Abstract
Background. When compared with Mycobacterium tuberculosis, individuals that live in the same household as an active case of smear-positive pulmonary tuberculosis exposed to M. africanum progress less frequently to active disease within 2 years. A putative ESX-1 secretion apparatus member, Rv3879c, is mutated in M. africanum, and individuals infected with M. africanum less frequently demonstrate T-cell responses to the ESX-1–secreted virulence factor ESAT-6 than those infected with M. tuberculosis. We hypothesized that less frequent progression is caused by impaired secretion of ESAT-6.
Methods. We analyzed in vivo growth and in vitro secretion of ESAT-6 and CFP-10, comparing M. tuberculosis to M. africanum and a strain of M. africanum complemented with M. tuberculosis Rv3879c.
Results. ESAT-6 and CFP-10 secretion were similar for all strains, although these were enriched in M. africanum cell lysates, suggesting a modest ESX-1 secretion defect unrelated to the Rv3879c mutation. In mice, M. africanum demonstrated smaller bacterial population sizes than M. tuberculosis but similar numbers and frequencies of ESAT-6–responsive T cells in the lungs.
Conclusions. These results confirm impaired fitness of M. africanum in vivo and indicate that Rv3879c is not required for secretion of ESAT-6 or for its presentation as an antigen to T cells in vivo.
Mycobacterium africanum causes up to 50% of pulmonary tuberculosis in West Africa [1, 2]. A distinct lineage within the M. tuberculosis complex, M. africanum is classified by spoligotype analysis and large-sequence polymorphisms (LSPs) [3–5]. Strains previously classified biochemically as West African M. africanum (type I) share a ΔRD9 LSP and cluster phylogenetically with animal strains, including M. bovis and M. microti. West African–type strains are further subclassified into MAF1 (ΔRD711) and MAF2 (ΔRD7, RD8, RD10, and RD702). The present study concerns West African strains of M. africanum, utilizing the MAF2 clinical isolate GM041182, which has recently undergone genome sequencing.
In West Africa, the prevalence of M. africanum MAF1 and MAF2 varies geographically. In The Gambia, a study of 386 smear-positive tuberculosis cases revealed a prevalence of M. africanum of 38.4%, with all M. africanum isolates categorized as MAF2 [3]. We previously reported that, although transmission rates of M. tuberculosis and M. africanum were similar, Gambian patients infected with M. africanum were less likely to develop active pulmonary disease than M. tuberculosis–infected patients during a 2-year follow-up [6]. This and the finding that M. africanum is more prevalent among malnourished, human immunodeficiency virus (HIV)–infected, and older individuals suggest that M. africanum is less virulent and more opportunistic than M. tuberculosis [1].
Mycobacterium africanum–infected individuals also have lower-frequency T-cell responses to the secreted antigen and virulence factor ESAT-6 (EsxA/Rv3875), secreted by the ESX-1 type VII secretion system [7]. In contrast, T-cell responses to another ESX-1 product, CFP-10 (EsxB/Rv3874), generally thought to be cosecreted with ESAT-6 [8], are similar among those with M. africanum and M. tuberculosis [7]. The components of ESX-1 are encoded in the RD1 locus (Rv3871–Rv3879c) and are absent from M. bovis Bacille Calmette-Guérin [9]. Because of its role in virulence [10], ESX-1 is the subject of genetic and biochemical studies that have suggested a functional model [11] and revealed the genes within the RD1 locus required for ESAT-6 and CFP-10 secretion [10, 12, 13]. The present study investigated the role of the RD1-encoded protein Rv3879c/EspK in ESX-1 secretion by M. africanum, which carries a single nucleotide deletion at base pair 427 of the Rv3879c open reading frame, resulting in a premature stop codon [7]. We hypothesized that the Rv3879c mutation results in defective secretion of ESAT-6 by M. africanum, underlying the differences in ESAT-6–specific T-cell responses and disease outcome among M. africanum–infected patients.
MATERIAL AND METHODS
Mycobacteria
M. tuberculosis H37Rv was obtained from American Type Culture Colletion; M. africanum GM041182 was obtained from an HIV-uninfected male with pulmonary tuberculosis in The Gambia. Mycobacterial strains were grown in Middlebrook 7H9 liquid or 7H11 solid media supplemented with 10% v/v albumin, dextrose, and catalase. For liquid growth, cultures were grown at 37°C for >7 days with shaking and samples taken daily to measure optical density (OD). Samples were plated on solid media to calculate the correlation between OD and colony forming units (CFUs)/mL. Plates were routinely incubated for 21 days with longer incubations (approximately 28 days) for M. africanum.
Complementation of M. africanum With M. tuberculosis Rv3879c
A 3200-base-pair region containing the Rv3879c open reading frame and 438 base pairs upstream from the start codon was amplified by polymerase chain reaction (PCR) from M. tuberculosis genomic DNA, ligated into the pMV306 integrating plasmid, and electroporated into M. africanum. Kanamycin-resistant clones were verified for presence of the complementing construct by PCR.
In Vitro Secretion Assays
To quantitate secretion of specific proteins, liquid cultures were grown to early-midlogarithmic phase as measured by OD and centrifuged at 2000g for 10 minutes. Supernatants containing secreted proteins were passed through 0.2 μm syringe filters to obtain culture filtrate (CF). Cell pellets were washed twice in cold phosphate-buffered saline to eliminate residual extracellular proteins, then underwent bead beating with 0.1 mm zirconia beads to lyse bacterial cells. Bead-beating supernatants were obtained as cell lysates (CLs), and total protein was quantified. CF or CL were mixed with sodium dodecyl sulfate (SDS) gel loading buffer, heated at 95°C for 5 minutes, and stored at −80°C.
Immunoblots
Samples were separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE; 10% acrylamide for Ag85 and GroEL, 18% for ESAT-6 and CFP-10) with loading normalized to the culture OD at which the sample was obtained. After SDS-PAGE, proteins were transferred to 0.2 μm nitrocellulose membranes, and membranes were probed with the following antibodies: ESAT-6: mouse monoclonal Ab HYB076-08 (Abcam); CFP-10: 05-CFP10.10; GroEL: 03.IT.29.65; and Ag85 rabbit polyclonal generated against recombinant Ag85B (our laboratory). Horseradish peroxidase–conjugated goat antimouse or antirabbit immunoglobulin G (IgG) was used to detect bound primary antibodies. For CF blots, equal loading of samples was verified by the intensity of the Ag85 A/C band (non–ESX-1 mediated secretion), and for CL blots, it was verified by the intensity of GroEL1 (intracellular protein). To ensure that the protein loaded in final blots did not exceed the linear range of detection, preliminary blots with serial dilutions of CF were performed. Specificity of CF blots for detection of secreted proteins as opposed to products of bacterial cell lysis was verified by the absence of detectable GroEL1 in CF samples.
Electron Microscopy
Mycobacteria were centrifuged and resuspended in 2% paraformaldehyde and 2% glutaraldehyde in 50 mM cacodylate buffer (pH 7.2) overnight at room temperature. Electron microscopy was carried out by staff of the New York University School of Medicine Electron Microscopy core facility. Average bacterial cell size was determined using ImageJ software by analysis of >5 fields of negative-stained bacteria from M. tuberculosis and M. africanum samples, with at least 10 bacilli per field measured along the long axis.
Aerosol Infection of C57BL/6 Mice, Tissue Processing, and Flow Cytometry
Aerosol infection of C57BL/6 mice, tissue processing, and flow cytometry were performed as previously described [14, 15]. All animal experiments were done in accordance with procedures approved by the New York University School of Medicine Institutional Animal Care and Use Committee of the National Institutes of Health under the Assurance of Compliance Number A3435-01. Cell suspensions were stained using the following fluorescently labeled antibodies (Biolegend, BD Pharmingen, or eBioscience): anti-CD3 PerCP, anti-CD8 PE, anti-CD4 (L3T4) Pacific Blue, anti–interferon γ (IFN-γ; XMG1.2) APC, and rat IgG1 APC isotype control. Flow cytometry was performed and analyzed as described [14, 15].
T-Cell Restimulation
To quantitate antigen-specific T-cell responses in the lungs of mice infected with M. tuberculosis or M. africanum, 3 × 106 lung cells were resuspended in Roswell Park Memorial Institute (RPMI) 1640 medium with 10% fetal calf serum and cultured at 37°C with 5.0% carbon dioxide for 4 hours in 96-well plates. Cultures were supplemented with Brefeldin A (10 μg/mL) and either anti-CD3/anti-CD28 or peptides corresponding to C57BL/6 mouse T-cell epitopes ESAT-61–20 or Ag85B240–254.
Statistical Analyses
To determine statistical significance when comparing 2 groups of mice, the 2-tailed Student t test was routinely used. To compare the growth rate of M. tuberculosis and M. africanum in vitro, a nonlinear regression analysis (curve fit) with F test was used to determine whether a single curve could account for both data sets.
RESULTS
In Vitro Growth of M. tuberculosis and M. africanum
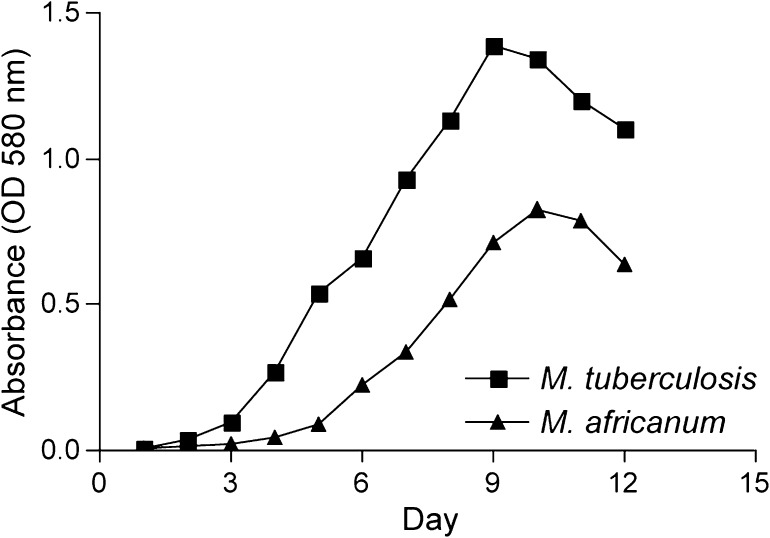
To test the hypothesis that truncation of the Rv3879c protein in M. africanum causes defective secretion of ESAT-6, we complemented M. africanum strain GM041182 with an integrating plasmid containing full-length M. tuberculosis Rv3879c under control of its native promoter (M. africanum Rv3879c complement). We first compared ESAT-6 secretion by this strain, by the parental M. africanum GM041182, and M. tuberculosis (H37Rv) during conditions of shaking liquid culture. We inoculated Middlebrook 7H9 with either M. tuberculosis or M. africanum and measured bacterial growth by OD (absorbance at 580 nm) daily for 14 days (Figure 1). In accordance with reports using solid medium [16], we observed slower growth during in vitro culture for M. africanum than M. tuberculosis (calculated doubling times: M. africanum, 23.9 hours; M. tuberculosis, 18.15; n = 2 cultures for each lineage) as well as entry into stationary phase at a lower optical density (M. africanum, approximately 0.7; M. tuberculosis, approximately 1.3). In vitro growth of the M. africanum Rv3879c complement strain was indistinguishable from wild-type M. africanum GM041182 (not shown). The in vitro growth defect of the M. africanum strains is likely to be at least partially explained by a mutation in the pyruvate kinase encoding gene pykA, which is shared by all members of the mycobacterial lineage carrying the ΔRD9 LSP, including M. bovis [17]. Although M. africanum strains grew more slowly than M. tuberculosis, all strains demonstrated characteristic growth with identifiable lag phase, exponential (log) phase, stationary phase, and death phase.
Figure 1.
In vitro growth of Mycobacterium tuberculosis and M. africanum. Growth kinetics of M. tuberculosis H37Rv and M. africanum GM041182 grown in Middlebrook 7H9 liquid medium, as measured by optical density (OD) of culture (absorbance at 580 nm) over time. Graph depicts a representative culture of each lineage from n > 3 cultures.
Comparison of ESAT-6 Secretion by M. africanum and M. tuberculosis
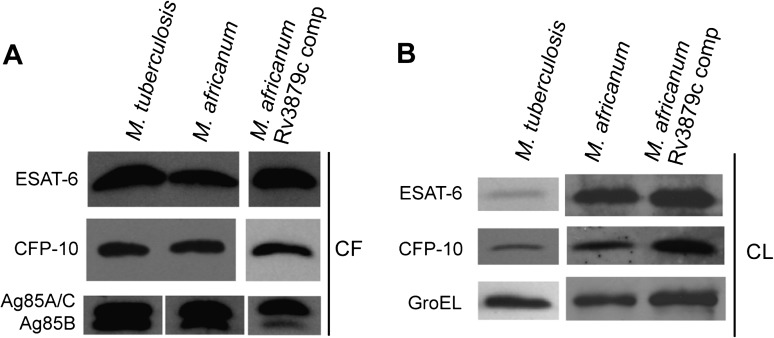
To compare secretion of ESAT-6 in vitro by these strains with different growth kinetics, we generated culture filtrates and bacterial lysates at points that corresponded to logarithmic phase growth of each strain (M. africanum, 0.2–0.5; M. tuberculosis, 0.5–1.0). On CF, we probed immunoblots with antibodies to the ESX-1 secretion products ESAT-6 and CFP-10. Because albumin in the culture medium prevented quantitation of total protein in CF, the amount of CF loaded for each strain was normalized to the OD of the culture at the time point when the CF sample was obtained. Equal loading of protein between strains was verified by immunoblot of Ag85A, -B, and -C, exported by the SecA secretion apparatus. ESAT-6 and CFP-10 blots revealed indistinguishable quantities of these proteins in CF for all 3 strains (Figure 2A), indicating that ESX-1–dependent secretion is comparable for M. tuberculosis and both M. africanum strains. For Ag85A and Ag85C, whose similar size result in a single band migrating at approximately 32 kDa, secretion was also comparable for all strains examined (Figure 2A), confirming equivalent CF loading between strains. In contrast, we observed less Ag85B, migrating at approximately 30 kDa, in CF of both strains of M. africanum when compared with M. tuberculosis (Figure 2A). We confirmed that proteins detected in CF samples were products of secretion and not cell lysis by probing CF blots with an antibody to the cell-associated protein GroEL1, which was below the limit of detection for all strains (not shown).
Figure 2.
Secretion of antigenic proteins by Mycobacterium tuberculosis and M. africanum. A, Immunoblot of ESAT-6, CFP-10, and Ag85 family members in the culture filtrate (CF), comparing H37Rv, M. africanum GM041182, and the M. africanum Rv3879c complemented strain. Gel loading for CF blots was normalized to culture optical density. B, Immunoblot of ESAT-6, CFP-10, and GroEL of cell lysates (CL) from culture cell pellets, comparing the same strains as in (A). Gel loading for CL blots was normalized to intensity of band for GroEL, a cell-associated housekeeping gene.
In CL of both M. africanum strains, we detected enrichment of the ESX-1 products ESAT-6 and CFP-10 when compared with M. tuberculosis, despite similar amounts of GroEL (Figure 2B). That similar quantities of ESAT-6 and CFP-10 are secreted by M. africanum and M. tuberculosis while these proteins are enriched in M. africanum bacterial cells suggests that M. africanum may synthesize higher quantities of these proteins in order to assure their adequate secretion, which is consistent with a lower efficiency of secretion. However, contrary to our initial hypothesis, because the M. africanum Rv3879c complemented strain behaved similarly to wild type M. africanum in both CF and CL assays, this apparent lower efficiency of ESAT-6 and CFP-10 secretion is not the result of truncation of Rv3879c.
M. africanum Bacilli Grown in Broth Are Larger Than M. tuberculosis Bacilli
M. africanum strains demonstrated substantially different in vitro growth characteristics than M. tuberculosis as measured by culture OD. Because gel loading in the previous experiments was normalized to the optical density of cultures, we investigated the possibility that size differences between M. tuberculosis and M. africanum might result in underestimation of the number of M. africanum bacilli contributing to protein secretion at a given OD. If this were the case, normalization to OD could mask an ESAT-6 secretion defect in M. africanum. To test this, we cultured M. africanum and M. tuberculosis in liquid media, obtained samples at various OD readings, and plated the samples on 7H11 solid media to enumerate CFUs. This analysis revealed an average bacterial concentration (CFUs/mL) to OD ratio of 2.8 ± 0.27 × 107 (mean ± standard error of mean [SEM]) for M. africanum and 1.1 ± 0.03 × 108 (mean ± SEM) for M. tuberculosis (P < .001). This indicates that when gel loading is normalized to OD, fewer bacteria contribute to protein secretion in M. africanum cultures than in M. tuberculosis cultures.
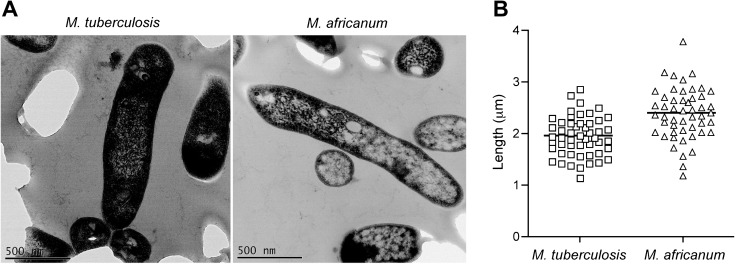
This result suggested that a given concentration of bacteria would correspond to a higher OD observed for M. africanum. Consistent with this observation, electron micrographs revealed a larger mean length of M. africanum bacilli (2.39 ± 0.07 μm) when compared with M. tuberculosis (1.95 ± 0.05 μm; P < .001) (Figure 3A and 3B). This indicates that the similarity of CFP-10 and ESAT-6 band intensity in CF blots is not attributable to a greater number of M. africanum bacilli contributing to protein secretion. Rather, these results suggest that M. africanum may actually secrete more ESAT-6 per bacillus than M. tuberculosis, contrary to the hypothesis that ESAT-6 secretion is defective in M. africanum.
Figure 3.
Size comparison of Mycobacterium tuberculosis and M. africanum. A, Electron micrographs of M. tuberculosis and M. africanum grown in liquid culture, depicting the larger size (B) of M. africanum bacilli. Bacterial size comparison was performed using ImageJ software to measure the lengths of >50 bacteria from each lineage.
Impaired Fitness of M. africanum in Mice
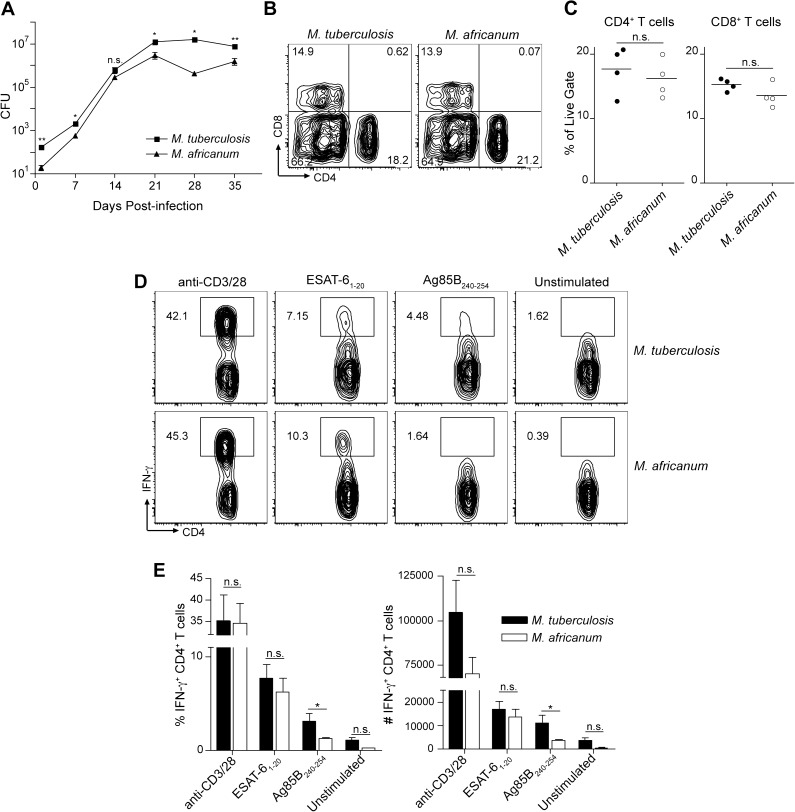
Patients infected with M. africanum are less likely to progress to active tuberculosis than patients infected with M. tuberculosis when assessed for the first 2 years after infection. To understand the underlying mechanisms, we infected immunocompetent C57BL/6 mice with a low (approximately 100 CFUs) aerosol dose of either M. africanum or M. tuberculosis. Despite a similar initial number of bacteria present in the lungs of both groups (M. africanum, 65 ± 14; M. tuberculosis, 44 ± 10; P = .29), 30 days postinfection, we found 4.7-fold (P = .017) fewer bacteria in lungs of M. africanum–infected mice (not shown). The number of bacteria in the spleen, although also lower for M. africanum, was not statistically different from that of M. tuberculosis. In a time course, ranging from day 1 to day 35 postinfection, we also observed slower rates of M. africanum growth, resulting in a smaller bacterial population size in the lungs throughout the infection (Figure 4A). These results suggest that M. africanum is attenuated for growth and/or persistence in vivo and may help explain the difference in disease progression among patients infected with either lineage. The results are also consistent with our previous observations that M. africanum behaves more opportunistically than M. tuberculosis; impaired fitness of M. africanum may prevent this species from causing disease in otherwise healthy individuals as frequently as does M. tuberculosis.
Figure 4.
Impaired fitness of Mycobacterium africanum in vivo. A, C57BL/6 mice were infected with either M. tuberculosis or M. africanum, and the number of bacteria in the lungs throughout infection was determined by diluting lung homogenates and plating on 7H11 agar plates. *P <.05; **P < .005. B and C, The frequency of CD4+ or CD8+ T cells among the live, leukocyte gate in the lungs of mice infected with either M. tuberculosis or M. africanum. Flow cytometry plots show a representative mouse from n = 4 mice per group. D, Cells from the lungs of mice infected with M. tuberculosis or M. africanum were restimulated ex vivo with either the pan–T cell agonist anti-CD3/28, ESAT-61–20 peptide, Ag85B peptide 25, or media alone. Flow cytometry plots show CD3+, CD4+ cells from the lungs of 1 representative well from each infection/restimulation group. E, Graphs indicate the mean frequency and total number of IFN-γ± CD4± cells from the lungs of n = 4 mice per condition. Error bars indicate mean ± SEM. Asterisks indicate statistical significance of differences in frequency of IFN-γ+ cells detected among 1 cell type between different treatment groups: *P < .05; **P < .005. Abbreviations: CFUs, colony-forming units; IFN-γ, interferon γ; NS, not significant; SEM, standard error of the mean.
T-Cell Responses to Mycobacterial Antigens in M. africanum Infection of Mice
Because M. africanum–infected patients are less likely to have positive enzyme-linked immunosorbent spot (ELISPOT) assay results for ESAT-6 [7], we evaluated T cells from infected mice for responses to ESAT-6 and Ag85B. Recruitment of effector T cells to the lungs of mice infected with M. africanum or M. tuberculosis was similar, with a larger number of CD4+ than CD8+ T cells in both groups of mice (Figure 4B and 4C), confirming that the immune response to both species was qualitatively comparable. Furthermore, CD4+ T cells from the lungs in both infections were similar in terms of baseline unstimulated activation and responses to nonspecific stimulation via the T-cell receptor agonist anti-CD3/CD28, as measured by intracellular IFN-γ staining (Figure 4D and 4E). We also observed similar responses in CD4+ T cells from both infections to ESAT-6 (Figure 4D and 4E), consistent with our in vitro observation that similar quantities of ESAT-6 are secreted by M. tuberculosis and M. africanum (Figure 2A). Also consistent with our in vitro secretion studies, which showed less Ag85B in CF from M. africanum (Figure 2A), we observed a lower frequency and number of Ag85B responsive CD4+ T cells in the M. africanum infection group than the M. tuberculosis infection group (Figure 4D and 4E). The similar response of CD4+ T cells to ESAT-6 in both groups implies that host factors account for the difference in T-cell responses to this antigen in human patients. These in vivo results verify our in vitro secretion assays and further suggest that differences in ESAT-6 secretion do not underlie the impaired fitness of M. africanum in vivo.
DISCUSSION
In this study, we compared M. africanum and M. tuberculosis in vitro and in vivo to understand the differences in the epidemiology and clinical course of tuberculosis caused by these closely related pathogens. We specifically investigated the role of ESX-1–dependent secretion of ESAT-6 and CFP-10 in the virulence of these lineages and as a determinant of the magnitude of adaptive immune responses. Despite our observation that patients infected with M. africanum have lower frequency ELISPOT responses to ESAT-6 [7], we observed similar in vitro secretion of ESAT-6 in both lineages and a similar frequency of CD4+ T cells specific for ESAT-6 in the lungs of mice infected with either lineage. Consistent with evidence that M. africanum–infected individuals are less likely to progress to active disease within 2 years of exposure [6], M. africanum was attenuated during in vivo mouse infection, with a smaller bacterial burden in the lungs.
Contrary to our hypothesis that a mutation in Rv3879c causes defective ESAT-6 secretion by M. africanum, we observed indistinguishable levels of ESAT-6 and CFP-10 in culture supernatants from M. tuberculosis and M. africanum. Moreover, we found no difference in ESAT-6 secretion by wild-type M. africanum or M. africanum complemented with the functional M. tuberculosis allele of Rv3879c. Although our findings are consistent with evidence that Rv3879c is dispensable for ESAT-6 secretion in M. bovis [18], other studies demonstrated that the Rv3879c homolog is required for secretion of ESAT-6 in M. marinum [12]. The M. marinum Rv3879c homolog was also observed to interact with the secreted protein EspB and was required for its secretion [19].
We indirectly verified secretion of ESAT-6 by M. africanum in vivo by comparing the magnitude of T-cell responses specific for this antigen in the lungs of mice infected with M. africanum or M. tuberculosis and observed no difference in the 2 groups. These findings weigh against the possibility that differences in ESAT-6 secretion account for the difference in ESAT-6 ELISPOT responses among patients infected with M. tuberculosis or M. africanum. However, we did observe a difference in secretion of Ag85B, with M. africanum secreting less of this protein than M. tuberculosis. The lower frequency of Ag85B-specific T-cell responses in mice infected with M. africanum is consistent with a model in which differences in antigen secretion influence the immune response. In contrast to Ag85B, levels of Ag85A and Ag85C secretion were similar between the 2 strains. Because Ag85A, Ag85B, and ESAT-6 are all targets of novel vaccines against tuberculosis, these new data suggest that although vaccines targeting Ag85B may be less effective in preventing M. africanum infection, those targeting Ag85A and/or ESAT-6 could be similarly effective.
Although we found that the extent of protein secretion can influence immunogenicity, our results also imply that other factors underlie the impaired ESAT-6-specific T-cell response in M. africanum–infected patients. For instance, although patients infected with M. africanum or M. tuberculosis had similar numbers of bacteria in sputum [2], impaired fitness of M. africanum may result in lower total bacterial burdens, as we observed in mice. Alternatively, one recent study suggests that ancient lineages within the M. tuberculosis complex induce hyperinflammatory responses when compared with modern lineages [20]. Mycobacterium africanum may be better controlled by innate immunity, leading to fewer bacteria and lower adaptive immune responses. Furthermore, the lower-frequency ELISPOT response to ESAT-6 has not been observed in all M. africanum cases and contacts and could therefore be limited to subtypes within the M. africanum lineage. Although the Rv3879c mutation is common to all M. africanum isolates examined and clearly does not explain the difference, comparison of secretion of ESAT-6 by M. africanum strains with distinct phenotypes may clarify the mechanism responsible.
If M. africanum is attenuated in vivo, it is unclear why the prevalence of M. africanum is as high as 38% in The Gambia and remains relatively constant [2]. Our new data suggest that host-intrinsic factors among M. africanum–infected individuals result in differences in the immune response to ESAT-6 and the rate of progression to active disease. These include a preference of M. africanum for hosts with certain genetic backgrounds or with impaired immunity. Although the former hypothesis could help explain the regional exclusivity of M. africanum, it is not supported by current epidemiology, which has yet to detect a specific West African ethnicity with enriched prevalence of M. africanum infection (authors’ unpublished data). Regarding the latter hypothesis, our previous studies identified a preference of M. africanum for those with advanced age, malnutrition, and HIV infection. However, the last of these was excluded as a contributor to the difference in T-cell response to ESAT-6 among M. africanum–infected patients [7]. Furthermore, although differences in host genetics and immunity may underlie phenotypes observed in patients, we observed moderately impaired fitness of M. africanum in inbred mice, which minimized host-intrinsic contributions. These data suggest that host variability may contribute to, but is unlikely to fully account for, the differences between tuberculosis caused by these 2 distinct lineages.
In conclusion, we have determined that M. africanum is attenuated relative to M. tuberculosis in vivo, but we excluded the possibility that differences in ESAT-6 secretion account for this and the observation that patients infected with M. africanum are less likely to progress to active disease than patients infected with M. tuberculosis. Future studies should identify the reasons for the geographical isolation of M. africanum, as well as the bacterial and host factors that contribute to the differences between the 2 lineages in tuberculosis pathogenesis.
Notes
Acknowledgments.
We thank Peter Lopez at the New York University Cancer Institute Flow Cytometry and Cell Sorting facility for expert assistance.
Financial support.
This work was supported in part by grants from the National Institutes of Health (R01 AI051242, R01 AI084041, and R01 AI090928 to J. D. E; 5K01 TW006083-06 to B. C. D. J.; and F30 HL096342 to T. D. B.) and the American Lung Association (RG-167675 to B. C. D. J.).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.de Jong BC, Adetifa I, Walther B, et al. Differences between tuberculosis cases infected with Mycobacterium africanum, West African type 2, relative to Euro-American Mycobacterium tuberculosis: an update. FEMS Immunol Med Microbiol. 2010;58:102–5. doi: 10.1111/j.1574-695X.2009.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Jong BC, Antonio M, Gagneux S. Mycobacterium africanum—review of an important cause of human tuberculosis in West Africa. PLoS Negl Trop Dis. 2010;4:e744. doi: 10.1371/journal.pntd.0000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Jong BC, Antonio M, Awine T, et al. Use of spoligotyping and large sequence polymorphisms to study the population structure of the Mycobacterium tuberculosis complex in a cohort study of consecutive smear-positive tuberculosis cases in The Gambia. J Clin Microbiol. 2009;47:994–1001. doi: 10.1128/JCM.01216-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mostowy S, Onipede A, Gagneux S, et al. Genomic analysis distinguishes Mycobacterium africanum. J Clin Microbiol. 2004;42:3594–9. doi: 10.1128/JCM.42.8.3594-3599.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hershberg R, Lipatov M, Small PM, et al. High functional diversity in Mycobacterium tuberculosis driven by genetic drift and human demography. PLoS Biol. 2008;6:e311. doi: 10.1371/journal.pbio.0060311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jong BC, Hill PC, Aiken A, et al. Progression to active tuberculosis, but not transmission, varies by Mycobacterium tuberculosis lineage in The Gambia. J Infect Dis. 2008;198:1037–43. doi: 10.1086/591504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jong BC, Hill PC, Brookes RH, et al. Mycobacterium africanum elicits an attenuated T cell response to early secreted antigenic target, 6 kDa, in patients with tuberculosis and their household contacts. J Infect Dis. 2006;193:1279–86. doi: 10.1086/502977. [DOI] [PubMed] [Google Scholar]
- 8.Simeone R, Bottai D, Brosch R. ESX/type VII secretion systems and their role in host-pathogen interaction. Curr Opin Microbiol. 2009;12:4–10. doi: 10.1016/j.mib.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Abdallah AM, Gey van Pittius NC, Champion PA, et al. Type VII secretion—mycobacteria show the way. Nat Rev Microbiol. 2007;5:883–91. doi: 10.1038/nrmicro1773. [DOI] [PubMed] [Google Scholar]
- 10.Brodin P, Majlessi L, Marsollier L, et al. Dissection of ESAT-6 system 1 of Mycobacterium tuberculosis and impact on immunogenicity and virulence. Infect Immun. 2006;74:88–98. doi: 10.1128/IAI.74.1.88-98.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bitter W, Houben EN, Bottai D, et al. Systematic genetic nomenclature for type VII secretion systems. PLoS Pathog. 2009;5:e1000507. doi: 10.1371/journal.ppat.1000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao LY, Guo S, McLaughlin B, Morisaki H, Engel JN, Brown EJ. A mycobacterial virulence gene cluster extending RD1 is required for cytolysis, bacterial spreading and ESAT-6 secretion. Mol Microbiol. 2004;53:1677–93. doi: 10.1111/j.1365-2958.2004.04261.x. [DOI] [PubMed] [Google Scholar]
- 13.Guinn KM, Hickey MJ, Mathur SK, et al. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol Microbiol. 2004;51:359–70. doi: 10.1046/j.1365-2958.2003.03844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf AJ, Linas B, Trevejo-Nunez GJ, et al. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol. 2007;179:2509–19. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- 15.Bold TD, Banaei N, Wolf AJ, Ernst JD. Suboptimal activation of antigen-specific CD4 effector cells enables persistence of M. tuberculosis in vivo. PLoS Pathog. 2011;7:e1002063. doi: 10.1371/journal.ppat.1002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grosset J, Decroix G, Sors C. Tuberculosis due to Mycobacterium africanum in African negroes in the Paris area. Rev Tuberc Pneumol (Paris) 1971;35:430–6. [PubMed] [Google Scholar]
- 17.Keating LA, Wheeler PR, Mansoor H, et al. The pyruvate requirement of some members of the Mycobacterium tuberculosis complex is due to an inactive pyruvate kinase: implications for in vivo growth. Mol Microbiol. 2005;56:163–74. doi: 10.1111/j.1365-2958.2005.04524.x. [DOI] [PubMed] [Google Scholar]
- 18.Inwald J, Jahans K, Hewinson RG, Gordon SV. Inactivation of the Mycobacterium bovis homologue of the polymorphic RD1 gene Rv3879c (Mb3909c) does not affect virulence. Tuberculosis (Edinb) 2003;83:387–93. doi: 10.1016/j.tube.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin B, Chon JS, MacGurn JA, et al. A mycobacterium ESX-1–secreted virulence factor with unique requirements for export. PLoS Pathog. 2007;3:e105. doi: 10.1371/journal.ppat.0030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Portevin D, Gagneux S, Comas I, Young D. Human macrophage responses to clinical isolates from the Mycobacterium tuberculosis complex discriminate between ancient and modern lineages. PLoS Pathog. 2011;7:e1001307. doi: 10.1371/journal.ppat.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]