Abstract
The “four core genotypes” (FCG) model comprises mice in which sex chromosome complement (XX vs. XY) is unrelated to the animal's gonadal sex. The four genotypes are XX gonadal males or females, and XY gonadal males or females. The model allows one to measure (1) the differences in phenotypes caused by sex chromosome complement (XX vs. XY), (2) the differential effects of ovarian and testicular secretions, and (3) the interactive effects of (1) and (2). Thus, the FCG model provides new information regarding the origins of sex differences in phenotype that has not been available from studies that manipulate gonadal hormone levels in normal XY males and XX females. Studies of the FCG model have uncovered XX vs. XY differences in behaviors (aggression, parenting, habit formation, nociception, social interactions), gene expression (septal vasopressin), and susceptibility to disease (neural tube closure and autoimmune disease) not mediated by gonadal hormones. Some sex chromosome effects are mediated by sex differences in dose of X genes or their parental imprint. Future studies will identify the genes involved and their mechanisms of action.
Keywords: Sex chromosome, X chromosome, Y chromosome, Sex differences, Sexual differentiation, Nociception, Neural tube closure, Autoimmune disease, Addiction
1. Introduction
The vast majority of natural phenomena cannot be explained by experimental studies of those phenomena themselves, because the number of phenomena greatly exceeds the number of scientists. Instead, scientists study a restricted set of phenomena, from which they extract principles that are generalized to explain other phenomena. The model systems, if chosen appropriately, can yield generally useful rules that are found to govern the function of other systems. Individual model systems often provide only a limited perspective, however, so that new models and approaches can lead to modifications of old dogma. Here we review the impact of a recently developed mouse model system that offers some advantages for studying proximate signals that cause sex differences in phenotypes.
In mammals, the most obvious sex differences—the external and internal genitalia—were the first to be studied to determine which agents cause differences between males and females [54,51]. Because disruption of gonadal secretions interfered with normal masculine or feminine development, these secretions were identified as the direct causal agents of sexual dimorphism. That principle then suggested the idea that the brain might also be sexually differentiated by the same gonadal signals, an idea that was tested successfully in the classic 1959 experiment of Phoenix et al. [68]. They found that exposing female guinea pigs to testosterone in the fetal period permanently masculinized and defeminized their sexual behavior. This experiment, and many others since, established that testicular secretions have permanent (“organizational” or differentiating) effects directly on the brain, during critical periods of development in fetal and neonatal life [8]. Female phenotypes (e.g., vagina, clitoris, uterus, female patterns of behavior) were envisioned as being the “default” condition, which were hypothesized to differentiate permanently in the absence of gonadal hormonal effects. A second class of sex-specific signals, which cause sex differences in numerous phenotypes, are the reversible “activational” or acute effects of gonadal steroids operating at many life stages [13]. Sex differences in brain and behavioral phenotypes are caused by the different hormones secreted by the male and female gonad throughout life. Sex differences caused by activational effects are abolished by gonadectomy of adult animals.
Although the first phenotypes studied were mostly those involved in reproduction, where sex differences are large and adaptive, the organizational/activational theory was eventually applied to many brain and behavioral systems that are not so directly involved in reproduction: cognition, pain, feeding, etc. [53,26]. In many cases involving diverse physiological, anatomical, or behavioral variables, sexually differentiated phenotypes have been found to be influenced by gonadal secetions. The dominant perception is that the organizational-activational concept has had a great deal of power to explain sex differences across the board. Standard experimental approaches were developed for studying each type of hormonal effect [13]. To study organizational effects of gonadal steroids, one often measures masculinization or defeminization of the phenotype caused by injection of neonatal females with testosterone, or demasculinization or feminization of the phenotype in males deprived perinatally of their normal testicular secretions. To study activational effects of hormones, one manipulates levels of hormones acutely, for example by gonadectomy and hormone replacement, to determine if the sex difference is abolished and reinstated by the manipulations [13]. These model experiments have been found to cause sex-reversal of many phenotypes, thus establishing that many sex differences are controlled by differences in the levels of gonadal hormones at different life stages. To date, the primary approach for studying factors that cause sexual differentiation is the manipulation of gonadal hormone levels (or their receptors or metabolic enzymes, etc.) at various times of life.
Based on extensive evidence supporting the critical importance of gonadal hormones, the study of sex differences in the brain became a subdiscipline of the field of neuroendocrinology, so that this review is appropriately published in a journal of that field. To the extent that many neuroscientists perceived that the action of gonadal hormones was on restricted brain regions, sex differences themselves were arguably thought to be a side issue in the field of neuroscience. Many neuroscientists study only one sex (usually males), or fail to record or report the sex of their experimental animals, based on the idea that basic principles of neuroscience are similar in both sexes. However, sex has a large effect on diverse neural and behavioral phenotypes, and principles learned in male model systems do not necessarily transfer to females [64,19]. One thesis of the present paper is that the study of sex differences in the brain should no longer be seen as a subdiscipline of neuroendocrinology, but rather as its own field that requires working knowledge of genetics (especially of the sex chromosomes), endocrinology, and neuroscience.
The overwhelming evidence in favor of gonadal hormone control of sex differences made it seem to be difficult to overemphasize their role [65]. Eventually, however, a few neural and non-neural phenotypes were found in which sex differences were not explained by the action of gonadal hormones [71,70,18,78,3,1]. In these instances, sex differences were found at developmental stages before the onset of sex differences in levels of gonadal hormones, or the classic hormonal manipulations failed to cause sex-reversal [9]. At first, these departures from a hormone-only view of sexual differentiation were reactive, in the sense that they emphasized that the classic theories were incomplete (e.g., [4]). In this reactive mode, the main point would seem to be that some sex differences are not caused by gonadal hormones. That conclusion is not enough, however, because the primary goal is to identify the factors causing sex differences, not to identify the factors that are not causal. The primary alternative to gonadal hormones as causal agents are the direct effects of genes encoded on the sex chromosomes. These factors, the X and Y genes, are the only factors known to be represented differently in the male and female zygote. Such direct sex-specific actions of sex chromosome genes are well-known to be the proximate agents inducing sex differences throughout the body in invertebrate systems such as Drosophila [77]. In both birds and mammals, the same imbalance of sex chromosome factors triggers sexual differentiation of the gonads. Historically the imbalance of sex chromosomes was thought not to be responsible for sex differences in non-gonadal tissues, because experiments testing the role of hormones showed large effects in both XX and XY animals so that sex chromosome complement was thought to be minor or irrelevant (e.g., [16]). A strong test of the role of sex chromosome complement (XX vs. XY) required direct manipulation of that factor without changing gonadal secretions.
Manipulations of gene expression on the X chromosome are as tractable as for any of the autosomes, using standard methods of gene knock-out and transgenic overexpression. Manipulation of individual gene expression from the Y chromosome, however, is not as easy because knock-outs of Y genes have not been successful, and studies of Y gene overexpression by themselves may not yield reliable information about gene function. Importantly, however, manipulation of X and Y genes one by one is not an attractive first strategy for determining if XX and XY cells differ because of differences in X and Y gene expression. In the absence of candidate genes, it is difficult to know where to start, and which genes to manipulate. Moreover, the question at hand is not whether an X or Y gene is involved in a trait, but rather if the difference in gene expression found in XX and XY cells is responsible for a sex difference in phenotype. Thus, it becomes important to compare XX and XY cells (or animals) while at the same time trying to arrange the gonadal hormone levels so that they do not cause the differences between groups. In practice no one has ever produced XX and XY animals that are known to be hormonally equivalent (especially because proving hormonal equivalence requires proving a null hypothesis), so achieving perfection is elusive. However, perfection is not required to produce important new information on the differential effects of XX and XY genomes, as is discussed further below.
This review focuses on the “four core genotypes” (FCG) mouse model, an interesting and tractable genetic model, which like the classic endocrine manipulations discussed above, is becoming a standard in the investigation of sex differences caused by sex chromosome complement [37,7]. This model produces XX and XY gonadal males, and XX and XY gonadal females, so that one can contrast the effect of an XX and XY genotype in mice with the same gonadal type (Fig. 1). The model also has the advantage that it tests simultaneously for hormonal effects, and the interaction of sex chromosome and hormonal effects. Other mouse models are also useful for manipulating sex chromosomes or sex chromosome complement to test for sex chromosome effects [31,30,29,32,48,39,58,17,45,67], but are beyond the scope of this review.
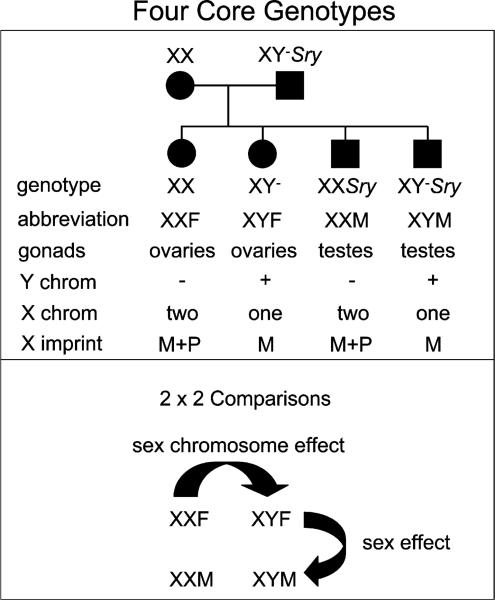
Fig. 1.
FCG mice are produced by breeding XX gonadal females with XY–Sry gonadal males, producing the four genotypes shown. The figure shows the genetic differences among the four genotypes, in presence/absence of the Y chromosome, number of X chromosomes, and genomic imprint on X genes. Below, FCG mice allow a 2 × 2 comparison to detect the phenotypic effects of sex (Sry present or absent) or sex chromosome (XX vs. XY).
2. How FCG mice were made
FCG mice involve two critical genetic components: the deletion of the testis-determining gene Sry from the Y chromosome, first reported by Lovell-Badge and Robertson [55], and the insertion of an Sry transgene onto an autosome in the same mice, achieved by Burgoyne and colleagues [56]. The Y chromosome of these mice sustained a spontaneous deletion that removed the entire Sry gene, yielding the “Y minus” chromosome (Y–) that not longer is testis-determining. XY– mice thus are gonadal females (Fig. 1). When a functional Sry transgene is present on an autosome, the mice are gonadal males (XY–Sry). Thus, testis determination resides on an autosome, so that XX and XY mice can be gonadal males or females. When XY–Sry gonadal males are mated with XX females, four core genotypes are produced, XX females, XY– females, XXSry males, and XY–Sry males. Because we define sex by the type of gonads, our shorthand refers to the four genotypes as XXF, XYF, XXM, and XYM.
The four genotypes yield a 2X2 comparison that fits the 2-way ANOVA (Fig. 1), with factors of sex (Sry present or absent) and sex chromosome complement (XX vs. XY). Three important tests are performed: (A) Testing the sex factor: Comparing males and females (XXF vs. XXM, and XYF vs. XYM), one tests the phenotypic effects of gonadal type. Significant differences in these comparisons are called effects of sex, since sex is defined by gonadal type. More precisely, these comparisons test for the direct and indirect effects of the Sry transgene, since that is the only gene that differs in the comparison. Although Sry has known direct effects on the brain [38], the majority of differences in this comparison are likely explained by the different effects of ovarian and testicular secretions. (B) Testing the factor of sex chromosome complement (“sex chromosome effects”): Comparing XX and XY mice of the same gonadal type (XXM vs. XYM, and XXF vs. XYF), one tests for the phenotypic effects of sex chromosome complement (Fig. 1). Because the genetic differences between groups mimics (except for the Sry gene) the genetic difference between normal XX females and XY males, we infer that sex chromosome effects that occur in the FCG model reflect sex chromosome effects that occur in normal males and females. (C) Testing interactions of sex and sex chromosome complement: The two-way ANOVA tests for an interaction, for example if the difference in effect of XX vs. XY is different in males and females. Stated differently, the model tests if the difference between gonadal males and gonadal females is significantly different in XX and XY mice. Testing for this interaction is a major advantage of the FCG model.
The gonads of XX and XY mice of the same gonadal sex are known to differ in morphology and function. For example, spermatogenesis requires non-Sry Y genes, so XXM lack sperm and have smaller testes than XYM. It is not known if XXM and XYM differ in the level of testicular secretions at specific times of life. Numerous traits that respond to organizational effects of testosterone are similar in XXM and XYM [37,57,80,42], suggesting that these two groups experience similar levels of testosterone. Measurements of circulating levels of testosterone in adult mice so far show no difference between XXM and XYM [42,66]. XYF differ from XXF in that they lose germ cells and stop estrous cycling early in life. Thus, phenotypes that respond to cyclic ovarian hormones are expected to differ in gonadally intact XXF and XYF mice.
Because we are interested in sex chromosome effects, and wish to discriminate those from the effects of gonadal hormones, to date we have usually compared FCG mice that have been gonadectomized, or gonadectomized and treated equally with specific gonadal hormones. These manipulations remove group differences created by acute (activational) effects of gonadal hormones. An important question is whether sex chromosome effects, measured by comparing gonadectomized same-sex XX and XY mice, might be caused by group differences in gonadal hormones, especially differences that might have occurred prior to gonadectomy. This possibility usually cannot be ruled out, although some experimental results make it unlikely in several cases, as is discussed below.
3. Genetic differences in XX vs. XY FCG mice
When same-sex XX and XY mice differ in the FCG model, we conclude that the difference is caused by a “sex chromosome effect”. There are numerous possible genetic origins for such effects (Fig. 1). (1) Y gene dose: XY mice are influenced by Y genes, but XX mice are not. (2) X gene dose: XX mice experience a higher expressed dose of X genes by virtue of the double genomic dose of X genes, in contrast to the single dose in XY mice. Although most dosage-related sex differences in X gene expression are essentially abolished by the sex-specific process of X-inactivation [49], which transcriptionally silences one X chromosome in each XX cell, some X genes are incompletely inactivated [20,81,83,84]. Thus, inherent functional differences in XX vs. XY cells might stem from differences in X gene expression resulting from differences in the copy number of X genes. (3) X gene imprint: XX and XY mice differ in the parental imprint of X genes. XY mice receive only a paternal imprint, and XX mice inherit both parental imprints although they are expressed in different cells depending on which X chromosome is active in the cell. XX vs. XY differences in X gene expression may result from the differences in parent of origin. (4) Buffering because of allelic mosaicism: XX and XY mice differ in the mosaic quality of their tissues. In outbred strains of mice, the parents of each mouse will have a different set of X alleles. XX mice will express the maternal X alleles in about half of their cells but paternal alleles in the other half of cells, whereas XY mice will express the maternal X alleles in all cells. The mosaic nature of the XX human has been suggested to lead to sex differences in mortality and susceptibility to disease, because the greater mixture of X genetic background in XX females could buffer against environmental challenges more than in XY males [63,6]. This is also a possible explanation for XX vs. XY phenotypic differences in some studies of FCG mice, when outbred mice were used [37]. However, some studies of FCG mice utilized inbred strains (e.g., C57BL/6, SJL) that are homozygous for all X alleles [42,43], in which case there is no XX mosaicism caused by differences in X alleles. Some studies with outbred MF1 mice also utilized mice that have identical X chromosomes (produced by breeding an XY male with his XO mother) [44,23], so that there was no allelic variation on the X chromosome. Even in these cases, however, the XX mouse is mosaic for X parental imprint, in contrast to the XY mouse with uniform imprint. The mosaicism itself could lead to XX vs. XY differences in phenotype. (5) Different life histories of XX and XY cells: XX cells must undergo X-inactivation, whereas XY mice do not. This process, which occurs early in development, requires the commitment of cellular resources that are not needed in XY cells. The difference in genetic resource allocation could make development of XX and XY cells fundamentally different, and could make them differentially susceptible to specific variations or abnormalities in developmental processes [23,27].
FCG mice are used to test whether the difference in XX and XY genomes has an effect on specific phenotypes that are measured. When a sex chromosome effect is found, the goal becomes to identify the X or Y genes that could be responsible for these effects, and their mechanisms of action. One next step is to compare XO with XX and XY. Under conditions that eliminate group differences caused by gonadal hormones, if a phenotype of XO mice is equivalent to that of XY mice but different than that of XX mice (e.g., XO = XY < XX), then we suspect that the number of X chromosomes or the parental X imprint causes the sex chromosome effect. If XO mice are similar to XX but different to XY (XO = XX < XY), then we suspect that one or more Y-linked genes cause the sex chromosome effect. An extensive discussion of strategies for identifying the critical X or Y genes is beyond the scope of this review, and important details are not discussed here.
4. The first use of FCG mice in studies of the brain and behavior
When FCG mice were first used to study sex differences in brain and behavior, the goal was to investigate well-known sexually dimorphic phenotypes that had previously shaped the literature [37,57,80]. Mice were gonadectomized as adults and then treated with equal levels of testosterone. The first question was whether sex chromosome complement played any role in sexual differentiation. For most phenotypes studied, the answer was “no”. The phenotypes selected for analysis had all been previously shown to be sexually differentiated by organizational and/or activational effects of gonadal hormones, and many showed a sex (gonadal male vs. gonadal female) difference in testosterone-treated adults. In most of the phenotypes, there was no significant effect of XX vs. XY sex chromosome complement. Thus, the FCG model confirmed that most of these phenotypes are sexually differentiated because of the action of gonadal hormones. These phenotypes included male copulatory behavior (M > F), social exploration behavior, the number of tyrosine hydroxylase neurons in the AVPV (anteroventral periventricular nucleus of the preoptic region, F > M), the number of motoneurons in the spinal nucleus of the bulbocavernosus (M > F), and thickness of the cerebral cortex (M > F). In addition, FCG mice analyzed at birth showed sex differences (M > F) in the number of progesterone receptor containing cells in three areas of the diencephalon, the AVPV, the medial preoptic nucleus, and the ventromedial nucleus, but no sex chromosome effects [80]. These results once again validate the long-standing conclusion that sex differences in these regions are controlled by gonadal hormones.
One of the well-established sexual dimorphisms, however, showed a sex chromosome effect in addition to the gonadal effects [37]. Male rodents have more vasopressin fibers in the lateral septum, which is known to be caused by organizational and activational effects of androgens and estrogens [46,36]. In gonadectomized adult mice treated with testosterone, XY mice had more vasopressin fibers than did XX mice (XYM > XXM, XYF > XXF). A subsequent study using C57BL/6 FCG mice rather than MF1 mice used by De Vries et al. [37], found a larger effect of sex chromosome complement on septal vasopressin independent of gonadal sex (XY:XX ratio approaching 2:1) as well as an effect of gonadal sex (M > F) that was independent of sex chromosome complement [42].
At the time of these first neurobehavioral measurements of FCG mice, one rodent phenotype had already been reported to differentiate in male and female cells prior to the onset of sex differences in gonadal secretions. When cells from the mesencephalon of mouse embryos were dissociated and cultured, they eventually developed differences in phenotype (more cells expressing tyrosine hydroxylase in XY than XX cultures) even if the cells were harvested at embryonic day 14, before sex differences in gonadal secretions were detected [75]. It was important, therefore, to test this trait also in FCG mice, to determine if the sex chromosome effect could be confirmed. We found that under a variety of conditions, XYM and XYF FCG mouse mesencephalic cultures showed more tyrosine hydroxylase cells than XXM and XXF cultures (Fig. 2) [21]. In contrast to the adult phenotypes measured [37], the early development of mesencephalic cells, at least in vitro, reflected a sex difference induced by sex chromosome complement, not hormones. Importantly, the dopaminergic nigrostriatal neurons are also influenced directly by the Y-linked gene Sry, which regulates the level of tyrosine hydroxylase in adult mesencephalon, and also regulates motor coordination in a male-specific manner [38]. The sex chromosome effect that differentiates XX and XY dopamine cells in vitro, however, cannot be explained by a direct effect of Sry. In the FCG model, XX and XY cells are compared that either both have Sry (XXM vs. XYM) or do not have Sry (XXF vs. XYF). The difference thus cannot be explained by an Sry effect. Nevertheless, small differences in the number of tyrosine hydroxylase cells were also found in groups that did or did not possess Sry [21], which could reflect a direct or indirect (e.g., hormonally mediated) effect of Sry.
Fig. 2.

Sex chromosome effect on embryonic mesencephalic dopamine cells in vitro. Dissociated mesencephalic cells were harvested from embryonic day 14 mice, then grown in vitro for 11 days. The graph shows that the number of tyrosine hydroxylase (TH) neurons, as a ratio to number of MAP2-positive neurons, was higher in XY than XX FCG mice (**p < 0.000001). Sex had no significant effect. From [21]. Reprinted by permission from Macmillan Publishers Ltd.; from Nature Neuroscience 5:933, copyright 2002.
5. More sex chromosome effects
In recent years FCG mice have been utilized to test for sex differences in diverse phenotypes, and large sex chromosome effects have been found in neural and non-neural systems.
5.1. Sex differences in aggression, parenting, and social interactions
Aggression is closely related to reproduction, since aggression likely evolved, at least in part, to enable the animal to protect its access to limited resources required for reproduction. Access to a female is required by males, which may account for the wide-spread testosterone-dependence of aggression in male vertebrates and the higher level of aggression found in male vs. female rodents in reproductive contexts. In mice, males show much higher levels of offensive aggression in various laboratory tests, and both organizational and activational effects of testosterone contribute to the large sex differences [45,50]. However, gonadally intact male mice with different Y alleles show differences in aggression, suggesting that Y genes also influence the level of aggression [59], although a gonadal hormonal mediation of this effect has not been ruled out. To shed further light on sex chromosome effects on aggression, Rissman and colleagues used FCG mice tested for offensive aggression in home cage intruder tests [42]. Intruders were gonad-intact male mice from which the olfactory bulb had been removed, which ensured that they were submissive and did not initiate any attacks on the resident mouse. FCG mice of all groups were gonadectomized as adults, and then treated with equal amounts of testosterone prior to the tests. Three groups showed similar levels of aggression on the intruder: XXM, XYM, and XYF, whereas XXF showed less aggression. The results suggest that sex chromosome complement has effects on mice with ovaries, such that XYF are more aggressive than XXF. In the presence of testes, however, there appeared to be no differential effect of sex chromosome complement. In this case, the effects of testicular secretions or an XY genome seem to dominate.
FCG mice were also tested in a pup-retrieval test, in which mouse pups are offered to the FCG mice before or after they were gonadectomized as adults [42]. The retrieval of the pups is thought to reflect parenting behavior. XXF mice retrieved pups more than any other group, indicating a sex chromosome effect that appears only in gonadal females. Thus, the presence of testes during development, or an XY genome, appears to interfere with the expression of this behavior.
The Rissman laboratory has also tested FCG mice in a wide variety of standard tests of olfaction (latency to find hidden food), motor coordination (rotorod), learning to avoid shock, open field activity, thresholds for flinch in response to footshock, anxiety (elevated plus maze), and social interaction in response to a non-aggressive intruder male [62]. In these tests, all mice were gonadectomized as adults. FCG mice did not differ on most of these tests, indicating that the genetic differences among these mice seem not to influence numerous behavioral systems mediated by diverse neural systems. Mice showed a sex effect on avoidance behavior: females responded faster than males. Sex chromosome effects were seen in measures of social interaction. XX mice sniffed and groomed the intruder mouse less than did XY mice, irrespective of gonadal sex. Moreover, XXF showed more digging behavior in response to the intruder mouse, relative to XYF, whereas XXM and XYM did not differ. The digging was interpreted as an asocial response reflecting a lower probability of social interaction among the XXF compared to XYF.
5.2. Sex chromosome effects on nociception
Because we detected sex chromosome effects on expression of genes related to opioid receptor systems in the brain (Chen et al., in preparation), we used FCG mice to investigate sex chromosome effects on nociception, which involves opiate systems. Gonadectomized adult FCG C57BL/6 mice were placed on a hotplate, and the latency to lick their hind paws was recorded. XX mice showed faster latency than XY mice, irrespective of gonadal sex [43]. Morphine injection caused an increase in latency of the same magnitude in XX and XY. A second experimental paradigm was used to test for sex chromosome effects on the development of tolerance to morphine. In this experiment, mice were injected with morphine twice daily for 6 days, with or without an NMDA antagonist suspected to interfere with development of tolerance, and then tested on the hotplate before and after a test injection of morphine (Fig. 3). Again, XX mice across all drug conditions had considerably shorter latencies to respond to the thermal nociceptive stimulus, both before and after the injection of morphine [43]. The effect of morphine was greater in XY than XX. To test whether a sex chromosome effect was also found in response to a noxious chemical stimulus, mice were observed after injection of formalin into the paw. XX mice licked the paw in the first 5 min more than XY mice [43]. Thus, under a variety of testing conditions and in response to different nociceptive stimuli, XX mice show a greater or faster response. A similar sex chromosome effect is found in newborn FCG mice. Gonad-intact neonatal XX mice have a shorter latency to withdraw the foot from a hotplate, although the difference is small [44]. The direction of these sex chromosome effects is similar to that in gonad-intact humans and mice, in which females normally show a greater response or sensitivity to painful stimuli, although the result is highly variable and depends on testing conditions [14,60,25]. Although gonadal steroids have both organizational and activational effects that cause sex differences in pain [26], sex chromosome effects also contribute to sex differences.
Fig. 3.
Sex chromosome effect on response to nociceptive stimuli. FCG mice were injected daily for six days with morphine or saline, with or without an NMDA blocker. On the seventh day, mice were placed on a hotplate and tested for their response before (at 0 min) and after an injection of morphine. The graph shows the latency to lick the hindpaw, for each of the four genotypes. All drug treatment groups are combined in this graph to illustrate the overall main effect of sex chromosome complement. XX mice of both sexes showed considerably shorter latencies to respond (*p < 0.00001), before or after the injection of morphine after the zero time point. Latencies were increased after morphine injection more in the XY than XX groups (p < 0.05). From [43]. Reprinted from Hormones and Behavior [43], copyright 2008, with permission of Elsevier, Inc.
5.3. Sex differences in habit formation potentially related to drug abuse
Human females use drugs of abuse at lower doses than males, and are reported to escalate usage to the point of addiction more rapidly than males [12]. In rodent models, mice will learn an instrumental response for food or drugs. At first the response is sensitive to the response-reward contingencies, but with time the behavior becomes habitual and (in contrast to earlier stages) continues even if the reward value is reduced. Using food reward, FCG mice of all groups learned an operant response equally. When the reward was devalued by associating the taste of the food with an aversive stimulus, XX mice persisted in responding for food, more than XY mice, irrespective of gonadal sex [69]. The results suggest that mice with different sex chromosomes have differences in the rate at which they learn a habit. That finding suggests the hypothesis that sex differences in forming drug-reinforced habits could be partly due to a sex chromosome effect as well.
5.4. X-linked factors cause sex differences in neural tube closure
Neural tube defects are responsible for a wide variety of developmental abnormalities, including spina bifida, exencephaly (growth of the brain outside of the cranial vault) leading to anencephaly (major loss of brain tissue), and oral-facial midline defects [24]. In humans, anterior neural tube defects occur more among females than males [74]. Successful closure of the neural tube requires the coordinated action of hundreds of genes that control critical timing of cell proliferation, movement, adhesion, and death. Over two hundred mouse mutations are associated with abnormal neural tube closure [47], some of which show dramatic sex differences because one sex is more affected. For example, in inbred strains, mice lacking the tumor suppressor gene p53 experience failure of anterior neural tube closure, exencephaly and anencephaly, and die by the day of birth [73,2]. The p53 mutation disproportionately affects females, paralleling the sex bias in humans. In C53BL/6 mice, litters contain almost no p53 nullizygous females on the day of birth. Because neural tube closure precedes the differentiation of the gonads, the sex difference in NTD has been correctly assumed to be a sex chromosome effect rather than a hormonal effect. We confirmed this conclusion using p53 nullizygous FCG mice, and found that XX mice showed more prenatal or perinatal mortality than XY mice, irrespective of gonadal sex [23]. Comparison of mice with different numbers of X chromosomes, with or without the Y chromosome, indicated that the sex chromosome effect was an X effect, explained by sex differences in expression of X genes caused by differences in genomic dose and/or parental imprint [23]. The Y chromosome did not protect from neural tube defects. This case is unusual. Usually X-linkage of disease susceptibility genes results in greater disease in males, in which the mutant allele is exposed because of X chromosome hemizygosity. In contrast, in this case the female is more susceptible. One potential explanation is that XX cells (but not XY cells) must undergo X-inactivation shortly before the onset of cellular events leading to neural tube closure, and thus might be especially susceptible to deletion of p53 if that results in improper X-inactivation and X chromosome replication [23,28]. If the p53 deletion accurately models the female bias in neural tube closure defects, then the greater female susceptibility may reside in X genes related to X-inactivation or cell death.
5.5. Sex chromosome effects in sexually dimorphic autoimmune diseases: Multiple Sclerosis (MS) and Systemic Lupus Erythematosus (SLE)
Almost all autoimmune diseases occur more in females than in males. In general, women are more immunologically reactive than men, for evolutionary reasons that are not immediately apparent. A greater rate of a life-threatening disease in females cannot be seen as a female-specific adaptation that increases their fitness, nor can it be seen as the result of male-specific adaptations that increase their fitness by protecting them from disease. If the protective mechanisms have evolved in one sex, they should have easily been favored (selected for) in the other sex as well. Rather, sex differences in disease susceptibility are likely “side effects” of other sex-specific adaptations. Because many genes have pleiotropic effects, selection may alter genes or genomes to produce important sex-specific effects, which may have some disadvantages that have not been completed mitigated by subsequent evolution for a variety of reasons [72,40]. For example, the Y chromosome evolved in males along specific pathways because of its dominant function to promote testis development and production of sperm [22]. Males are thus constrained to possess Y genes, even if those Y genes have pleiotropic effects on other tissues that are not advantageous. Similarly, females are constrained to undergo adaptive female-specific X-inactivation, even if it makes them susceptible to some developmental perturbations.
Women are diagnosed with Multiple Sclerosis (MS) more often and at an earlier age than males, with an approximate 3:1 ratio [79]. At later ages, men suffer from MS at greater rates. MS is caused by an autoimmune attack on myelin-producing oligodendrocytes, and produces deficits in numerous neural functions depending on the brain regions affected. The cellular events of MS have been studied using the mouse model Experimental Autoimmune Encephalomyelitis (EAE), in which mice are induced to produce an immune reaction to myelin proteins. Similar to MS, EAE produces sclerotic degenerative plaques in the CNS, loss of motor function, and sometimes death. In some mouse strains, female mice are more affected by EAE than male mice. Androgens protect females from the disease and account in part for the sex differences [79]. However, using FCG mice of the SJL strain, Voskuhl and colleagues [66,76] have found that gonadectomized XX mice show greater EAE-induced motor deficits in EAE than XY mice (Fig. 4), irrespective of their gonadal type. XX mice also show greater inflammation of the spinal cord.
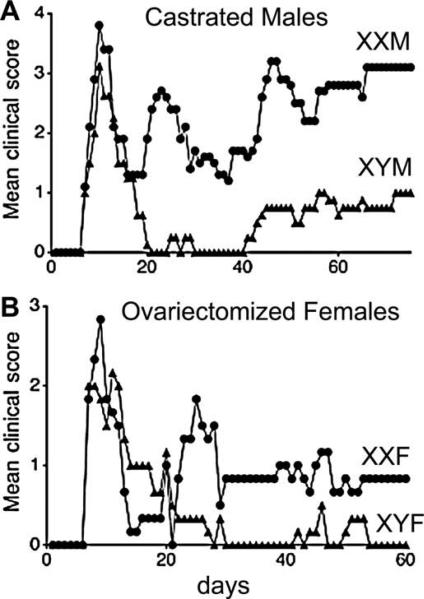
Fig. 4.
Effect of sex chromosome complement on response of mice to active EAE (Experimental Autoimmune Encephalomyelitis). Gonadectomized FCG mice were induced to develop an immune response to an injected myelin autoantigen. The graph shows the clinical scores of males (A) and females (B) over time in days after injection of the autoantigen. Higher clinical score indicates more severed motor impairment: 0 indicates no impairment, 1 and 2 mild and moderate limb weakness, 3 hind limb paresis, 4 total hind limb paralysis, 5 pre-moribund. In each experiment, XX mice showed significantly more severe disease (p < 0.0001). From [76], copyright by the authors and published by the Rockefeller University Press.
Among the most sex-biased diseases is SLE, which affects about nine times more women than men. SLE is an autoimmune disease characterized by widespread inflammation that can affect joints, heart, kidney, skin, lung, and brain. One mouse model of SLE involves the injecting the mouse with pristane, a saturated terpenoid alkane, which causes female-biased pathology of the kidney and other organs, and ultimately death. When gonadectomized FCG mice were treated with pristane, XY were less affected and survived longer than XX mice, irrespective of gonadal sex (Fig. 5) [76]. XX mice had higher levels of anti-DNA antibodies than XY mice, and much greater kidney pathology. These studies, in models of MS and SLE, suggest a large effect of sex chromosome complement leading to sex differences in autoimmune diseases.
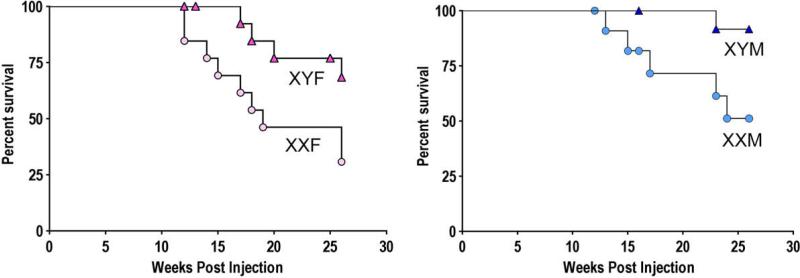
Fig. 5.
Effect of sex chromosome complement in a mouse model of Systemic Lupus Erythematosus (SLE). Mice were injected with pristane, and developed SLE-like symptoms including severe renal pathology. The graphs shows greater survival of XY than XX gonadectomized mice of each sex (females on left p < 0.04, males on right p < 0.03). From [76], copyright by the authors and published by the Rockefeller University Press.
6. Experimental designs and interpretation
The FCG mouse model has been applied so far mostly as a test of the hypothesis that some sex differences in phenotype result from the action of sex chromosome complement (XX vs. XY). In part, these experiments are a reaction to previous theories that attributed all sex differences to the effects of gonadal hormones. Because of this emphasis, we and other investigators have usually sought to reduce effects of gonadal hormones as much as possible, so that sex chromosome effects could be detected. FCG mice have been gonadectomized as adults to eliminate group differences caused by activational effects of gonadal hormones. Adult gonadectomy does not eliminate all effects of gonadal hormones, however, because some effects are long-lasting and could outlast the removal of gonads. However, the FCG model has a built-in assessment of the organizational effects of gonadal hormones. By comparing gonadal males and females after gonadectomy, one can test whether having testes or ovaries causes a long-lasting difference in the phenotype. In the case of the sex chromosome effect on adult nociception, for example, a robust sex chromosome effect was found in gonadectomized adults but no sex effect (i.e., no difference between gonadal males and females) (Fig. 3). The lack of a sex effect suggests that major sex differences in gonadal secretions, which cause normal sexual differentiation of numerous sexual dimorphisms in the brain and spinal cord of FCG mice [37,57,80], do not create differences in the nociceptive behaviors under the conditions of the study [43]. This finding suggests that differences between XX and XY mice of the same gonadal type might not be mediated by more minor within-sex differences in the levels of gonadal hormones. Moreover, because the sex chromosome effects are often seen in mice of both gonadal sexes, it is unlikely that a common within-sex difference in gonadal secretions accounts for the sex chromosome affect. Under these conditions, we feel comfortable in concluding that the sex chromosome affect is not mediated by gonadal secretions. That conclusion does not mean that the sex chromosome effect is not hormonal, since XX vs. XY mice might differ in levels of hormones produced in the brain or other non-gonadal tissues. Thus, we avoid labeling sex chromosome effects as “non-hormonal”, but prefer terms such as “direct genetic” or “sex chromosome effects”. Ultimately, the mechanisms mediating sex chromosome effects can be established only when the relevant genes are identified, and their mechanisms of action determined.
An important attribute of the FCG model is that it models the sex differences in sex chromosome complement found in normal males and females. Thus, when a sex chromosome effect is found, it is possible to hypothesize that the same sex chromosome effect is found in normal males and females and could contribute to sex differences in phenotype.
Because of the focus on finding sex chromosome effects, there has been little progress to date in using FCG mice to analyze the interaction of sex chromosome and gonadal hormone effects. Do gonadal steroids have the same effects in XX and XY? Is the difference in effects of XX vs. XY genomes the same in males and females? To analyze these effects, it will be important to manipulate steroid levels, at various life stages of FCG mice, to measure the interaction of genes and gonadal hormones.
7. Changes in the dogma required by outcomes of studies of FCG mice
The study of sex differences has been changed by the realization that XX and XY cells are not equivalent. The change has been slow to develop, and is ongoing.
7.1. The female is not the default sex
According to the classic Jost–Young dogma, secretions of the testes cause development to deviate from its default track, which is inherently feminine [52]. A female phenotype was thought to be the form that develops in the absence of gonadal secretions. The “default female” concept was always somewhat problematic, however, because scientists rarely, if ever, studied the phenotype of a mouse lacking gonadal secretions, so there was no accurate determination of the default phenotype. Indeed, various studies have suggested that fully feminine behavior requires ovarian secretions, not just to activate the behaviors, but also during development to differentiate the behavior [41]. Among the most intriguing studies are those in aromatase knock-out mice that show that development of feminine behaviors requires the aromatase gene [10,11], although those studies do not indicate whether the estrogens required for feminine differentiation of the brain are gonadal or made by the brain itself.
If an XX and XY genome have different effects on cells, however, then the XX condition cannot be considered default, but rather just the main alternative to the XY condition. It remains true that even XY mice lacking testicular secretions throughout life have numerous feminine traits, most prominent among them the feminine external genitalia and a uterus and oviducts. However, the XY mouse lacking testicular secretions would appear to lack numerous feminine traits including a fully feminine susceptibility to autoimmune disease or neural tube defects, and a feminine response to nociceptive stimuli, Thus, if there are any investigators still wedded to the notion of the female default, that concept should be abandoned in favor of a regrettably more complex model in which a variety of gonadal and non-gonadal proximate sex-specific forces are recognized.
7.2. Multiple sex chromosome signals lead to sex differences
To date, Sry is the only sex chromosome gene that has been identified that acts directly on the brain in a sex-specific manner [38]. Yet, we know that other genes must be in this class, because X genes are implicated in causing sex differences in neural tube defects in the p53 model [23], and because all sex chromosome effects detected with FCG mice are not explained by differences in action of Sry. We also know that parent of origin of X genes has effects on cognitive behavior of mice [30], which theoretically could have an impact on sex differences in cognition. Thus, it is likely that several or even many X or Y genes have direct sex-specific effects on the brain and other tissues. Numerous X genes are expressed higher in XX than XY mice [81,83,84,82]. We look forward to future studies that will identify which genes account for various sex chromosome effects.
7.3. Sex-specific factors can make or break sex differences
The classic Jost–Young dogma was heuristically pleasing, because it identified a single tissue, the testes, as the source of all signals that permanently sexually differentiate somatic (non-gonadal) tissues. It made sense that the control of somatic sex was given over to a single organ and to a few specific secretions, because they could coordinate the numerous developmental changes that lead to masculine or feminine differentiation as a whole [52,5]. Importantly, in the old dogma, all male-specific factors were pushing the animal to be more masculine, and female-specific factors were pushing in the female direction. However, if sex chromosome genes join gonadal secretions on the list of factors that act directly on tissues to create sex differences, then it becomes more likely that sex-specific forces can either push in the same direction or act in opposite directions and cancel each other out [66,35,33,34]. The most significant example of a sex-specific factor that reduces sex differences in phenotype is Xist, the X gene expressed only in females, which reduces what would otherwise be a widespread sexual discrepancy in X gene expression [49,15]. However, other cases occur [35]. The evolution of sex-specific forces to counteract sexual dimorphism underscores the idea that all sex differences are not adaptive. There is significant selection pressure to counteract some sex differences, as discussed above.
8. Future prospects
Three major developments will improve our understanding of direct sex chromosome effects on sex differences in phenotypes. First, as the number of studies increases, we will be better able to judge how widespread sex chromosome effects are, and how important they are. To date, only a limited number of neural and behavioral phenotypes have been tested. Second, currently we almost completely lack information about how sex chromosome complement interacts with the effects of hormones, for example whether hormonal effects swamp out sex chromosome effects, counteract them, or are different depending on whether the animal is XX or XY. Progress on that front is required to help understand the coordinated development of sex differences in phenotype when all sex-specific factors are in play. Third, progress is needed to identify the X and Y genes responsible for sex chromosome effects, and to determine their sites and mechanisms of action. Existing mouse lines and transgenic technologies are sufficient to allow one to determine whether the sex chromosome effect is an X or Y effect, and then narrow the list of candidate genes until the relevant genes are identified (e.g., [30,32,61] so that the mechanisms of action of the genes can be studied. Use of the FCG model is thus seen not as the end of the process of understanding sex chromosome effects, but as a beginning.
Acknowledgments
The development and testing of the FCG mice has been the result of a large collaborative effort across numerous labs. Paul Burgoyne and colleagues had the insight to combine the Y– chromosome with the Sry autosomal transgene, which led to a breakthrough in the study of sexually dimorphic phenotypes. We are indebted to the following people for many critical discussions of the ideas and results discussed here: Paul Burgoyne, Robin Lovell-Badge, Emilie Rissman, Geert De Vries, Amanda Swain, Richard Simerly, Rhonda Voskuhl, Eric Vilain, Margaret McCarthy, Jun Xu, Christine Wagner, Janice Juraska, Laura Gioiosa, Camron Bryant, Chris Evans, Jane Taylor, Jennifer Quinn, Yuichiro Itoh, and Laura Carruth. Supported by NIH grants MH59268, NS043196, NS045966, AI/AR50839, AI070306, AT002681, DK41310, and a grant from the Iris Cantor – UCLA Women's Health and Resource Center.
References
- 1.Agate RJ, Grisham W, Wade J, Mann S, Wingfield J, Schanen C, Palotie A, Arnold AP. Neural not gonadal origin of brain sex differences in a gynandromorphic finch. Proc. Natl. Acad. Sci. USA. 2003;100:4873–4878. doi: 10.1073/pnas.0636925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong JF, Kaufman MH, Harrison DJ, Clarke AR. High-frequency developmental abnormalities in p53-deficient mice. Curr. Biol. 1995;5:931–936. doi: 10.1016/s0960-9822(95)00183-7. [DOI] [PubMed] [Google Scholar]
- 3.Arnold AP. Genetically triggered sexual differentiation of brain and behavior. Horm. Behav. 1996;30:495–505. doi: 10.1006/hbeh.1996.0053. [DOI] [PubMed] [Google Scholar]
- 4.Arnold AP. Sexual differentiation of the Zebra Finch song system: Positive evidence, negative evidence, null hypotheses, and a paradigm shift. J. Neurobiol. 1997;33:572–584. [PubMed] [Google Scholar]
- 5.Arnold AP. Concepts of genetic and hormonal induction of vertebrate sexual differentiation in the twentieth century, with special reference to the brain. In: Pfaff DW, Arnold AP, Etgen A, Fahrbach S, Rubin R, editors. Hormones, Brain, and Behavior. Academic Press; San Diego: 2002. pp. 105–135. [Google Scholar]
- 6.Arnold AP. Sex chromosomes and brain gender. Nat. Rev. Neurosci. 2004;5:701–708. doi: 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]
- 7.Arnold AP, Burgoyne PS. Are XX and XY brain cells intrinsically different? Trends Endocrinol Metab. 2004;15:6–11. doi: 10.1016/j.tem.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the CNS. Annu. Rev. Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- 9.Arnold AP, Rissman EF, De Vries GJ. Two perspectives on the origin of sex differences in the brain. Steroids Nerv. Syst. 2003;1007:176–188. doi: 10.1196/annals.1286.018. [DOI] [PubMed] [Google Scholar]
- 10.Bakker J, Baum MJ. Role for estradiol in female-typical brain and behavioral sexual differentiation. Front Neuroendocrinol. 2008;29:1–16. doi: 10.1016/j.yfrne.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakker J, Honda S, Harada N, Balthazart J. The aromatase knockout (ArKO) mouse provides new evidence that estrogens are required for the development of the female brain. Ann. NY Acad. Sci. 2003;1007:251–262. doi: 10.1196/annals.1286.024. [DOI] [PubMed] [Google Scholar]
- 12.Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 14.Berkley KJ. Sex differences in pain. Behav. Brain Sci. 1997;20:371–380. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- 15.Boumil RM, Lee JT. Forty years of decoding the silence in X-chromosome inactivation. Hum. Mol. Genet. 2001;10:2225–2232. doi: 10.1093/hmg/10.20.2225. [DOI] [PubMed] [Google Scholar]
- 16.Breedlove SM, Arnold AP. Hormonal control of a developing neuromuscular system: I. Complete demasculinization of the male rat spinal nucleus of the bulbocavernosus using the antiandrogen flutamide. J. Neurosci. 1983;3:417–423. doi: 10.1523/JNEUROSCI.03-02-00417.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Budefeld T, Grgurevic N, Tobet SA, Majdic G. Sex differences in brain developing in the presence or absence of gonads. Dev. Neurobiol. 2008;68:981–995. doi: 10.1002/dneu.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgoyne PS. A Y-chromosomal effect on blastocyst cell number in mice. Development. 1993;117:341–345. doi: 10.1242/dev.117.1.341. [DOI] [PubMed] [Google Scholar]
- 19.Cahill L. Why sex matters for neuroscience. Nat. Rev. Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 20.Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 21.Carruth LL, Reisert I, Arnold AP. Sex chromosome genes directly affect brain sexual differentiation. Nat. Neurosci. 2002;5:933–934. doi: 10.1038/nn922. [DOI] [PubMed] [Google Scholar]
- 22.Charlesworth B. The evolution of sex chromosomes. Science. 1991;251:1030–1033. doi: 10.1126/science.1998119. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Watkins R, Delot E, Reliene R, Schiestl RH, Burgoyne PS, Arnold AP. Sex difference in neural tube defects in p53-null mice is caused by differences in the complement of X not Y genes. Dev. Neurobiol. 2008;68:265–273. doi: 10.1002/dneu.20581. [DOI] [PubMed] [Google Scholar]
- 24.Copp AJ, Greene NDE, Murdoch JN. The genetic basis of mammalian neurulation. Nat. Rev. Genet. 2003;4:784–793. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- 25.Craft RM. Sex differences in opioid analgesia: “from mouse to man”. Clin. J. Pain. 2003;19:175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur. J. Pain. 2004;8:397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Cranston A, Fishel R. Female embryonic lethality in Msh2-Trp53 nullizygous mice is strain dependent. Mamm. Genome. 1999;10:1020–1022. doi: 10.1007/s003359901151. [DOI] [PubMed] [Google Scholar]
- 28.Cranston A, Bocker T, Reitmair A, Palazzo J, Wilson T, Mak T, Fishel R. Female embryonic lethality in mice nullizygous for both Msh2 and p53. Nat. Genet. 1997;17:114–118. doi: 10.1038/ng0997-114. [DOI] [PubMed] [Google Scholar]
- 29.Davies W, Isles A, Burgoyne P, Wilkinson L. Evidence for X-linked imprinted genes affecting cognition in the mouse. Genet. Res. 2004;84:119. [Google Scholar]
- 30.Davies W, Isles A, Smith R, Karunadasa D, Burrmann D, Humby T, Ojarikre O, Biggin C, Skuse D, Burgoyne P, Wilkinson L. Xlr3b is a new imprinted candidate for X-linked parent-of-origin effects on cognitive function in mice. Nat. Genet. 2005;37:625–629. doi: 10.1038/ng1577. [DOI] [PubMed] [Google Scholar]
- 31.Davies W, Isles AR, Burgoyne PS, Wilkinson LS. X-linked imprinting: effects on brain and behaviour. BioEssays. 2006;28:35–44. doi: 10.1002/bies.20341. [DOI] [PubMed] [Google Scholar]
- 32.Davies W, Humby T, Isles AR, Burgoyne PS, Wilkinson LS. X-monosomy effects on visuospatial attention in mice. a candidate gene and implications for Turner syndrome and attention deficit hyperactivity disorder. Biol. Psychiatry. 2007;61:1351–1360. doi: 10.1016/j.biopsych.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 33.De Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145:1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- 34.De Vries GJ. Sex steroids and sex chromosomes at odds? Endocrinology. 2005;146:3277–3279. doi: 10.1210/en.2005-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Vries GJ, Boyle PA. Double duty for sex differences in the brain. Behav. Brain Res. 1998;92:205–213. doi: 10.1016/s0166-4328(97)00192-7. [DOI] [PubMed] [Google Scholar]
- 36.De Vries GJ, Duetz W, Buijs RM, Van Heerikhuize J, Vreeburg JTM. Effects of androgens and estrogens on the vasopressin and oxytocin innervation of the adult rat brain. Brain Res. 1986;399:296–302. doi: 10.1016/0006-8993(86)91519-2. [DOI] [PubMed] [Google Scholar]
- 37.De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J. Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dewing P, Chiang CWK, Sinchak K, Sim H, Fernagut PO, Kelly S, Chesselet MF, Micevych PE, Albrecht KH, Harley VR, Vilain E. Direct regulation of adult brain function by the male-specific factor SRY. Curr. Biol. 2006;16:415–420. doi: 10.1016/j.cub.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 39.Didier-Erickson A, Maxson SC, Ogawa S. Differential effect of the DBA1 and C57BL10 Y chromosomes on the response to social or other stimuli for offense. Behav. Genet. 1989;19:675–683. doi: 10.1007/BF01066030. [DOI] [PubMed] [Google Scholar]
- 40.Ellegren H, Parsch J. The evolution of sex-biased genes and sex-biased gene expression. Nat. Rev. Genet. 2007;8:689–698. doi: 10.1038/nrg2167. [DOI] [PubMed] [Google Scholar]
- 41.Fitch RH, Denenberg VH. A role for ovarian hormones in sexual differentiation of the brain. Behav. Brain Sci. 1998;21:311–327. doi: 10.1017/s0140525x98001216. [DOI] [PubMed] [Google Scholar]
- 42.Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J. Neurosci. 2006;26:2335–2342. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gioiosa L, Chen X, Watkins R, Klanfer N, Bryant CD, Evans CJ, Arnold AP. Sex chromosome complement affects nociception in tests of acute and chronic exposure to morphine in mice. Horm. Behav. 2008;53:124–130. doi: 10.1016/j.yhbeh.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gioiosa L, Chen X, Watkins R, Umeda EA, Arnold AP. Sex chromosome complement affects nociception and analgesia in newborn mice. J. Pain. 2008;9:962–964. doi: 10.1016/j.jpain.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grgurevic N, Budefeld T, Rissman EF, Tobet SA, Majdic G. Aggressive behaviors in adult SF-1 knockout mice that are not exposed to gonadal steroids during development. Behav. Neurosci. 2008;122:876–884. doi: 10.1037/0735-7044.122.4.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han TM, De Vries GJ. Organizational effects of testosterone, estradiol, and dihydrotestosterone on vasopressin mRNA expression in the bed nucleus of the stria terminalis. J. Neurobiol. 2003;54:502–510. doi: 10.1002/neu.10157. [DOI] [PubMed] [Google Scholar]
- 47.Harris MJ, Juriloff DM. Mouse mutants with neural tube closure defects and their role in understanding human neural tube defects. Birth Defects Res. Part A—Clin. Mol. Teratol. 2007;79:187–210. doi: 10.1002/bdra.20333. [DOI] [PubMed] [Google Scholar]
- 48.Isles AR, Davies W, Burrmann D, Burgoyne PS, Wilkinson LS. Effects on fear reactivity in XO mice are due to haploinsufficiency of a non-PAR X gene: implications for emotional function in Turner's syndrome. Hum. Mol. Genet. 2004;13:1849–1855. doi: 10.1093/hmg/ddh203. [DOI] [PubMed] [Google Scholar]
- 49.Itoh Y, Melamed E, Yang X, Kampf K, Wang S, Yehya N, Van Nas A, Replogle K, Band MR, Clayton DF, Schadt EE, Lusis AJ, Arnold AP. Dosage compensation is less effective in birds than in mammals. J. Biol. 2007;6:2. doi: 10.1186/jbiol53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson F, Whalen RE. Testicular hormones reduce individual differences in the aggressive behavior of male mice. A theory of hormone action. Neurosci. Biobehav. Rev. 1988;12:93–99. doi: 10.1016/s0149-7634(88)80001-0. [DOI] [PubMed] [Google Scholar]
- 51.Jost A. Reserches sur la différenciation sexuelle de l'embryon de lapin. Arch. Anat. Microsc. Morphol. Exp. 1947;36:271–315. [Google Scholar]
- 52.Jost A, Vigier B, Prepin J, Perchellet JP. Studies on sex differentiation in mammals. Rec. Prog. Horm. Res. 1973;29:1–41. doi: 10.1016/b978-0-12-571129-6.50004-x. [DOI] [PubMed] [Google Scholar]
- 53.Juraska JM. Sex differences in “cognitive” regions of the rat brain. Psychoneuroendocrinology. 1991;16:105–119. doi: 10.1016/0306-4530(91)90073-3. [DOI] [PubMed] [Google Scholar]
- 54.Lillie FR. The theory of the freemartin. Science. 1916;43:611–613. doi: 10.1126/science.43.1113.611. [DOI] [PubMed] [Google Scholar]
- 55.Lovell-Badge R, Robertson E. XY female mice resulting from a heritable mutation in the primary testis-determining gene, Tdy. Development. 1990;109:635–646. doi: 10.1242/dev.109.3.635. [DOI] [PubMed] [Google Scholar]
- 56.Mahadevaiah SK, Odorisio T, Elliott DJ, Rattigan A, Szot M, Laval SH, Washburn IL, McCarrey JR, Cattanach BM, Lovell-Badge R, Burgoyne PS. Mouse homologues of the human AZF candidate gene RBM are expressed in spermatogonia and spermatids, and map to a Y chromosome deletion interval associated with a high incidence of sperm abnormalities. Hum. Mol. Genet. 1998;7:715–727. doi: 10.1093/hmg/7.4.715. [DOI] [PubMed] [Google Scholar]
- 57.Markham JA, Jurgens HA, Auger CJ, De Vries GJ, Arnold AP, Juraska JM. Sex differences in mouse cortical thickness are independent of the complement of sex chromosomes. Neuroscience. 2003;116:71–75. doi: 10.1016/s0306-4522(02)00554-7. [DOI] [PubMed] [Google Scholar]
- 58.Maxson SC. Potential genetic models of aggression and violence in males. In: Driscoll P, editor. Genetically Defined Animal Models of Neurobehavioral Dysfunctions. Birkhauser; Boston, Basel. Berlin: 1992. pp. 174–188. [Google Scholar]
- 59.Maxson SC, Didier- Erickson A, Ogawa S. The Y chromosome, social signals, and offense in mice. Behav. Neural Biol. 1989;52:251–259. doi: 10.1016/s0163-1047(89)90369-5. [DOI] [PubMed] [Google Scholar]
- 60.Mayer EA, Berman S, Lin C, Naliboff BD. Sex-based differences in gastrointestinal pain. Eur. J. Pain. 2004;8:451–463. doi: 10.1016/j.ejpain.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 61.Mazeyrat S, Saut N, Grigoriev V, Mahadevaiah SK, Ojarikre OA, Rattigan A, Bishop C, Eicher EM, Mitchell MJ, Burgoyne PS. A Y-encoded subunit of the translation initiation factor Eif2 is essential for mouse spermatogenesis. Nat. Genet. 2001;29:49–53. doi: 10.1038/ng717. [DOI] [PubMed] [Google Scholar]
- 62.McPhie-Lalmansingh AA, Tejada LD, Weaver JL, Rissman EF. Sex chromosome complement affects social interactions in mice. Horm. Behav. 2008;54:565–570. doi: 10.1016/j.yhbeh.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Migeon BR. Females are Mosaic: X Inactivation and Sex Differences in Disease. Oxford University Press; Oxford: 2007. [DOI] [PubMed] [Google Scholar]
- 64.Mogil JS, Wilson SG, Chesler EJ, Rankin AL, Nemmani KVS, Lariviere WR, Groce MK, Wallace MR, Kaplan L, Staud R, Ness TJ, Glover TL, Stankova M, Mayorov A, Hruby VJ, Grisel JE, Fillingim RB. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc. Natl. Acad. Sci. USA. 2003;100:4867–4872. doi: 10.1073/pnas.0730053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat. Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- 66.Palaszynski KM, Smith DL, Kamrava S, Burgoyne PS, Arnold AP, Voskuhl RR. A Yin-Yang effect between sex chromosome complement, sex hormones on the immune response. Endocrinology. 2005;146:3280–3285. doi: 10.1210/en.2005-0284. [DOI] [PubMed] [Google Scholar]
- 67.Park JH, Burns-Cusato M, Dominguez-Salazar E, Riggan A, Shetty S, Arnold AP, Rissman EF. Effects of sex chromosome aneuploidy on male sexual behavior. Genes Brain Behav. 2008;7:609–617. doi: 10.1111/j.1601-183X.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 69.Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR. Sex chromosome complement regulates habit formation. Nat. Neurosci. 2007;10:1398–1400. doi: 10.1038/nn1994. [DOI] [PubMed] [Google Scholar]
- 70.Reisert I, Pilgrim C. Sexual differentiation of monoaminergic neurons- genetic or epigenetic. Trends Neurosci. 1991;14:467–473. doi: 10.1016/0166-2236(91)90047-x. [DOI] [PubMed] [Google Scholar]
- 71.Renfree MB, Short RV. Sex determination in marsupials: evidence for a marsupial-eutherian dichotomy. Phil. Trans. R. Soc. London. B: Biol. Sci. 1988;322:41–53. doi: 10.1098/rstb.1988.0112. [DOI] [PubMed] [Google Scholar]
- 72.Rice WR. Sexually antagonistic genes—experimental evidence. Science. 1992;256:1436–1439. doi: 10.1126/science.1604317. [DOI] [PubMed] [Google Scholar]
- 73.Sah VP, Attardi LD, Mulligan GJ, Williams BO, Bronson RT, Jacks T. A subset of p53-deficient embryos exhibit exencephaly. Nat. Genet. 1995;10:175–180. doi: 10.1038/ng0695-175. [DOI] [PubMed] [Google Scholar]
- 74.Seller MJ. Sex, neural tube defects, and multisite closure of the human neural tube. Am. J. Med. Genet. 1995;58:332–336. doi: 10.1002/ajmg.1320580406. [DOI] [PubMed] [Google Scholar]
- 75.Sibug R, Küppers E, Beyer C, Maxson SC, Pilgrim C, Reisert I. Genotype-dependent sex differentiation of dopaminergic neurons in primary cultures of embryonic mouse brain. Dev. Brain Res. 1996;93:136–142. doi: 10.1016/0165-3806(96)00024-7. [DOI] [PubMed] [Google Scholar]
- 76.Smith-Bouvier DL, Divekar AA, Sasidhar M, Du S, Tiwari-Woodruff SK, King JK, Arnold AP, Singh RR, Voskuhl RR. A role for sex chromosome complement in the female bias in autoimmune disease. J. Exp. Med. 2008;205:1099–1108. doi: 10.1084/jem.20070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taylor BJ, Villella A, Ryner LC, Baker BS, Hall JC. Behavioral and neurobiological implications of sex-determining factors in Drosophila. Dev. Genet. 1994;15:275–296. doi: 10.1002/dvg.1020150309. [DOI] [PubMed] [Google Scholar]
- 78.Thornhill AR, Burgoyne PS. A paternally imprinted X chromosome retards the development of the early mouse embryo. Development. 1993;118:171–174. doi: 10.1242/dev.118.1.171. [DOI] [PubMed] [Google Scholar]
- 79.Voskuhl R. Sex differences in autoimmune disease. In: Pfaff D, Arnold AP, Etgen A, Fahrbach S, Ruben R, editors. Academic Press; in press. [Google Scholar]
- 80.Wagner CK, Xu J, Pfau JL, Quadros PS, De Vries GJ, Arnold AP. Neonatal mice possessing an Sry transgene show a masculinized pattern of progesterone receptor expression in the brain independent of sex chromosome status. Endocrinology. 2004;145:1046–1049. doi: 10.1210/en.2003-1219. [DOI] [PubMed] [Google Scholar]
- 81.Xu J, Burgoyne PS, Arnold AP. Sex differences in sex chromosome gene expression in mouse brain. Hum. Mol. Genet. 2002;11:1409–1419. doi: 10.1093/hmg/11.12.1409. [DOI] [PubMed] [Google Scholar]
- 82.Xu J, Taya S, Kaibuchi K, Arnold AP. Sexually dimorphic expression of Usp9x is related to sex chromosome complement in adult mouse brain. Eur. J. Neurosci. 2005;21:3017–3022. doi: 10.1111/j.1460-9568.2005.04134.x. [DOI] [PubMed] [Google Scholar]
- 83.Xu J, Deng X, Disteche CM. Sex-specific expression of the X-linked histone demethylase gene Jarid1c in brain. PLoS ONE. 2008;3:e2553. doi: 10.1371/journal.pone.0002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu J, Deng X, Watkins R, Disteche CM. Sex-specific differences in expression of histone demethylases Utx and Uty in mouse brain and neurons. J. Neurosci. 2008;28:4521–4527. doi: 10.1523/JNEUROSCI.5382-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]