Abstract
(See the editorial commentary by Bagni and Whitby, on pages 873–4.)
Background. Infection with Epstein–Barr virus (EBV) early in life and repeated malaria exposure have been proposed as risk factors for endemic Burkitt lymphoma (eBL).
Methods. Infants were enrolled from 2 rural sites in Kenya: the Kisumu District, where malaria transmission is holoendemic and risk for eBL is high, and the Nandi District, where malaria transmission is limited and the risk for eBL is low. Blood samples were taken from infants through 2 years of age to measure EBV viral load, EBV antibodies, and malaria parasitemia.
Results. We observed a significantly younger age at time of primary EBV infection in children from Kisumu compared with children from Nandi (mean age, 7.28 months [±0.33 SEM] in Kisumu vs 8.39 months [±0.26 SEM] in Nandi), with 35.3% of children in Kisumu infected before 6 months of age. To analyze how different predictors affected EBV viral load over time, we performed multilevel mixed modeling. This modeling revealed that residence in Kisumu and younger age at first EBV infection were significant predictors for having a higher EBV viral load throughout the period of observation.
Conclusions. Children from a region at high risk for eBL were infected very early in life with EBV, resulting in higher viral loads throughout infancy.
Endemic Burkitt lymphoma (eBL), the most common childhood malignancy in Equatorial Africa, is rapidly fatal if untreated. This cancer has been etiologically associated with both Epstein–Barr virus (EBV) infection and holoendemic malaria transmission [1–3]. In the 1970s, de-Thé et al performed a prospective study on >40 000 children in Uganda and found very high antibody titers against the EBV viral capsid antigen (VCA) in children who subsequently developed eBL [4]. The elevated and stable VCA titers over time led de-Thé et al to hypothesize that infection of infants with EBV early in life could result in an infection that was poorly controlled by the host and thus increased the risk for eBL [4, 5]. However, subsequent to this study, Biggar et al followed a much smaller cohort of infants from an urban area of Ghana from birth through 2 years of age [6, 7]. Most infants were not infected until after 8 months of age and <50% were infected by 12 months of age. Because this study found maternal antibodies in all newborns tested and no EBV infection until after the waning of maternal antibodies, the investigators concluded that EBV infections could not occur in early infancy. Other studies on primary EBV infection in infants living in Hong Kong [8] and urban areas of the United States [9] also found that a later age at time of primary EBV infection supports a model in which primary EBV infection in infants <6 months of age is rare. None of these studies accounted for malaria transmission as a factor in primary EBV infection or analyzed infants living in a rural setting.
In Kenya, malaria transmission dynamics vary in different geographic locales such that people living in some areas experience limited (unstable, less intense) exposure, whereas those who reside in areas where transmission is intense experience stable transmission characterized by repeated or chronic malaria infections throughout the year (ie, holoendemic malaria) [10, 11]. These differences in malaria transmission also correlate with differences in eBL incidence rates [1, 12] as well as differences in EBV viral loads in children 1–4 years of age [13]. These findings suggest that malaria transmission intensity can influence EBV infection in infants and contribute to the increase in risk of eBL associated with living in areas of higher malaria transmission.
In the current study, we followed infants from 2 areas of rural Kenya with divergent malaria exposure and differential risk for eBL to address the question of site of residence and the effects of age at time of primary EBV infection on EBV persistence. We found that infants living in high malaria transmission regions were infected with EBV significantly earlier in life than has been previously reported and that the younger age at time of infection led to more frequent detection of EBV and higher EBV viral loads over time. Together, these data support de-Thé’s hypothesis that younger age at time of EBV infection is a risk factor for eBL [14].
METHODS
Study Population
Two cohorts of children were established at the Chulaimbo Sub-District Hospital in Kisumu District (Nyanza Province) and at the Mosoriot Sub-District Hospital in Nandi District (Rift Valley Province), Kenya. These cohorts will be referred to as Kisumu (ie, from stable/high malaria transmission region, high risk for eBL) and Nandi (ie, from unstable/low malaria transmission region, low risk for eBL). We have previously described these study sites [1, 12, 13]; both serve patients from a predominantly rural area. Ethical approval for this study was given by the Kenya Medical Research Institute Ethical Review Committee and the Institutional Review Board at State University of New York Upstate Medical University. All children who were enrolled in the study were born to human immunodeficiency virus (HIV)–seronegative mothers, as determined through the mother’s participation in antenatal HIV screening performed independently at Voluntary Counseling and Testing centers. The first sampling and data collection were performed when the children were approximately 1 month of age, then every month during the first 12 months, and every 4 months after that through 2 years of age.
Blood Collection and Processing
At least 150 μL of finger or heel prick blood was collected in EDTA microtainers (BD). Blood was centrifuged to isolate the plasma fraction from the cell pellet, and an equivalent volume of phosphate-buffered saline was added to replace plasma volume removed. Plasma and blood samples were stored at −80°C until further analysis.
Quantitative Polymerase Chain Reaction to Quantify EBV and Plasmodium falciparum DNA
DNA was extracted from up to 200 μL of blood using a Qiagen DNAeasy kit (Qiagen) according to the manufacturer’s protocol. DNA was eluted off the column in an equivalent volume of water and stored at −20°C. EBV DNA levels were determined as previously described using primers and probes designed to detect a 70-basepair region of the EBV BALF5 gene and β-actin gene as a control for DNA input [13, 15]. The limit of detection is 2 copies EBV/μL. The few samples that were not β-actin positive after 2 polymerase chain reaction (PCR) attempts (<1% of all samples) were excluded from further analysis. Plasmodium falciparum DNA was detected using PCR primer and probes as described elsewhere [16].
Detection of EBV-Specific Antibodies
Two synthetic peptides covering immunodominant epitopes of VCA P18 and EBV nuclear antigen 1 (EBNA1) were used to detect EBV-specific antibodies in a luminex bead–based array assay as described elsewhere [17–19]. The results of the assay were expressed as the median fluorescence intensity (MFI) of at least 75 beads for each EBV antigen. EBV-specific immunoglobulin M (IgM) was detected using enzyme-linked immunosorbent assay as described [17], using the VCA P18 peptide.
Statistical Analysis
All statistical analyses were conducted using Stata IC software (version 11.1 [20]), setting 2-tailed α to reject the null hypotheses at 0.05. Comparisons between groups (Kisumu and Nandi cohorts) on single observations of continuous variables (eg, age [months] of first EBV infection, age [months] of last detected maternal antibodies) were made with Student t test, following successful homogeneity of variance assumption testing. Fisher exact tests were used for comparisons of frequencies by group (eg, number of children infected with EBV prior to 6 months of age). Mann–Whitney tests were used to compare median EBV loads. Ordinary least squares regression (OLS) was used to assess relationships among the age at time of the last observed maternal antibodies and group their interaction on the age at time of first EBV infection.
Modeling of Longitudinal EBV DNA Levels and Malaria Burden
EBV viral load data collected longitudinally from children at multiple times during the course of the study were highly variable, containing zeros where viral load was below the limit of detection and contrasted with samples where very high levels were sometimes observed. As such, we performed log10 transformations of the nonzero data to help normalize the distributions of data. Next, we calculated subjects’ time-averaged cumulative area under the curve (AUC) on the log-normalized data over their age at time of observations using the trapezoidal method to represent their cumulative viral load encountered during the period under investigation. Time-averaged AUC measures were calculated for all subjects (n = 136) who had sufficient log10 transformed data (nonzero with at least 2 data points) from which an area (trapezoidal method) could be calculated. These time-averaged AUC data were then submitted to an OLS that included indicator variables to evaluate the effects of site of residence (Kisumu vs Nandi) and sex. The OLS also included a continuous covariate to assess the effect of the age of a child at the time of primary EBV infection on his or her cumulative EBV viral load observations; this was assessed 1735 times from the 136 children over the course of the study.
We log10-transformed the nonzero data related to malaria parasitemia (determined by quantitative PCR [qPCR]) and submitted these 486 observations to a mixed-effects regression model to evaluate the effects of group, sex, and age on malaria parasitemia observations.
RESULTS
Establishment and Characterization of the Longitudinal Infant Cohort
From April to June 2006, 108 infants were enrolled in Kisumu and 116 were enrolled in Nandi. There were no significant differences in the frequency of males in each group (Table 1). At the end of 2 years, we retained 64% participation in Kisumu and 78% participation in Nandi. In Kisumu, 10 children died during the study due to illness (n = 9) or accident (n = 1). In contrast, only 2 children died in Nandi, both of illness. A few study participants (n = 7) who had follow-up samples until 2 years of age, but with gaps of >3 months in their follow-up prior to evidence of EBV infection, were excluded from further analysis because we could not determine age at time of primary EBV infection within the same time frame as other study participants. We analyzed data obtained from 150 infants who had complete follow-up throughout the study period (Table 1). There were 68 infants in Kisumu and 82 infants in Nandi, representing 786 samples from Kisumu and 949 samples from Nandi analyzed for EBV viral load, EBV serology, and P. falciparum DNA.
Table 1.
Demographic and Clinical Characteristics of Study Participants by Region
| Characteristic | Kisumu | Nandi | P Value |
| Enrolled, No. | 108 | 116 | |
| Retention through 24 months, No. | 68 | 82 | |
| Sex, male, No. | 29 (42.6%) | 45 (54.9%) | .14 |
| Mean (SE) age at time of first Epstein–Barr virus infection (months) | 7.28 (.33) | 8.39 (.26) | .0072 |
To confirm previously reported differences in malaria exposure between these 2 sites [13], we analyzed the DNA samples from each cohort for P. falciparum DNA using qPCR, allowing for increased sensitivity compared with thick blood smear [21]. Although we were able to detect P. falciparum–positive blood samples in the children from both Kisumu and Nandi, we found a significantly higher number of children with asymptomatic P. falciparum parasitemia in Kisumu at any given time (Mann–Whitney test, P < .0001). Using a mixed-effects regression model to analyze malaria parasitemia burden, there was a significant effect for both age and site of residence (Kisumu vs Nandi), indicating higher malaria parasitemia for younger children and for children from Kisumu relative to Nandi (Table 2).
Table 2.
Malaria Burden in Kisumu and Nandi Cohortsa
| Explanatory Variable | Coefficient | Standard Error | 95% Confidence Interval | P Value |
| Nandi (vs Kisumu) | −0.3858 | 0.0619 | −.5072, −.2644 | .000 |
| Age (mo) at time of observation | −0.0126 | 0.0038 | −.0200, −.0052 | .001 |
| Sex (male) | −0.0758 | 0.0616 | −.1965, .0450 | .219 |
Malaria burden determined by quantitative polymerase chain reaction, followed by log10 transformations to normalize 486 nonzero observations submitted to a mixed-effects linear regression model with child-level random intercepts.
Infants From Malaria-Endemic Regions Are Younger at Time of Primary EBV Infection
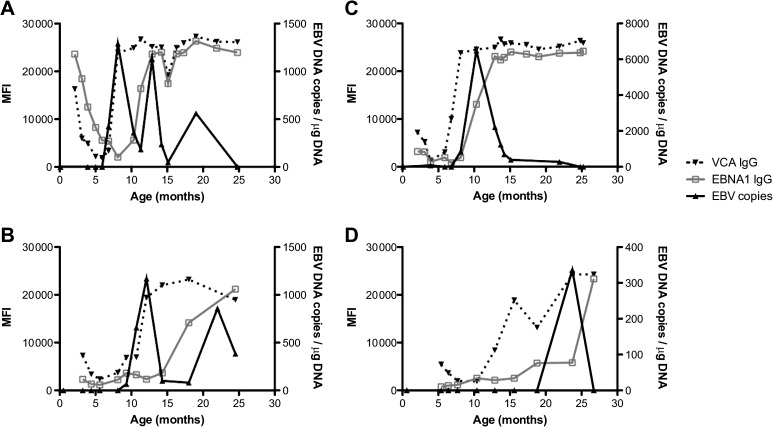
In our cohort study, we determined the age at time of primary EBV infection in each study participant to be the age when any marker of EBV infection (IgM to VCA, immunoglobulin G [IgG] to VCA or EBNA, or viral DNA) was first detected. To account for the presence of maternal antibodies, which would confound our determinations, we generated plots of each study participant, overlaying EBV viral load and EBV antibody responses over time. This allowed us to determine the last age at detection of maternal antibody. For EBV serology to be considered an indicator of primary infection, there had to be a >2-fold increase in the MFI over the previous sample. Representative examples of EBV serology and DNA profiles are shown in Figure 1. As shown in Figure 1A (Kisumu) and Figure 1B (Nandi), maternal antibody to VCA is readily observed and declines over time, infection is evidenced by the appearance of EBV DNA before EBV-specific IgG (and IgM, not shown), and EBNA1-specific IgG levels increase during later months. In contrast, Figure 1C (Kisumu) and Figure 1D (Nandi) indicate that EBV infection was first evidenced by the appearance of VCA-specific IgG (and IgM, not shown) in 2 children. There was a wide variety in patterns of EBV DNA levels over time, ranging from a single peak gradually decreasing over time (Figure 1C) to repeated peaks over >1 year after primary infection (Figure 1A and 1B). As summarized in Table 3, primary EBV infection was detected by the presence of EBV DNA in approximately half of the study participants regardless of where they were from (Kisumu or Nandi; 50.0% vs 52.4%, respectively; Fisher exact test, P = .87). The median first EBV load detected was not significantly different between Kisumu and Nandi infants (Table 3).
Figure 1.
Representative figures of primary infection detected by first appearance of Epstein–Barr virus (EBV) DNA or by first appearance of EBV-specific antibodies. Data are shown for infants with primary infection with appearance of DNA before and at the same time as antibodies in Kisumu (A) and Nandi (B), as well as infants who have antibodies appear before EBV DNA in Kisumu (C) and Nandi (D). The left y-axis shows the median fluorescence intensity (MFI) of at least 75 beads for the response against each EBV antigen studied. The right y-axis plots EBV viral load. EBV DNA levels, solid black circle and solid black line; viral capsid antigen (VCA) IgG, solid black triangle and dotted line; and EBNA1 IgG, light gray open square and dotted line.
Table 3.
Markers and Age at Time of Primary Epstein–Barr Virus (EBV) Infection and EBV Load in Infants in Kisumu and Nandi
| Kisumu | Nandi | P Value | |
| EBV DNA as first indicator of infection, No. | 34/68 (50.0%) | 43/82 (52.4%) | .87a |
| EBV infection before 6 months, No. | 24/68 (35.3%) | 10/82 (12.2%) | <.0001a |
| First detectable EBV load (copies/μg)b | 536 (130; 1408) | 414 (193; 1523) | .7614c |
| EBV load (log10 copies/μg)d | 1.28 (0.84; 1.68) | 0.78 (0.39; 1.23) | <.002c |
Abbreviation: EBV, Epstein–Barr virus.
Indicates P values determined using Fisher exact test comparing values of Kisumu and Nandi.
Median EBV load (25th percentile; 75th percentile).
Indicates P values determined using the Mann–Whitney test.
Based on time-averaged area under the curve.
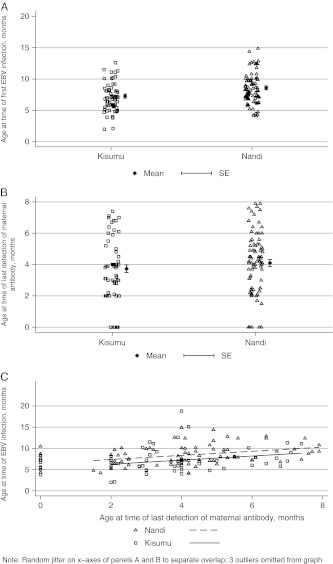
We observed a significantly earlier age at time of primary EBV infection in children from Kisumu compared to those from Nandi (P < .006), with a mean age of 7.28 months (±0.33 SEM) in Kisumu vs 8.39 months (±0.26 SEM) in Nandi (Figure 2A). Of note, the proportion of children infected before 6 months of age was significantly higher in Kisumu (35.3%) in comparison with Nandi (12.2%; Fisher exact test, P < .0001; Table 3). These numbers were much higher than in the earlier studies by Biggar et al [6], who found very few children infected before 6 months of age (<5%) and protective effects of maternal antibodies for at least 2 months after they became undetectable.
Figure 2.
A, Younger age at time of primary Epstein–Barr virus (EBV) infection in children from Kisumu compared with children in Nandi. Shown is the mean age at time of EBV in infants from Kisumu and Nandi (7.28 vs 8.39 months, respectively); each dot represents individual ages at time of infection. B, Comparable age at time of waning of maternal antibodies to EBV antigens in infants from Kisumu and Nandi. Shown is the mean age at which EBV-specific IgG (to viral capsid antigen or EBV nuclear antigen 1, depending on which was last detected) became undetectable in infants from Kisumu and Nandi (3.7 and 4.1 months, respectively; P = .33); each dot represents individual ages at time of waning. C, Correlation between age at time of last detection of maternal antibodies and age at time of infection by EBV for children from Kisumu and Nandi (R = 0.3240, P = .0001). Of note, there were group differences in age at time of EBV infection even after factoring out the effect of the age at time of last observed maternal antibody (P = .043), as shown by the parallel lines for the correlation between age at time of infection and age at time of last detection of maternal antibodies for both sites separately.
Differences in Age at Time of EBV Infection Are Not Explained by Maternal Antibodies
Next, we determined whether earlier loss of maternal antibodies could explain the younger age at time of infection in Kisumu compared with Nandi. As shown in Figure 2B, there was no significant difference in age at time of last detection between Kisumu and Nandi children (mean age, 3.7 and 4.1 months, respectively; t test, P = .33). Of note, in both sites VCA-specific maternal IgG was the last detected in three-quarters of cases and EBNA1-specific IgG was detected in one-quarter of cases (data not shown). The frequency of infants without detectable maternal antibodies was comparable for both sites: 9.7% in Kisumu and 7.3% in Nandi (Fisher exact test, P = .76; data not shown). There was a significant correlation between the age at which maternal antibodies were last detected and the age at time of infection by EBV (R = 0.3240; P = .0001; Figure 2C). Interestingly, there was no interaction effect (P = .743), meaning that the effect of maternal antibodies appears similar for both groups (parallel lines, Figure 2C). Furthermore, even after factoring out the effect of the age at the time of last observed maternal antibody, there was still an effect of site of residence on age at time of first EBV infection (P = .043). Together these results indicate that although maternal antibodies do appear to play a role in delaying infection by EBV in infants from both Kisumu and Nandi, they do not explain the difference in age at time of infection between the sites. Of note, at both sites a few infants were infected by EBV, as indicated by detection of EBV viral load in peripheral blood before maternal antibodies became undetectable (6 cases in Kisumu, 3 cases in Nandi) [22].
Infection With EBV Earlier in Life and Residence in Kisumu Predicts Higher Viral Load Over Time
We modeled the effects of age at time of EBV infection, site of residence (Nandi, Kisumu), and sex based on the cumulative EBV viral load using subjects’ time-averaged cumulative area under the log10 EBV viral load curve as the dependent variable in an OLS regression analysis. Our model revealed significant contributions to higher EBV viral load based on study participants’ site of residence (Kisumu vs Nandi; P = .011) and younger age at time of EBV infection (P = .001), but not sex (P = .282; Table 4). Analysis of the median viral load also indicated higher median viral loads in infants living in Kisumu compared with those in Nandi (P < .00001; Table 3).
Table 4.
Time-Averaged Area Under the Curve Analysis of Longitudinal Observations of Epstein–Barr Virus Loada
| Explanatory Variable | Coefficient | SE | 95% CI | P Value |
| Nandi (vs Kisumu) | −0.27 | 0.10 | −.48, −.06 | .011 |
| Age (mo) of first EBV infection | −0.07 | 0.02 | −.11, −.03 | .001 |
| Male (vs female) | −0.11 | 0.10 | −.30, .09 | .282 |
Abbreviations: CI, confidence interval; EBV, Epstein–Barr virus; SE, standard error.
Log10 transformed (n = 136; observations = 1735).
DISCUSSION
In 1977, de-Thé postulated that EBV infection in infants during the first few months of life was a risk factor for eBL [4, 14]. However, subsequent longitudinal studies of primary EBV infection did not find a significant proportion of infants infected prior to 8 months of age (Table 5), and this hypothesis was discarded [6, 8, 23]. All of these prior studies were based on urban populations and only 1 study was performed in Africa [4, 6, 7] where the malaria burden was not described. A major finding of our study was that almost 1 in 5 infants from a rural population in Kenya with high levels of malaria transmission and risk for eBL was infected with EBV by 5 months of age (Table 5). Also, overall, there was a significantly younger age at time of EBV infection in infants living in a malaria-holoendemic region relative to infants living in a region with low malaria transmission. The consequence for infants who were infected early in life was more frequent detection of EBV and higher EBV viral load throughout the period of observation, suggesting that infants infected earlier in life had poor control of the viral infection. Together, our data support the model that early age at time of infection with EBV is a risk factor for eBL.
Table 5.
Comparison of Rates of Epstein–Barr Virus Infection in Infants in Longitudinal Cohorts
| Kenya (n = 150) (this paper) |
Ghana [6] |
Zambia [31] |
Hong Kong [8] |
United States [9] |
|||
| Rural (n = 68) | Rural (n = 82) | Urban (n = 32) | Urban (n = 677) | Urban (n = 66) | Urbana (n = 80) | Urbana (n = 384) | |
| Infectious disease status of population | Malariahi | Malarialo | nd | HIV+ and HIV− | malarianeg | HIV+ | HIV− |
| Age, months | (%) | (%) | (%) | (%) | (%) | (%) | (%) |
| 5 | 17.90 | 1.20 | 0 | 0 | <3 | <3 | |
| 8 | 68.70 | 48.10 | 1.50 | ||||
| 12 | 97.00 | 88.90 | 44 | 59 | 18.40 | 23.20 | 31.70 |
| 15–16 | 99.00 | 100 | 62 | 40.60 | |||
| 20–21 | 100 | 81 | 64.40 | ||||
| 24 | 83.20 | 54.70 | 64.90 | ||||
Values highlighted in bold were determined in this study and represent the cumulative percent of infants infected over time.
Abbreviation: nd, malaria status not determined.
Boston, Los Angeles, Houston, New York; all infants born to human immunodeficiency virus (HIV)–infected mothers.
The observation that infants infected with EBV at <6 months of age had more frequently detected and elevated viral loads over time is consistent with studies following infection of neonates with another gammaherpesvirus, murine gammaherpesvirus-68. In that study, infection of mice during the neonatal period led to chronic persistence in the lungs long after adult mice were able to clear the virus [24]. Other viral infections that occur in infancy, such as hepatitis viruses [25] and cytomegalovirus [26], are also associated with chronic infection, suggesting that differences between the infant and adult immune systems could lead to variation in viral pathogenesis.
We observed that infants from Kisumu had more malaria exposure than Nandi infants, as indicated by the presence of malaria parasitemia at time of sampling. In addition, living in Kisumu predicted higher viral loads over time. This is consistent with our study as well as those of others who found higher viral load and more EBV reactivation over time in children from malaria-holoendemic regions [13, 27, 28], as well as evidence that malaria can directly interact with EBV-infected B cells and induce viral reactivation [29]. What remains to be determined is how a higher viral load would increase risk for eBL. We do know that children with eBL have elevated viral loads compared with healthy age-matched children [13, 30, 31]. Elevated viral load could result in an increasing pool of cells that provide an anti-apoptotic environment because of the presence of EBV and could tolerate the c-myc translocation that is the hallmark of Burkitt lymphoma [32]. A second but not exclusive possibility is that chronic viral loads can result in exhausted T-cell response to EBV, consistent with the observations by Moormann et al [15], providing an opportunity for escape of a malignant clone from immune surveillance.
In our study, we utilized a wide array of markers to assess infection by EBV (DNA, IgG, and IgM) and monthly sampling that may influence the rate of detection of infection and thus could account for our detection of earlier age at time of EBV infection compared with previous studies. Due to monthly sampling, in general, IgM and IgG EBV responses appeared simultaneously and at the latest 1 month after appearance of DNA. This could reflect the fact that monthly sampling is unlikely to be frequent enough to discriminate between the time at which the virus becomes detectable in peripheral blood and the time at which the immune response is measurable. This may also explain why we did not always find DNA as the first marker of infection; DNA levels may have fallen from the first peak levels to below the limit of detection. This latter point is interesting relative to the studies of Hochberg [33], who found a slow decay of EBV-infected B cells over several months in young adult patients with infectious mononucleosis. In contrast, in many of our study participants, we observed multiple peaks of EBV viral load over time rather than a single peak and decay. One challenge in studying primary EBV infection in infants is that we were limited by the sample volume we had for analysis. In the studies by Hochberg et al, by studying primary EBV infection in young adults, it was possible to obtain sufficient sample volume and perform very elegant limiting dilution studies to determine the frequencies of latently infected cells.
Interestingly, it was observed earlier that infants from an urban setting in Ghana were not infected before disappearance of maternal antibodies, as measured by VCA-specific antibodies [6]. Consistent with this, we observed a correlation between loss of VCA antibodies and infection with EBV, suggesting that maternal antibodies are protective against infection. However, our data also indicate that independent of the loss of maternal antibodies, there is an additional effect of living in a malaria-holoendemic area as a predictor of younger age at time of infection.
In conclusion, these data demonstrate that infants from a region in Kenya with high malaria exposure were infected with EBV earlier in life and that younger age at time of infection led to poor control of the virus after primary infection. In contrast to earlier longitudinal studies indicating a later age at time of EBV infection in infants (Table 5) [6, 8, 9, 23], the current study was conducted in rural areas with a high malaria burden and eBL incidence and directly compared areas with low malaria burden and low eBL incidence. Therefore, the data presented are consistent with the hypothesis that infection by EBV in infancy may set the stage for eBL development [4, 5]. Our data strongly argue that we need to revisit this model and ask the questions: What are the underlying mechanisms that predispose infants to EBV infection before 6 months of age in a region with high malaria transmission? Could infectious diseases during pregnancy such as malaria lead to reduced resistance of the infant to subsequent infections?
Notes
Acknowledgments.
We acknowledge the Chulaimbo Sub-District Hospital and Mosoriot Sub-District Hospital for allowing us to use their facilities to perform this study. We also thank our clinical officers, medical officers, and data entry and field staff who were involved in the project. We are particularly grateful to the mothers and children for their participation in this study.
Financial support.
This work was supported by the National Cancer Institute at the National Institutes of Health (CA102667 to R. R. and CA134051 to A. M. M.) as well as a grant from Alex’s Lemonade Stand (to R. R.).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Rainey JJ, Mwanda WO, Wairiumu P, Moormann AM, Wilson ML, Rochford R. Spatial distribution of Burkitt’s lymphoma in Kenya and association with malaria risk. Trop Med Int Health. 2007;12:936–43. doi: 10.1111/j.1365-3156.2007.01875.x. [DOI] [PubMed] [Google Scholar]
- 2.Facer CA, Playfair JH. Malaria, Epstein-Barr virus, and the genesis of lymphomas. Adv Cancer Res. 1989;53:33–72. doi: 10.1016/s0065-230x(08)60278-x. [DOI] [PubMed] [Google Scholar]
- 3.Morrow RH. Epidemiological evidence for the role of falciparum malaria in the pathogenesis of Burkitt’s lymphoma. In: Lenoir G, O'Connor G, Olweny C, editors. Burkitt’s lymphoma: a human cancer model. Lyon, France: IARC Press; 1985. pp. 177–85. [PubMed] [Google Scholar]
- 4.de-Thé G, Geser A, Day NE, et al. Epidemiological evidence for causal relationship between Epstein-Barr virus and Burkitt’s lymphoma from Ugandan prospective study. Nature. 1978;274:756–61. doi: 10.1038/274756a0. [DOI] [PubMed] [Google Scholar]
- 5.de-Thé G. Epstein-Barr virus and Burkitt’s lymphoma worldwide: the causal relationship revisited. IARC Sci Publ. 1985;60:165–76. [PubMed] [Google Scholar]
- 6.Biggar RJ, Henle W, Fleisher G, Bocker J, Lennette ET, Henle G. Primary Epstein-Barr virus infections in African infants. I. Decline of maternal antibodies and time of infection. Int J Cancer. 1978;22:239–43. doi: 10.1002/ijc.2910220304. [DOI] [PubMed] [Google Scholar]
- 7.Biggar RJ, Henle G, Bocker J, Lennette ET, Fleisher G, Henle W. Primary Epstein-Barr virus infections in African infants. II. Clinical and serological observations during seroconversion. Int J Cancer. 1978;22:244–50. doi: 10.1002/ijc.2910220305. [DOI] [PubMed] [Google Scholar]
- 8.Chan KH, Tam JS, Peiris JS, Seto WH, Ng MH. Epstein-Barr virus (EBV) infection in infancy. J Clin Virol. 2001;21:57–62. doi: 10.1016/s1386-6532(01)00149-4. [DOI] [PubMed] [Google Scholar]
- 9.Jenson H, McIntosh K, Pitt J, et al. Natural history of primary Epstein-Barr virus infection in children of mothers infected with human immunodeficiency virus type 1. J Infect Dis. 1999;179:1395–404. doi: 10.1086/314764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snow RW, Omumbo JA, Lowe B, et al. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet. 1997;349:1650–4. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- 11.Mbogo CN, Snow RW, Khamala CP, et al. Relationships between Plasmodium falciparum transmission by vector populations and the incidence of severe disease at nine sites on the Kenyan coast. Am J Trop Med Hyg. 1995;52:201–6. doi: 10.4269/ajtmh.1995.52.201. [DOI] [PubMed] [Google Scholar]
- 12.Rainey JJ, Omenah D, Sumba PO, Moormann AM, Rochford R, Wilson ML. Spatial clustering of endemic Burkitt’s lymphoma in high-risk regions of Kenya. Int J Cancer. 2007;120:121–7. doi: 10.1002/ijc.22179. [DOI] [PubMed] [Google Scholar]
- 13.Moormann AM, Chelimo K, Sumba OP, et al. Exposure to holoendemic malaria results in elevated Epstein-Barr virus loads in children. J Infect Dis. 2005;191:1233–8. doi: 10.1086/428910. [DOI] [PubMed] [Google Scholar]
- 14.de-Thé G. Is Burkitt’s lymphoma related to perinatal infection by Epstein-Barr virus? Lancet. 1977;1:335–8. doi: 10.1016/s0140-6736(77)91137-0. [DOI] [PubMed] [Google Scholar]
- 15.Moormann AM, Chelimo K, Sumba PO, Tisch DJ, Rochford R, Kazura JW. Exposure to holoendemic malaria results in suppression of Epstein-Barr virus-specific T cell immunosurveillance in Kenyan children. J Infect Dis. 2007;195:799–808. doi: 10.1086/511984. [DOI] [PubMed] [Google Scholar]
- 16.Hermsen CC, Telgt DS, Linders EH, et al. Detection of Plasmodium falciparum malaria parasites in vivo by real-time quantitative PCR. Mol Biochem Parasitol. 2001;118:247–51. doi: 10.1016/s0166-6851(01)00379-6. [DOI] [PubMed] [Google Scholar]
- 17.Fachiroh J, Paramita DK, Hariwiyanto B, et al. Single-assay combination of Epstein-Barr virus (EBV) EBNA1- and viral capsid antigen-p18-derived synthetic peptides for measuring anti-EBV immunoglobulin G (IgG) and IgA antibody levels in sera from nasopharyngeal carcinoma patients: options for field screening. J Clin Microbiol. 2006;44:1459–67. doi: 10.1128/JCM.44.4.1459-1467.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Middeldorp JM, Meloen RH. Epitope-mapping on the Epstein-Barr virus major capsid protein using systematic synthesis of overlapping oligopeptides. J Virol Methods. 1988;21:147–59. doi: 10.1016/0166-0934(88)90061-4. [DOI] [PubMed] [Google Scholar]
- 19.van Grunsven WM, Spaan WJ, Middeldorp JM. Localization and diagnostic application of immunodominant domains of the BFRF3-encoded Epstein-Barr virus capsid protein. J Infect Dis. 1994;170:13–9. doi: 10.1093/infdis/170.1.13. [DOI] [PubMed] [Google Scholar]
- 20.Stata statistical software. Release 11 ed. College Station, TX: StataCorp, LP; 2009. [Google Scholar]
- 21.Menge DM, Ernst KC, Vulule JM, Zimmerman PA, Guo H, John CC. Microscopy underestimates the frequency of Plasmodium falciparum infection in symptomatic individuals in a low transmission highland area. Am J Trop Med Hyg. 2008;79:173–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Rickinson AB, Kieff E. Epstein-Barr virus. In: Knipe DM, Howley PM, editors. Fields virology. 5th ed. Vol. 2. Philadelphia, PA: Lippincott & Williams; 2006. pp. 2655–700. [Google Scholar]
- 23.Huang LM, Lee CY, Chang MH, Wang JD, Hsu CY. Primary infections of Epstein-Barr virus, cytomegalovirus, and human herpesvirus-6. Arch Dis Child. 1993;68:408–11. doi: 10.1136/adc.68.3.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ptaschinski C, Rochford R. Infection of neonates with murine gammaherpesvirus 68 results in enhanced viral persistence in lungs and absence of infectious mononucleosis syndrome. J Gen Virol. 2008;89:1114–21. doi: 10.1099/vir.0.83470-0. [DOI] [PubMed] [Google Scholar]
- 25.Cote PJ, Korba BE, Miller RH, et al. Effects of age and viral determinants on chronicity as an outcome of experimental woodchuck hepatitis virus infection. Hepatology. 2000;31:190–200. doi: 10.1002/hep.510310128. [DOI] [PubMed] [Google Scholar]
- 26.Tu W, Chen S, Sharp M, et al. Persistent and selective deficiency of CD4+ T cell immunity to cytomegalovirus in immunocompetent young children. J Immunol. 2004;172:3260–7. doi: 10.4049/jimmunol.172.5.3260. [DOI] [PubMed] [Google Scholar]
- 27.Rasti N, Falk KI, Donati D, et al. Circulating Epstein-Barr virus in children living in malaria-endemic areas. Scand J Immunol. 2005;61:461–5. doi: 10.1111/j.1365-3083.2005.01589.x. [DOI] [PubMed] [Google Scholar]
- 28.Piriou E, Kimmel R, Chelimo K, et al. Serological evidence for long-term Epstein-Barr virus reactivation in children living in a holoendemic malaria region of Kenya. J Med Virol. 2009;81:1088–93. doi: 10.1002/jmv.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chene A, Donati D, Guerreiro-Cacais AO, et al. A molecular link between malaria and Epstein-Barr virus reactivation. PLoS Pathog. 2007;3:e80. doi: 10.1371/journal.ppat.0030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asito AS, Piriou E, Odada PS, et al. Elevated anti-Zta IgG levels and EBV viral load are associated with site of tumor presentation in endemic Burkitt’s lymphoma patients: a case control study. Infect Agent Cancer. 2010;5:13. doi: 10.1186/1750-9378-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens SJ, Pronk I, Middeldorp JM. Toward standardization of Epstein-Barr virus DNA load monitoring: unfractionated whole blood as preferred clinical specimen. J Clin Microbiol. 2001;39:1211–6. doi: 10.1128/JCM.39.4.1211-1216.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly GL, Rickinson AB. Burkitt lymphoma: revisiting the pathogenesis of a virus-associated malignancy. Hematol Am Soc Hematol Educ Program. 2007:277–84. doi: 10.1182/asheducation-2007.1.277. [DOI] [PubMed] [Google Scholar]
- 33.Hochberg D, Souza T, Catalina M, Sullivan JL, Luzuriaga K, Thorley-Lawson DA. Acute infection with Epstein-Barr virus targets and overwhelms the peripheral memory B-cell compartment with resting, latently infected cells. J Virol. 2004;78:5194–204. doi: 10.1128/JVI.78.10.5194-5204.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]