Abstract
In murine histoplasmosis, tumor necrosis factor α (TNF-α) antagonism increases the number of regulatory T cells (Tregs) in lungs, and these cells profoundly hinder protective immunity. Because CCR5 mediates Treg homing and proliferation, we determined the outcome of antagonizing TNF-α in CCR5–/– mice infected with Histoplasma capsulatum. The absence of CCR5 attenuated the severity of infection associated with TNF-α neutralization. Infected controls given anti–TNF-α had a 10-fold increase in the number of Tregs in lungs compared with a <2-fold increase in CCR5–/– lungs. This difference was partially attributable to impaired homing in the absence of CCR5. Neutralization of TNF-α–enhanced CCR5 ligands in wild-type lungs thus promotes a gradient between lungs and the thymus. This study elucidates the interplay between TNF-α and CCR5 in histoplasmosis. The data suggest that targeting CCR5 may improve host immunity in individuals receiving TNF-α antagonists during infection.
Protection from the dimorphic fungus Histoplasma capsulatum requires a robust T-helper (Th) 1 immune response. Infection causes upregulation of several proinflammatory cytokines and chemokines and, subsequently, the influx of inflammatory cells to the lungs [1, 2]. The organism is internalized by phagocytes and can replicate within macrophages (Mφ) prior to activation of adaptive immunity. Tumor necrosis factor α (TNF-α) is fundamental for protection in both murine and human histoplasmosis [3–6]. Mice treated with a monoclonal antibody (mAb) to TNF-α or that lack either TNF receptor (TNFR) 1 or TNFR2 manifest an elevated fungal burden and ultimately succumb to H. capsulatum infection [3, 4]. TNF-α regulates the emergence of suppressor CD4+ CD25+ cells, and TNF-α antagonism dampens protective immunity by enhancing the proportion and number of antigen-specific regulatory T cells (Tregs) in the lungs during H. capsulatum infection [5].
Regulatory T cells are fundamental in controlling immunity to both self and foreign antigen. Defective function or the absence of Tregs is often associated with autoimmune diseases, and extensive experimental studies have demonstrated that the presence of Tregs prevents or cures such diseases [7]. TNF-α antagonists ameliorate inflammatory diseases such as rheumatoid arthritis and Crohn’s disease by enhancing the number and function of Tregs [8, 9]. Although H. capsulatum infection is typically asymptomatic or associated with mild flulike symptoms in healthy individuals, exposure to or reactivation of H. capsulatum can be lethal in immunocompromised individuals [10, 11]. The use of TNF-α antagonists for the treatment of inflammatory diseases has detrimental consequences if an individual has previously been infected or is subsequently exposed to H. capsulatum [6]. CCR5 dictates resolution of H. capsulatum infection by influencing homing and local proliferation of Tregs [12]. CCR5–/– mice have fewer Tregs in the lungs, which, in turn, promotes an amplified Th17 response that accelerates fungal clearance. In this study, we examined whether the absence of CCR5 imparts a beneficial effect on the course of H. capsulatum infection after TNF-α antagonism.
METHODS
Mice and Neutralization of TNF-α
C57BL/6 (wild-type [WT]), CCR5–/–, TNFR1–/–, and TNFR2–/– mice were purchased from The Jackson Laboratory and maintained by the Department of Laboratory Animal Medicine, University of Cincinnati, which is accredited by the American Association for Accreditation of Laboratory Animal Medicine. All animal experiments were done in accordance with the Animal Welfare Act guidelines of the National Institutes of Health. For neutralization of TNF-α, mice were given 1 mg/mL rat antimouse TNF-α (from cell line XT-22.1) at the time of infection and once a week thereafter [4].
H. capsulatum Infection and Organ Culture
Mice aged 5–6 weeks were intranasally inoculated with 2 × 106 H. capsulatum yeasts (strain G217B) diluted in Hanks balanced salt solution (HBSS). Lungs were homogenized and serially diluted onto mycosel blood agar plates to assess fungal burden. Results are presented as the mean colony-forming units per lung ± SEM.
Isolation of Leukocytes
Thymi and lymph nodes were teased apart in HBSS using the ends of 2 frosted glass slides. Lungs were homogenized in HBSS using a gentleMACS Dissociator (Miltenyi Biotec), and Lympholyte M (Cedarlane Laboratories) was used to isolate leukocytes. All cell solutions were filtered through 60-μm nylon mesh (Spectrum Laboratories).
Flow Cytometry
The following mAbs were purchased from BD Biosciences: allophycocyanin-conjugated CD8α, peridin-chlorophyll protein-conjugated CD4, and fluorescein isothiocyanate–conjugated CD3ϵ. For surface staining, cells were washed with 1% bovine serum albumin in HBSS (pH 7.4) and were stained at 4°C for 15 minutes. To characterize Foxp3 expression, cells were incubated with Cytofix/Cytoperm (BD Biosciences), washed in permeabilization buffer (BD Biosciences), and stained for 1 hour with phycoerythrin-conjugated Foxp3 (eBioscience). Cells were characterized using a FACSCalibur flow cytometer (BD Biosciences) and FCS Express Software.
Measurement of Nitric Oxide
To measure nitric oxide (NO) production in the lungs, leukocytes were isolated from infected lungs and incubated overnight in Dulbecco’s modified Eagle’s medium supplemented with 5% fetal bovine serum (HyClone).Total nitrate and nitrite was measured using a nitrate/nitrite colorimetric assay kit (Cayman Chemical).
Bromodeoxyuridine Labeling and Detection
To measure Treg proliferation in the thymus, an FITC flow kit was purchased from BD Biosciences. Bromodeoxyuridine (BrdU) (1 mg/mL) was administered intraperitoneally for 2 consecutive days before mice were killed. Cells were stained as previously described [12].
Quantitative Real-Time Polymerase Chain Reaction
TRIzol reagent (Invitrogen) was utilized to extract RNA from the lungs. A reverse transcription systems kit (Promega) was used to synthesize complementary DNA. Cytokine expression was characterized by quantitative real-time polymerase chain reaction (PCR) using TaqMan Fast Universal Master Mix and primer/probe sets from Applied Biosystems. Samples were normalized to hypoxanthine-guanine phosphoribosyltransferase (Applied Biosystems) and analyzed on an ABI Prism 7500 instrument (Applied Biosystems).
Statistical Analysis
Analysis of variance (ANOVA) was used to compare groups. The log rank sum test was used to analyze survival. Statistical significance was characterized by a P value < .05.
RESULTS
CCR5–/– Mice Display Prolonged Survival in the Absence of TNF-α
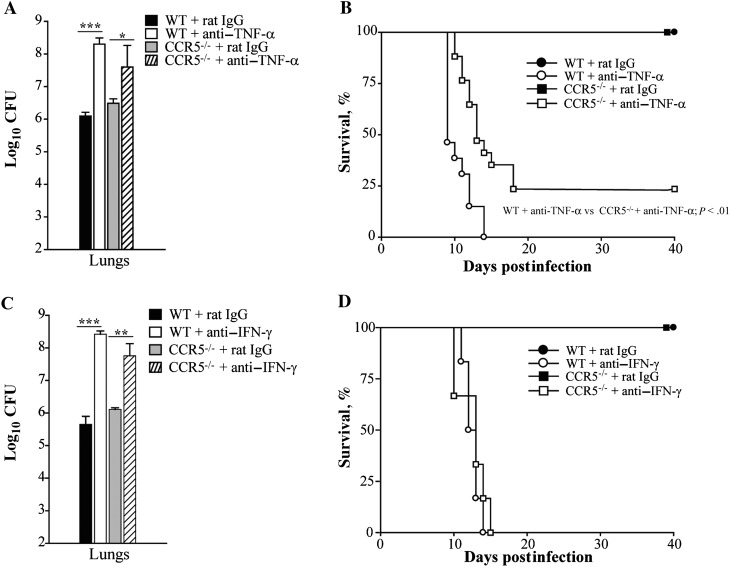
Tumor necrosis factor α antagonism increases the number of Tregs in the lungs of mice infected with H. capsulatum and causes them to succumb to infection [5]. CCR5 signaling promotes the homing and proliferation of Tregs in this fungal infection and consequently the fungal burden [12]. This prompted us to characterize the effect of TNF-α antagonism in CCR5–/– mice in murine histoplasmosis. Wild-type and CCR5–/– mice were infected with 2 × 106 H. capsulatum yeasts and treated with rat immunoglobulin G (IgG) or mAb to TNF-α to assess fungal burden and survival. At day 7 postinfection, the mean number of colony forming units was significantly elevated in lungs from WT mice treated with anti–TNF-α compared with rat IgG. Although TNF-α antagonism raised fungal burden in CCR5–/– lungs, it was not to the same extent as observed in WT lungs (Figure 1A). In survival studies, all WT mice given anti–TNF-α died between days 8 and 14 postinfection. In contrast, TNF-α–neutralized CCR5–/– mice displayed significantly prolonged survival, with >30% survival at the end of the experiment (P < .01, log rank sum test). One hundred percent survival was observed in mice given rat IgG (Figure 1B). To determine if CCR5–/– mice displayed prolonged survival in the absence of another critical cytokine, interferon γ (IFN-γ) was neutralized in WT and CCR5–/– mice. Both WT and CCR5–/– mice had a significantly higher fungal burden in their lungs in the absence of IFN-γ on day 7 postinfection and died by day 15 (Figure 1C and 1D).
Figure 1.
CCR5–/– mice display prolonged survival in the absence of tumor necrosis factor α (TNF-α). Wild-type (WT) and CCR5–/– mice were given a monoclonal antibody to TNF-α, interferon γ (IFN-γ), or rat immunoglobulin G (IgG) and intranasally infected with 2 × 106 yeasts to determine fungal burden and survival. Lungs were harvested at day 7 postinfection to determine colony-forming units (CFU) (A and C). For survival studies, animals were given anti–TNF-α, anti–IFN-γ, or rat IgG once a week and monitored for 40 days (B and D). Data represent the mean ± SEM (n = 6–12) from 2–3 experiments. *P < .05; **P < .01; ***P < .001.
To characterize if extended survival in CCR5–/– mice was caused by an augmented proinflammatory response, control and antibody-treated mice were infected with 2 × 106 yeasts to characterize immune cell infiltration and NO production in the lungs. At day 7 postinfection, WT and CCR5–/– mice lacking TNF-α manifested a significantly higher proportion of neutrophils, monocytes, and Mφ in the lungs compared with controls. The percentage of dendritic cells, CD4+, and CD8+ T cells was diminished in the absence of TNF-α signaling (data not shown). In WT animals given anti–TNF-α, the absolute number of neutrophils, monocytes, Mφ, and CD4+ and CD8+ T cells was elevated in the lungs compared with IgG controls. Although the number of neutrophils, monocytes, and Mφ was also elevated in CCR5–/– lungs in the absence of TNF-α, the increase was less dramatic than observed in WT mice (Table 1). Nitric oxide production is stimulated by TNF-α and is required to control fungal growth in Mφ [4]. We postulated that elevated NO in CCR5–/– mice contributed to prolonged survival in the absence of TNF-α. Mice lacking endogenous TNF-α had nearly a 10-fold reduction in NO in the lungs on day 7 postinfection (29.5 ± 1.8 μM vs 4.0 ± 1.0 μM; P < .001, ANOVA). Although NO production is slightly elevated in CCR5–/– lungs at day 7 postinfection [12], a similar reduction in NO was observed in lungs harvested from CCR5–/– mice given anti–TNF-α (50.8 ± 4.7 μM vs 7.0 ± 2.3 μM; P < .001, ANOVA).
Table 1.
Inflammatory Cell Populations in Histoplasma capsulatum–Infected Lungs
| Mean No. of Leukocytes (±SEM) × 105 |
||||
| Phenotype | WT + Rat IgG | WT + anti–TNF-α | CCR5–/– + Rat IgG | CCR5–/– + anti–TNF-α |
| CD4+ T cell | 4.8 ± 1.1 | 14.0 ± 1.8a | 4.9 ± 1.5 | 4.9 ± 1.2 |
| CD8+ T cell | 3.9 ± 0.8 | 14.0 ± 1.7a | 3.2 ± 1.0 | 3.5 ± 0.9 |
| Neutrophil | 2.1 ± 0.5 | 44.1 ± 5.5a | 0.8 ± 0.3 | 13.0 ± 3.2a |
| Monocyte | 3.5 ± 0.7 | 58.0 ± 7.1a | 2.6 ± 0.9 | 16.2 ± 4.0a |
| Macrophage | 3.6 ± 0.7 | 60.3 ± 7.5a | 2.3 ± 0.7 | 15.3 ± 3.9a |
| Dendritic cell | 5.6 ± 1.1 | 6.0 ± 0.7 | 2.3 ± 0.7 | 1.6 ± 0.4 |
WT and CCR5–/– mice given rat IgG or anti–TNF-α were infected with 2 × 106 yeasts intranasally and lung leukocytes were stained and characterized by flow cytometry at day 7 postinfection. Cell populations were phenotypically characterized by the following surface markers: CD4+ T cells were CD3+ CD4+; CD8+ T cells were CD3+ CD8+; neutrophils were Ly6Ghi CD11b+ CD11c–; monocytes were Ly6Chi CD11b+ CD11c–; macrophages were Mac3+ CD11b+; and DCs were I-Abhi CD11c+. Data represent the mean ± SEM (n = 6–12) from 2–3 experiments.
Abbreviations: IgG, immunoglobulin G; SEM, standard error of the mean; TNF-α, tumor necrosis factor α; WT, wild type.
P < .001 vs WT or CCR5–/– + rat IgG control.
TNF-α Antagonism Increases the Number of Tregs in the Lungs
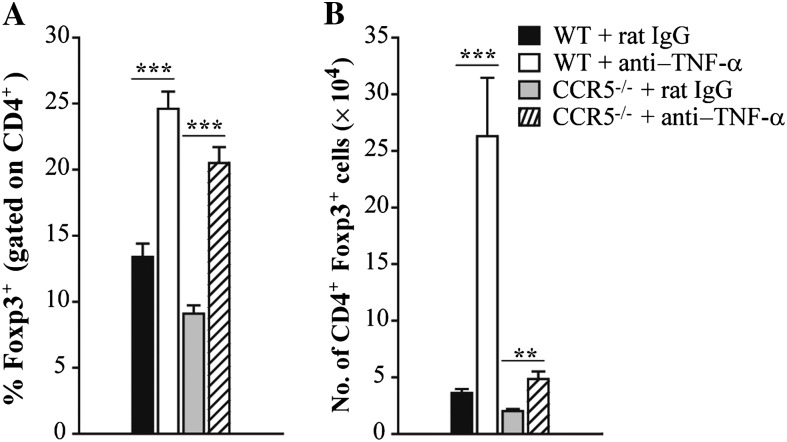
Having demonstrated that the absence of CCR5 was salutary to TNF-α antagonism, we sought to determine if this finding was attributable to differences in the number of Tregs cells in the lungs. WT and CCR5–/– mice were infected with a sublethal inoculum of H. capsulatum and treated with rat IgG or a mAb to TNF-α at the time of infection. Control and TNF-α–neutralized animals were killed 7 days postinfection to characterize the proportion and number of CD4+ Foxp3+ cells in the lungs. CCR5–/– mice manifested a reduced proportion and number of Tregs in their lungs compared with WT at day 7 postinfection. Compared to IgG controls, the proportion of CD4+ cells that was Foxp3+ doubled in WT and CCR5–/– lungs upon treatment with anti–TNF-α (Figure 2A). However, the absolute number of Tregs was dramatically different between the 2 groups. TNF-α antagonism resulted in a 5-fold increase in the total number of leukocytes in WT mice (3.2 × 106 ± 0.5 vs 1.7 × 107 ± 0.2; P < .001, ANOVA). In contrast, the number of leukocytes was unchanged in CCR5–/– lungs after TNF-α antagonism (2.2 × 106 ± 0.7 vs 3.3 × 106 ± 0.5). Thus, the number of Tregs in WT lungs was significantly elevated compared with CCR5–/– lungs in the absence of endogenous TNF-α (Figure 2B).
Figure 2.
Tumor necrosis factor α (TNF-α) antagonism increases the number of regulatory T cells (Tregs) in the lungs. Wild-type (WT) and CCR5–/– mice were given a monoclonal antibody to TNF-α or rat immunoglobulin G (IgG) and intranasally infected with 2 × 106 yeasts. At day 7 postinfection, the proportion (A) and absolute number (B) of Tregs were characterized in the lungs by flow cytometry. Tregs were defined as CD4+ Foxp3+ T cells. Data represent the mean ± SEM (n = 8–12) from 2–3 experiments. **P < .01; ***P < .001.
TNF-α antagonism also altered the number of Tregs in the mediastinal lymph nodes at day 7 postinfection. WT mice lacking endogenous TNF-α had a 2-fold increase in the number of CD4+ Foxp3+ T cells in the mediastinal lymph nodes compared with IgG controls (1.1 × 105 ± 0.1 vs 2.2 × 105 ± 0.2; P < .01, ANOVA). The absence of CCR5 is associated with accumulation of Tregs in the mediastinal lymph nodes during H. capsulatum infection [12]. In contrast to WT, the number of CD4+ Foxp3+ T cells was unaltered upon neutralization of TNF-α (2.2 × 105 ± 0.3 vs 1.9 × 105 ± 0.2).
Neutralization of TNF-α Enhances Treg Proliferation in WT and CCR5–/– Lungs
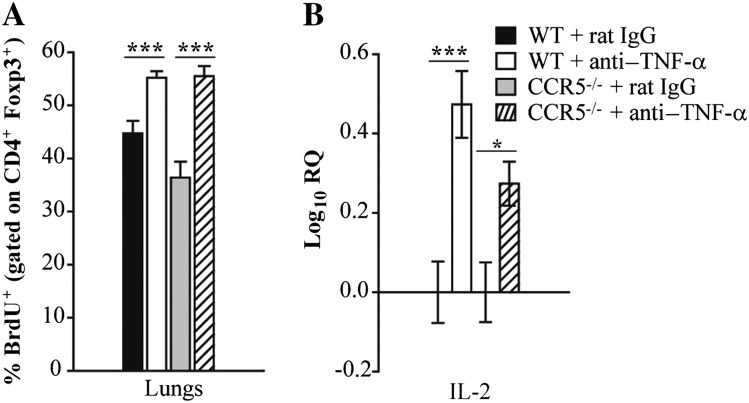
We sought to determine if the increase in Tregs in lungs was a result of augmented proliferation. Control and TNF-α–neutralized mice were treated with BrdU for 2 consecutive days prior to being killed. At day 7, the percent of Foxp3+ cells that incorporated BrdU was elevated in the lungs of WT and CCR5–/– mice treated with anti–TNF-α compared with IgG controls (Figure 3A). Because interleukin 2 (IL-2) is critical for Treg peripheral expansion and maintenance [13], we examined its expression by quantitative real-time PCR. At day 7 postinfection, enhanced Treg proliferation correlated with a modest increase in IL-2 transcript in lung leukocytes isolated from both groups (Figure 3B).
Figure 3.
Neutralization of tumor necrosis factor α (TNF-α) enhances regulatory T-cell (Treg) proliferation in wild-type (WT) and CCR5–/– lungs. WT and CCR5–/– mice were given a monoclonal antibody to TNF-α or rat immunoglobulin G (IgG) and intranasally infected with 2 × 106 yeasts. Mice were intraperitoneally given bromodeoxyuridine (BrdU) for 2 consecutive days before being killed. At day 7 postinfection, the percentage of Tregs (CD4+ Foxp3+ T cells) that incorporated BrdU was measured by flow cytometry (A). RNA was extracted from lung leukocytes at day 7 postinfection and interleukin 2 (IL-2) expression was measured by quantitative real-time polymerase chain reaction. Hypoxanthine-guanine phosphoribosyltransferase was used as an endogenous control, and values represent log increase compared with respective IgG controls (B). Data represent the mean ± SEM (n = 6–12) from 2–3 experiments. **P < .01; ***P < .001.
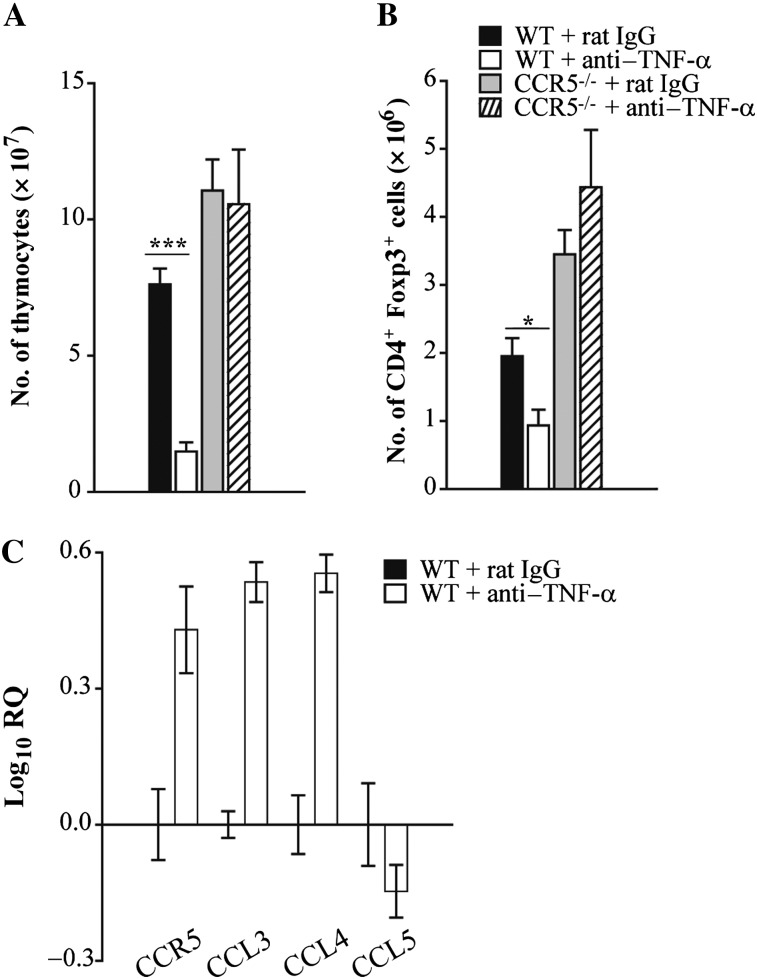
TNF-α Antagonism Alters Thymic Cellularity in WT and, to a Lesser Extent, CCR5–/– Mice During Infection
In WT and CCR5–/– mice given anti–TNF-α, a greater number of Tregs in the lungs was associated with enhanced proliferation. However, the total number of Tregs was dramatically different between the 2 groups. Whereas WT mice given anti–TNF-α had a 10-fold increase in the number of Tregs in the lungs, a <2-fold increase was observed in CCR5–/– lungs. Because CCR5 has been implicated in Treg exiting from the thymus and subsequent homing to H. capsulatum–infected lungs [14], we next explored whether the difference in Treg number was attributable to impaired trafficking in the absence of CCR5 upon neutralization of TNF-α. At day 7, CCR5–/– thymi had a slight increase in the total number of thymocytes compared with WT. TNF-α antagonism resulted in thymic involution and/or lack of expansion in WT and, to a lesser extent, CCR5–/– mice (Figure 4A). Similar to lungs, blockade of TNF-α signaling elevated the proportion of CD4+ single positive cells that expressed Foxp3. However, due to a 10-fold reduction in total number of thymocytes, the absolute number of Tregs was significantly reduced in WT mice treated with anti–TNF-α compared with IgG controls. In contrast, neutralization of TNF-α did not change the absolute number of CD4+ Foxp3+ cells in CCR5–/– thymi (Figure 4B). The involvement of CCR5 in Treg homing was further supported by alterations in expression of CCR5 and its ligands after TNF-α antagonism. Transcript-encoding CCR5, as well as CCL3 and CCL4, was more abundant in lungs of mice given anti–TNF-α compared with rat IgG (Figure 4C).
Figure 4.
Tumor necrosis factor α (TNF-α) antagonism alters thymic cellularity in wild-type (WT) and, to a lesser extent, CCR5–/– mice during infection. Wild-type and CCR5–/– mice were given a monoclonal antibody to TNF-α or rat immunoglobulin G (IgG) and intranasally infected with 2 × 106 yeasts. At day 7 postinfection, thymi were isolated from mice to determine the total number of thymocytes (A) and number of CD4+ Foxp3+ cells (B). RNA was extracted from lung leukocytes at day 7 postinfection and CCR5, CCL3, CCL4, and CCL5 expression was measured by quantitative real-time polymerase chain reaction. Hypoxanthine-guanine phosphoribosyltransferase was used as an endogenous control, and values represent log increase compared with IgG control lungs (C). Data represent the mean ± SEM (n = 6–12) from 2–3 experiments. **P < .01; ***P < .001.
The Population of Tregs That Emerges in the Absence of TNF-α Does Not Suppress T-Cell Proliferation or Proinflammatory Cytokine Production In Vivo
Regulatory T cells control the magnitude of a Th1 response by regulating T-cell proliferation and proinflammatory cytokine production [7]. To determine if the population of Tregs that emerged in the lungs inhibited proliferation of conventional T cells upon neutralization of TNF-α, mice were treated with BrdU and killed on day 7 postinfection. Neutralization of TNF-α enhanced proliferation of CD4+ T cells compared with IgG controls in WT (18.0 ± 0.6% vs 34.0 ± 1.7%; P < .001, ANOVA) and CCR5–/– (12.7 ± 0.8% vs 30.2 ± 1.8%; P < .001, ANOVA) mice at day 7 postinfection. In contrast, proliferation of CD8+ T cells was unaltered after TNF-α antagonism (data not shown). Analysis of the cytokine profile in lungs revealed that transcription of pro- and anti-inflammatory cytokines, including IFN-γ, granulocyte macrophage colony-stimulating factor, interleukin 17F, interleukin 4, and interleuking 10, were elevated to a similar extent in both WT and CCR5–/– mice given anti–TNF-α (Table 2). To confirm cytokine expression was enhanced at the protein level, IFN-γ in lung homogenates was measured by enzyme-linked immunosorbent assay. Consistent with real-time PCR data, TNF antagonism elevated expression of IFN-γ in WT (16.0 ± 2.7 ng/mL vs 201.3 ± 23.3 ng/mL) and CCR5–/– lungs (13.7 ± 2.8 ng/mL vs 192.3 ng/mL) at day 7 postinfection.
Table 2.
Cytokine Expression in Histoplasma capsulatum–Infected Lungs
| Log10 RQ |
||||
| Cytokine | WT + rat IgG | WT + anti–TNF-α | CCR5–/– + rat IgG | CCR5–/– + anti–TNF-α |
| IL-12 | 1.80 ± 0.08 | 1.63 ± 0.20 | 1.68 ± 0.10 | 1.83 ± 0.10 |
| IFN-γ | 2.78 ± 0.08 | 3.51 ± 0.04c | 2.96 ± 0.06 | 3.37 ± 0.10a |
| GM-CSF | 0.28 ± 0.05 | 0.64 ± 0.05c | 0.26 ± 0.06 | 0.57 ± 0.07b |
| IL-17A | 3.50 ± 0.08 | 4.13 ± 0.06c | 3.89 ± 0.11 | 4.03 ± 0.13 |
| IL-17F | 1.68 ± 0.21 | 2.67 ± 0.14b | 1.86 ± 0.25 | 2.72 ± 0.15b |
| IL-4 | 1.02 ± 0.12 | 1.88 ± 0.16c | 0.79 ± 0.29 | 1.48 ± 0.15a |
| IL-10 | 1.16 ± 0.09 | 1.45 ± 0.07a | 1.21 ± 0.10 | 1.58 ± 0.08b |
RNA was extracted from lung leukocytes from WT and CCR5–/– mice given rat IgG or anti–TNF-α at day 7 postinfection, and cytokine expression was measured by quantitative real-time polymerase chain reaction. Hypoxanthine-guanine phosphoribosyltransferase was used as an endogenous control, and values represent log increase compared with uninfected lungs. Data represent the mean ± SEM (n = 6–12) from 2–3 experiments. aP < .05; bP < .01; cP < .001 vs WT or CCR5–/– plus rat IgG control.
Abbreviations: GM-CSF, granulocyte macrophage colony-stimulating factor; IFN, interferon; IgG, immunoglobulin G; IL, interleukin; RQ, relative quantification; TNF-α, tumor necrosis factor α; WT, wild type.
TNF-α Signals via TNFR1 to Regulate Treg Emergence in Infected Lungs
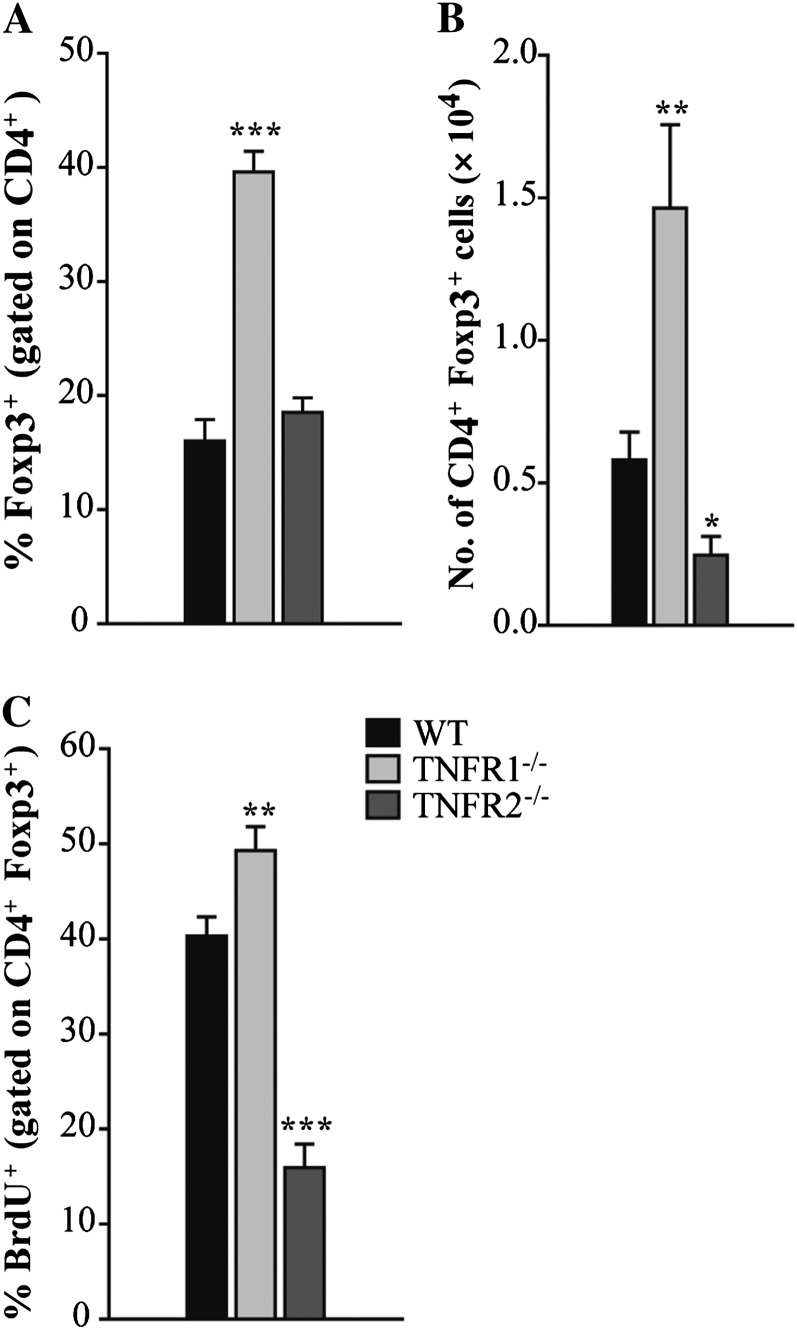
To determine if TNF-α regulated the emergence of Foxp3+ cells by signaling through TNFR1 or TNFR2, WT and TNFR1–/– mice were infected with 2 × 106 H. capsulatum and killed on day 7 postinfection. Similar to anti–TNF-α, the proportion of CD4+ that were Foxp3+ in TNFR1–/– lungs was significantly elevated compared with WT controls (Figure 5A). In contrast, the proportion of CD4+ cells that were Foxp3+ was unaltered in the absence of TNFR2 (Figure 5A). The absolute number of CD4+ Foxp3+ cells was elevated in TNFR1–/– and reduced in TNFR2–/– lungs (Figure 5B). To examine Treg expansion, mice were given BrdU. Consistent with an enhanced proportion, CD4+ Foxp3+ proliferation was augmented in TNFR1–/– lungs. The percent of CD4+ Foxp3+cells that was BrdU+ was significantly diminished in TNFR2–/– lungs (Figure 5C).
Figure 5.
Tumor necrosis factor α (TNF-α) signals via TNF receptor (TNFR) 1 to regulate regulatory T cell (Treg) emergence in infected lungs. Wild-type (WT), TNFR1–/–, and TNFR2–/– mice were intranasally infected with 2 × 106 yeasts. Mice were intraperitoneally given bromodeoxyuridine (BrdU) for 2 consecutive days before being killed. At day 7 postinfection, the percentage of CD4+ cells that were Foxp3+ (A), the absolute number of CD4+ Foxp3+ cells (B), and the percentage of CD4+ Foxp3+ that incorporated BrdU (C) in the lungs were measured by flow cytometry. Data represent the mean ± SEM (n = 8–12) from 2–3 experiments. *P < .05; **P < .01; ***P < .001.
DISCUSSION
The proinflammatory cytokine TNF-α exerts a variety of functions in host defense and autoimmune diseases [15]. TNF-α is synthesized by hemopoietic and nonhemopoietic cells and signals via TNFR1 or TNFR2 expressed on a variety of cell types [16]. Depending on which receptor TNF-α binds, it can induce apoptosis, T-cell proliferation, and chemokine production [17, 18]. During primary infection with H. capsulatum, TNF-α is required for host protection. TNF-α stimulates the production of NO in the lungs, inhibiting intracellular replication of H. capsulatum in Mφ. Blockade of TNF-α signaling results in diminished production of NO as well as altered cytokine expression and inflammatory cell infiltration in the lungs [4, 19]. More recently, the importance of TNF-α in the generation and function of Tregs has been described [5, 20, 21].
In this study, we have elucidated the immunoregulatory role of TNF-α in H. capsulatum infection and demonstrated that the absence of CCR5 ameliorates the outcome of TNF-α antagonism. Neutralization of TNF-α drastically increased the number of Tregs in lungs of WT and, to a lesser extent, CCR5–/– mice. In both groups, TNF-α antagonism increased proliferation of Tregs in the lungs. IL-2, which is required for the expansion and maintenance of peripheral Tregs [22], was elevated in WT and CCR5–/– lungs from mice treated with anti–TNF-α. These results suggest TNF-α can regulate IL-2 expression and upregulation of IL-2 after TNF-α antagonism may facilitate Treg emergence. In addition to enhanced proliferation, improved homing likely contributed to more Tregs in the lungs after neutralization of TNF-α.
CCR5 promotes the migration of Tregs to infectious sites [12, 23, 24]. In the absence of CCR5, fewer Tregs in the lungs is associated with accumulation in the thymus and lymph nodes. Neutralization of TNF-α resulted in thymic involution in WT mice during H. capsulatum infection. TNF-α antagonism resulted in upregulation of CCR5, as well as CCL3 and CCL4, in the lungs. Because CCL4 has specifically been identified as a potent mediator of Treg chemotaxis [25], these results suggest enhanced ligand expression may facilitate the migration of Tregs to infected lungs in TNF-α–neutralized mice. Although Treg proliferation was enhanced in CCR5–/– mice, the absence of TNF-α failed to alter thymic cellularity in CCR5–/– mice and nearly one-third of the mice resolved H. capsulatum infection. Thus, the enhanced number of Tregs and, consequently, the lethal outcome upon TNF-α antagonism in WT mice is likely a result of enhanced Treg proliferation and improved ability to exit the thymus.
One explanation for the improved outcome in CCR5–/– mice given anti–TNF-α is that these mice produce much more TNF-α than WT and therefore neutralization of this cytokine may be incomplete. Production of TNF-α in lungs of WT and CCR5–/– mice is similar [12], therefore excluding the possibility of differences in neutralizing capacity of the mAb. Hence our results highlight the importance of Tregs in dictating survival of CCR5–/– mice in the absence of TNF-α. This argument was supported by studies in which IFN-γ was neutralized. IFN-γ is required for host protection in H. capsulatum infection, but its absence is not associated with perturbations in the numbers of Tregs in the lungs of mice with histoplasmosis [2, 5]. Therefore, we postulated that the absence of CCR5 would not confer protection in mice that lack IFN-γ. Indeed, administration of mAb to IFN-γ to WT and CCR5–/– mice elevated fungal burden and caused mice to succumb by day 15 postinfection. The salutary effect of the absence of CCR5 appears to be restricted to conditions in which Tregs are largely, but perhaps not exclusively, responsible for dampening protective immunity.
TNFR2 is preferentially expressed on the surface of Tregs, and TNF-α has been reported to signal through TNFR2 to induce Treg expansion and function [26–28]. In our studies, the use of TNFR1–/– mice recapitulated the results observed in TNF-α–neutralized mice. TNFR1–/–, but not TNFR2–/–, mice exhibited an enhanced proportion and number of Tregs in the lungs at day 7 postinfection. Consistent with previous findings, Treg proliferation was diminished in TNFR2–/– lungs, highlighting the significance of this receptor in promoting Treg expansion. These data indicate that, in an inflammatory environment induced by an intracellular pathogen, signaling through both TNFR1 and TNFR2 dictates the magnitude of the Treg response.
Targeting TNF-α is beneficial for the treatment of inflammatory diseases. Tregs isolated from individuals with rheumatoid arthritis lack the ability to suppress proinflammatory cytokine secretion by conventional T cells. Antagonizing TNF-α restores Treg suppressor function and increases the number of Tregs in the peripheral blood, ultimately improving the severity of disease [29]. In H. capsulatum infection, the Tregs that emerge upon TNF-α antagonism suppress immune responses in vitro and dampen protective immunity in vivo [5]. Interestingly, the population of Tregs that emerged upon neutralization of TNF-α did not depress T-cell proliferation or the production of proinflammatory cytokines in vivo during infection. Rather, anti–TNF-α treatment enhanced CD4+ T-cell proliferation and elevated expression of both Th1 and Th17 cytokines in WT and CCR5–/– lungs.
One concern is why the absence of CCR5 only provided a moderate level of protection. The likely reason is that the presence of even a small number of Tregs is sufficient to significantly dampen immunity. Previously we reported that elimination of Tregs rescues mice from the deleterious effects of TNF-α neutralization [5]. Even though the number of Tregs is diminished in CCR5–/– mice, the numbers that are present are sufficient that they impair immunity. Moreover, the Tregs that are present in CCR5–/– are functional [12].
The finding that the absence of CCR5 confers protection in the absence of TNF-α has major therapeutic implications. Although antagonizing TNF-α is beneficial for controlling inflammatory diseases, a major adverse consequence is infection with diverse intracellular pathogens including H. capsulatum. Most often, infection with this fungal pathogen in recipients of TNF-α antagonists results in progressive disseminated disease that is life threatening [6, 30, 31]. Our studies raise the possibility that interruption of CCR5 signaling through the use of a receptor antagonist may be critically useful as adjunctive therapy in this clinical setting.
Over a decade ago, CCR5 was identified as a major coreceptor for human immunodeficiency virus (HIV) entry into human cells [32, 33]. Individuals that express a truncated form of CCR5 that is not expressed on the cell surface are highly resistant to HIV infection. This mutation is common in white individuals and is the result of a 32–base pair deletion in the coding region of the CCR5 gene [34]. The absence of expressed CCR5 in these individuals raises the possibility that such individuals who receive anti–TNF-α therapy may be genetically protected from disseminated histoplasmosis. Because CCR5 is a key element in the development, migration, and function of Tregs in mice, individuals expressing this mutation may not respond to TNF-α antagonism or do so in a mechanistically different manner. These data suggest targeting CCR5 in parallel with TNF-α may provide further protection in these patients.
Notes
Financial support.
This work was supported by a Merit Review from the Department of Veterans Affairs and by the National Institutes of Health (AI-073337 and AI-083313).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Cain JA, Deepe GS., Jr Evolution of the primary immune response to Histoplasma capsulatum in murine lung. Infect Immun. 1998;66:1473–81. doi: 10.1128/iai.66.4.1473-1481.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P, Sieve MC, Bennett J, et al. IL-12 prevents mortality in mice infected with Histoplasma capsulatum through induction of IFN-γ. J Immunol. 1995;155:785–95. [PubMed] [Google Scholar]
- 3.Allendoerfer R, Deepe GS., Jr Regulation of infection with Histoplasma capsulatum by TNFR1 and -2. J Immunol. 2000;165:2657–64. doi: 10.4049/jimmunol.165.5.2657. [DOI] [PubMed] [Google Scholar]
- 4.Allendoerfer R, Deepe GS., Jr Blockade of endogenous TNF-α exacerbates primary and secondary pulmonary histoplasmosis by differential mechanisms. J Immunol. 1998;160:6072–82. [PubMed] [Google Scholar]
- 5.Deepe GS, Jr, Gibbons RS. TNF-α antagonism generates a population of antigen-specific CD4+CD25+ T cells that inhibit protective immunity in murine histoplasmosis. J Immunol. 2008;180:1088–97. doi: 10.4049/jimmunol.180.2.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood KL, Hage CA, Knox KS, et al. Histoplasmosis after treatment with anti-tumor necrosis factor-α therapy. Am J Respir Crit Care Med. 2003;167:1279–82. doi: 10.1164/rccm.200206-563OC. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 8.Moreland LW, Baumgartner SW, Schiff MH, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997;337:141–7. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- 9.Scallon B, Cai A, Solowski N, et al. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther. 2002;301:418–26. doi: 10.1124/jpet.301.2.418. [DOI] [PubMed] [Google Scholar]
- 10.Graybill JR. Histoplasmosis and AIDS. J Infect Dis. 1988;158:623–6. doi: 10.1093/infdis/158.3.623. [DOI] [PubMed] [Google Scholar]
- 11.Deepe GS, Bullock WE. Histoplasmosis: a granulomatous inflammatory response. New York: Raven Press; 1992. [Google Scholar]
- 12.Kroetz DN, Deepe GS., Jr CCR5 dictates the equilibrium of proinflammatory IL-17+ and regulatory Foxp3+ T cells in fungal infection. J Immunol. 2010;184:5224–31. doi: 10.4049/jimmunol.1000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–78. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 14.Kroetz DN, Deepe GS., Jr An aberrant thymus in CCR5-/- mice is coupled with an enhanced adaptive immune response in fungal infection. J Immunol. 2011;186:5949–55. doi: 10.4049/jimmunol.1003876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–56. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 16.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–52. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 17.Grell M, Douni E, Wajant H, et al. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 18.Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331–67. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 19.Zhou P, Miller G, Seder RA. Factors involved in regulating primary and secondary immunity to infection with Histoplasma capsulatum: TNF-α plays a critical role in maintaining secondary immunity in the absence of IFN-γ. J Immunol. 1998;160:1359–68. [PubMed] [Google Scholar]
- 20.Wu AJ, Hua H, Munson SH, McDevitt HO. Tumor necrosis factor-α regulation of CD4+CD25+ T cell levels in NOD mice. Proc Natl Acad Sci U S A. 2002;99:12287–92. doi: 10.1073/pnas.172382999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–61. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–51. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 23.Moreira AP, Cavassani KA, Massafera Tristao FS, et al. CCR5-dependent regulatory T cell migration mediates fungal survival and severe immunosuppression. J Immunol. 2008;180:3049–56. doi: 10.4049/jimmunol.180.5.3049. [DOI] [PubMed] [Google Scholar]
- 24.Yurchenko E, Tritt M, Hay V, Shevach EM, Belkaid Y, Piccirillo CA. CCR5-dependent homing of naturally occurring CD4+ regulatory T cells to sites of Leishmania major infection favors pathogen persistence. J Exp Med. 2006;203:2451–60. doi: 10.1084/jem.20060956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2:1126–32. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Baumel M, Mannel DN, Howard OM, Oppenheim JJ. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J Immunol. 2007;179:154–61. doi: 10.4049/jimmunol.179.1.154. [DOI] [PubMed] [Google Scholar]
- 27.Annunziato F, Cosmi L, Liotta F, et al. Phenotype, localization, and mechanism of suppression of CD4+CD25+ human thymocytes. J Exp Med. 2002;196:379–87. doi: 10.1084/jem.20020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Subleski JJ, Kopf H, Howard OM, Mannel DN, Oppenheim JJ. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J Immunol. 2008;180:6467–71. doi: 10.4049/jimmunol.180.10.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrenstein MR, Evans JG, Singh A, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFα therapy. J Exp Med. 2004;200:277–85. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hage CA, Bowyer S, Tarvin SE, Helper D, Kleiman MB, Joseph Wheat L. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis. 2010;50:85–92. doi: 10.1086/648724. [DOI] [PubMed] [Google Scholar]
- 31.Smith JA, Kauffman CA. Endemic fungal infections in patients receiving tumour necrosis factor-alpha inhibitor therapy. Drugs. 2009;69:1403–15. doi: 10.2165/00003495-200969110-00002. [DOI] [PubMed] [Google Scholar]
- 32.Reynes J, Portales P, Segondy M, et al. CD4+ T cell surface CCR5 density as a determining factor of virus load in persons infected with human immunodeficiency virus type 1. J Infect Dis. 2000;181:927–32. doi: 10.1086/315315. [DOI] [PubMed] [Google Scholar]
- 33.Reynes J, Portales P, Segondy M, et al. CD4 T cell surface CCR5 density as a host factor in HIV-1 disease progression. AIDS. 2001;15:1627–34. doi: 10.1097/00002030-200109070-00004. [DOI] [PubMed] [Google Scholar]
- 34.Stephens JC, Reich DE, Goldstein DB, et al. Dating the origin of the CCR5-Delta32 AIDS-resistance allele by the coalescence of haplotypes. Am J Hum Genet. 1998;62:1507–15. doi: 10.1086/301867. [DOI] [PMC free article] [PubMed] [Google Scholar]