Abstract
Nitric oxide, which is produced endogenously within cardiac myocytes by three distinct isoforms of nitric oxide synthase, is a key regulator of myocardial function. This review will focus on the regulation of myocardial function by each nitric oxide synthase isoform during health and disease, with a specific emphasis on the proposed end-targets and signaling pathways.
Keywords: NOS, Peroxynitrite, cGMP, Excitation-contraction coupling, L-type Ca2+ channel, Phospholamban, Ryanodine receptor
1.1 Nitric oxide and the myocardium
The role of nitric oxide (NO) signaling has been well defined in such processes as neural transmission and the dilation of blood vessels. Although the function of NO remains less well defined in the heart, NO has been shown to be a key regulator of excitation-contraction coupling (ECC) [1]. The process of ECC underlies myocardial contraction [2]. The β-adrenergic receptor (β-AR) signaling pathway is also a critical modulator of ECC and produces positive inotropic and lusitropic effects upon activation [3]. Balligand et al. first demonstrated that endogenous NO plays a role in the mediation of β-AR signaling as well [4].
NO is synthesized upon the cleavage of L-arginine into L-citrulline by three distinct isoforms of NO synthase (NOS) within the myocardium [5, 6]. Neuronal NOS (nNOS, NOS1) and endothelial NOS (eNOS, NOS3) are constitutively expressed in cardiac myocytes. These two isoforms are considered to be low output enzymes and produce NO in phase with myocyte contraction due to Ca-calmodulin regulation. In early studies, the use of NO donors or nonspecific NOS inhibitors made it difficult to distinguish between NOS1 and NOS3 signaling. However, recent studies have found that although NO is a highly diffusible signaling molecule, signaling via NOS1 and NOS3 is compartmentalized, and NOS1 and NOS3 differentially modulate cardiac function [5, 7, 8]. Inducible NOS (iNOS, NOS2), on the other hand, is only expressed during inflammatory responses and has been shown to be present during many pathophysiological conditions of the myocardium (e.g. ischemia-reperfusion injury, septicemia, aging, heart failure, etc.). When expressed, NOS2 produces much higher levels of NO independent of [Ca2+]i, compared to the constitutive NOS isoforms [6, 9].
1.2 Nitric Oxide Signaling
NO has been shown to signal through at least two distinct pathways: cGMP-dependent and cGMP-independent [10]. The cGMP-dependent effects of NO result from the NO-induced activation of guanylate cyclase, leading to increased cGMP levels, which modulate the activity of protein kinase G (PKG), as well as cGMP-regulated phosphodiesterases (PDE; cGMP-stimulated: PDE2; cGMP-inhibited: PDE3). cGMP-independent effects occur mainly via S-nitrosylation, an important protein modification related to cell signaling [11]. NO can also directly activate adenylate cyclase, thus increasing cAMP levels and myocardial contractility [12]. Additionally, NO may couple with other reactive oxygen and nitrogen species, leading to the formation of related congeners, such as peroxynitrite (ONOO−). These related species may also influence cardiac contractility, and in some cases produce markedly differing effects from those observed with NO alone. Therefore, it is not surprising that paradoxical results have been reported in the literature, as both positive and negative effects of NO and related congeners have been observed. However, recent studies are resolving these apparent contradictions by determining that the contractile effects of NO are greatly influenced by NOS isoform localization [5, 6], and the activation of distinct cGMP-dependent and cGMP-independent signaling pathways which target individual ECC proteins in the cardiac myocyte. Additional studies have determined that these contractile effects are further confounded by such factors as gender [13], site of production [14, 15], species produced [16-18], concentration [19, 20], and cardiac myocyte contractile state [17, 20]. These factors are relevant to the contractile effects of NO and related congeners during both health and disease and are sensitive to cellular redox state.
2.1 NOS1 expression in the myocardium
The neuronal isoform of NOS (NOS1) was originally characterized in the forebrain [21], but has also been found to be constitutively expressed in cardiac myocytes. NOS1 has been shown to be localized to the sarcoplasmic reticulum (SR), and co-immunoprecipitates with the SR Ca2+ release channel or ryanodine receptor (RyR) under physiological conditions [5, 7]. Although sex hormones such as estradiol have been demonstrated to increase NOS1 mRNA levels [22], the effect of gender on the expression of NOS1 remains less well defined. One study demonstrated higher levels of NOS1 expression in female hearts compared to male [23], while another study found no difference between male and female hearts [24]. However, this discrepancy may result from species differences (rat vs. mouse) and/or female estrus cycle variance.
2.2 Contractile effects of NOS1-derived NO
The force frequency response (FFR) is an important mediator of contractility [25], and is partly modulated by NOS1 signaling. For instance, in vivo measurements have demonstrated that NOS1 knockout (NOS1−/−) mice exhibit a blunted FFR (contraction and relaxation), which was also apparent in isolated NOS1−/− trabeculae and myocytes [5, 26, 27].
Several studies have shown that NOS1 is also capable of regulating the β-AR pathway. Specifically, in vivo and whole heart experiments demonstrated that the knockout of NOS1 leads to a reduced contractile response to β-AR stimulation [5, 28, 29]. We have recently demonstrated that myocytes isolated from NOS1−/− hearts also had a blunted response to β-AR stimulation, observed as a decrease in [Ca2+]i transient and cell shortening amplitudes compared to WT [27].
NOS1 expression and activity may also be upregulated in certain disease states. For example, one study noted gender-dependent changes in NOS1 activity following pressure overload [30]. NOS1 has also been shown to translocate to the sarcolemma and localize with caveolin-3 during disease states [31, 32], or with conditional overexpression [33]. Conditional cardiac-specific overexpression of NOS1 resulted in decreased contractile function, while NOS1−/− mice exhibited increased mortality, hypertrophy, and left-ventricular dilation after myocardial infarction [28, 34]. Although NOS1 appears to be cardioprotective, the mechanism(s) for these effects are unknown.
2.3 End-targets and signaling pathways of NOS1
Phospholamban (PLB) is a key ECC protein which modulates SR Ca2+-ATPase activity (SERCA). As such, PLB is a participant in the FFR and is also the major phosphoprotein in the β-AR pathway [35]. We and others have demonstrated that PLB is a key target of NOS1 signaling [27, 36]. In WT myocytes, acute NOS1 inhibition resulted in decreased basal and β-AR-stimulated contraction, and slowed [Ca2+]i decline (Fig. 1B; similar to NOS1−/−). However, with acute NOS1 inhibition in PLB−/− myocytes, we noted no effect on contraction or [Ca2+]i decline. We further examined the effects of NOS1 signaling on PLB, and observed that NOS1 inhibition decreased PLB phosphorylation [27], which was shown to be due to enhanced protein phosphatase activity [36]. We also observed a decreased SR Ca2+ load, an important determinant of myocyte contraction [37], with NOS1 knockout or inhibition. Interestingly, NOS1−/− hearts have decreased expression of PLB and increased expression of RyR and calsequestrin [26, 38]. These changes appear to be compensatory in an attempt to increase SR Ca2+ uptake, SR Ca2+ load, and SR Ca2+ release. During β-AR stimulation, PLB phosphorylation levels are similar between NOS1−/− and WT myocytes [36]. This normalized PLB phosphorylation leads to similar [Ca2+]i decline and myocyte re-lengthening rates between NOS1−/− and WT myocytes [27, 36]. However, we have shown that there is still a reduced contractile response to β-AR stimulation in NOS1−/− myocytes [27], suggesting additional protein targets.
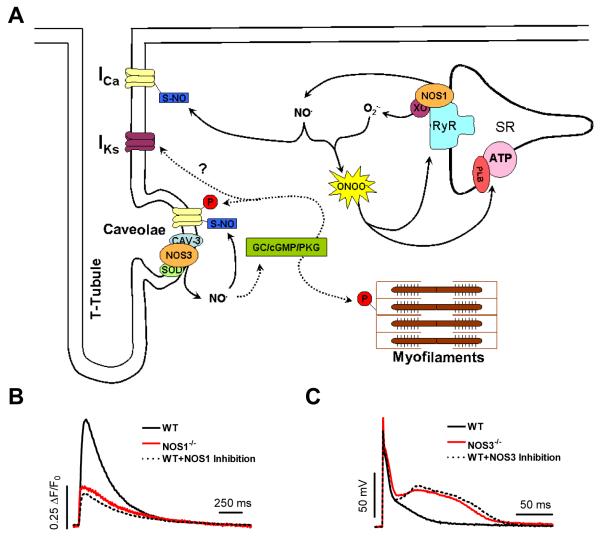
Figure 1. Physiological (non-pathophysiological) signaling of constitutive NOS isoforms in the myocardium.
A.) Proposed cGMP-dependent (dashed lines) and cGMP-independent (solid lines) signaling pathways and end targets of NOS1 and NOS3 in the normal functioning myocardium. B.) Basal cardiac myocyte [Ca2+]i transients in WT, NOS1−/−, and WT+NOS1 Inhibition (S-methyl-L-thiocitrulline). C.) Cardiac myocyte action potential waveform during perfusion with 1 μmol/L isoproterenol (ISO, non-specific β-AR agonist) in WT, NOS3−/−, and WT+NOS3 Inhibition (N5-(1-Iminoethyl)-L-ornithine dihydrochloride). NOTE: Data modified from references [27, 61].
NOS1 has also been shown to target RyR [39], as one study demonstrated that NOS1−/− myocytes had an enhanced diastolic leak via RyR. This enhanced leak could also contribute to the reduction in SR Ca2+ load. NOS1 signaling has also been found to target the L-type Ca2+ current (ICa). Interestingly, this NOS1-mediated decrease in ICa led to decreased basal and β-AR-stimulated contraction [38]. NOS1 also interacts with non-ECC proteins, as NOS1 has been shown to bind with sarcolemmal Ca2+ pump 4b (PMCA) [40], which regulates NOS1 activity by modulating [Ca2+]i levels. Overexpression of PMCA was shown reduce NOS1 activity, and decrease the response to β-AR stimulation, with a trend toward decreased basal contraction (similar to NOS1−/− ). Notably, several of these studies showed that these effects were through cGMP-independent signaling pathways (Fig. 1A, solid lines) [27, 36, 39].
Since NOS1 co-immunoprecipitates with xanthine oxidoreductase [41], a superoxide (O2.−) producing enzyme, low levels of peroxynitrite may be formed. Additionally, it is possible for NOS1 to produce both NO and superoxide [42], although this is more likely to occur with uncoupling in disease states [43]. Thus peroxynitrite, produced by the reaction of NO and superoxide, may be a potential signaling molecule for NOS1. We investigated the role of peroxynitrite in NOS1 signaling and upon perfusion with FeTPPS, a peroxynitrite decomposition catalyst, we were able to mimic the effects of NOS1 knockout or acute inhibition (decreased contraction, slowed [Ca2+]i decline, decreased PLB phosphorylation) [27]. In another study, we demonstrated that a low concentration of peroxynitrite increased basal contraction and further increased the response to β-AR stimulation in WT myocytes through a PLB-dependent mechanism [20]. These data are consistent with NOS1 signaling occurring via peroxynitrite.
RyR activity is also regulated by S-nitrosylation, which increases RyR open probability [44]. Xu et al. confirmed reversible S-nitrosylation of up to 12 cysteines of the RyR tetramer, which led to progressive channel activation [45]. More recently, Gonzalez et al. [39] reported that NOS1 knockout decreased RyR S-nitrosylation levels. They also observed increased RyR oxidation, which can lead to enhanced RyR activity. However, prolonged oxidation of RyR leads to irreversible inactivation [46]. As a result, NOS1−/− myocytes may also have decreased RyR activity. Indeed, our preliminary data suggests that RyR activity is decreased in NOS1−/− myocytes [47]. In summary, NOS1 modulates cardiac myocyte contraction mainly through cGMP-independent signaling pathways by targeting multiple ECC proteins, including PLB, RyR, and the L-type Ca2+ channel (Fig. 1A).
3.1 NOS3 expression in the myocardium
As with NOS1, the endothelial isoform of NOS (NOS3) is also constitutively expressed in cardiac myocytes. NOS3 has been shown to be localized to the caveolaeand co-immunoprecipitates with caveolin-3 (Fig. 1A) [5, 48]. NOS3 expression also appears to be graded in the myocardium, such that NOS3 expression in left ventricular epicardial myocytes is significantly increased compared to left ventricular endocardial myocytes [49]. Additionally, the expression of NOS3 in the myocardium is not limited to the cardiac myocyte, as NOS3 is also expressed in endothelial cells [50], where NOS3-dervied NO serves to regulate vascular tone and contraction [15, 51]. Sex hormones have also been shown to increase NOS3 mRNA levels [22, 52], but the effects of gender on NOS3 expression remain inconsistent [23, 24]. This is likely due the preparation used (myocyte vs. whole heart) and/or variances in the female estrus cycle. Phosphorylation of NOS3 by protein kinase AKT can also increase the enzyme activity of NOS3 [53]. Interestingly, studies have also shown that estrogen can bind to membrane receptors, leading to the phosphorylation and activation of NOS3 [54, 55]. However, gender-based differences in NOS3 phosphorylation in the myocardium are currently unknown.
3.2 Contractile effects of NOS3-derived NO
In contrast to NOS1, NOS3 signaling does not regulate the FFR or basal function, as studies have found no difference in isolated cardiac myocytes with NOS3 knockout (NOS3−/−) or overexpression compared to WT [5, 26, 56, 57]. Conversely, some in vivo and whole heart studies have found NOS3 to modulate basal function [5, 58, 59]. However, endothelial cell-derived NO may cause indirect effects on cardiac myocytes through diffusion [15]. NOS3 signaling has also been shown to play a pivotal role in the stretch-dependent enhancement of [Ca2+]i transients and cardiac myocytecontraction [8]. This phenomenon allows the heart to increase contraction in response to an increase in preload beyond that achieved by ordinary length-dependent activation mechanisms.
Studies have consistently reported that NOS3 signaling decreases the cardiac functional response to β-AR stimulation [5, 58-61]. For example, NOS3−/− myocytes exhibited a greater β-AR-stimulated [Ca2+]i transient and cell shortening amplitude compared to WT [5, 61]. Additionally, cardiac specific NOS3 overexpression resulted in a reduced response to β-AR-stimulation [62-64]. In contrast, one study observed no difference in the response to β-AR stimulation in NOS3−/− myocytes compared to myocytes from WT littermate hearts [56]. However, we observed that acute NOS3 inhibition in WT myocytes increased the contractile response to β-AR stimulation [61].
NOS3 signaling may also play a cardioprotective role. We observed that the largest effector of NOS3 signaling was action potential (AP) waveform, as β-AR-stimulated myocytes with NOS3 knockout or inhibition exhibited a substantially larger increase in AP duration (measured as time to 90% repolarization – APD90) compared to WT myocytes (Fig. 1C) [61]. This greatly prolonged APD in NOS3−/− 90 myocytes may contribute to arrhythmias. Previous studies have found that NOS3−/− mice had a greater incidence of digoxin-induced premature ventricular beats and ventricular tachycardia, and increased oubain-induced aftercontractions in isolated myocytes [65, 66]. We also observed greater spontaneous activity in NOS3−/− myocytes during β-AR stimulation, manifested as a higher incidence of early and delayed afterdepolarizations in NOS3−/− myocytes [61]. Early and delayed afterdepolarizations occur from increased Ca2+ influx, which subsequently produces SR Ca2+ overload and spontaneous release [67, 68]. However, NOS3 appears to protect the myocardium from this arrhythmogenic activity during β-AR stimulation.
In addition to protecting the heart from arrhythmias, it appears NOS3 signaling is also beneficial by limiting remodeling. NOS3−/− mice showed increased hypertrophy, fibrosis, and contractile dysfunction after chronic pressure overload compared to WT [69]. Conversely, cardiac-specific NOS3 overexpression limited hypertrophy and contractile dysfunction following chronic pressure overload or myocardial infarction [62, 70]. Therefore, the anti-adrenergic effects of NOS3 signaling are beneficial, acting as an endogenous β-blocker. Unfortunately, it appears that NOS3 expression and activity is decreased in pathophysiological states (e.g., heart failure) [30, 31]. Further, NOS3 can become uncoupled during such disease states as pressure overload, thus leading to the formation of supraphysiological levels of superoxide and additional pathophysiological remodeling [71]. Therefore, NOS3 may represent a novel therapeutic target [72].
3.3 End-Targets and Signaling Pathways of NOS3
The L-type Ca2+ channel is a key ECC protein. Ca2+ influx via the L-type Ca2+ current (I ) provides the trigger Ca2+ for the release of additional Ca2+ Ca from the SR (Ca2+-induced Ca2+-release). I -derived Ca2+ can also increase SR Ca2+ Ca load, and may directly activate the myofilaments to contract. NOS3 localizes to the caveolae, along with the L-type Ca2+ channel [48, 73]. Importantly, the L-type Ca2+ channel is a target for the β-AR pathway as we observed that NOS3 signaling has only anti-adrenergic effects. ICa also contributes to the development of early and delayed afterdepolarizations, and the activation of apoptotic and hypertrophic signaling pathways [67, 74, 75]. Our recent data demonstrated that NOS3 signaling is able to limit β-AR-stimulated ICa [61]. Consistent with these data, as well as that of others, a NOS3-induced reduction in β-AR-stimulated ICa will decrease contraction, and protect against arrhythmias and hypertrophic signaling. In addition, the L-type Ca2+ channels located in the caveolae may play a minor role in ECC, and this may be the reason only modest effects were observed on contraction. NOS3 signaling has also been demonstrated to affect the slow component of the delayed rectifier K+ current (IKs). Bai et al. showed that NOS3 activation resulted in the depression of ICa, the enhancement of IKs, and the shortening of AP duration [76]. Altered ICa and IKs are both known to play critical roles in the genesis of arrhythmias.
NOS3 signaling can also directly alter cardiac myocyte contraction by targeting myofilament proteins. For example, NOS3 has been shown to target TnI, thus decreasing myofilament Ca2+ sensitivity [77]. This decrease in myofilament sensitivity will also decrease myocyte shortening amplitude.
Studies have yet to completely elucidate the signaling pathway(s) for NOS3. However, since NOS3 has been shown to co-localize with superoxide dismutase [49], an enzyme which catalyzes the decomposition of superoxide, the reaction of NO with superoxide is prevented. Thus, NOS3-derived NO leads to the enhanced activation of guanylate cyclase and increased cGMP production (Fig. 1A, dashed lines). The cGMP-dependent pathway in cardiac myocytes is primarily through the activation of PKG [78]. α1C subunit of the L-type Ca2+ channel at Ser533 within cardiac myocytes [79, 80]. PKG is also able to phosphorylate TnI [81]. Interestingly, a study reported that inhibition of the cGMP-specific phosphodiesterase (PDE5A) depressed β-AR-stimulated cardiac contraction through increased PKG activity in WT mice, with no effect in NOS3−/− [82]. We also observed that PDE5 inhibition decreased β-AR-stimulated ICa through increased PKG activity [83]. These data suggest that NOS3 and PDE5 co-localize, further implicating cGMP as the signaling molecule of NOS3.
NOS3 may also signal independent of cGMP (Fig. 1A, solid lines). Sun et al. demonstrated that NOS3 signaling in female myocytes had decreased ICa via increased S-nitrosylation of the α1C subunit of the L-type Ca2+ channel compared to male myocytes [84]. Thus, NOS3 modulates cardiac myocyte contraction through cGMP-dependent and cGMP-independent signaling pathways, primarily by targeting ICa, IKs, and TnI.
4.1 NOS2 expression in the myocardium
The inducible isoform of NOS (NOS2) is only expressed during immune responses [85], and therefore during pathophysiological conditions of the myocardium that are associated with an upregulated inflammatory response. These include such conditions as ischemia-reperfusion injury [86], septicemia [87-89], aging [90], and heart failure [91]. NOS2 is widely considered to be a cytosolic protein [6].
4.2 Contractile effects of NOS2 expression
Numerous studies have shown that in many pathophysiological conditions of the myocardium, the observed cardiac dysfunction is partly due to NOS2. For example, NOS2 expression following ischemia-reperfusion injury was shown to contribute to basal cardiac dysfunction and an increase in infarct size [86, 92]. Additionally, genetic deletion or inhibition of NOS2 afforded protection against myocardial dysfunction in sepsis [87, 88]. In the aged myocardium, whole-heart function was shown to be decreased compared to young hearts, but was normalized with NOS2 inhibition [90]. Furthermore, we have demonstrated that β-AR-stimulated [Ca2+]i transients and cell shortening were reduced in failing human cardiac myocytes expressing NOS2 [91]. Similarly, we observed dysfunctional contraction in myocytes isolated from rejecting transplanted hearts that was reversible with NOS2 inhibition [9, 93].
4.3 End-targets and signaling pathways of NOS2
NOS2 has been shown to target several Ca2+-handling proteins within the cardiac myocyte, including troponin I [94], RyR [89], and ICa [9]. Many cGMP-dependent effects of NOS2 signaling have been observed through the activation of PKG. Yasuda et al. demonstrated that NOS2 induced a reduction in myofilament Ca2+ sensitivity [94], likely mediated by troponin I phosphorylation via PKG [81], thus decreasing myocardial contraction (Fig. 2A). The dysfunction that we observed with NOS2 expression in myocytes isolated from rejecting transplanted hearts resulted from a reduction in basal ICa, that was reversible upon NOS2 inhibition [9]. This effect was shown to be dependent upon the phosphorylation of the L-type Ca2+ channel by PKG. Other cGMP-dependent effects of NOS2 have been shown to be mediated through alterations in phosphodiesterase activity (Fig. 2A). Joe et al. demonstrated that NOS2 attenuated the response to β-AR stimulation by decreasing cAMP levels [95], possibly via PDE2 activation.
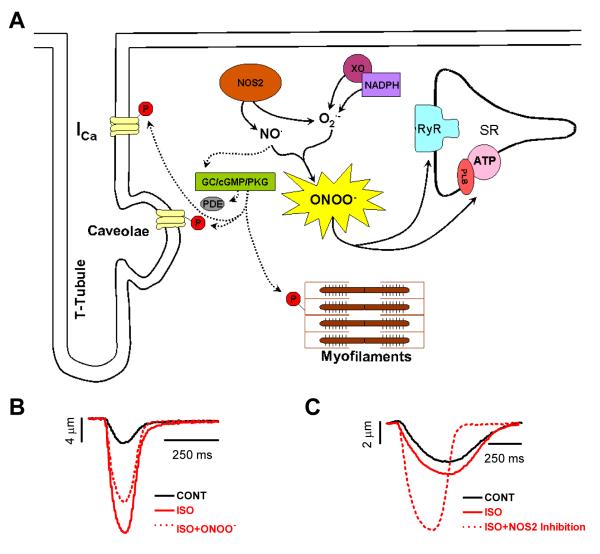
Figure 2. NOS2 signaling in the myocardium.
A.) Proposed cGMP-dependent (dashed lines) and cGMP-independent (solid lines) signaling pathways and end targets of NOS2. B.) Cell shortening in a mouse cardiac myocyte during perfusion with control, 1 μmol/L isoproterenol (ISO, non-specific β-AR agonist), and ISO+SIN-1 (peroxynitrite donor). C.) Cell shortening in a failing human cardiac myocyte during perfusion with control, 1 μmol/L isoproterenol, and ISO+NOS2 inhibition (L-N6-[1-Iminoethyl]-lysine dihydrochloride). NOTE: Data modified from references [16, 91].
cGMP-independent effects of NOS2 expression have also been demonstrated. Since NOS2 is a high output isoform, NOS2 can easily become uncoupled, leading to the production of both NO and superoxide , which couple to form high levels of peroxynitrite [96, 97]. At high concentrations, peroxynitrite is a potent oxidant capable of causing cellular damage, often by nitrating tyrosine residues [98], and is most likely the major signaling molecule of NOS2 [99]. Additionally, NADPH oxidase and xanthine oxidoreductase can increase superoxide production during pathophysiological conditions of the myocardium [100, 101]. Studies examining peroxynitrite have demonstrated dysfunctional effects via direct exposure or using peroxynitrite donors [99, 102, 103]. Previous studies have also shown that peroxynitrite can directly inactivate SERCA at high concentrations [104, 105], thus reducing SR Ca2+ load and myocardial contraction. Peroxynitrite has also been demonstrated to affect other ECC proteins (Fig. 2A), including RyR [89], and PLB [16]. In a previous study, we demonstrated a reduction in the β-AR response, observed as a decrease in myocyte [Ca2+]i transients and shortening, upon perfusion with a high concentration of peroxynitrite with no effects during basal stimulation (Fig. 2B) [16]. We further demonstrated that high peroxynitrite exerted anti-adrenergic effects by reducing cAMP-dependent PLBSerine16 phosphorylation via activation of protein phosphatases, resulting in reduced SERCA uptake of Ca2+ and decreased myocardial contraction. These results are strikingly similar to the effects that we observed in failing human myocytes expressing NOS2, where β-AR-stimulated myocyte [Ca2+]i transients and shortening were increased following NOS2 inhibition with no effect during basal stimulation (Fig. 2C) [91]. The expression of NOS2 in heart failure may be a key component of the observed β-AR dysfunction, as studies have demonstrated increased peroxynitrite production [99, 106, 107], increased protein phosphatase activity [108, 109], and decreased PLBSerine16 phosphorylation in heart failure [110, 111]. Thus, NOS2 modulates cardiac myocyte contraction through cGMP-dependent (Fig. 2A, dashed lines) and cGMP-independent (Fig. 2A, solid lines) signaling pathways by targeting multiple ECC proteins, as well as many components of the β-AR pathway. NOS2 is likely capable of producing these vast effects through the production of such high levels of NO compared to the constitutive NOS isoforms, thus resulting in signaling that is not compartmentalized. In addition, it has been hypothesized that the high NO production of NOS2 may result in the loss of local NOS1 and NOS3 signaling during many of these pathophysiological states [112].
5. Conclusions
NO signaling plays a critical role in the modulation of myocardial function. The resulting functional effects of NO signaling in the myocardium, as many studies have demonstrated, are multifaceted and highly dependent on such factors as NOS isoform localization, the activated signaling pathway, species produced, concentration, gender, site of production, and myocyte contractile state. These effects can also be influenced by additional factors, including the redox state of the myocyte and disease states. Therefore, it comes as no surprise that results contrary to those detailed herein have been previously reported in the literature [36, 38, 56, 72, 113-118]. However, recent studies are beginning to resolve these apparent controversies, and although conflicting reports regarding NO signaling exist, these results indicate that NO signaling does indeed play a key role, albeit complex, in the regulation of myocardial function.
ACKNOWLEDGEMENTS
Supported by the American Heart Association (Pre-doctoral Fellowship 0715159B, MJK; Post-doctoral Fellowship, 0725560B HW) and the National Institutes of Health (R01HL079283, MTZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- [3].Bers DM, Ziolo MT. When is cAMP not cAMP? Effects of compartmentalization. Circ Res. 2001;89(5):373–5. [PubMed] [Google Scholar]
- [4].Balligand JL, Kelly RA, Marsden PA, Smith TW, Michel T. Control of cardiac muscle cell function by an endogenous nitric oxide signaling system. Proc Natl Acad Sci U S A. 1993;90(1):347–51. doi: 10.1073/pnas.90.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, et al. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416(6878):337–9. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- [6].Ziolo MT, Bers DM. The real estate of NOS signaling: location, location, location. Circ Res. 2003;92(12):1279–81. doi: 10.1161/01.RES.0000080783.34092.AF. [DOI] [PubMed] [Google Scholar]
- [7].Xu KY, Huso DL, Dawson TM, Bredt DS, Becker LC. Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1999;96(2):657–62. doi: 10.1073/pnas.96.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Petroff MG, Kim SH, Pepe S, Dessy C, Marban E, Balligand JL, et al. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat Cell Biol. 2001;3(10):867–73. doi: 10.1038/ncb1001-867. [DOI] [PubMed] [Google Scholar]
- [9].Ziolo MT, Harshbarger CH, Roycroft KE, Smith JM, Romano FD, Sondgeroth KL, et al. Myocytes isolated from rejecting transplanted rat hearts exhibit a nitric oxide-mediated reduction in the calcium current. J Mol Cell Cardiol. 2001;33(9):1691–9. doi: 10.1006/jmcc.2001.1420. [DOI] [PubMed] [Google Scholar]
- [10].Ziolo MT. The fork in the nitric oxide road: Cyclic GMP or nitrosylation? Nitric Oxide. 2008;18(3):153–6. doi: 10.1016/j.niox.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stamler JS, Lamas S, Fang FC. Nitrosylation. the prototypic redox-based signaling mechanism. Cell. 2001;106(6):675–83. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- [12].Vila-Petroff MG, Younes A, Egan J, Lakatta EG, Sollott SJ. Activation of distinct cAMP-dependent and cGMP-dependent pathways by nitric oxide in cardiac myocytes. Circ Res. 1999;84(9):1020–31. doi: 10.1161/01.res.84.9.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sun J, Picht E, Ginsburg KS, Bers DM, Steenbergen C, Murphy E. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel alpha1 subunit and reduced ischemia/reperfusion injury. Circ Res. 2006;98(3):403–11. doi: 10.1161/01.RES.0000202707.79018.0a. [DOI] [PubMed] [Google Scholar]
- [14].Katori T, Donzelli S, Tocchetti CG, Miranda KM, Cormaci G, Thomas DD, et al. Peroxynitrite and myocardial contractility: in vivo versus in vitro effects. Free Radic Biol Med. 2006;41(10):1606–18. doi: 10.1016/j.freeradbiomed.2006.08.023. [DOI] [PubMed] [Google Scholar]
- [15].Walsh EK, Huang H, Wang Z, Williams J, de Crom R, van Haperen R, et al. Control of myocardial oxygen consumption in transgenic mice overexpressing vascular eNOS. Am J Physiol Heart Circ Physiol. 2004;287(5):H2115–21. doi: 10.1152/ajpheart.00267.2004. [DOI] [PubMed] [Google Scholar]
- [16].Kohr MJ, Wang H, Wheeler DG, Velayutham M, Zweier JL, Ziolo MT. Targeting of phospholamban by peroxynitrite decreases {beta}-adrenergic stimulation in cardiomyocytes. Cardiovasc Res. 2008;77(2):353–61. doi: 10.1093/cvr/cvm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ziolo MT, Katoh H, Bers DM. Positive and negative effects of nitric oxide on Ca(2+) sparks: influence of beta-adrenergic stimulation. Am J Physiol Heart Circ Physiol. 2001;281(6):H2295–303. doi: 10.1152/ajpheart.2001.281.6.H2295. [DOI] [PubMed] [Google Scholar]
- [18].Tocchetti CG, Wang W, Froehlich JP, Huke S, Aon MA, Wilson GM, et al. Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum Ca2+ cycling. Circ Res. 2007;100(1):96–104. doi: 10.1161/01.RES.0000253904.53601.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kojda G, Kottenberg K, Nix P, Schluter KD, Piper HM, Noack E. Low increase in cGMP induced by organic nitrates and nitrovasodilators improves contractile response of rat ventricular myocytes. Circ Res. 1996;78(1):91–101. doi: 10.1161/01.res.78.1.91. [DOI] [PubMed] [Google Scholar]
- [20].Kohr MJ, Wang H, Wheeler DG, Velayutham M, Zweier JL, Ziolo MT. Biphasic effect of SIN-1 is reliant upon cardiomyocyte contractile state. Free Radic Biol Med. 2008;45(1):73–80. doi: 10.1016/j.freeradbiomed.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Knowles RG, Palacios M, Palmer RM, Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989;86(13):5159–62. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Weiner CP, Lizasoain I, Baylis SA, Knowles RG, Charles IG, Moncada S. Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc Natl Acad Sci U S A. 1994;91(11):5212–6. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen J, Petranka J, Yamamura K, London RE, Steenbergen C, Murphy E. Gender differences in sarcoplasmic reticulum calcium loading after isoproterenol. Am J Physiol Heart Circ Physiol. 2003;285(6):H2657–62. doi: 10.1152/ajpheart.00557.2003. [DOI] [PubMed] [Google Scholar]
- [24].Cross HR, Murphy E, Steenbergen C. Ca(2+) loading and adrenergic stimulation reveal male/female differences in susceptibility to ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2002;283(2):H481–9. doi: 10.1152/ajpheart.00790.2001. [DOI] [PubMed] [Google Scholar]
- [25].Janssen PM, Periasamy M. Determinants of frequency-dependent contraction and relaxation of mammalian myocardium. J Mol Cell Cardiol. 2007;43(5):523–31. doi: 10.1016/j.yjmcc.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Khan SA, Skaf MW, Harrison RW, Lee K, Minhas KM, Kumar A, et al. Nitric oxide regulation of myocardial contractility and calcium cycling: independent impact of neuronal and endothelial nitric oxide synthases. Circ Res. 2003;92(12):1322–9. doi: 10.1161/01.RES.0000078171.52542.9E. [DOI] [PubMed] [Google Scholar]
- [27].Wang H, Kohr MJ, Traynham CJ, Wheeler DG, Janssen PM, Ziolo MT. Neuronal Nitric Oxide Synthase Signaling within Cardiac Myocytes Targets Phospholamban. Am J Physiol Cell Physiol. 2008;294(6):C1566–C75. doi: 10.1152/ajpcell.00367.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dawson D, Lygate CA, Zhang MH, Hulbert K, Neubauer S, Casadei B. nNOS gene deletion exacerbates pathological left ventricular remodeling and functional deterioration after myocardial infarction. Circulation. 2005;112(24):3729–37. doi: 10.1161/CIRCULATIONAHA.105.539437. [DOI] [PubMed] [Google Scholar]
- [29].Vandsburger MH, French BA, Helm PA, Roy RJ, Kramer CM, Young AA, et al. Multi-parameter in vivo cardiac magnetic resonance imaging demonstrates normal perfusion reserve despite severely attenuated beta-adrenergic functional response in neuronal nitric oxide synthase knockout mice. Eur Heart J. 2007;28(22):2792–8. doi: 10.1093/eurheartj/ehm241. [DOI] [PubMed] [Google Scholar]
- [30].Loyer X, Oliviero P, Damy T, Robidel E, Marotte F, Heymes C, et al. Effects of sex differences on constitutive nitric oxide synthase expression and activity in response to pressure overload in rats. Am J Physiol Heart Circ Physiol. 2007;293(5):H2650–8. doi: 10.1152/ajpheart.00883.2007. [DOI] [PubMed] [Google Scholar]
- [31].Damy T, Ratajczak P, Shah AM, Camors E, Marty I, Hasenfuss G, et al. Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet. 2004;363(9418):1365–7. doi: 10.1016/S0140-6736(04)16048-0. [DOI] [PubMed] [Google Scholar]
- [32].Damy T, Ratajczak P, Robidel E, Bendall JK, Oliviero P, Boczkowski J, et al. Up-regulation of cardiac nitric oxide synthase 1-derived nitric oxide after myocardial infarction in senescent rats. Faseb J. 2003;17(13):1934–6. doi: 10.1096/fj.02-1208fje. [DOI] [PubMed] [Google Scholar]
- [33].Burkard N, Rokita AG, Kaufmann SG, Hallhuber M, Wu R, Hu K, et al. Conditional neuronal nitric oxide synthase overexpression impairs myocardial contractility. Circ Res. 2007;100(3):e32–44. doi: 10.1161/01.RES.0000259042.04576.6a. [DOI] [PubMed] [Google Scholar]
- [34].Saraiva RM, Minhas KM, Raju SV, Barouch LA, Pitz E, Schuleri KH, et al. Deficiency of neuronal nitric oxide synthase increases mortality and cardiac remodeling after myocardial infarction: role of nitroso-redox equilibrium. Circulation. 2005;112(22):3415–22. doi: 10.1161/CIRCULATIONAHA.105.557892. [DOI] [PubMed] [Google Scholar]
- [35].Frank K, Kranias EG. Phospholamban and cardiac contractility. Ann Med. 2000;32(8):572–8. doi: 10.3109/07853890008998837. [DOI] [PubMed] [Google Scholar]
- [36].Zhang YH, Zhang MH, Sears CE, Emanuel K, Redwood C, El-Armouche A, et al. Reduced phospholamban phosphorylation is associated with impaired relaxation in left ventricular myocytes from neuronal NO synthase-deficient mice. Circ Res. 2008;102(2):242–9. doi: 10.1161/CIRCRESAHA.107.164798. [DOI] [PubMed] [Google Scholar]
- [37].Bassani JW, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol. 1995;268:C1313–9. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- [38].Sears CE, Bryant SM, Ashley EA, Lygate CA, Rakovic S, Wallis HL, et al. Cardiac neuronal nitric oxide synthase isoform regulates myocardial contraction and calcium handling. Circ Res. 2003;92(5):e52–9. doi: 10.1161/01.RES.0000064585.95749.6D. [DOI] [PubMed] [Google Scholar]
- [39].Gonzalez DR, Beigi F, Treuer AV, Hare JM. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc Natl Acad Sci U S A. 2007;104(51):20612–7. doi: 10.1073/pnas.0706796104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Oceandy D, Cartwright EJ, Emerson M, Prehar S, Baudoin FM, Zi M, et al. Neuronal nitric oxide synthase signaling in the heart is regulated by the sarcolemmal calcium pump 4b. Circulation. 2007;115(4):483–92. doi: 10.1161/CIRCULATIONAHA.106.643791. [DOI] [PubMed] [Google Scholar]
- [41].Khan SA, Lee K, Minhas KM, Gonzalez DR, Raju SV, Tejani AD, et al. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc Natl Acad Sci U S A. 2004;101:15944–8. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pou S, Pou WS, Bredt DS, Snyder SH, Rosen GM. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem. 1992;267(34):24173–6. [PubMed] [Google Scholar]
- [43].Sun J, Druhan LJ, Zweier JL. Dose dependent effects of reactive oxygen and nitrogen species on the function of neuronal nitric oxide synthase. Arch Biochem Biophys. 2008;471(2):126–33. doi: 10.1016/j.abb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Stoyanovsky D, Murphy T, Anno PR, Kim YM, Salama G. Nitric oxide activates skeletal and cardiac ryanodine receptors. Cell Calcium. 1997;21(1):19–29. doi: 10.1016/s0143-4160(97)90093-2. [DOI] [PubMed] [Google Scholar]
- [45].Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279(5348):234–7. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- [46].Xie H, Zhu PH. Biphasic modulation of ryanodine receptors by sulfhydryl oxidation in rat ventricular myocytes. Biophys J. 2006;91(8):2882–91. doi: 10.1529/biophysj.106.087338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wang H, Viatchenko-Karpinski S, Gyorke I, Kohr MJ, Wheeler DG, Gyorke S, et al. Neuronal nitric oxide synthase regulates the activity of cardiac myocyte ryanodine receptors. Circulation. 2007;116(19):II–30. (ABSTRACT) [Google Scholar]
- [48].Feron O, Dessy C, Opel DJ, Arstall MA, Kelly RA, Michel T. Modulation of the endothelial nitric-oxide synthase-caveolin interaction in cardiac myocytes. Implications for the autonomic regulation of heart rate. J Biol Chem. 1998;273(46):30249–54. doi: 10.1074/jbc.273.46.30249. [DOI] [PubMed] [Google Scholar]
- [49].Brahmajothi MV, Campbell DL. Heterogeneous basal expression of nitric oxide synthase and superoxide dismutase isoforms in mammalian heart : implications for mechanisms governing indirect and direct nitric oxide-related effects. Circ Res. 1999;85(7):575–87. doi: 10.1161/01.res.85.7.575. [DOI] [PubMed] [Google Scholar]
- [50].Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333(6174):664–6. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- [51].Rees DD, Palmer RM, Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989;86(9):3375–8. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nuedling S, Kahlert S, Loebbert K, Doevendans PA, Meyer R, Vetter H, et al. 17 Beta-estradiol stimulates expression of endothelial and inducible NO synthase in rat myocardium in-vitro and in-vivo. Cardiovasc Res. 1999;43(3):666–74. doi: 10.1016/s0008-6363(99)00093-0. [DOI] [PubMed] [Google Scholar]
- [53].Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399(6736):597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Russell KS, Haynes MP, Sinha D, Clerisme E, Bender JR. Human vascular endothelial cells contain membrane binding sites for estradiol, which mediate rapid intracellular signaling. Proc Natl Acad Sci U S A. 2000;97(11):5930–5. doi: 10.1073/pnas.97.11.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, et al. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res. 2000;87(8):677–82. doi: 10.1161/01.res.87.8.677. [DOI] [PubMed] [Google Scholar]
- [56].Martin SR, Emanuel K, Sears CE, Zhang YH, Casadei B. Are myocardial eNOS and nNOS involved in the beta-adrenergic and muscarinic regulation of inotropy? A systematic investigation. Cardiovasc Res. 2006;70(1):97–106. doi: 10.1016/j.cardiores.2006.02.002. [DOI] [PubMed] [Google Scholar]
- [57].Han X, Kubota I, Feron O, Opel DJ, Arstall MA, Zhao YY, et al. Muscarinic cholinergic regulation of cardiac myocyte ICa-L is absent in mice with targeted disruption of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1998;95(11):6510–5. doi: 10.1073/pnas.95.11.6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Champion HC, Georgakopoulos D, Takimoto E, Isoda T, Wang Y, Kass DA. Modulation of in vivo cardiac function by myocyte-specific nitric oxide synthase-3. Circ Res. 2004;94(5):657–63. doi: 10.1161/01.RES.0000119323.79644.20. [DOI] [PubMed] [Google Scholar]
- [59].Godecke A, Heinicke T, Kamkin A, Kiseleva I, Strasser RH, Decking UK, et al. Inotropic response to beta-adrenergic receptor stimulation and anti-adrenergic effect of ACh in endothelial NO synthase-deficient mouse hearts. J Physiol. 2001;532:195–204. doi: 10.1111/j.1469-7793.2001.0195g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gyurko R, Kuhlencordt P, Fishman MC, Huang PL. Modulation of mouse cardiac function in vivo by eNOS and ANP. Am J Physiol Heart Circ Physiol. 2000;278(3):H971–81. doi: 10.1152/ajpheart.2000.278.3.H971. [DOI] [PubMed] [Google Scholar]
- [61].Wang H, Kohr MJ, Wheeler DG, Ziolo MT. Endothelial nitric oxide synthase decreases {beta}-adrenergic responsiveness via inhibition of the L-type Ca2+ current. Am J Physiol Heart Circ Physiol. 2008;294(3):H1473–80. doi: 10.1152/ajpheart.01249.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Janssens S, Pokreisz P, Schoonjans L, Pellens M, Vermeersch P, Tjwa M, et al. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circ Res. 2004;94(9):1256–62. doi: 10.1161/01.RES.0000126497.38281.23. [DOI] [PubMed] [Google Scholar]
- [63].Brunner F, Andrew P, Wolkart G, Zechner R, Mayer B. Myocardial contractile function and heart rate in mice with myocyte-specific overexpression of endothelial nitric oxide synthase. Circulation. 2001;104(25):3097–102. doi: 10.1161/hc5001.101966. [DOI] [PubMed] [Google Scholar]
- [64].Massion PB, Dessy C, Desjardins F, Pelat M, Havaux X, Belge C, et al. Cardiomyocyte-restricted overexpression of endothelial nitric oxide synthase (NOS3) attenuates beta-adrenergic stimulation and reinforces vagal inhibition of cardiac contraction. Circulation. 2004;110(17):2666–72. doi: 10.1161/01.CIR.0000145608.80855.BC. [DOI] [PubMed] [Google Scholar]
- [65].Kubota I, Han X, Opel DJ, Zhao YY, Baliga R, Huang P, et al. Increased susceptibility to development of triggered activity in myocytes from mice with targeted disruption of endothelial nitric oxide synthase. J Mol Cell Cardiol. 2000;32(7):1239–48. doi: 10.1006/jmcc.2000.1158. [DOI] [PubMed] [Google Scholar]
- [66].Rakhit A, Maguire CT, Wakimoto H, Gehrmann J, Li GK, Kelly RA, et al. In vivo electrophysiologic studies in endothelial nitric oxide synthase (eNOS)-deficient mice. J Cardiovasc Electrophysiol. 2001;12(11):1295–301. doi: 10.1046/j.1540-8167.2001.01295.x. [DOI] [PubMed] [Google Scholar]
- [67].January CT, Riddle JM. Early afterdepolarizations: mechanism of induction and block. A role for L-type Ca2+ current. Circ Res. 1989;64(5):977–90. doi: 10.1161/01.res.64.5.977. [DOI] [PubMed] [Google Scholar]
- [68].Tweedie D, Harding SE, MacLeod KT. Sarcoplasmic reticulum Ca content, sarcolemmal Ca influx and the genesis of arrhythmias in isolated guinea-pig cardiomyocytes. J Mol Cell Cardiol. 2000;32(2):261–72. doi: 10.1006/jmcc.1999.1070. [DOI] [PubMed] [Google Scholar]
- [69].Ruetten H, Dimmeler S, Gehring D, Ihling C, Zeiher AM. Concentric left ventricular remodeling in endothelial nitric oxide synthase knockout mice by chronic pressure overload. Cardiovasc Res. 2005;66(3):444–53. doi: 10.1016/j.cardiores.2005.01.021. [DOI] [PubMed] [Google Scholar]
- [70].Buys ES, Raher MJ, Blake SL, Neilan TG, Graveline AR, Passeri JJ, et al. Cardiomyocyte-restricted restoration of nitric oxide synthase 3 attenuates left ventricular remodeling after chronic pressure overload. Am J Physiol Heart Circ Physiol. 2007;293(1):H620–7. doi: 10.1152/ajpheart.01236.2006. [DOI] [PubMed] [Google Scholar]
- [71].Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, et al. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005;115(5):1221–31. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Moens AL, Takimoto E, Tocchetti CG, Chakir K, Bedja D, Cormaci G, et al. Reversal of cardiac hypertrophy and fibrosis from pressure overload by tetrahydrobiopterin: efficacy of recoupling nitric oxide synthase as a therapeutic strategy. Circulation. 2008;117(20):2626–36. doi: 10.1161/CIRCULATIONAHA.107.737031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac L-type Ca(2+) channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation. Proc Natl Acad Sci U S A. 2006;103(19):7500–5. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, et al. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res. 2005;97(10):1009–17. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- [75].Fiedler B, Lohmann SM, Smolenski A, Linnemuller S, Pieske B, Schroder F, et al. Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proc Natl Acad Sci U S A. 2002;99(17):11363–8. doi: 10.1073/pnas.162100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Bai CX, Namekata I, Kurokawa J, Tanaka H, Shigenobu K, Furukawa T. Role of nitric oxide in Ca2+ sensitivity of the slowly activating delayed rectifier K+ current in cardiac myocytes. Circ Res. 2005;96(1):64–72. doi: 10.1161/01.RES.0000151846.19788.E0. [DOI] [PubMed] [Google Scholar]
- [77].Kaye DM, Wiviott SD, Kelly RA. Activation of nitric oxide synthase (NOS3) by mechanical activity alters contractile activity in a Ca2+-independent manner in cardiac myocytes: role of troponin I phosphorylation. Biochem Biophys Res Commun. 1999;256(2):398–403. doi: 10.1006/bbrc.1999.0346. [DOI] [PubMed] [Google Scholar]
- [78].Wegener JW, Nawrath H, Wolfsgruber W, Kuhbandner S, Werner C, Hofmann F, et al. cGMP-dependent protein kinase I mediates the negative inotropic effect of cGMP in the murine myocardium. Circ Res. 2002;90(1):18–20. doi: 10.1161/hh0102.103222. [DOI] [PubMed] [Google Scholar]
- [79].Jiang LH, Gawler DJ, Hodson N, Milligan CJ, Pearson HA, Porter V, et al. Regulation of cloned cardiac L-type calcium channels by cGMP-dependent protein kinase. J Biol Chem. 2000;275(9):6135–43. doi: 10.1074/jbc.275.9.6135. [DOI] [PubMed] [Google Scholar]
- [80].Yang L, Liu G, Zakharov SI, Bellinger AM, Mongillo M, Marx SO. Protein kinase G phosphorylates Cav1.2 alpha1c and beta2 subunits. Circ Res. 2007;101(5):465–74. doi: 10.1161/CIRCRESAHA.107.156976. [DOI] [PubMed] [Google Scholar]
- [81].Layland J, Li JM, Shah AM. Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J Physiol. 2002;540:457–67. doi: 10.1113/jphysiol.2001.014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Takimoto E, Champion HC, Belardi D, Moslehi J, Mongillo M, Mergia E, et al. cGMP catabolism by phosphodiesterase 5A regulates cardiac adrenergic stimulation by NOS3-dependent mechanism. Circ Res. 2005;96(1):100–9. doi: 10.1161/01.RES.0000152262.22968.72. [DOI] [PubMed] [Google Scholar]
- [83].Ziolo MT, Lewandowski SJ, Smith JM, Romano FD, Wahler GM. Inhibition of cyclic GMP hydrolysis with zaprinast reduces basal and cyclic AMP-elevated L-type calcium current in guinea-pig ventricular myocytes. Br J Pharmacol. 2003;138(5):986–94. doi: 10.1038/sj.bjp.0705112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Sun J, Picht E, Ginsburg KS, Bers DM, Steenbergen C, Murphy E. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel alpha1 subunit and reduced ischemia/reperfusion injury. Circ Res. 2006;98(3):403–11. doi: 10.1161/01.RES.0000202707.79018.0a. [DOI] [PubMed] [Google Scholar]
- [85].Balligand JL, Ungureanu-Longrois D, Simmons WW, Pimental D, Malinski TA, Kapturczak M, et al. Cytokine-inducible nitric oxide synthase (iNOS) expression in cardiac myocytes. Characterization and regulation of iNOS expression and detection of iNOS activity in single cardiac myocytes in vitro. J Biol Chem. 1994;269(44):27580–8. [PubMed] [Google Scholar]
- [86].Wildhirt SM, Weismueller S, Schulze C, Conrad N, Kornberg A, Reichart B. Inducible nitric oxide synthase activation after ischemia/reperfusion contributes to myocardial dysfunction and extent of infarct size in rabbits: evidence for a late phase of nitric oxide-mediated reperfusion injury. Cardiovasc Res. 1999;43(3):698–711. doi: 10.1016/s0008-6363(99)00080-2. [DOI] [PubMed] [Google Scholar]
- [87].Ichinose F, Hataishi R, Wu JC, Kawai N, Rodrigues AC, Mallari C, et al. A selective inducible NOS dimerization inhibitor prevents systemic, cardiac, and pulmonary hemodynamic dysfunction in endotoxemic mice. Am J Physiol Heart Circ Physiol. 2003;285(6):H2524–30. doi: 10.1152/ajpheart.00530.2003. [DOI] [PubMed] [Google Scholar]
- [88].Ullrich R, Scherrer-Crosbie M, Bloch KD, Ichinose F, Nakajima H, Picard MH, et al. Congenital deficiency of nitric oxide synthase 2 protects against endotoxin-induced myocardial dysfunction in mice. Circulation. 2000;102(12):1440–6. doi: 10.1161/01.cir.102.12.1440. [DOI] [PubMed] [Google Scholar]
- [89].Ziolo MT, Katoh H, Bers DM. Expression of inducible nitric oxide synthase depresses beta-adrenergic-stimulated calcium release from the sarcoplasmic reticulum in intact ventricular myocytes. Circulation. 2001;104(24):2961–6. doi: 10.1161/hc4901.100379. [DOI] [PubMed] [Google Scholar]
- [90].Yang B, Larson DF, Watson RR. Modulation of iNOS activity in age-related cardiac dysfunction. Life Sci. 2004;75(6):655–67. doi: 10.1016/j.lfs.2003.09.076. [DOI] [PubMed] [Google Scholar]
- [91].Ziolo MT, Maier LS, Piacentino V, 3rd, Bossuyt J, Houser SR, Bers DM. Myocyte nitric oxide synthase 2 contributes to blunted beta-adrenergic response in failing human hearts by decreasing Ca2+ transients. Circulation. 2004;109(15):1886–91. doi: 10.1161/01.CIR.0000124231.98250.A8. [DOI] [PubMed] [Google Scholar]
- [92].Parlakpinar H, Ozer MK, Acet A. Effect of aminoguanidine on ischemia-reperfusion induced myocardial injury in rats. Mol Cell Biochem. 2005;277(1-2):137–42. doi: 10.1007/s11010-005-5779-9. [DOI] [PubMed] [Google Scholar]
- [93].Ziolo MT, Dollinger SJ, Wahler GM. Myocytes isolated from rejecting transplanted rat hearts exhibit reduced basal shortening which is reversible by aminoguanidine. J Mol Cell Cardiol. 1998;30(5):1009–17. doi: 10.1006/jmcc.1998.0665. [DOI] [PubMed] [Google Scholar]
- [94].Yasuda S, Lew WY. Lipopolysaccharide depresses cardiac contractility and beta-adrenergic contractile response by decreasing myofilament response to Ca2+ in cardiac myocytes. Circ Res. 1997;81(6):1011–20. doi: 10.1161/01.res.81.6.1011. [DOI] [PubMed] [Google Scholar]
- [95].Joe EK, Schussheim AE, Longrois D, Maki T, Kelly RA, Smith TW, et al. Regulation of cardiac myocyte contractile function by inducible nitric oxide synthase (iNOS): mechanisms of contractile depression by nitric oxide. J Mol Cell Cardiol. 1998;30(2):303–15. doi: 10.1006/jmcc.1997.0593. [DOI] [PubMed] [Google Scholar]
- [96].Mungrue IN, Gros R, You X, Pirani A, Azad A, Csont T, et al. Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest. 2002;109(6):735–43. doi: 10.1172/JCI13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Xia Y, Zweier JL. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc Natl Acad Sci U S A. 1997;94(13):6954–8. doi: 10.1073/pnas.94.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Cai L, Wang J, Li Y, Sun X, Wang L, Zhou Z, et al. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes. 2005;54(6):1829–37. doi: 10.2337/diabetes.54.6.1829. [DOI] [PubMed] [Google Scholar]
- [99].Ferdinandy P, Danial H, Ambrus I, Rothery RA, Schulz R. Peroxynitrite is a major contributor to cytokine-induced myocardial contractile failure. Circ Res. 2000;87(3):241–7. doi: 10.1161/01.res.87.3.241. [DOI] [PubMed] [Google Scholar]
- [100].Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, et al. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol. 2003;41(12):2164–71. doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- [101].Minhas KM, Saraiva RM, Schuleri KH, Lehrke S, Zheng M, Saliaris AP, et al. Xanthine oxidoreductase inhibition causes reverse remodeling in rats with dilated cardiomyopathy. Circ Res. 2006;98(2):271–9. doi: 10.1161/01.RES.0000200181.59551.71. [DOI] [PubMed] [Google Scholar]
- [102].Ma XL, Lopez BL, Liu GL, Christopher TA, Ischiropoulos H. Peroxynitrite aggravates myocardial reperfusion injury in the isolated perfused rat heart. Cardiovasc Res. 1997;36(2):195–204. doi: 10.1016/s0008-6363(97)00179-x. [DOI] [PubMed] [Google Scholar]
- [103].Stojanovic MO, Ziolo MT, Wahler GM, Wolska BM. Anti-adrenergic effects of nitric oxide donor SIN-1 in rat cardiac myocytes. Am J Physiol Cell Physiol. 2001;281(1):C342–9. doi: 10.1152/ajpcell.2001.281.1.C342. [DOI] [PubMed] [Google Scholar]
- [104].Knyushko TV, Sharov VS, Williams TD, Schoneich C, Bigelow DJ. 3-Nitrotyrosine modification of SERCA2a in the aging heart: a distinct signature of the cellular redox environment. Biochemistry. 2005;44(39):13071–81. doi: 10.1021/bi051226n. [DOI] [PubMed] [Google Scholar]
- [105].Lokuta AJ, Maertz NA, Meethal SV, Potter KT, Kamp TJ, Valdivia HH, et al. Increased nitration of sarcoplasmic reticulum Ca2+-ATPase in human heart failure. Circulation. 2005;111(8):988–95. doi: 10.1161/01.CIR.0000156461.81529.D7. [DOI] [PubMed] [Google Scholar]
- [106].Mihm MJ, Coyle CM, Schanbacher BL, Weinstein DM, Bauer JA. Peroxynitrite induced nitration and inactivation of myofibrillar creatine kinase in experimental heart failure. Cardiovasc Res. 2001;49(4):798–807. doi: 10.1016/s0008-6363(00)00307-2. [DOI] [PubMed] [Google Scholar]
- [107].Zhang P, Xu X, Hu X, van Deel ED, Zhu G, Chen Y. Inducible nitric oxide synthase deficiency protects the heart from systolic overload-induced ventricular hypertrophy and congestive heart failure. Circ Res. 2007;100(7):1089–98. doi: 10.1161/01.RES.0000264081.78659.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Gupta RC, Mishra S, Rastogi S, Imai M, Habib O, Sabbah HN. Cardiac SR-coupled PP1 activity and expression are increased and inhibitor 1 protein expression is decreased in failing hearts. Am J Physiol Heart Circ Physiol. 2003;285(6):H2373–81. doi: 10.1152/ajpheart.00442.2003. [DOI] [PubMed] [Google Scholar]
- [109].Neumann J, Eschenhagen T, Jones LR, Linck B, Schmitz W, Scholz H, et al. Increased expression of cardiac phosphatases in patients with end-stage heart failure. J Mol Cell Cardiol. 1997;29(1):265–72. doi: 10.1006/jmcc.1996.0271. [DOI] [PubMed] [Google Scholar]
- [110].Bartel S, Stein B, Eschenhagen T, Mende U, Neumann J, Schmitz W, et al. Protein phosphorylation in isolated trabeculae from nonfailing and failing human hearts. Mol Cell Biochem. 1996;157(1-2):171–9. doi: 10.1007/BF00227896. [DOI] [PubMed] [Google Scholar]
- [111].Schwinger RH, Munch G, Bolck B, Karczewski P, Krause EG, Erdmann E. Reduced Ca(2+)-sensitivity of SERCA 2a in failing human myocardium due to reduced serin-16 phospholamban phosphorylation. J Mol Cell Cardiol. 1999;31(3):479–91. doi: 10.1006/jmcc.1998.0897. [DOI] [PubMed] [Google Scholar]
- [112].Feldman DS, Elton TS, Sun B, Martin MM, Ziolo MT. Mechanisms of disease: detrimental adrenergic signaling in acute decompensated heart failure. Nat Clin Pract Cardiovasc Med. 2008;5(4):208–18. doi: 10.1038/ncpcardio1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Ashley EA, Sears CE, Bryant SM, Watkins HC, Casadei B. Cardiac nitric oxide synthase 1 regulates basal and beta-adrenergic contractility in murine ventricular myocytes. Circulation. 2002;105(25):3011–6. doi: 10.1161/01.cir.0000019516.31040.2d. [DOI] [PubMed] [Google Scholar]
- [114].Vandecasteele G, Eschenhagen T, Scholz H, Stein B, Verde I, Fischmeister R. Muscarinic and beta-adrenergic regulation of heart rate, force of contraction and calcium current is preserved in mice lacking endothelial nitric oxide synthase. Nat Med. 1999;5(3):331–4. doi: 10.1038/6553. [DOI] [PubMed] [Google Scholar]
- [115].Paulus WJ. Beneficial effects of nitric oxide on cardiac diastolic function: ’the flip side of the coin’. Heart Fail Rev. 2000;5(4):337–44. doi: 10.1023/a:1026511229882. [DOI] [PubMed] [Google Scholar]
- [116].Heymes C, Vanderheyden M, Bronzwaer JG, Shah AM, Paulus WJ. Endomyocardial nitric oxide synthase and left ventricular preload reserve in dilated cardiomyopathy. Circulation. 1999;99(23):3009–16. doi: 10.1161/01.cir.99.23.3009. [DOI] [PubMed] [Google Scholar]
- [117].Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, et al. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci U S A. 1999;96(20):11507–12. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Li Q, Guo Y, Tan W, Ou Q, Wu WJ, Sturza D, et al. Cardioprotection afforded by inducible nitric oxide synthase gene therapy is mediated by cyclooxygenase-2 via a nuclear factor-kappaB dependent pathway. Circulation. 2007;116(14):1577–84. doi: 10.1161/CIRCULATIONAHA.107.689810. [DOI] [PMC free article] [PubMed] [Google Scholar]