Abstract
(See the article by Delwart et al, on pages 875–85, and see the editorial commentary by Katz, on pages 867–9.)
Background. There have been anecdotal reports of influenza viremia since the 1960s. We present an assessment of the prevalence of seasonal and 2009 H1N1 influenza viremia (via RNA testing) in blood donor populations using multiple sensitive detection assays.
Methods. Several influenza RNA amplification assays, including transcription-mediated amplification (TMA) and 2 reverse-transcription polymerase chain reaction (RT-PCR) assays, were evaluated and used to test donor samples. Retrospective samples from 478 subjects drawn at sites with high influenza activity were tested. Prospective samples were collected from 1004 blood donors who called their donation center within 3 days of donation complaining of influenza-like illness (ILI). The plasma collected on the day of donation for these subjects was tested.
Results. Of the repository samples, 2 of 478 plasma samples were initially reactive but not repeat reactive by influenza TMA. Of blood donors reporting ILI symptoms postdonation, 1 of 1004 samples was TMA initially reactive but not repeat reactive; all samples were nonreactive by RT-PCR testing.
Conclusions. Targeting blood donor populations most likely to have influenza infection, we failed to detect influenza RNA in 1482 donor samples, with most tested by 3 different RNA assays. Seasonal influenza does not appear to pose a significant contamination threat to the blood supply.
The 2009 H1N1 influenza outbreak illustrates that the virus remains a global threat with serious sequellae in some affected individuals. Circulating seasonal strains can spread easily from human to human, rapidly covering the globe during the initial phase of a pandemic. In addition to seasonal influenza and the recently introduced triple reassortant 2009 H1N1 strain, highly pathogenic avian influenza (H5N1) circulates in Asian bird populations, and humans have been infected in multiple countries [1, 2]. Fortunately, sustained human-to-human transmission of H5N1 influenza virus has not been seen [3, 4], as mortality can reach 50% [5, 6]. The recent introduction of the novel H1N1 reassortant influenza virus, and the potential for high mortality after infection with highly pathogenic avian influenza virus, have focused attention on the epidemiology and pathophysiology of influenza infection.
During the 2009 H1N1 influenza outbreak, the prime concern of most institutions was to maintain operational capability in the face of heightened employee absenteeism due to illness of the worker or family member, as well as the obvious concern regarding an absence of blood donors. In addition to these concerns, blood collectors also had to consider whether blood collected during the outbreak might contain infectious influenza virus. Transfusion transmission of influenza would not likely play a major role in spreading a virus that is primarily transmitted by aerosolization/droplet infection. However, transfusion recipients are likely at increased risk of serious sequellae of influenza infection. Immune compromised subjects are susceptible to severe disease, and influenza virus-infected bone marrow transplant patients have shown ∼25% mortality after infection [7, 8]. In the recent 2009 H1N1 outbreak it was also noted that pregnant women were at risk for more severe disease and death than the general population [9]. Although influenza viremia has infrequently been observed [10–17] (reviewed in [18]), the potential remains that influenza viremia can occur during presymptomatic or asymptomatic infection [19].
After introduction of an antigenically novel influenza virus, attack rates can be extraordinarily high, reaching 15%–30% in some areas [20], with baseline infection rates fluctuating between 5% and 10% [21]. To study whether donated blood contained influenza virus, we capitalized on historical knowledge of influenza incidence and the collection of repository samples from blood donors linked to location and time of donation. In a second phase of testing, we prospectively surveyed blood from donors who reported symptoms consistent with influenza-like illness (ILI) within 3 days of blood donation, with the hypothesis that these donors would be most likely to harbor viremia at the earliest stage of infection. Here we present an analysis of commercially available and research-phase influenza RNA detection assays and the results of screening repository and prospectively collected samples for influenza RNA.
MATERIALS AND METHODS
Study Subjects
Two cohorts of subjects were studied, a retrospective and a prospective cohort.
Retrospective Cohort
The first group of 478 subjects was selected from the Retrovirus Epidemiology Donor Study (REDS) Allogeneic Donor and Recipient (RADAR) repository, composed of 120 000 whole blood and plasma specimens collected in ethylenediaminetetraacetic acid that are linked to donor zip code and date of blood donation during the years 2000 through 2003.
Prospective Cohort
The second cohort of 1004 subjects was drawn from donors to Blood Systems and the American Red Cross (ARC) whose blood was collected prior to April 2009 (n = 256) and coincident with the recent H1N1 influenza outbreak from April 2009 through 2010, with both collection periods occurring during high national influenza activity as reported by the Centers for Disease Control and Prevention (CDC) (n = 748) [22]. These donors were selected on the basis of contacting the blood collection center within 3 days of donation and offering postdonation information (PDI) of symptoms consistent with ILI. The frozen plasma component was obtained in citrate phosphate dextrose from each donor reporting PDI and maintained frozen until aliquots were prepared for testing. A subset of 21 prospectively collected blood donors provided 2 samples separated by 8 weeks (acute and convalescent) for antibody testing.
Ferret Samples and Processing
Three ferrets were infected with influenza A virus (H3N2) intranasally at a titer of 106 egg infectious dose (EID)50. Serial blood and serum samples (2 mL) were collected preinfection and days 1, 2, 4, and 6 postinfection. Directly after collection, RNA was extracted from 2-mL aliquots using the PAXgene Blood RNA kit (Qiagen) according to the manufacturer’s instructions.
Virus Stock and Sample Preparation
For assay evaluation experiments, replicate samples spiked with varied amounts of influenza virus were prepared and sent blindly to each laboratory involved in retrospective testing for this study. Influenza A WS/33 (H1N1) stock was obtained at a TCID50 of 105.75 (catalog VR-1520, National Institutes of Health Biodefense and Emerging Infections Research Repository). Serial 10-fold dilutions of the virus were performed in phosphate-buffered saline (PBS) and added to 50-mL aliquots of whole blood. One-milliliter aliquots of influenza-spiked whole blood were prepared, and the remaining volume was spun at 1200 rpm followed by preparation of 1-mL plasma aliquots.
Influenza Detection Assays
Retrospective Cohort and Ferret Sample Testing
A number of different influenza RNA detection assays were evaluated prior to testing RADAR donor samples, all of which were previously shown to detect commonly circulating contemporary influenza viruses (data not shown). (1) The MGB Alert system (Epoch Biosciences) uses RNA extraction followed by real-time reverse-transcription polymerase chain reaction (RT-PCR) RNA amplification and detection using probe oligonucleotides targeting either influenza A or B matrix genes with a fluorophore and quencher linked by the probe sequence. After probe hybridization to the target nucleic acid sequence, the fluorophore and quencher are separated, resulting in fluorescence of the probe. (2) The ProFlu-1 assay (Prodesse) uses RNA extraction followed by a TaqMan-based, real-time RT-PCR amplification targeting the influenza A matrix gene [23]. (3) Transcription-mediated amplification (Gen-Probe) uses a target-capture probe for influenza A matrix gene during RNA extraction followed by isothermal amplification using bacteriophage T7 RNA polymerase and Moloney murine leukemia virus reverse transcriptase [24]. An internal control RNA is included to calculate a signal-to-cutoff ratio and ensure assay validity, with valid samples having the internal control signal above the cutoff determined for each run. Positive control samples consist of a detergent solution spiked with in vitro synthesized RNA transcripts corresponding to a portion of the influenza A matrix gene (quantitated by absorbance at 260 nm). Ferret samples were tested using the ProFlu-1 and TMA assays.
Prospective Cohort Testing
Prospectively collected samples were tested using TMA plus 2 additional RT-PCR assays, also able to detect commonly circulating contemporary influenza viruses. First, we used an RT-PCR test developed at the US Food and Drug Administration (FDA) using influenza A matrix gene primers and probes in an automated TaqMan ABI 7500 Analyzer using a QuantiTect Probe RT-PCR kit (Qiagen). The analytical sensitivity was determined using serial dilutions of viral RNA (from 1 ng to 1 fg) from the A/California/04/2009 strain. One femtogram of viral RNA is equivalent to ∼130 copies [25]. Six TaqMan reactions were performed in 3 experiments, and 10 fg (∼1300 copies/reaction) of viral RNA was detected.
The second RT-PCR was the Centers for Disease Control and Prevention (CDC) Human Influenza Virus Real-Time RT-PCR Detection and Characterization Panel (rRT-PCR Flu Panel). This assay, designed and developed by the CDC, uses primer sets directed at the influenza A M gene and an influenza B NS gene, and can detect seasonal and novel H1N1 influenza [26]. Permission was obtained from the Influenza Division at the CDC to use these reagents for this study. Nucleic acid extraction was performed on a bioMerieux easyMAG, and the assay performed on ABI 7500 FAST Dx real-time PCR instruments. The limits of detection for the CDC RT-PCR Flu Panel have been assessed by the CDC with 1 or 2 strains of each subtype of influenza A and 2 subtypes of influenza B; one each of the Victoria and Yamagata lineages. Serial, 10-fold dilutions revealed limits of detection ranging from 10 to 102.2 EID50 for influenza A strains and 1–100.5 EID50 for influenza B strains [27]. Finally, influenza immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies were assayed by enzyme-linked immunosorbent assay (ELISA; American Research Products).
Statistical Analysis
Exact confidence intervals around the influenza viremia prevalence estimates were calculated using the method of Blyth-Still-Casella [28, 29].
RESULTS
Relative Sensitivity of Influenza RNA Detection Assays
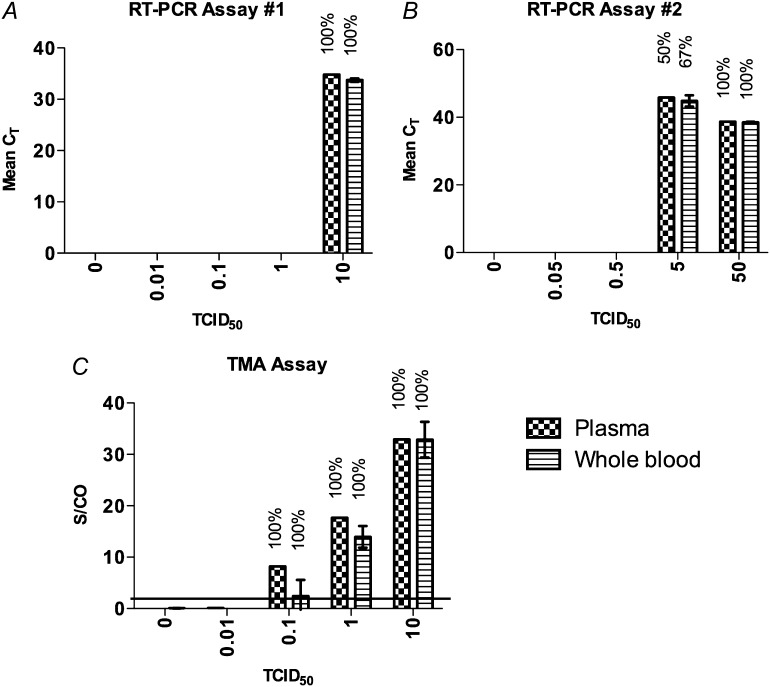
Prior to testing the retrospective samples, the relative sensitivities of the MGB Alert, ProFlu-1 RT-PCR, and the TMA assays were tested using influenza RNA in spiked samples. Nucleic acid amplification is more difficult using a whole blood sample; however, there are theoretical reasons to suspect that the virus might preferentially compartmentalize in the red blood cell fraction of blood through interaction of hemagglutinin with the surface of red blood cells and platelets [30, 31]. Therefore, in an attempt to maximize assay sensitivity, influenza-spiked blood aliquots were separated into the plasma fraction for testing or tested as a whole blood aliquot. Both RT-PCR based assays had similar sensitivity (Figure 1A and 1B), between 5 and 10 TCID50 of influenza virus spiked in plasma. The TMA assay was ∼1- to 2-log10 more sensitive than the 2 RT-PCR-based assays (Figure 1C). In a second experiment TMA detected 2 of 2 samples spiked with 0.05 TCID50 of influenza virus (data not shown). Thus, TMA was selected for testing the retrospective sample set. Of note, testing of whole blood samples was not more sensitive than testing plasma samples in these in vitro experiments.
Figure 1.
Relative sensitivity of influenza A RNA detection assays. Blood samples spiked with influenza virus RNA were prepared and tested in a blinded fashion using reverse-transcription polymerase chain reaction (RT-PCR) assay (A) 1, (B) 2, or (C) transcription-mediated amplification (TMA). Results for RT-PCR are described as the cycle threshold (CT) at which signal was detected and for TMA as a signal-to-cutoff (S/CO) ratio. The percentage of replicates that tested positive is shown above each bar. Two replicates were tested in (A) and (C) in 2 separate experiments (1 representative experiment shown), and 6 replicates were tested in (B) in 1 experiment. Line denotes S/CO = 1, error bars represent standard error.
Influenza Viremia Detection in an Animal Model
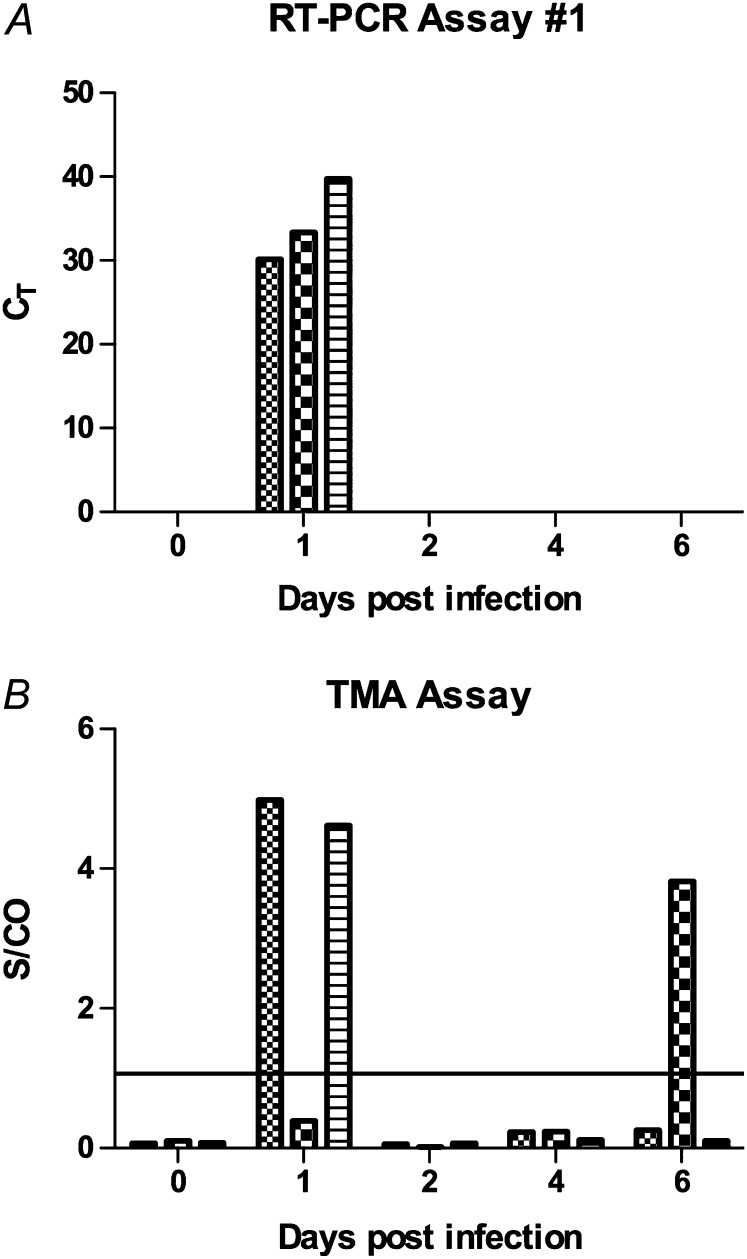
Although the above data indicated that the RNA detection assays could detect virus in a spiked blood sample, it was unclear whether the assays would detect virus after in vivo infection. To test for viremia, the ferret model of influenza infection was used, which is an accepted animal model of influenza virus infection in humans [32–34]. In total, 3 of 3 intranasally infected animals had detectable influenza RNA in the blood 1 day after inoculation using RT-PCR and/or TMA assays, and 1 animal showed RNA 6 days after infection by TMA (Figure 2). Serum samples were available for TMA testing, and none showed detectable influenza RNA. These results indicate that influenza viremia resulting from intranasal infection could be detected using two different assays, though the viral load in the serum in ferrets did not appear high enough for detection of RNA.
Figure 2.
Detection of influenza viremia in influenza H3N2-infected ferrets. Three ferrets were infected with 106 egg infectious dose (EID50) of influenza A H3N2 virus intranasally and followed up for 6 days, with bleeds prior to infection and at days 1, 2, 4, and 6. RNA was extracted from whole blood samples and was tested for influenza RNA by (A) reverse-transcription polymerase chain reaction (RT-PCR) assay #1 or (B) transcription-mediated amplification (TMA). Line denotes S/CO = 1. The experiments were each performed once.
Influenza Virus Detection in a Retrospective Cohort
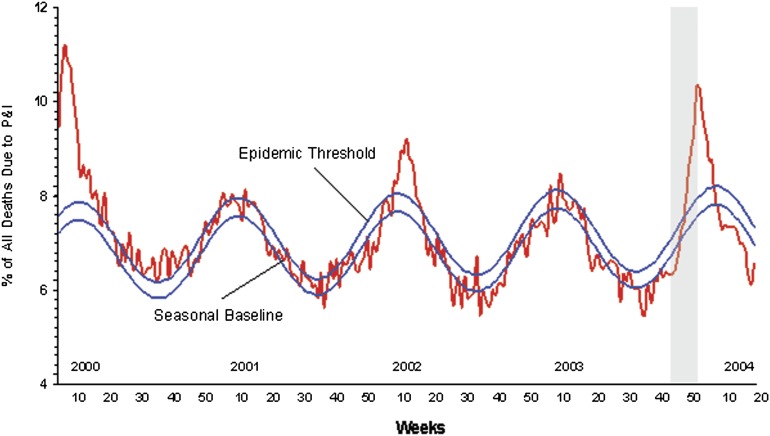
We questioned whether blood donors who gave blood during periods of high transmission in the community would be viremic, either by donating during asymptomatic infection or during the early, presymptomatic phase of infection. To identify a random community sample of blood donors whose samples were collected during the highest risk period, we matched data generated by the Centers for Disease Control and Prevention (CDC) with the RADAR repository sample database. The period of highest influenza transmission during RADAR accrual occurred at the end of 2003 (Figure 3); samples collected in weeks 45–51 were tested by TMA. A total of 484 whole blood samples were available from collection sites in Detroit (n = 224) and Pittsburg (n = 260); 478 matched plasma aliquots were also available. Testing of whole blood yielded nearly 50% invalid reactions with no reactive results observed, so whole blood testing was terminated after 350 samples (Table 1). Only 3 of 478 plasma samples yielded invalid results, and no repeat reactive samples were seen (2 were low-level reactive on initial testing, signal-to-cutoff ratio [S/CO <10]). The negative RNA results suggest that influenza viremia occurs rarely, if at all, in an unselected blood donor population, even during periods of high activity in the community.
Figure 3.
Pneumonia and influenza mortality for 122 US cities. Each week, the vital statistics offices of 122 cities reported the total number of death certificates received and the number of those for which pneumonia or influenza was listed as the underlying or contributing cause of death by age group. The percentage of all deaths due to pneumonia and influenza (P&I) were compared with a seasonal baseline and epidemic threshold value calculated for each week. The seasonal baseline of P&I deaths was calculated using a periodic regression model that incorporates a robust regression procedure applied to data from the previous five years. An increase of 1645 standard deviations above the seasonal baseline of P&I deaths is considered the “epidemic threshold,” ie, the point at which the observed proportion of deaths attributed to pneumonia or influenza was significantly higher than would be expected at that time of the year in the absence of substantial influenza-related mortality. Figure from the Centers for Disease Control and Prevention (CDC) website at http://www.cdc.gov/flu/weekly/weeklyarchives2003–2004/03–04summary.htm, accessed 20 September 2011. The gray shaded area represents the time period during which the tested repository samples were collected.
Table 1.
TMA Testing of RADAR Repository Samples
| Test Result | Plasma | Whole Blood |
| Invalid | 3 | 168 |
| Nonreactive | 473 | 182 |
| Initial reactive | 2 | 0 |
| Repeat reactive | 0 | 0 |
Abbreviations: RADAR, Retrovirus Allogeneic Donor and Recipient; TMA, transcription-mediated amplification.
Identification of Blood Donors With ILI
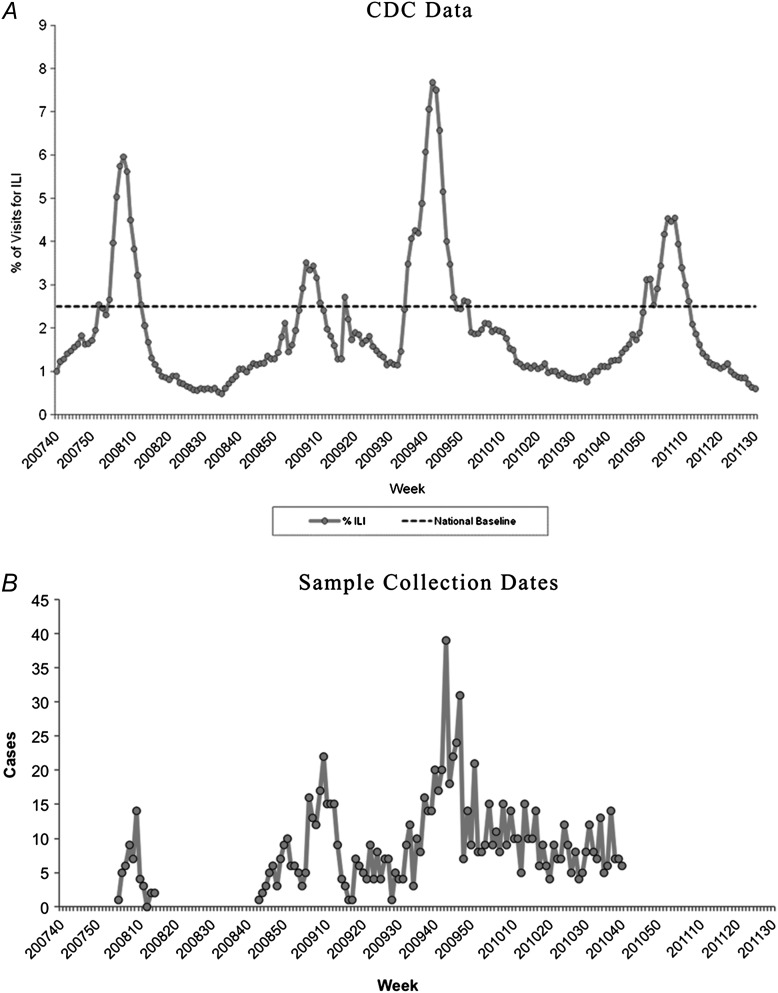
Although the above data demonstrate that influenza viremia is at most an uncommon occurrence, the power of the retrospective study was limited. Standard procedures in blood centers involve recording information from donors who contact the blood center following their donation to report subsequent illness or disease symptoms; this is termed postdonation information (PDI). Two PDI cohorts of subjects were accrued, the first from Blood Systems, Inc (BSI), in 2008 (n = 48) and a second from the ARC spanning the 2008–2009 and 2009–2010 influenza seasons (n = 956); although collections occurred continuously from 2008 to 2010, the peaks of subject enrollments were during the influenza seasons of those years (Figure 4). A questionnaire was developed to assess whether the PDI donors’ symptoms were consistent with ILI (Table 2), and plasma from subjects reporting ≥2 symptoms on the questionnaire within 3 days of donation was tested for influenza RNA using 3 different research-use assays in 3 different laboratories. Although the study was designed to capture those at highest risk of being infected with influenza virus (reporting ILI symptoms shortly after donation), the true incidence of influenza infection in the cohort was not known. Subjects in the BSI cohort were asked to return 2 months after the initial donation to obtain a serum sample to test for development of influenza-specific antibodies. Study of acute and convalescent samples from 21 subjects collected in early 2008 with available follow-up samples revealed 3 subjects with influenza A-specific IgM titers from 1:16 to 1:64 on acute and convalescent specimens; corresponding IgG measurements showed a 4-fold rise in titers in 2 of the 3 subjects. The same 3 subjects had influenza B-specific IgM detectable at titers of 1:4 to 1:64 on acute and convalescent samples, and 2 of these subjects had a 4-fold rise in IgG titers. An additional 6 subjects had ≥4-fold rise in influenza B-specific IgG without corresponding IgM increases. Although the effects of undocumented influenza vaccination cannot be ruled out, these data suggest that 43% (9 of 21) of blood donors complaining of ILI symptoms shortly after blood donation were potentially infected with influenza virus.
Figure 4.
Comparison of Centers for Disease Control and Prevention (CDC) influenza-like illness (ILI) surveillance data with prospective sample collection dates. A, Percentage of visits for ILI reported by the US Outpatient ILI surveillance Network (ILINet), weekly national summary, 30 September 2007 to 30 July 2011. Figure from the CDC website at http://www.cdc.gov/flu/weekly/weeklyarchives2010–2011/picILI30.htm, accessed 20 September 2011. B, The number of samples collected at Blood Systems, Inc (BSI) sites (early 2008) or American Red Cross (ARC) sites (late 2008 through late 2010). The peaks in sample collection for both organizations corresponded to reported high levels of ILI activity in the community (panel A).
Table 2.
Postdonation Information ILI Symptoms
| Symptoms | Fever | Headache | Cough | Muscle Aches | Sore Throat | Chills | Nausea Vomiting | Weakness |
| Yes | 981(98%) | 622 (62%) | 496 (49%) | 694 (69%) | 453 (45%) | 839 (84%) | 416 (41%) | 884 (88%) |
| No | 14 (1%) | 366 (36%) | 502 (50%) | 303 (30%) | 545 (54%) | 158 (16%) | 588 (59%) | 116 (12%) |
| Do not know | 9 (0.9%) | 16 (2%) | 6 (0.6%) | 7 (0.7%) | 5 (0.5%) | 5 (0.5%) | 0 | 4 (0.4%) |
Abbreviation: ILI, influenza-like illness.
Influenza Virus Detection in Donors With ILI
Of 48 plasma samples derived from Blood Systems donors tested in duplicate by TMA for the presence of influenza RNA, 1 was initially reactive in 1 of 2 aliquots (S/CO <10, low-level positive) but negative on repeat testing. The second group of donors from the ARC was tested using TMA and the FDA and CDC RT-PCR assays. A total of 956 plasma samples was tested, with 748 (78%) of those collected after the introduction of the novel H1N1 virus in April 2009 [35]. TMA testing yielded invalid results for 1 sample and negative results for the remaining 955 samples. The positive controls run alongside the test samples showed 95% reactivity at 42 copies/mL (n = 39 samples) and 85% reactivity at 17 copies/mL (n = 40 samples), with copy number calculated using data generated using transcript quantitated by absorbance at 260 nm (data not shown). All 956 ARC samples were also negative when tested using the 2 RT-PCR assays. For the CDC assay, all but one sample produced cycle threshold (Ct) values within the acceptable range (<37) for the assay internal control (termed inconclusive). For the FDA assay, one sample yielded an inconclusive test result.
Including the retrospective and prospective cohorts, a total of 1482 blood donor samples tested negative for the presence of influenza viremia (following retesting of 3 initially reactive samples), yielding 95% confidence intervals of 0–2.5 influenza viremia cases per 1000 blood donations from donors collected during the peak season, or from those reporting postdonation flulike illness, which also were collected primarily during the peak season.
DISCUSSION
The current study represents an analysis of samples collected from blood donors at the estimated highest risk for influenza infection, based on historical data during known influenza outbreaks or by blood donors reporting symptoms consistent with influenza infection within 3 days of blood donation. In spite of testing with a variety of sensitive and validated influenza virus RNA assays, none of the samples yielded repeat-reactive results. Furthermore, use of whole blood samples did not increase sensitivity of the most sensitive assay used (TMA); however, this assessment was limited due to technical issues of assay performance using whole blood.
The results of the current study are reassuring to those whose patients require blood transfusion and are consistent with recently published work in the field. A study of 96 blood donors in Japan who developed symptoms within 7 days of donation (42% within 2 days of donation) also revealed no influenza viremia [36], with a second study of 579 donors also revealing no influenza viremia [37]. The limit of detection for the RT-PCR assay used in the Japanese study was 300 genome equivalents/mL, ∼7-fold less sensitive than TMA (at 95% sensitivity of 42 copies/mL). Screening of German blood donors using a slightly less sensitive assay on pools of ∼90 donors per pool also revealed no evidence of influenza RNA in over 10000 blood donations, albeit the sample dilution due to pooled testing would have required higher-level RNA for detection [38]. Influenza viremia in subjects with fatal highly pathogenic H5N1 influenza infection has been described elsewhere [6, 39], and our data of influenza infection in a ferret model demonstrated detectable RNA using both RT-PCR and TMA. A more recent study of the novel 2009 H1N1 virus revealed viral RNA in the blood from 3 of 23 patients who developed acute respiratory distress syndrome (ARDS) or died, 1 of 14 patients who required supplemental oxygen but did not develop ARDS, and 0 of 37 patients with mild disease [40], and another study demonstrated viral RNA in the blood of 2 subjects with severe disease [41].
Although our findings, and those of others, suggest that influenza viremia is rare, there are numerous examples of viruses spread by the respiratory route that cause viremia. Human bocavirus 1 and respiratory syncytial virus have been detected in children with respiratory tract infections [42, 43], and children under age 2 are more likely to be bocavirus viremic, with viremia also associated with lower respiratory tract infection and higher viral load in a nasopharyngeal aspirate. The mechanism of virus entry has been explored in animal models, with an immune carrier cell (possibly mucosal dendritic cells) implicated in ushering equine herpesvirus 1 in ponies and measles virus in macaques into the bloodstream [44, 45]. Study of varicella virus in a macaque model suggests infection of a broad array of immune cells (including B and T cells) as the route of viremic spread [46]. However, not all invasive respiratory viruses pass the respiratory epithelium via immune cells, as the pig pathogen pseudorabies virus can directly invade through the basement membrane to reach underlying tissues [47]. In spite of carrying both sialic acid and other influenza receptors such as macrophage mannose receptor and macrophage galactose-type lectin [48], infection of macrophages does not appear to lead to dissemination and viremia in most subjects. One possibility is that infected pulmonary macrophages do not produce progeny virus, suggested by work in the mouse model [49], which is consistent with the surprisingly low frequency of influenza viremia compared with other respiratory viruses.
One of the limitations of the current study is that we did not attempt to document influenza infection of prospectively enrolled subjects by viral isolation. Acute and convalescent testing of a small subset of 21 donors with available convalescent samples revealed that approximately 40% showed serological evidence of influenza infection or vaccination. Of the 956 American Red Cross donors, 38% reported receipt of the seasonal influenza vaccine (2008–2010) with 16% reporting receipt of the A/H1N1/2009 vaccine within the year prior to donation. A proportion of the vaccinees would likely have been protected from subsequent influenza infection. Given that the subjects were recruited with symptoms consistent with influenza [50] during periods of increased community transmission, it is probable that many of the subjects with ILI symptoms in our study were infected with influenza virus.
We failed to detect influenza A viremia using very sensitive tests in blood samples from donors collected during periods of high influenza transmission in the community, with the majority of the tested donors reporting symptoms of influenza-like illness. The preponderance of evidence from this and other studies thus suggests that influenza viremia is at most an exceedingly rare event in blood donors, who are healthy at the time of donation, and that influenza viremia only occurs in subjects with moderate to severe disease. An important limitation of this study is that influenza virus may be present at levels below the limit of detection of the assays used and could be present bound to cellular components of blood, as the bulk of testing was limited to the plasma samples. Additionally, only the MGB Alert and CDC assays would have detected influenza B viremia, potentially limiting sensitivity for detection of influenza B viremia. Furthermore, these data were generated during periods of seasonal influenza activity and may not apply to infection with highly pathogenic avian influenza, although viremia has been described only in subjects with severe disease from H5N1 influenza infection [6, 39–41]. These findings indicate that influenza viremia does not appear to normally occur during the presymptomatic phase of influenza infection in healthy individuals; thus, there would be insignificant yield from screening the blood supply for influenza virus. In addition, the infectivity of RNA-positive units is unknown, but our animal data suggest the influenza RNA levels are low and transient.
Notes
Acknowledgments.
We would like to thank Michael Popowich and Daryl Lamson for excellent technical assistance. And ARC staff, Colleen Winton, Pam Sledge; Ann Kaldun and Beth Hewitt who coordinated and executed obtaining donor samples for testing. The Retrovirus Epidemiology Donor Study–II (REDS-II Study Group) was the responsibility of the following:
Blood Centers
American Red Cross Blood Services, New England Region
R. Cable, J. Rios, and R. Benjamin
American Red Cross Blood Services, Southern Region/Department of Pathology and Laboratory Medicine, Emory University School of Medicine
J. D. Roback
Hoxworth Blood Center, University of Cincinnati Academic Health Center
R. A. Sacher, S. L. Wilkinson, and P. M. Carey
Blood Centers of the Pacific, University of California San Francisco, Blood Systems Research Institute
E. L. Murphy, B. Custer, and N. Hirschler
The Institute for Transfusion Medicine
D. Triulzi, R. Kakaiya, and J. Kiss
Blood Center of Wisconsin
J. Gottschall and A. Mast
Coordinating Center: Westat, Inc
J. Schulman and M. King
National Heart, Lung, and Blood Institute, National Institutes of Health
G. Nemo
Central Laboratory: Blood Systems Research Institute
M. P. Busch and P. Norris.
Financial support.
This work was supported by NHLBI contract N01-HB-57181.
Potential conflicts of interest.
The following authors are employed at companies: C. C., T. M., K. H., J. M. L. (Gen-Probe), N. M. J. V. (Epoch Biosciences). M. P. B. serves on the scientific advisory board of Gen-Probe. No other authors reported potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Yu H, Shu Y, Hu S, et al. The first confirmed human case of avian influenza A (H5N1) in Mainland China. Lancet. 2006;367:84. doi: 10.1016/S0140-6736(05)67894-4. [DOI] [PubMed] [Google Scholar]
- 2.Tran TH, Nguyen TL, Nguyen TD, et al. Avian influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med. 2004;350:1179–88. doi: 10.1056/NEJMoa040419. [DOI] [PubMed] [Google Scholar]
- 3.Outbreak news. Avian influenza, Indonesia–update. Wkly Epidemiol Rec. 2006;81:233. [PubMed] [Google Scholar]
- 4.Ungchusak K, Auewarakul P, Dowell SF, et al. Probable person-to-person transmission of avian influenza A (H5N1) N Engl J Med. 2005;352:333–40. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- 5.Yuen KY, Chan PK, Peiris M, et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–71. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 6.de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–7. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Englund JA, Champlin RE, Wyde PR, et al. Common emergence of amantadine- and rimantadine-resistant influenza A viruses in symptomatic immunocompromised adults. Clin Infect Dis. 1998;26:1418–24. doi: 10.1086/516358. [DOI] [PubMed] [Google Scholar]
- 8.Raboni SM, Nogueira MB, Tsuchiya LR, et al. Respiratory tract viral infections in bone marrow transplant patients. Transplantation. 2003;76:142–6. doi: 10.1097/01.TP.0000072012.26176.58. [DOI] [PubMed] [Google Scholar]
- 9.Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517–25. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naficy K. Human influenza infection with proved viremia. Report of a case. N Engl J Med. 1963;269:964–6. doi: 10.1056/NEJM196310312691807. [DOI] [PubMed] [Google Scholar]
- 11.Oseasohn R, Adelson L, Kaji M. Clinicopathologic study of thirty-three fatal cases of Asian influenza. N Engl J Med. 1959;260:509–18. doi: 10.1056/NEJM195903122601101. [DOI] [PubMed] [Google Scholar]
- 12.Khakpour M, Saidi A, Naficy K. Proved viraemia in Asian influenza (Hong Kong variant) during incubation period. Br Med J. 1969;4:208–9. doi: 10.1136/bmj.4.5677.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minuse E, Willis PW, 3rd, Davenport FM, Francis T., Jr An attempt to demonstrate viremia in cases of Asian influenza. J Lab Clin Med. 1962;59:1016–19. [PubMed] [Google Scholar]
- 14.Poliakova TG, Ketiladze ES, Zhilina NN, Stakhanova VM. [Viremia in influenza A2 (Hong Kong)] Vopr Virusol. 1970;15:724–8. [PubMed] [Google Scholar]
- 15.Lehmann NI, Gust ID. Viraemia in influenza. A report of two cases. Med J Aust. 1971;2:1166–9. doi: 10.5694/j.1326-5377.1971.tb92768.x. [DOI] [PubMed] [Google Scholar]
- 16.Roberts GT, Roberts JT. Postsplenectomy sepsis due to influenzal viremia and pneumococcemia. Can Med Assoc J. 1976;115:435–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuruoka H, Xu H, Kuroda K, et al. Detection of influenza virus RNA in peripheral blood mononuclear cells of influenza patients. Jpn J Med Sci Biol. 1997;50:27–34. doi: 10.7883/yoken1952.50.27. [DOI] [PubMed] [Google Scholar]
- 18.Likos AM, Kelvin DJ, Cameron CM, Rowe T, Kuehnert MJ, Norris PJ. Influenza viremia and the potential for blood-borne transmission. Transfusion. 2007;47:1080–8. doi: 10.1111/j.1537-2995.2007.01264.x. [DOI] [PubMed] [Google Scholar]
- 19.Stanley ED, Jackson GG. Viremia in Asian influenza. Trans Assoc Am Physicians. 1966;79:376–87. [PubMed] [Google Scholar]
- 20.Glezen WP, Couch RB. Interpandemic influenza in the Houston area, 1974–76. N Engl J Med. 1978;298:587–92. doi: 10.1056/NEJM197803162981103. [DOI] [PubMed] [Google Scholar]
- 21.Bueving HJ, van der Wouden JC, Berger MY, Thomas S. Incidence of influenza and associated illness in children aged 0–19 years: a systematic review. Rev Med Virol. 2005;15:383–91. doi: 10.1002/rmv.477. [DOI] [PubMed] [Google Scholar]
- 22. Weekly report: influenza summary update: center for disease control and prevention. http://www.cdc.gov/flu/weekly/weeklyarchives2009-2010/09-10summary.htm. Accessed 20 September 2011.
- 23.Liao RS, Tomalty LL, Majury A, Zoutman DE. Comparison of viral isolation and multiplex real-time reverse transcription-PCR for confirmation of respiratory syncytial virus and influenza virus detection by antigen immunoassays. J Clin Microbiol. 2009;47:527–32. doi: 10.1128/JCM.01213-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giachetti C, Linnen JM, Kolk DP, et al. Highly sensitive multiplex assay for detection of human immunodeficiency virus type 1 and hepatitis C virus RNA. J Clin Microbiol. 2002;40:2408–19. doi: 10.1128/JCM.40.7.2408-2419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang R, Sheng ZM, Taubenberger JK. Detection of novel (swine origin) H1N1 influenza A virus by quantitative real-time reverse transcription-PCR. J Clin Microbiol. 2009;47:2675–7. doi: 10.1128/JCM.01087-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jernigan DB, Lindstrom SL, Johnson JR, et al. Detecting 2009 pandemic influenza A (H1N1) virus infection: availability of diagnostic testing led to rapid pandemic response. Clin Infect Dis. 2011;52(Suppl 1):S36–43. doi: 10.1093/cid/ciq020. [DOI] [PubMed] [Google Scholar]
- 27. 510(k) Summary for centers for disease control and prevention human influenza virus real-time RT-PCR detection and characterization panel. http://www.accessdata.fda.gov/cdrh_docs/pdf8/k080570.pdf. Accessed 20 September 2011. [Google Scholar]
- 28.Blyth CR, Still HA. Binomial confidence intervals. J Am Stat Assoc. 1983;78:108–16. [Google Scholar]
- 29.Casella G. Refining binomial confidence intervals. Can J Stat. 1986;14:113–29. [Google Scholar]
- 30.Buzzell A, Hanig M. The mechanism of hemagglutination by influenza virus. Adv Virus Res. 1958;5:289–346. doi: 10.1016/s0065-3527(08)60676-4. [DOI] [PubMed] [Google Scholar]
- 31.Jerushalmy Z, Kohn A, De Vries A. Interaction of myxoviruses with human blood platelets in vitro. Proc Soc Exp Biol Med. 1961;106:462–6. doi: 10.3181/00379727-106-26370. [DOI] [PubMed] [Google Scholar]
- 32.Toms GL, Bird RA, Kingsman SM, Sweet C, Smith H. The behaviour in ferrets of two closely related clones of influenza virus of differing virulence for man. Br J Exp Pathol. 1976;57:37–48. [PMC free article] [PubMed] [Google Scholar]
- 33.Zitzow LA, Rowe T, Morken T, Shieh WJ, Zaki S, Katz JM. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J Virol. 2002;76:4420–9. doi: 10.1128/JVI.76.9.4420-4429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cameron CM, Cameron MJ, Bermejo-Martin JF, et al. Gene expression analysis of host innate immune responses during lethal H5N1 infection in ferrets. J Virol. 2008;82:11308–17. doi: 10.1128/JVI.00691-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dawood FS, Jain S, Finelli L, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto C, Sobata R, Uchida S, et al. Risk for transmission of pandemic (H1N1) 2009 virus by blood transfusion. Emerg Infect Dis. 2010;16:722–3. doi: 10.3201/eid1604.091795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sobata R, Matsumoto C, Igarashi M, et al. No viremia of pandemic (H1N1) 2009 was demonstrated in blood donors who had donated blood during the probable incubation period. Transfusion. 2011;51:1949–56. doi: 10.1111/j.1537-2995.2011.03109.x. [DOI] [PubMed] [Google Scholar]
- 38.Hourfar MK, Themann A, Eickmann M, et al. Blood screening for influenza. Emerg Infect Dis. 2007;13:1081–3. doi: 10.3201/eid1307.060861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Jong MD, Bach VC, Phan TQ, et al. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N Engl J Med. 2005;352:686–91. doi: 10.1056/NEJMoa044307. [DOI] [PubMed] [Google Scholar]
- 40.To KK, Hung IF, Li IW, et al. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin Infect Dis. 2010;50:850–9. doi: 10.1086/650581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oughton M, Dascal A, Laporta D, Charest H, Afilalo M, Miller M. Evidence of viremia in 2 cases of severe pandemic influenza A H1N1/09. Diagn Microbiol Infect Dis. 2011;70:213–17. doi: 10.1016/j.diagmicrobio.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 42.Christensen A, Nordbo SA, Krokstad S, Rognlien AG, Dollner H. Human bocavirus in children: mono-detection, high viral load and viraemia are associated with respiratory tract infection. J Clin Virol. 2010;49:158–62. doi: 10.1016/j.jcv.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rohwedder A, Keminer O, Forster J, Schneider K, Schneider E, Werchau H. Detection of respiratory syncytial virus RNA in blood of neonates by polymerase chain reaction. J Med Virol. 1998;54:320–7. doi: 10.1002/(sici)1096-9071(199804)54:4<320::aid-jmv13>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 44.Gryspeerdt AC, Vandekerckhove AP, Garre B, Barbe F, Van de Walle GR, Nauwynck HJ. Differences in replication kinetics and cell tropism between neurovirulent and non-neurovirulent EHV1 strains during the acute phase of infection in horses. Vet Microbiol. 2010;142:242–53. doi: 10.1016/j.vetmic.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 45.Lemon K, de Vries RD, Mesman AW, et al. Early target cells of measles virus after aerosol infection of non-human primates. PLoS Pathog. 2011;7:e1001263. doi: 10.1371/journal.ppat.1001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Messaoudi I, Barron A, Wellish M, et al. Simian varicella virus infection of rhesus macaques recapitulates essential features of varicella zoster virus infection in humans. PLoS Pathog. 2009;5:e1000657. doi: 10.1371/journal.ppat.1000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glorieux S, Favoreel HW, Meesen G, de Vos W, Van den Broeck W, Nauwynck HJ. Different replication characteristics of historical pseudorabies virus strains in porcine respiratory nasal mucosa explants. Vet Microbiol. 2009;136:341–6. doi: 10.1016/j.vetmic.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Upham JP, Pickett D, Irimura T, Anders EM, Reading PC. Macrophage receptors for influenza A virus: role of the macrophage galactose-type lectin and mannose receptor in viral entry. J Virol. 2010;84:3730–7. doi: 10.1128/JVI.02148-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodgers B, Mims CA. Interaction of influenza virus with mouse macrophages. Infect Immun. 1981;31:751–7. doi: 10.1128/iai.31.2.751-757.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza? JAMA. 2005;293:987–97. doi: 10.1001/jama.293.8.987. [DOI] [PubMed] [Google Scholar]