Abstract
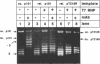
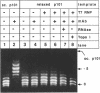
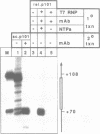
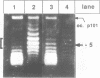
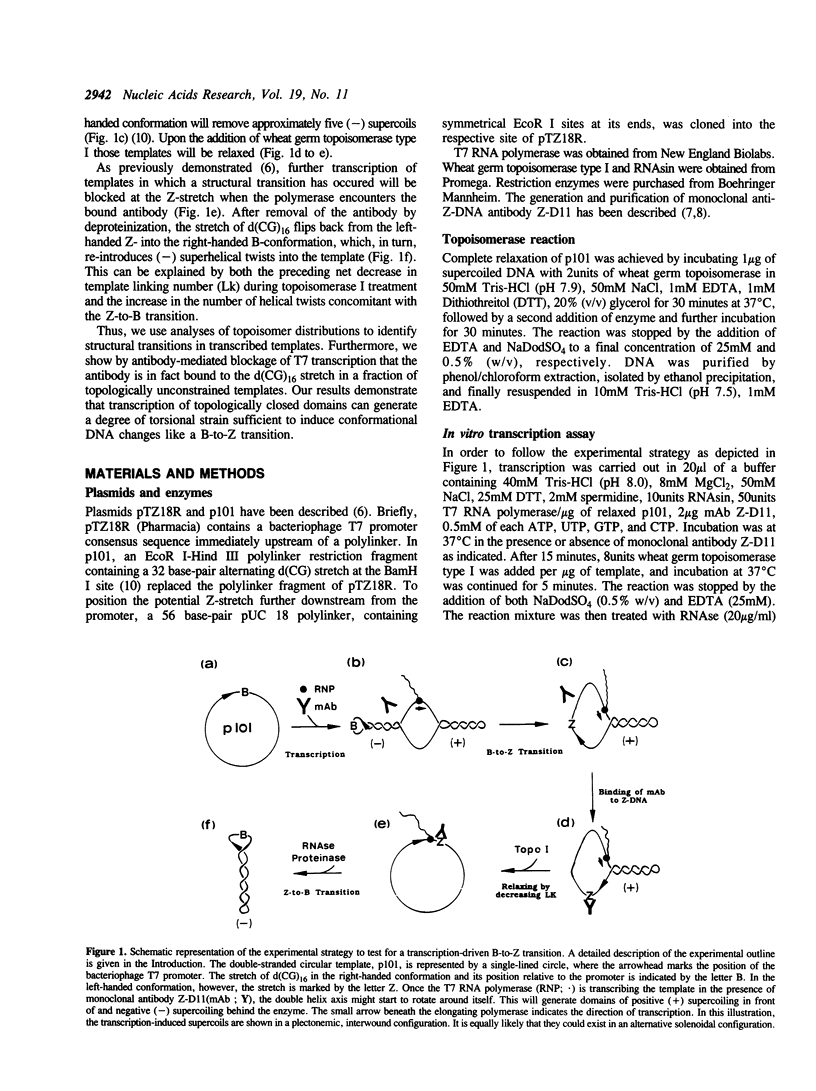
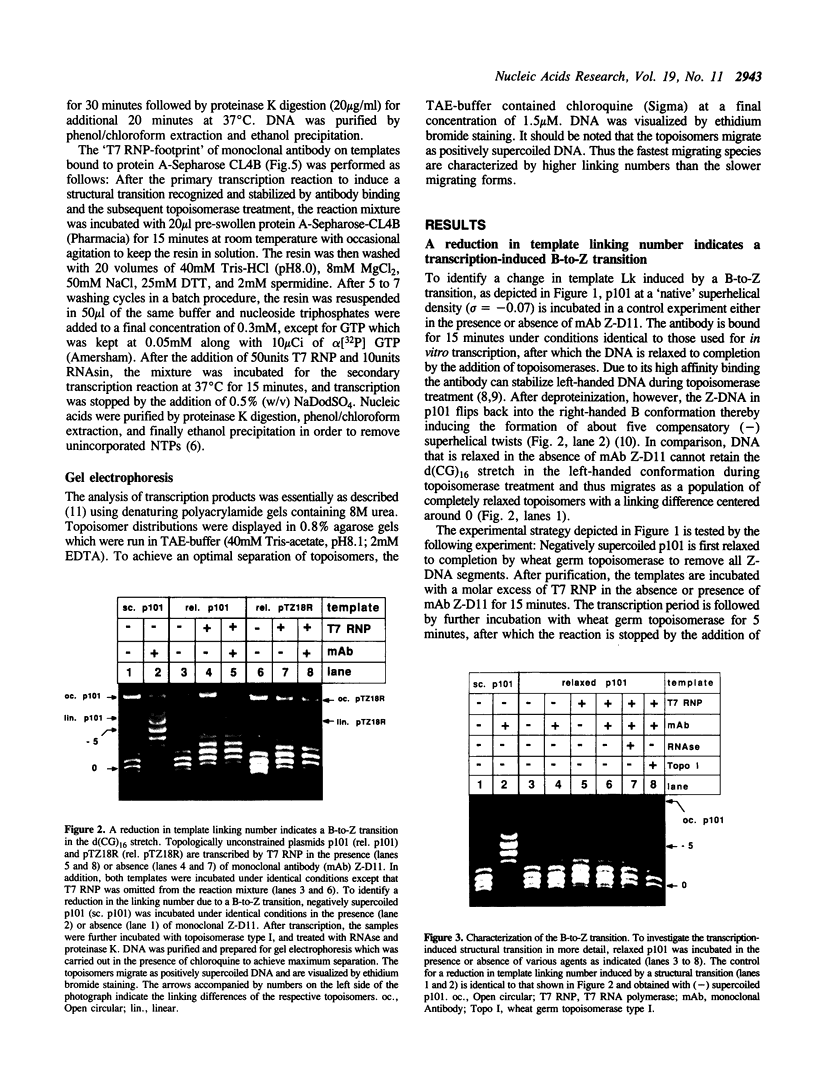
We have tested in vitro the occurrence of a B-to-Z transition in a region of alternating purines and pyrimidines as a consequence of transcription-induced negative supercoiling. By using a monoclonal antibody as a specific Z-DNA stabilizing agent, we demonstrate that the formation of left-handed DNA can transiently occur when a topologically unconstrained template is transcribed. The B-to-Z transition, observed in a subpopulation of templates, appears to be induced by negative supercoiling generated in the wake of an elongating T7 RNA polymerase. Consistent with this, the presence of topoisomerases during the transcription period prevents the change in DNA conformation. These data agree with the 'twin-supercoiled-domain' model for transcription of Liu and Wang (1). Interestingly, our results suggest that the diffusion rate of transcription-induced superhelical twists must be relatively slow compared to their generation, and that under in vitro conditions localized transient supercoiling can reach unexpectedly high levels.
Full text
PDF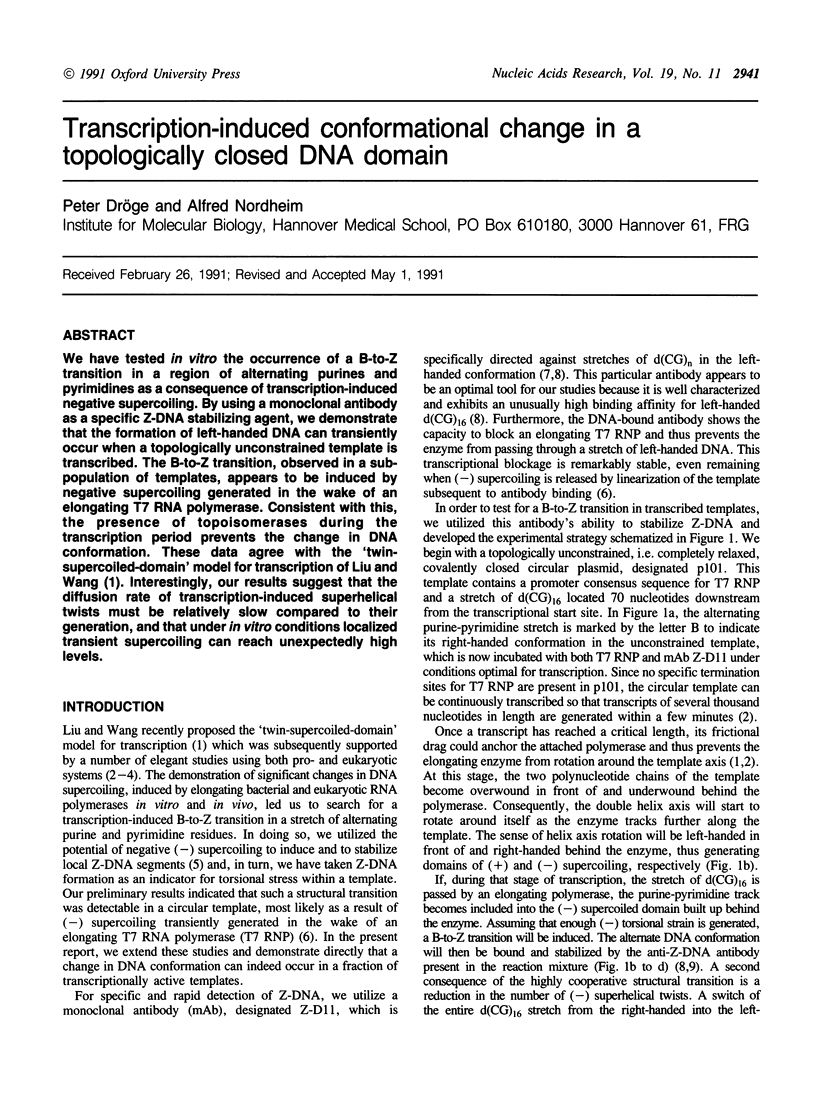



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brill S. J., Sternglanz R. Transcription-dependent DNA supercoiling in yeast DNA topoisomerase mutants. Cell. 1988 Jul 29;54(3):403–411. doi: 10.1016/0092-8674(88)90203-6. [DOI] [PubMed] [Google Scholar]
- Gamper H. B., Hearst J. E. A topological model for transcription based on unwinding angle analysis of E. coli RNA polymerase binary, initiation and ternary complexes. Cell. 1982 May;29(1):81–90. doi: 10.1016/0092-8674(82)90092-7. [DOI] [PubMed] [Google Scholar]
- Giaever G. N., Snyder L., Wang J. C. DNA supercoiling in vivo. Biophys Chem. 1988 Feb;29(1-2):7–15. doi: 10.1016/0301-4622(88)87020-0. [DOI] [PubMed] [Google Scholar]
- Giaever G. N., Wang J. C. Supercoiling of intracellular DNA can occur in eukaryotic cells. Cell. 1988 Dec 2;55(5):849–856. doi: 10.1016/0092-8674(88)90140-7. [DOI] [PubMed] [Google Scholar]
- Heck M. M., Hittelman W. N., Earnshaw W. C. Differential expression of DNA topoisomerases I and II during the eukaryotic cell cycle. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1086–1090. doi: 10.1073/pnas.85.4.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafer E. M., Sousa R., Rich A. Anti-Z-DNA antibody binding can stabilize Z-DNA in relaxed and linear plasmids under physiological conditions. EMBO J. 1985 Dec 30;4(13B):3655–3660. doi: 10.1002/j.1460-2075.1985.tb04131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley D. M. DNA opens up--supercoiling and heavy breathing. Trends Genet. 1988 Apr;4(4):111–114. doi: 10.1016/0168-9525(88)90099-6. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Nordheim A., Rich A., Wang J. C. Flipping of cloned d(pCpG)n.d(pCpG)n DNA sequences from right- to left-handed helical structure by salt, Co(III), or negative supercoiling. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4560–4564. doi: 10.1073/pnas.79.15.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Energetics of B-to-Z transition in DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6206–6210. doi: 10.1073/pnas.80.20.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C., Nordheim A., Rich A. Rate of B to Z structural transition of supercoiled DNA. J Mol Biol. 1986 Jul 5;190(1):125–127. doi: 10.1016/0022-2836(86)90082-3. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Dynamics of the B-to-Z transition in supercoiled DNA. Proc Natl Acad Sci U S A. 1986 Jul;83(14):4983–4987. doi: 10.1073/pnas.83.14.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni A. R., Wells R. D. Stabilization of Z DNA in vivo by localized supercoiling. Science. 1989 Oct 20;246(4928):358–363. doi: 10.1126/science.2678475. [DOI] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Stewart A. F., Herrera R. E., Nordheim A. Rapid induction of c-fos transcription reveals quantitative linkage of RNA polymerase II and DNA topoisomerase I enzyme activities. Cell. 1990 Jan 12;60(1):141–149. doi: 10.1016/0092-8674(90)90724-s. [DOI] [PubMed] [Google Scholar]
- Tsao Y. P., Wu H. Y., Liu L. F. Transcription-driven supercoiling of DNA: direct biochemical evidence from in vitro studies. Cell. 1989 Jan 13;56(1):111–118. doi: 10.1016/0092-8674(89)90989-6. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Giaever G. N. Action at a distance along a DNA. Science. 1988 Apr 15;240(4850):300–304. doi: 10.1126/science.3281259. [DOI] [PubMed] [Google Scholar]
- Yang L., Jessee C. B., Lau K., Zhang H., Liu L. F. Template supercoiling during ATP-dependent DNA helix tracking: studies with simian virus 40 large tumor antigen. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6121–6125. doi: 10.1073/pnas.86.16.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]