ABSTRACT
Aim: To evaluate the incidence of bacterial infections (BI) in hepatic cirrhosis (HC), the pathogen agents involved, to define the risk factors and impact on prognosis.
Methods: There was a retrospective study that enrolled a total of 1046 patients with HC admitted in our clinic between 1.10.2008-31.03.2009 (6 months). Clinical, biological and bacteriological data were monitored.
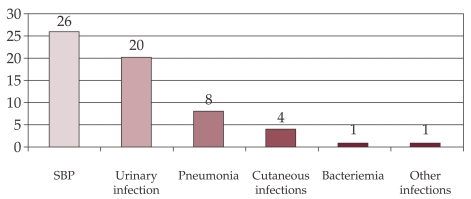
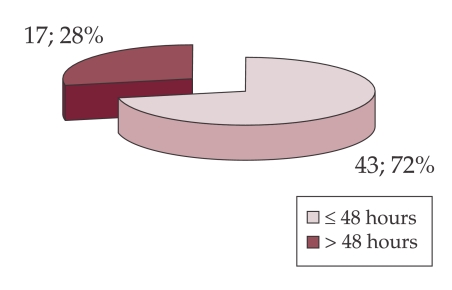
Results: 51 patients (4.9%) were found with BI. In one patient BI was located in three sites: peritoneal, blood and urine, and in 7 patients BI was located in 2 sites. BI location was: peritoneal-26 cases, urinary-20 cases, pneumonia – 8 cases, skin – 4 cases and bacteremia -1 case. 43 episodes were community acquired, while 17 episodes were - nosocomial (peritoneal – 3 cases, lung – 6 cases, skin – 2 cases, urinary – 5 cases). Of the 26 cases with bacterial peritonitis, the etiologic agent was identified in three: E. coli, Klebsiella, Alcaligenes. 18% of patients with HC and BI presented upper GI bleeding. 12 cases required admission to the Intensive Care Unit, where the death rate reached 83%. The risk factors for BI in HC were: decompensated HC OR=58,23 (95% CI 8.63÷1141.31), p-value 10-12, Child Pugh score C: OR =1.99 (95% CI 1.04÷3.8), p-value= 0.02.
Conclusions: In this study the rate of bacterial infections in HC is low compared with the literature (4.9% vs. 15-30%), because the study was retrospective, hence recorded only severe infections. We must actively seek infections in all hospitalized patients with HC, especially in the ones with decompensated cirrhosis and with upper GI bleeding.
Keywords: liver cirrhosis, bacterial infection, spontaneous bacterial peritonitis, upper gastrointestinal bleeding
INTRODUCTION
Bacterial infection is a complication that occurs frequently in patients with cirrhosis and it may be present at admission or it may appear during the hospital stay up to 30-60% of hospitalized patients (1,2). Several factors are known to facilitate a bacterial infection: hepatic disease stage, malnutrition, impairment of cutaneo-mucous barrier, associated pathology (diabetes mellitus, neoplasia), upper gastro-intestinal hemorrhage, invasive maneuvers (3,4).
The most frequent infectious complications are spontaneous bacterial peritonitis (25%), urinary tract infection (20%), pneumonia (15%), bacteremia (12%), cellulitis or other infections (3,4).
Cirrhotic patients are susceptible to infections due to abnormal immune response and bacterial translocation which enables alteration of local immunity and bacterial growth (4,5).
Malfunctions that lead to infection at these patients are: endothelial and reticular system alteration, leukocytes dysfunction, less bactericide activity of ascitic fluid and iatrogenic factors (6).
Hyperglobulinemia and cellular immune response inhibition are commonly found in chronic hepatic diseases as a response of hepatic damage (6).
The phagocytosis process is deeply altered at patients with liver cirrhosis due to rearrangement of antigens, affecting cellular and humoral immune response (7,8).
Bacterial translocation is the singular element that breaks the balance in host flora and determines the initiation of inflammatory response and afterwards the infection as a result of ineffective local and later systemic immune response (7).
The onset of infection opens the path to systemic inflammatory response syndrome (SIRS), with hypotension (severe sepsis), renal dysfunction, encephalopathy, coagulopathy, and finally multiple system organ failure (MSOF) (9,10).
Clinically, bacterial infection at cirrhotic patients can be asymptomatic or can have a paucity of sympyoms. So, bacterial infection must be sought in any patient cu liver cirrhosis with a sudden worsening of hepatic function. Treatment must be started immediately with bactericide doses of broad-spectrum antibiotics, excluding aminoglycosides (11-14).
Mortality by infection is 20 times higher in cirrhotic patients compared to general population (15).
Infection evolution and prognostic is related to accurate rapid diagnostic (15).
The aim of this study is to identify risk factors for bacterial infection in patients with liver cirrhosis, identify the bacterial make-up present in our tertiary center and evaluate impact of bacterial infection in the clinical outcome of the patients. ❑
MATERIAL AND METHOD
The research guidelines of the Fundeni Hepatology and Gastroenterology Clinic were followed in the clinical research of this report.
All the records of the patients admitted with the diagnoses of hepatic cirrhosis, fever, bacteremia, sepsis, systemic inflammatory syndrome, from October 1st 2008 to March 31st 2009, were reviewed. For all patients we reviewed the clinical and laboratory data entries in their medical records.
Criteria for infection diagnostic were set according to its localization.
The diagnosis of spontaneous bacterial peritonitis was made by: more than 250 neutrocytes in ascitic fluid and / or positive cultures for a certain germ and exclusion of other secondary causes of peritonitis (9).
The seeding of ascitic fluid in hemoculture bottle with aerobic and anaerobic media and the follow-up of the sample by incubation in Bactec authomatic system was done in 2 patients.
In all the other cases, ascites was centrifugated 20 min. 3000 rotations/min and out of the deposit, seedings were done on solid media, gelose blood and gelose lactose, with incubation at 37 degrees Celsius and follow-up of bacterial growth.
The diagnosis of urinary tract infection was made by: clinical symptoms and signs (dysuria, fever), >15 leukocytes in urinalysis, and/or positive urine culture (>100.000 CFU/ml) (16).
The diagnosis of respiratory tract infection was made by: clinical symptoms and signs (cough, expectoration, pulmonary sounds, fever), positive radiologic signs (patchy alveolar opacities), and/or positive bacteriologic exam (sputum or hemoculture) (17).
The diagnosis of cutaneous and soft tissues infection was made by: fever, local sign (blush, tumefaction, pain), leukocytosis with neutrophilia, positive cultures of wound secretion or hemoculture (18).
To diagnose bacteremia several criteria were used: positive hemoculture for known germ or germs from saprophyte flora at a patient with central line, signs like fever, shivers, hypotension to which the germ identified in hemoculture has no connection with an infection located elsewhere (19).
To diagnose sepsis and septic shock the following clinical criteria were used: fever >38°C or hypothermia (<36°C), tachycardia (pulse >90/min), tachypnea (>20/min), hypoxemia (<70 mmHg), metabolic acidosis, oliguria (diuresis <30 ml/min) (10).
Patients with unknown source of infection, with fever (>38°C) more than 24 hours and leukocytosis with neutrophilia, were recorded as having a bacterial infection.
From a total of 1579 patients, 533 patients with a hospital stay equal or shorter than 2 days were excluded from the study. So, 1046 cases were selected.
Statistical analysis was made with Epi Info 6.04 program, using Chi square as a correlation test, and p-value less than 0.05 as statistically significant. ❑
RESULTS
Applying selected criteria for the clinical follow-up of the cirrhotic patient, 51 from the total 1046 (4.9%) were diagnosed with bacterial infection.
Etiology and demographic distribution data from the patients with liver cirrhosis can be seen in Table 1.
Table 1.
Etiology and demographic distribution of patients with liver cirrhosis
| No infection (%) | With infection (%) | |
|---|---|---|
| Sex | ||
| Male | 552 (55.5) | 29 (57) |
| Female | 443 (44.5) | 22 (43) |
| Age (years)-mean | 54.2 | 54.7 |
| Etiology | ||
| Viral B | 162 (16.28) | 11 (22) |
| Viral C | 249 (25.02) | 13 (25) |
| Alcoholic | 327 (32.86) | 18 (35) |
| Combined | 195 (19.6) | 8 (16) |
| Other causes | 49 (4.9) | 1 (2) |
In both groups there is a mild male predominance, with a mean age in both groups of 54 years old (ranging from 23 to 77 years old).
Liver cirrhosis etiology was assessed as a potential risk factor for development of bacterial infection, but no statistically significant OR (odds ratio) was determined in any of the 4 etiology classes.
Table 2.
Patients distribution according to liver cirrhosis stage disease
| No infection (%) | With infection (%) | |
|---|---|---|
| Disease | ||
| Stage compensated | 535 (53.78) | 1 (2) |
| decompensated | 460 (46.22) | 50 (98) |
| Among decompensated | ||
| Score Child-Pugh A | 175 (38) | 14 (14) |
| Child-Pugh B | 78 (17) | 25 (26) |
| Child-Pugh C | 207 (45) | 59 (60) |
As noted in table one, although the alcoholic cirrhosis is the predominant etiology of the liver cirrhosis in our patient population, both in the patient with bacterial infections (35%) and those without bacterial infection (32.86%), overall there was no predisposition to sepsis given by the etiology of the liver disease.
The patients from the two groups were divided according to stage disease (compensated or decompensated cirrhosis), according to Child-Pugh score.
According to liver cirrhosis staging, decompensated disease was present at 98% of patients with concomitant bacterial infection, in comparison with 46,22% in the groups of cirrhotic patients with no bacterial infection. 60% of patients with infection had severely decompensated liver disease, corresponding to a Child-Pugh class C.
The degree of the severity of liver disease had a tremendous impact on the number of the bacterial infections found. While the mild hepatic cirrhosis, Child Pugh Class A, had only 14% of the patient with concomitant bacterial infections, 60% the patients with Child Pugh Class C had a bacterial infection.
Calculated OR for decompensated cirrhosis: OR=58, 23 (95% CI 8.63÷1141.31), p-value 10-12. OR Class C Child-Pugh: 1.99 (95% CI 1.04÷3.8), p-value= 0.02 (Chi square test).
Among the 1046 patients with liver cirrhosis, 51 (4, 9%) has bacterial infection present.
The number of bacterial infection episodes was 60, calculating a bacterial infection rate of 5.7%.
One patient was found with positive cultures at multiple sites (peritoneal, blood, urinary) and other (n=7) had two major infection sites: 3 patients had spontaneous bacterial peritonitis (SBP) and pneumonia, one had and SBP, one with acute urinary tract infection and SBP, one had complicated urinary tract infection with Fournier gangrene, and the last one had with SBP and vertebral osteomyelitis.
In our study, the most frequent infections were SBP (n=26), followed by urinary tract infection (n=20) and pneumonia (n=8) (Figure 1).
Figure 1. Frequency of infection types.
According to the time of admission, there were 43 episodes of bacterial infection diagnosed within 48 hours (community acquired) and 17 episodes after 48 hours from admission (nosocomial infections) (Figure 2).
Figure 2. Distribution of cases with infection according to admission moment.
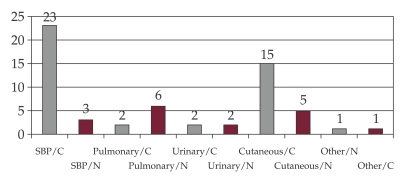
The SBP was community acquired in 89% of cases (n=23), and urinary tract infection in 75% (n=15).
The pulmonary infections (pneumonia) were nosocomial in 75 % cases (n=6), acquired during hospital stay (Figure 3).
Figure 3. Distribution of the cases with nosocomial and community acquired infections.
For other infection sites there were no statistically significant case distribution to nosocomial or community acquired infections.
The mean length of stay in the patients with bacterial infections was 14 days, compared to the patients with negative cultures, where the length of stay was in 6.8 days.
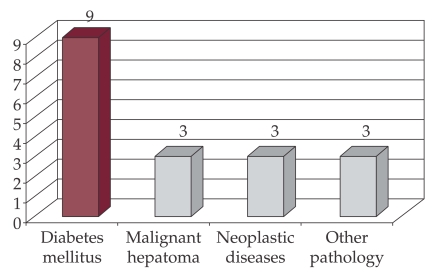
Associated pathology was assessed at cirrhotic patients with bacterial infection. 18 patients had other comorbid conditions: diabetes mellitus 50% (9), neoplasia 33% (Figure 4).
Figure 4. Distribution of patients with bacterial infection by associated pathology.
Spontaneous Bacterial Peritonitis
81% (n=21) from the patients had advanced liver disease, with Child Pugh score C.
Most of the patients presented with fever and abdominal pain, at the time of admission (n=17), and two more patients during hospital stay (73.1% in total).
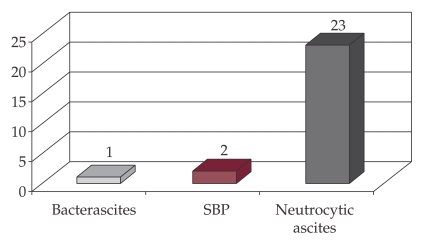
In the whole group, cultures were positive for germs at 3 patients (11.54%): E. Coli, Klebsiella, Alcaligenes faecalis.
One case (4%) had monomicrobial nonneutrocytic bacterascitis (MNBA); bacterial findings Alcaligenes faecalis, neutrophil count 240/mm3.
Two cases (8%) met criteria for spontaneous bacterial peritonitis: over 250 neutrophils /mm3; one culture with E. Coli, one with Klebsiella pneumoniae.
Twenty-three cases (88%) were diagnosed with culture negative neutrocytic ascites, over 250 neutrophils/mm3 (500-6500 leukocytes/mm3) and negative cultures (Figure 5).
Figure 5. Distribution of cases with spontaneous infection of ascites.
The identified bacteria were sensible to several antibiotics: cephalosporins, quinolones, aminopenicilins with beta-lactamase inhibitors.
Most important factors asociated with SBP development were assessed. 15 patients (58%) had a total protein level in ascitic fluid of <1 g / dl. There were 5 cases of upper gastrointestinal bleeding UGB (19.23%).
Mortality rate was 43% (11 cases). There were 4 deceased patients with SBP and UGH (80%), only one case having a favorable evolution.
The antimicrobial treatment was applied to all 26 cases and was conducted on a mean 10 days.
Urinary tract infection
There were 20 episodes of urinary tract infection (UTI) per 1046 patients, with an incidence of 1.9%. There was an equal sex distribution of cases 11 males / 9 females.
The incidence of UTI increased with the severity of the hepatic cirrhosis, whith 5 cases in Child A cirrhosis, 6 cases in patient with Child B and 9 cases in Child C cirrhosis.
Eleven cases (55%) had fever and positive cultures (>100,000 germs/ml urine). Two cases (10%) were diagnosed based on clinical signs (dysuria) and pyuria. Seven cases (35%) had asymptomatic bacteriuria.
17 cases had good evolution under antimicrobial treatment, 2 of which were nosocomial infections with Klebsiella spp. There were 3 deaths at patients with Class C Child Pugh liver cirrhosis and nosocomial infections: Klebsiella spp. – 2 cases, Myroides odoratum – 1 case. Among these, two had upper gastrointestinal bleeding during hospital stay.
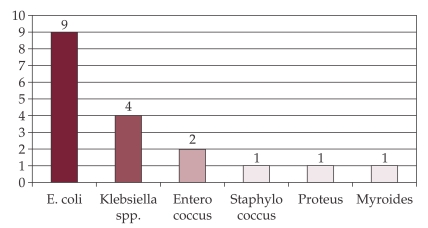
The bacteria isolated and identified in uroculture are presented in Figure 6.
Figure 6. Etiologic agents of urinary tract infection.
E.coli, Proteus, Enterococus and Stafilococus spp were sensitive to a large number of antibiotics.
Pneumonia was diagnosed in our group in 8 cases, of which 2 cases of community acquired disease. There was a good evolution under antimicrobial treatment with fourth generation cephalosporins (Cefepim, n=1) and beta-lactamase inhibitor (piperacillin/tazobactam n=1).
Other 6 cases were diagnosed as nosocomial pneumonia. The evolution was difficult in 5 cases which required artificial ventilation in the Intensive Care Unit. Among these, two cases had an episode of upper gastrointestinal bleeding at admission and developed later urinary infection. The etiologic diagnosis was established by sputum culture which isolated Klebsiella pneumoniae and broncho alveolar lavage which isolated Klebsiella pneumoniae and Methicillin-resistant Staphylococcus aureus. The antibiotherapy was with carbapenem in one patient, and in another, carbapenem with Linezolid.
There were 5 deaths in 8 cases (62.5% death rate), 3 of 5 patients with Class C Child - Pugh score (60%) and 2 of 3 patients with Class B Child - Pugh score (66.7%).
Cutaneous and soft tissues infections
There were 4 cases, 2 of erisipela and 2 of Fournier gangrene, in patients with decompensated liver cirrhosis, Child-Pugh class C.
The onset was in all cases with fever and leukocytosis (12,000-32,000 elements/sq mm) with local signs.
Bacteriologic examinations and cultures for wound secretion were positive for Fournier gangrene. The following aerobic germs were identified: Acinetobacter baumanii (n=1), E.coli and Klebsiella spp.(n=1). These patients developed also an episode of upper gastrointestinal hemorrhage. In one case, the evolution was worse, despite the well targeted antimicrobial treatment, with signs of multiple organ system failure and severe septic sock.
Other infections
Methicillin-resistant Staphylococcus aureus of unknown origin bacteremia was diagnosed in 1 patient who received cephalosporins for SBP,; ESBL- producing Klebsiella pneumoniae, betalactamase was also indentified later, and subsequesntly the patient required transfer to Intensive Care Unit with multi-organ system failure and expired, despite broad coverage with carbapenems and linezolid., tailored as per bacterial sensitivities.
Other patients characteristics
We found that UGI bleeding was present in 18% of our patients, however, the mortality of those patients was very high, over 50% (5 of 9 patients).
Most of the patient were anemic, 51% of the cases, with severe forms in 2 cases (hemoglobin less than 7 g/dl).
Fever was present in 34 of 51 patients (66.7%).
Renal failure with creatinine levels >1.5 mg/dl was found in 25 patients out of 51 (49.02%).
12 patients with decompensated liver cirrhosis Child –Pugh class C and 2 with Child-Pugh class B (in total 27.45% of the 51 with bacterial infection) needed admission to Intensive Care Unit due to complication following infections like SBP (n=4), pulmonary (n=5), urinary (n=2), soft tissues (1).
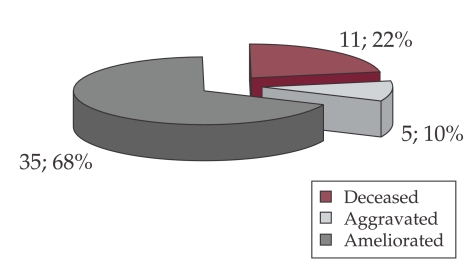
The death rate of this group was high 83%, despite the fact that all cases were treated with broad spectrum antibiotics.
Figure 7 displays the evolution and death rate of patients with bacterial infections. ❑
Figure 7. Distribution of patients by status at discharge from hospital.
DISCUSSION
In prospective recent studies bacterial infections were present at admission or during hospital stay in a third of patients (30%) (3,4,11,15,20).
The present study showed a low incidence of bacterial infections at hospitalized patients with hepatic cirrhosis of 5.7% (n=60). While low compared with the recent similar studies, our experience might reflect some differences compared with those studies above.
Firstly, due to the retrospective nature of our study, the total number of the infections might have been underestimated.
Secondly, a large number of the population studied were the patients admitted to the hospital for routine blood work and check-ups, due to the nature of the health care and the access to the providers in the tertiary care centers. For the patients coming from long distance, in order to provide routine blood work, imaging in the compensated cirrhosis of different etiologies, a hospital admission is required. More than that 51.24% (n = 536) of all cases were in compensated stage of liver cirrhosis and only 48.76% (n = 322) in decompensated stage. It is sometimes very difficult from a chart review to extract that paticular population.
Another observation was that the mean hospital stay for a cirrhotic patient with an infectious complication is two times longer than the mean hospital stay of a patient in the non-infected group (14 vs 6.8 days).
The data in literature concerning the frequence and distribution of bacterial infection suggest that SBP is the most common infection (over 25% of cases), followed by urinary tract infection (20%) and pneumonia (15%) (3,4,15).
Our results concerning cirrhotic patients with bacterial infections are similar in order and frequency: SPB (43.3%), urinary tract infection (33.3%) and pneumonia (13.3%).
SBP and urinary tract infections were more prevalent than in the literature.
The risk factors for the bacterial infections as quoted in the literature are upper digestive hemorrhage (UDH), hepatic dysfunction (class Child B and C) and malnutrition (11,14,16,18,27,29). This is also what we found in our study, with the main factor correlating to a bacterial infection being hepatic decompensation with OR 58.23.
We could not confirm that the alcoholic etiology is a risk factor, as suggested by other authors (21).
The strong link between UGI bleeding and infection in liver cirrhosis is probably explained by the cytokine activation in cascade with vasoactive compounds formation, increased variceal pressure and hemostasis dysfunction, which lead to haemorrhage (22-27) .
Bacterial infection was identified by Goulis J.in 1997 as and independent factor associated to failing of bleeding control in the first 5 days (22).
In our study, from the group of patients with bacterial infection (n=51), the GI bleeding was present in 9 cases (18%).
It increases the death rate in patients with SBP, our group having 4 deaths (80%).
Among patients with SBP without UGI bleeding (n=21), 7 deaths were encountered (33%).
All deaths happen in severe decompensated patients, with Child - Pugh score C.
As supported by the current literature,in order to prevent SBP and rebleding, antibiotics were administered in all patients with UGI bleed (24-27). We used third generation cephalosporines, quinolones, beta-lactamase inhibitor.
In our study, SBP and urinary infections were mostly community acquired infections, and all germs were sensitive to known antibiotics. E. Coli, according to other studies, dominated the etiologic spectrum, with preserved cephalosporins antibiotic sensitivity.
Etiologic agents of SBP were isolated in 11% of cases (n=3), versus 50% from other studies (9).
The low isolation rate can be explained by not inoculating the ascitic fluid directly in hemoculture recipients, but on solid media.
In the literature there are reports of increased prevalence of infections determined by antibiotic resistant gram positive cocci and gram negative bacilli. The explanations are the exposure of patients to hospital environment, invasive diagnosis manoeuvers and the usage of prophylactic antibiotherapy (28-32).
Most urinary infections were also community acquired, and our study demonstrates that, when these infections occurred during hospitalization, gram negative bacili were involved, multi resistant antibiotic phenotypes, with severe evolution to severe sepsis and death.
Nosocomial pneumonia (6 of 8 cases of pneumonia) was the main form present in our group leading to high mortality (63 %), irrespective of the state of hepatic decompensation.
The etiologic agents isolated were multi resistant Klebsiella pneumoniae or Methicillin-resistant Staphylococcus aureus.
It is known that pulmonary infections have as cause gram positive cocci (especially Pneumococcus), involved in community acquired infections, and gram negative or gram positive bacteria, involved in nosocomial infections (33).
Survival odds of this patients with multiple immunity dysfunctions, natural barrier alteration, are increased if the diagnosis is done as early as possible (34).
It is well known that some bacterial infections are asymptomatic and so they must be actively sought even if there is just a infection suspicion of infection, which might be a rapid decompensation with no apparent cause.
We believe the we need to have a high index of suspicion for an infectious process, despite the paucity of the signs and symptoms of an infections in all hospitalized patients with decompensated cirrhosis, and upper GI bleeding in any cirrhotic patient, given the observed high mortality. Prompt workup to determine the site of infection, along with early antibiotic therapy and supportive measure might lower the observed mortality.
References
- 1.Popescu I. Chirurgia Ficatului. Editura Universitara "Carol Davila"; 2004. [Google Scholar]
- 2.Gheorghe L, Iacob S, Simionov I, et al. Natural History of Compensated Viral B and D Cirrhosis. Rom J Gastroenterol. 2005;14:329–335. [PubMed] [Google Scholar]
- 3.Navasa M, Rodés J. Bacterial infections in cirrhosis. . Liver Intern. 2004;24:277–80. doi: 10.1111/j.1478-3231.2004.0934.x. [DOI] [PubMed] [Google Scholar]
- 4.Vilstrup H. Cirrhosis and bacterial infections. Rom J Gastroenterol. 2003;12:297–302. [PubMed] [Google Scholar]
- 5.Talwani R, Gilliam BL, Howell C. Infectious diseases and the liver. Clin Liver Dis. doi: 10.1016/j.cld.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422–33. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 7.Kalaitzakis E, Johansson JE, Bjarnason I, et al. Intestinal permeability in cirrhotic patients with and without ascites. Scand J Gastroenterol. 2006;4:326–30. doi: 10.1080/00365520510024278. [DOI] [PubMed] [Google Scholar]
- 8.Goez F, Ruiz P, Schreiber AD. Macrophage function in cirrhosis and the risk of bacterial infection. N Engl J Med. 1994;331:1122–8. doi: 10.1056/NEJM199410273311704. [DOI] [PubMed] [Google Scholar]
- 9.Caruntu L A, Benea L. Spontaneous Bacterial Peritonitis: Pathogenesis, Diagnosis, Treatment. J Gastrointest Liver Dis. 2006;15:51–56. [PubMed] [Google Scholar]
- 10.Lehner S, Stemmler HJ, Mück A, et al. Prognostic Parameters and Risk Stratification in Intensive Care Patients with Severe Liver Diseases. J Gastrointest Liver Dis. 2010;19:399–404. [PubMed] [Google Scholar]
- 11.Tandon P, Garcia Tsao G. Bacterial infections, sepsis and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28:26–42. doi: 10.1055/s-2008-1040319. [DOI] [PubMed] [Google Scholar]
- 12.Angelescu Mircea. Terapia cu antibiotice. Editura Medicala; 1998. [Google Scholar]
- 13.Ghassemi S, Garcia-Tsao G. Prevention and treatment of infections in patients with cirrhosis. Best Pract Res Clin Gastroenterol. 2007;21:77–93. doi: 10.1016/j.bpg.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Garcia Tsao G. Bacterial infections in cirrhosis: treatment and prophylaxis. J Hepatol. 2005;42(Suppl 1):S85–92. doi: 10.1016/j.jhep.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Borzio M, Salerno F, Piantoni L, et al. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001;33:41–8. doi: 10.1016/s1590-8658(01)80134-1. [DOI] [PubMed] [Google Scholar]
- 16.Cadranel JF, Denis J, Pauwels A, et al. Prevalence and risk factors of bacteriuria in cirrhotic patients: a prospective case control multicenter study in 244 patients. J Hepatol. 1999;31:464–8. doi: 10.1016/s0168-8278(99)80038-5. [DOI] [PubMed] [Google Scholar]
- 17.Fernández J, Navasa M, Gómez J, et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–8. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 18.Mohan P, Ramu B, Bhaskar E, et al. Prevalence and risk factors for bacterial skin infection and mortality in cirrhosis. Ann Hepatol. 2011 Jan-Mar;10(1):15–20. [PubMed] [Google Scholar]
- 19.Ruiz-del-Arbor L, Urman J, Fermandez J, et al. Cardiovascular, renal and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2003;38:1210–8. doi: 10.1053/jhep.2003.50447. [DOI] [PubMed] [Google Scholar]
- 20.Fasolato S, Angeli P, Dallagnese L, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007 Jan;45(1):223–9. doi: 10.1002/hep.21443. [DOI] [PubMed] [Google Scholar]
- 21.Rosa H, Silverio AO, Perini RF, et al. Bacterial infection in cirrhotic patients and its relationship with alcohol. Am J Gastroenterol. 2000;95:1290–3. doi: 10.1111/j.1572-0241.2000.02026.x. [DOI] [PubMed] [Google Scholar]
- 22.Goulis J, Armonis A, Patch D, et al. Bacterial infection is independently associated with failure to control bleeding in cirrhosis patients with gastrointestinal hemorrhage. Hepatology. 1998;27:1207–22. doi: 10.1002/hep.510270504. [DOI] [PubMed] [Google Scholar]
- 23.Goulis J, Patch D, Burroughs AK. Bacterial infection in the pathogenesis of variceal bleeding. Lancet. 1999;353:139–42. doi: 10.1016/S0140-6736(98)06020-6. [DOI] [PubMed] [Google Scholar]
- 24.Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila Fl, et al. Antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding: Cochrane Database Syst Rev. 2010 Sep;8(9):CD002907–CD002907. doi: 10.1002/14651858.CD002907.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin YT, Lo GH, Lai KH, et al. Prophylactic antibiotics in cirrhotics with upper gastrointestinal hemorrhage: a prospective controlled trial. J Chin Med Assoc. 2002;65:365–71. [PubMed] [Google Scholar]
- 26.Yang MT, Chen HS, Lee HC, et al. Risk factors and survival of early bleeding after esophageal variceal ligation. Hepatogastroenterology. 2007 Sep;54(78):1705–9. [PubMed] [Google Scholar]
- 27.Prelipcean CC, Sporea I, Mihai C, et al. Variceal upper digestive bleeding- an ever new complication in liver cirrhosis. Rev Med Chir Soc Med Nat Iasi. 2007 Jan-Mar;111(1):19–26. [Review] [PubMed] [Google Scholar]
- 28.Campillo B, Dupeyron C, Richardet JP. Hospital-acquired infections in cirrhotic patients: effect of carriage of methicillinresistant Staphylococcus aureus and influence of previous antibiotic therapy and norfloxacin prophylaxis. Epidemiol Infect. 2001;127:443–50. doi: 10.1017/s0950268801006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merli M, Lucidi C, Giannelli V, et al. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol. 2010 Nov;8(11):979–85. doi: 10.1016/j.cgh.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 30.Runyon BA. Changing flora of bacterial infections in patients with cirrhosis. Liver Int. 2010 Oct;30(9):1245–6. doi: 10.1111/j.1478-3231.2010.02337.x. [DOI] [PubMed] [Google Scholar]
- 31.Frazee LA, Marinos AE, Ryharczyk AM, et al. Long-term prophylaxis of spontaneous bacterial peritonitis in patients with cirrhosis. Ann Pharmacother. 2005;39:908–12. doi: 10.1345/aph.1E585. [DOI] [PubMed] [Google Scholar]
- 32.Von Graevenitz. In B. Murray, E. Baron, M. Pfaller, F. Tenover, and H. Yolken (ed.), 6th ed. ASM Press; Washington, D.C.: 1995. Nonfermentative gram-negative bacteria; pp. 520–532. [Google Scholar]
- 33.Zilberberg MD, Shorr AF. Healthcare- associated pneumonia: the state of evidence to date. Curr Opin Pulm Med. 2011 May;17(3):142–7. doi: 10.1097/MCP.0b013e328343eb33. [DOI] [PubMed] [Google Scholar]
- 34.Christou L, Pappas G, Falagas ME. Bacterial infection-related morbidity and mortality in cirrhosis. Am J Gastroenterol. 2007 Jul;102(7):1510–7. doi: 10.1111/j.1572-0241.2007.01286.x. [DOI] [PubMed] [Google Scholar]