ABSTRACT
Background: Colorectal cancer (CRC) develops by accumulation of multiple genetic damages leading to genetic instability that can be evaluated by cytogenetic methods. In the current study we used Cytokinesis-Blocked Micronucleus Assay (CBMN) technique to assess the behavior of Nuclear Division Index(NDI) in peripheral lymphocytes of patients with CRC and polyps versus patients with normal colonoscopy.
Methods: Blood samples were collected from patients after informed consent. By CBMN technique we assessed the proportion of mono-nucleated, bi-nucleated, tri-nucleated and tetra-nucleated cells/500 cells, to calculate NDI. Data were statistically analyzed using the SPSS 11.0 package.
Results: 45 patients were available for analysis, 23 men and 22 women, with a mean age of 58.7±13.5. 17 had normal colonoscopy, 17 colonic polyps and 11 CRC. The mean NDI values were significantly smaller for patients with CRC or polyps than in patients with normal colonoscopy (1.57 vs 1.73, p=0.013). The difference persisted for patients with neoplastic lesions (adenomas and carcinomas) when compared with patients with normal colonoscopy or non neoplastic (hyperplastic) polyps (1.56 vs.1.71, p=0.018). The NDI cut-off value to predict the presence of adenomas or carcinomas was equal to 1.55 with a 54.2% sensitivity and 81% specificity of lower values (p=0.019). The NDI cut off value to predict the presence of advanced adenomas or cancer was 1.525 for a sensitivity of 56.3% and a specificity of 82.8% (p=0.048).
Conclusion: NDI may be useful in screening strategies for colorectal cancer as simple, noninvasive, inexpensive cytogenetic biomarker.
Keywords: nuclear division index, colorectal cancer, polyps
INTRODUCTION
Colorectal cancer (CRC) is an important health problem worldwide and a significant burden for healthcare systems. Indentifying and treating precursor and early lesions may be prevent CRC or improve its outcome. One of the main priorities is to stratify population in risk groups using accessible noninvasive methods.
It was previously showed that cancer develops by accumulation of multiple genetic damages leading to genetic instability (1). In the last decade some studies have shown that there is a positive link between cytogenetic biomarkers and cancer risk (2-6).
The Cytokinesis-Blocked Micronucleus Assay (CBMN) is a recent technique which assesses DNA damages. CBMN allows identification of specific biomarkers to be analyzed in cancer studies. In 2003, Fenech et al. published the scoring criteria for the main biomarkers detected by this method (7). Adding cytochalasin-B to a lymphocytic culture, most of the cells are blocked in a binucleated phase, so cells will either not divide or will divide faster.
Nuclear Division Index (NDI) is a marker of cell proliferation in cultures which is considered a measure of general cytotoxicity (8,9). The hypothesis behind it, is that cells with greater chromosomal damage will either die before cell division or may be less likely to enter this phase (10-13). The lowest NDI value is 1.0, which occurs if all of the viable cells have failed to divide during the cytokinesis-block period and so, all will be mononucleated. If all viable cells complete one division there will be all binucleated, the NDI value is 2.0. An NDI value can be greater than 2.0 if certain viable cells have completed more than one nuclear division during the cytokinesis-block phase and therefore contain more than two nuclei (14).
In the current observational study we evaluated the behavior of NDI in peripheral lymphocytes of patients with colorectal cancer and polyps versus patients with normal colonoscopy so as to assess whether it may predict the presence of these lesions. ❑
MATERIAL AND METHOD
Population study was made of all consecutive patients undergoing screening or diagnostic colonoscopies at an academic hospital.
The inclusion criteria were:
at least one non neoplastic or neoplastic lesion (non polypoid, polypoid lesion or tumor) biopsied or excised at colonoscopy or normal colonoscopy with or without uncomplicated diverticulitis or angiomas,
age above 18,
informed consent.
The exclusion criteria were:
presence of any other colorectal mucosal lesions except those described at inclusion criteria (e.g. inflammatory bowel disease lesions, any other acute or chronic colitis lesions),
presence of large number of lesions suggestive for familial polyposis syndromes,
history of previous colorectal surgery,
history of familial colorectal cancer such as familial adenomatous polyposis (FAP) or hereditary nonpolypid colorectal cancer (HNPCC),
history of any other malignant disease,
history of radiation exposure (professional or accidental) or radiotherapy,
age under 18,
absence of informed consent.
Overall, patients with lesions suggestive of colorectal cancer, any other colorectal polyps or normal colonoscopies were invited to participate in this study. The invitation was made by the gastroenterologist who performed the colonoscopy at the same day or the day after the colonoscopy.
Histological analysis was confirmed by a second pathologist.
When 2 or more lesions were synchronously present, only the more advanced neoplastic lesion was retained for statistical analysis. If an adenoma and a hyperplastic polyp were present then only the adenoma was retained. If multiple adenomas were present, then only the one with the largest diameter was saved in the data sheet.
Heparinized blood samples collected by venipuncture were obtained from patients. Blood was stored at 2-4°C until the samples were processed (within 4 hours). The total volume of each culture was 5 ml. A peripheral blood karyotyping medium with phytohemagglutinin (PHA) M was used (Biological Industries, Israel). Whole blood cultures were used, with a 10% ratio of whole blood to culture medium. For each sample duplicate cultures were set up. Cells were incubated at 37°C. Then, Cytochalasin-B (Sigma, USA; dissolved in dimethylsulphoxide) was added to cultures 44 hours after PHA stimulation, in a concentration of 6.0 µg Cyt-B/ml. Culture tubes were re-incubated afterwards.
Cells were harvested 70–72 hours after PHA stimulation. First, cells were treated with a hypotonic solution for 5 minutes, at room temperature. After that, cells were fixed with methanol and acetic acid at a 3:1 ratio. Cells were transferred to slides by dropping and they were stained with 10% Giemsa. Two slides were prepared from each of the duplicate cultures in order to obtain a measure of experimental variation, i.e., coefficient of variation.
For each slide, the proportion of mono-nucleated, bi-nucleated, tri-nucleated and tetra-nucleated cells per 500 cells scored was assessed. Scoring criteria for cell selection was made according to criteria proposed by Fenech (7,14), following recommendations from the International Collaborative Project on Micronucleus Frequency in Human Populations (HUMN Project).
Nuclear Division Index (NDI) formula was (9):
NDI = (M1 +2M2 +3M3 +4M4)/N,
M1, M2, M3 and M4 indicate the number of cells with one, two, three and four nuclei and N the total number of cells analyzed. N = 500.
Slides were prepared and scored by a single genetic specialist.
Data were statistically analyzed using the SPSS 11.0 package. Quantitative variables were expressed as means ± standard deviations. Categorical variables were presented and/or expressed as percentages. The comparison of means for quantitative variables between groups was done using U Mann-Whitney test for two groups and Kruskal Wallis test for multiple groups. A p value of less than 0.05 was considered statistically significant. ROC curves were constructed for measured quantitative variables so as to explore cut-off values for sensitivities and specificities.
The local ethic committee approved the study protocol. ❑
RESULTS
50 patients matching the inclusion criteria signed the informed consent. 5 of 50 patients were subsequently excluded form analysis: a patient with prior professionally radiation exposure (radiotherapy nurse), 2 patients with malignant diseases (non Hodgkin lymphoma, chronic lymphatic leukemia), one patient with possible attenuated FAP syndrome (brother with FAP and more than 10 colorectal polypoid lesions) and one patient with an inconclusive result in histological analysis which was further lost to follow up to repeat colorectal biopsy.
Of the 45 patients available for analysis, 23 were men and 22 were women, with a mean age of 58.7 ± 13.5.
17 patients had a normal colonoscopy, 17 patients had only colonic polyps and 11 patients had colorectal adenocarcinoma (of which 3 patients had also synchronous polyps).
Of the 17 patients with colonic polyps, 4 patients had their most advanced lesion as hyperplastic and 13 had adenomatous lesions: 9 had tubular adenomas, 3 villous adenomas and one sessile serrated adenoma. All adenomas had low grade dysplasia.
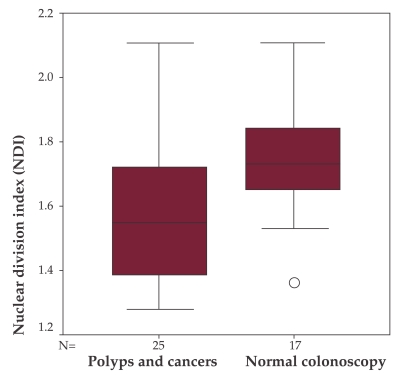
Significant difference regarding the mean NDI values of patients with a normal colonoscopy and patients with recto-colonic cancer and/or polyps was found (1.73 vs. 1.57, p = 0.013) (Figure 1).
Figure 1. NDI comparison between patients with normal colonoscopy and patients with colorectal hyperplastic polyps or neoplastic lesions (adenomas and carcinomas).
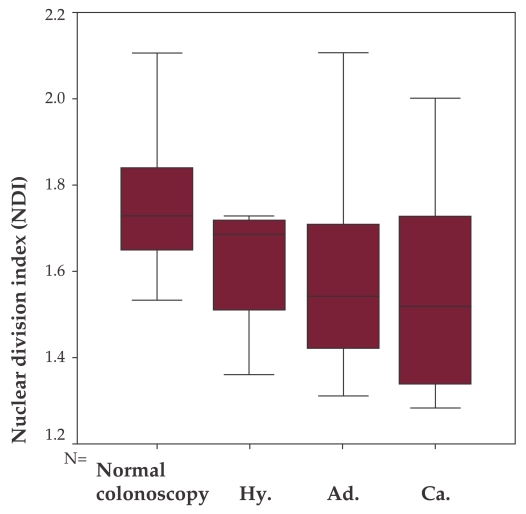
When analyzing each specific lesion, the mean NDI values were: normal colonoscopy 1.730, hyperplastic polyps 1.61, adenomas 1.5669 and carcinomas 1.5691 (p = 0.094). Although non significant, a tendency of decrease is noticed with progression towards carcinoma, from left to right (Figure 2).
Figure 2. NDI comparison between patients with normal colonoscopy, hyper plastic polyps (Hy), with adenomas (Ad.), and carcinomas (Ca.).
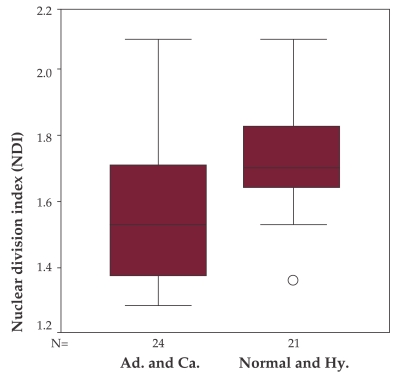
There is a significant difference between patients with either normal colonoscopy or non neoplastic polyps (hyperplastic) and patients with neoplastic lesions (adenomas and carcinomas) (1.71 vs. 1.56, p = 0.018) (Figure 3).
Figure 3. NDI comparison between patients with normal colonoscopy or hyperplastic polyps and patients with adenomas or carcinomas.
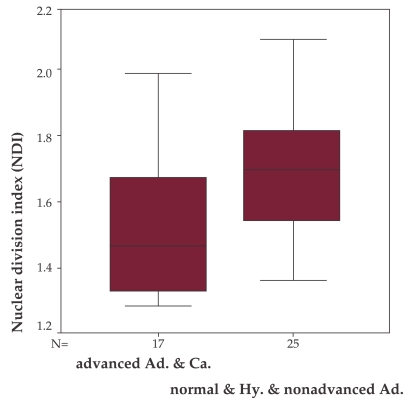
When the mean NDI of patients with advances adenomas (size over 10mm, villous or with high grade dysplasia) and carcinomas was compared with the mean NDI of patients with normal colonoscopy, hyperplastic polyps or less than 10mm tubular adenomas there was a significant statistical difference, with lower values for the first group (1.53 vs. 1.69, p = 0.015), (Figure 4).
Figure 4. NDI comparison between patients with advanced adenomas or carcinomas and patients with normal colonoscopy or hyperplastic polyps or non-advanced adenomas.
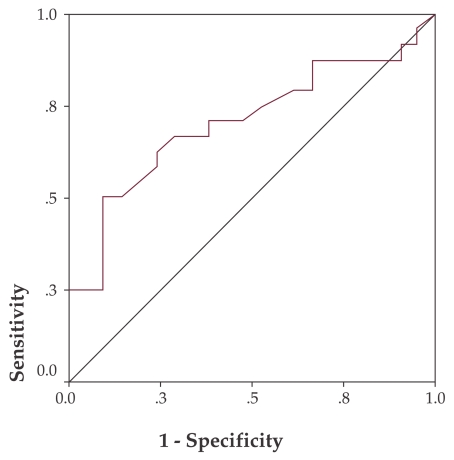
On the ROC curve, NDI values smaller than a 1.55 cut-off are predictive for adenomas or carcinomas with a sensitivity of 54.2% and a specificity of 81% (p = 0.019) (Figure 5).
Figure 5. NDI ROC curve for adenoma and carcinoma prediction.
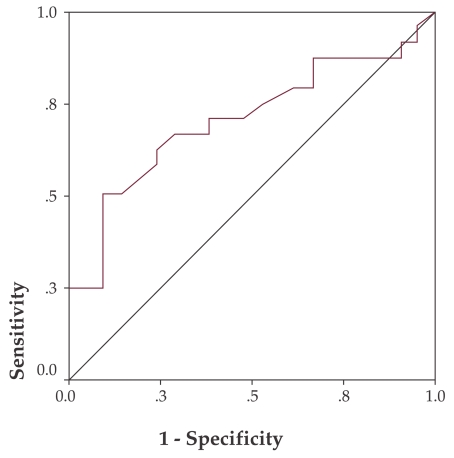
In a similar way, the ROC curve for prediction of advanced adenomas and carcinomas translates to a 1.525 cut-off with a sensitivity of 56.3% and a specificity of 82.8% (p=0.048) for NDI values lower than this point (Figure 6). ❑
Figure 6. NDI ROC curve for advanced adenoma and carcinoma prediction.
DISCUSSION
Colorectal cancer is the second major cause of cancer mortality worldwide in both men and women (15,16), with a mean age at diagnosis that varies between 65 to 71.5 years (17). Almost 75% of new diagnosed cases occur in asymptomatic individuals, with no apparent predisposing factor except the age above 50 (18). The outcome is favorable for early disease and poor in advanced stages.
Colonoscopy is the gold standard of CRC screening and it is also the method of choice in high risk groups. Nevertheless, the debate is still on whether colonoscopy is appropriate for average-risk individuals as high quality evidence randomized controlled trials are missing (19). As shown in several studies, polypectomy during colonoscopy significantly lowers the CRC incidence and mortality (20-24). However, concerns are raised about costs, morbidity and suboptimal sensitivity due to operator technique (missing lesions through lower cecal intubation rates or inappropriate withdrawal time) (19).
So, there is a permanent concern to improve screening strategies, including extensive research for simple inexpensive biomarkers. Recently, it was shown that CBMN assay of peripheral lymphocytes may allow CRC risk stratification. A good correlation between micronuclei frequencies (fMN) and CRC or adenoma presence was found (25).
In our paper, we tested NDI based on the fact that this marker estimates general toxicity (7,9,14). The proportion of bi-nucleated cells may be used as a biomarker of the lymphocytes mitogen response, immune functions and cytostatic effects of various studied agents (26).
NDI was already proposed as screening marker for lung cancer in smokers. Patients with lung cancer have a significantly lower mean NDI of peripheral lymphocytes than controls (1.52 versus 2.08, p <0.001) (2). As far we are aware of, this is the first study proving that lower NDI values may predict the presence of colorectal epithelial neoplastic lesions. We have proposed cut-offs firstly for adenomas and carcinomas and secondly for advanced adenomas and carcinomas. The ROC curves may be used for screening strategies.
The explanations for this behavior may come from recently published data showing that NDI is significantly lower in individuals occupationally exposed to lead (13). This is a metal known to have various types of toxic effects, including carcinogenesis (27-29). Based on other research papers (30-36) the authors offer some hypotheses to explain the effect. Firstly, circulating lymphocytes under the toxic effect of mutagenic agents (lead in this specific case), suffer DNA damages, cannot survive the division cellular cycle and enter in a process of necrosis or apoptosis before the finish of the first division. Secondly, there may be an induction of mitotic delay which, by not allowing the repair of genotoxic lesions, will modify the number of the cells entering mitosis and modify the proportion of mono-/bi-/tri- and tetra-nucleated cells. Thus, a lower NDI as fewer cells divide. Thirdly, there is the hypothesis of a clastogenic effect of mutagens with an aneugenic action, inducing some degree of blockade of the cell cycle. Therefore, more cells will not divide and NDI will again be low.
We can extrapolate these hypotheses in patients with neoplastic colorectal lesions. Mutagenic factors and sensible genetic terrain may reproduce such conditions and lymphocytes with greater chromosomal damage may die before cell division, may be more susceptible to necrosis and apoptosis or may be less likely to enter division. Subsequently, the number of division cycles will be lower resulting in a low NDI value.
In conclusion, NDI may be used in screening strategies for colorectal cancer as it may predict the presence of adenomas and carcinomas. This is a rather simple biomarker, evaluated by a noninvasive inexpensive cytogenetic method using Cytokinesis - Blocked Micronucleus Assay (CBMN). ❑
ACKNOLEDGENENTS
This work has been done with financial support from European Social Fond through POSDRU 2007–2013 program, within the POSDRU /6/1.5/S/17, "An European dimension of doctoral studies" ODEUS.
References
- 1.Solomon E, Borrow J, Goddard AD. Chromosome aberrations and cancer. . Science. 1991;254:1153–60. doi: 10.1126/science.1957167. [DOI] [PubMed] [Google Scholar]
- 2.El-Zein R.A, Fenech M, Lopez M.S. Cytokinesis-Blocked Micronucleus Cytome Assay biomarkers identify lung cancer cases amongst smokers. . Cancer Epidemiol Biomarkers Prev. 2008;17:1111–9. doi: 10.1158/1055-9965.EPI-07-2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonassi S, Hagmar L, Stromberg U. Chromosomal aberrations in lymphocytes predict human cancer independently of exposure to carcinogens. European Study Group on Cytogenetic Biomarkers and Health. . Cancer Res. 2000;60:1619–25. [PubMed] [Google Scholar]
- 4.Bonassi S, Znaor A, Norppa H. Chromosomal aberrations and risk of cancer in humans: an epidemiologic perspective. Cytogenet Genome Res. 2004;104:376–82. doi: 10.1159/000077519. [DOI] [PubMed] [Google Scholar]
- 5.Bonassi S, Ugolini D, Kirsch-Volders M. Human population studies with cytogenetic biomarkers: review of the literature and future prospective. Environ Mol Mutagen. 2005;45:258–70. doi: 10.1002/em.20115. [DOI] [PubMed] [Google Scholar]
- 6.Miller B, Pötter-Locher F, Seelbach A. Evaluation of the in vitro micronucleus test as an alternative to the in vitro chromosomal aberration assay: position of the GUM Working Group on the in vitro micronucleus test. Mutat Res. 1998;410:81–116. doi: 10.1016/s1383-5742(97)00030-6. [DOI] [PubMed] [Google Scholar]
- 7.Fenech M, Chang W.P, Kirsch-Volders M. HUMN project: detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutation Res. 2003;534:65–75. doi: 10.1016/s1383-5718(02)00249-8. [DOI] [PubMed] [Google Scholar]
- 8.Fenech M. The in vitro micronucleus technique. Mutat Res. 2000;455:81–95. doi: 10.1016/s0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 9.Eastmond D.A, Turcker J.D. Identification of aneuploidy inducing agents using cytokinesis-blocked human lymphocytes and anti-kinetochore antibody. Environ Mol Mutagen. 1989;13:34–43. doi: 10.1002/em.2850130104. [DOI] [PubMed] [Google Scholar]
- 10.Palus J, Rydzynski K, Dziubaltowska E. Genotoxic effects of occupational exposure to lead and cadmium. Mutat Res . 2003;540:19–28. doi: 10.1016/s1383-5718(03)00167-0. [DOI] [PubMed] [Google Scholar]
- 11.Santos-Mello R, Kwan D, Norman A. Chromosome aberrations and T-cell survival in human lymphocytes. Radiat Res. 1974;60:482–8. [PubMed] [Google Scholar]
- 12.Nath C.J, Ong T. Micronuclei assay in cytokinesis-blocked binucleated and conventional mononucleated methods in human peripheral lymphocytes. Teratog Carcinog Mutagen. 1990;10:273–9. doi: 10.1002/tcm.1770100310. [DOI] [PubMed] [Google Scholar]
- 13.Minozzo R, Deimling L.I, Petrucci Gigante L. Micronuclei in peripheral blood lymphocytes of workers exposed to lead. Mutation Research. 2004;565:53–60. doi: 10.1016/j.mrgentox.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Fenech M. Cytokinesis-block micronucleus cytome assay. Protocol Nature Protocols. 2007;2:1088–104. doi: 10.1038/nprot.2007.77. [DOI] [PubMed] [Google Scholar]
- 15.Ferlay J, Autier P, Boniol M. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol . 2007;18:581–92. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 16.Jemal A, Siegel R, Ward E. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 17.Sant M, Capocaccia R, Verdecchia A. Comparisons of colon-cancer survival among European countries: The Eurocare Study. Int J Cancer. 1995;63:43–8. doi: 10.1002/ijc.2910630109. [DOI] [PubMed] [Google Scholar]
- 18.Winawer S.J, Fletcher R.H, Miller L. Colorectal cancer screening – clinical guidelines and rationale. Gastroenterology. 1997;112:594–642. doi: 10.1053/gast.1997.v112.agast970594. [DOI] [PubMed] [Google Scholar]
- 19.Arditi C, Peytremann-Bridevaux I, Burnand B. Appropiateness of colonoscopy in Europe (EPAGE II). Screening for colorectal cancer. Endoscopy. 2009;41:200–8. doi: 10.1055/s-0028-1119626. [DOI] [PubMed] [Google Scholar]
- 20.Bertario L, Russo A, Sala P. Predictors of metachronous colorectal neoplasms in sporadic adenoma patients. Int J Cancer. 2003;105:82–7. doi: 10.1002/ijc.11036. [DOI] [PubMed] [Google Scholar]
- 21.Citarda F, Tomaselli G, Capocaccia R. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut. 2001;48:812–5. doi: 10.1136/gut.48.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jorgensen O.D, Kronborg O, Fenger C, et al. Influence of longterm colonoscopic surveillance on incidence of colorectal cancer and death from the disease in patients with precursors (adenomas). Acta Oncol. 2007;46:355–60. doi: 10.1080/02841860600897918. [DOI] [PubMed] [Google Scholar]
- 23.Thiis-Evensen E, Hoff G.S, Sauar J. Population-based surveillance by colonoscopy: effect on the incidence of colorectal cancer. Scand J Gastroenterol. 1999;34:414–20. doi: 10.1080/003655299750026443. [DOI] [PubMed] [Google Scholar]
- 24.Winawer SJ, Zauber AG, Ho MN. Prevention of colorectal cancer by colonoscopic polypectomy (The National Polyp Study Workgroup). N Engl J Med. 1993;329:1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 25.Karaman A, Binici DN, Kabalar ME. Micronucleus analysis in patients wih colorectal adenocarcinoma and colorectal polyps. World J Gastroenterol. 2008;28:6835–9. doi: 10.3748/wjg.14.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eastmond DA, Tucker JD. Kinetochore localization in micronucleated cytokinesis-blocked Chinese hamster ovary cells: a new and rapid assay for identifying aneuploidy-inducing agents. Mutat Res. 1989;224:517–25. doi: 10.1016/0165-1218(89)90079-7. [DOI] [PubMed] [Google Scholar]
- 27.Zelikoff JT, Li JH, Hartwing A. Genetic toxicology of lead compounds. Carcinogenesis. 1988;9:1727–32. doi: 10.1093/carcin/9.10.1727. [DOI] [PubMed] [Google Scholar]
- 28.Lin RH, Lee CH, Chen WK. Studies on cytotoxic and genotoxic effects of cadmium nitrate and lead nitrate in Chinese hamster ovary cells. Environ Mol Mutagen. 1994;23:143–9. doi: 10.1002/em.2850230212. [DOI] [PubMed] [Google Scholar]
- 29.Steenlan, S. Selevan, P. Landrigan A. The mortality of lead smelter workers: an update. Am J Public Health. 1992;82:1641–4. doi: 10.2105/ajph.82.12.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann M, Hagberg S, Karlsson A. Inorganic lead exposure does not affect lymphocyte micronuclei in car radiator repair workers. Hereditas. 1984;101:223–6. doi: 10.1111/j.1601-5223.1984.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 31.Forni A, Sciame A, Bertazzi PA. Chromosome and biochemical studies in women occupationally exposed to lead. Arch Environ Health. 1980;35:139–45. doi: 10.1080/00039896.1980.10667481. [DOI] [PubMed] [Google Scholar]
- 32.Vaglenov A, Lalchev SG, Nosko MS. Cytogenetic monitoring of exposed workers to lead. Cent Eur J Occup Environ Med. 1997;3:298–308. [Google Scholar]
- 33.Vaglenov A, Creus A, Lalchev SG. Occupational exposure to lead and induction of genetic damage. Environ Health Perspect. 2001;109:295–8. doi: 10.1289/ehp.01109295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinto D, Ceballo JM, Garcia G. Increased cytogenetic damage in outdoor painters. Mutat Res. 2000;467:105–11. doi: 10.1016/s1383-5718(00)00024-3. [DOI] [PubMed] [Google Scholar]
- 35.Duydu Y, Suzen HS, Aydin A. Correlation between lead exposure indicators and sister chromatid exchange (SCE) frequencies in lymphocytes from inorganic lead exposed workers. Arch Environ Contam Toxicol. 2001;41:241–6. doi: 10.1007/s002440010244. [DOI] [PubMed] [Google Scholar]
- 36.Fracasso M, Perbellini L, Solda S. Lead induced DNA strand breaks in lymphocytes of exposed workers: role of reactive oxygen species and protein kinase C. Mutat Res. 2002;515:159–69. doi: 10.1016/s1383-5718(02)00012-8. [DOI] [PubMed] [Google Scholar]