon YW, Chung K, Chung JM. Comparison of sympathetic sprouting in sensory ganglia in three animal models of neuropathic pain. Exp Brain Res. 1998;120:432–8. doi: 10.1007/s002210050416. [DOI] [PubMed] [Google Scholar]
- 31.Deng YS, Zhong JH, Zhou XF. Effects of endogenous neurotrophins on sympathetic sprouting in the dorsal root ganglia and allodynia following spinal nerve injury. Exp Neurol. 2000;164:344–50. doi: 10.1006/exnr.2000.7432. [DOI] [PubMed] [Google Scholar]
- 32.Deng YS, Zhong JH, Zhou XF. BDNF is involved in sympathetic sprouting in the dorsal root ganglia following peripheral nerve injury in rats. Neurotox Res. 2000;1:311–22. doi: 10.1007/BF03033260. [DOI] [PubMed] [Google Scholar]
- 33.Zhang JM, Li H, Munir MA. Decreasing sympathetic sprouting in pathologic sensory ganglia: a new mechanism for treating neuropathic pain using lidocaine. Pain. 2004;109:143–9. doi: 10.1016/j.pain.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 34.Lawson SN, Waddell PJ. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol. 1991;435:41–63. doi: 10.1113/jphysiol.1991.sp018497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawson SN, Harper AA, Harper EI, Garson JA, Anderton BH. A monoclonal antibody against neurofilament protein specifically labels a subpopulation of rat sensory neurones. J Comp Neurol. 1984;228:263–72. doi: 10.1002/cne.902280211. [DOI] [PubMed] [Google Scholar]
- 36.Robinson DR, McNaughton PA, Evans ML, Hicks GA. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol Motil. 2004;16:113–24. doi: 10.1046/j.1365-2982.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 37.Fried K, Govrin-Lippmann R, Rosenthal F, Ellisman MH, Devor M. Ultrastructure of afferent axon endings in a neuroma. J Neurocytol. 1991;20:682–701. doi: 10.1007/BF01187069. [DOI] [PubMed] [Google Scholar]
- 38.Zhou XF, Deng YS, Chie E, et al. Satellite-cell-derived nerve growth factor and neurotrophin-3 are involved in noradrenergic sprouting in the dorsal root ganglia following peripheral nerve injury in the rat. Eur J Neurosci. 1999;11:1711–22. doi: 10.1046/j.1460-9568.1999.00589.x. [DOI] [PubMed] [Google Scholar]
- 39.Ramer MS, Kawaja MD, Henderson JT, Roder JC, Bisby MA. Glial overexpression of NGF enhances neuropathic pain and adrenergic sprouting into DRG following chronic sciatic constriction in mice. Neurosci Lett. 1998;251:53–6. doi: 10.1016/s0304-3940(98)00493-5. [DOI] [PubMed] [Google Scholar]
- 40.Davis BM, Albers KM, Seroogy KB, Katz DM. Overexpression of nerve growth factor in transgenic mice induces novel sympathetic projections to primary sensory neurons. J Comp Neurol. 1994;349:464–74. doi: 10.1002/cne.903490310. [DOI] [PubMed] [Google Scholar]
- 41.Qiao LY, Grider JR. Colitis elicits differential changes in the expression levels of receptor tyrosine kinase TrkA and TrkB in colonic afferent neurons: a possible involvement of axonal transport. Pain. 2010;151:117–27. doi: 10.1016/j.pain.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson SW, Majithia AA. Leukemia inhibitory factor induces sympathetic sprouting in intact dorsal root ganglia in the adult rat in vivo. J Physiol. 1998;506:809–16. doi: 10.1111/j.1469-7793.1998.809bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie W, Strong JA, Li H, Zhang JM. Sympathetic sprouting near sensory neurons after nerve injury occurs preferentially on spontaneously active cells and is reduced by early nerve block. J Neurophysiol. 2007;97:492–502. doi: 10.1152/jn.00899.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Western blot analysis of TH immunoreactivity shows a single band indicating the specificity of the antibody used.
Table S1. Time-dependent changes in the degree of colonic inflammation induced by TNBS.