Abstract
Background. Although liver disease commonly causes morbidity and mortality among human immunodeficiency virus (HIV)–infected individuals, data are limited on its prevalence in HIV monoinfection. We used the aspartate aminotransferase-to-platelet ratio index (APRI) as a surrogate marker of hepatic fibrosis to characterize liver disease in the Multicenter AIDS Cohort Study.
Methods. Men were categorized based on their HIV and viral hepatitis status: uninfected (n = 1170), HIV monoinfected (n = 509), viral hepatitis monoinfected (n = 74), and HIV–viral hepatitis coinfected (n = 66).
Results. The median APRI in the HIV-monoinfected group was similar to that in the hepatitis-monoinfected group (0.42 vs 0.43; P > .05), higher than in the uninfected group (0.42 vs 0.27; P < .001) but lower than in the coinfected group (0.42 vs 1.0; P < .001). On multivariable analysis, HIV infection (1.39-fold increase [FI]; P < .001), viral hepatitis infection (1.52-FI; P < .001), and the interaction between HIV and viral hepatitis infections were independently associated with a higher APRI (1.57-FI; P < .001). Among the HIV-infected men, viral hepatitis coinfection (2.34-FI; P < .001), HIV RNA ≥100 000 copies/mL (1.26-FI; P = .007), and CD4 count ≤200 cells/mL (1.23-FI; P = .022) were independently associated with a higher APRI.
Conclusions. HIV and viral hepatitis are independently associated with an increased APRI. Further studies are needed to understand the biological basis for the association between HIV and liver disease.
Chronic liver disease is the most common non-AIDS-related cause of death among individuals with human immunodeficiency virus (HIV) type 1 infection [1]. Coinfection with HIV and hepatitis C virus (HCV) or hepatitis B virus (HBV) is responsible for a large proportion of liver disease among HIV-infected individuals [1–3]. However, HIV-monoinfected individuals are also at risk for liver disease from a variety of other factors including opportunistic infections, AIDS-related neoplasms, alcohol and other substance abuse, nonalcoholic fatty liver disease, and medication-related hepatotoxicity [4]. Indeed, previous studies have demonstrated that 20%–75% of HIV-monoinfected individuals have elevated liver enzymes [5–7]. Recent data suggest that HIV can activate hepatic stellate cells and may be able to infect hepatocytes [8, 9], potentially leading to liver disease. Despite this, there are limited data on the prevalence of and risk factors for liver disease in HIV-infected individuals without viral hepatitis infection [10–12].
Although liver biopsy is clinically the gold standard for diagnosing and staging liver disease, it is not possible to perform liver biopsies in large cohort studies because of the associated costs and risks to the patient [13]. Noninvasive surrogates of hepatic fibrosis are useful for evaluating liver disease when a liver biopsy is not feasible. The aspartate aminotransferase (AST)-to-platelet ratio index (APRI) is a validated marker of hepatic fibrosis among HCV-infected individuals with and without HIV infection [14–16]. APRI also predicts liver-related mortality among both HCV-monoinfected and HIV-HCV–coinfected individuals [17]. Using an APRI cutoff of >1.5, the sensitivity and specificity of APRI for biopsy-confirmed significant fibrosis are 41% and 95%, respectively [14].
The objective of this study was to determine the prevalence of and risk factors associated with liver disease as measured by APRI among highly active antiretroviral therapy (HAART)-naive HIV-monoinfected participants enrolled in the Multicenter AIDS Cohort Study (MACS), which is a cohort of men who have sex with men (MSM) and who either have or are at risk for HIV infection. For comparison, we also assessed APRI among 3 other groups of MACS participants according to their HIV and hepatitis status.
PATIENTS AND METHODS
Study Population and Design
The MACS is an ongoing prospective cohort study, established in 1984, that enrolled HIV-infected and -uninfected MSM from 4 metropolitan areas in the United States (Baltimore, Maryland/Washington, D.C.; Chicago, Illinois; Pittsburgh, Pennsylvania; and Los Angeles, California) during 3 recruitment periods: 1984–1985, 1987–1990, and 2001–2003. Details of the MACS participant recruitment, study design, and characteristics of the cohort have been described elsewhere [18, 19]. Participants were followed every 6 months for interview, physical examination, laboratory studies, and collection of biological specimens for repository storage. At entry into the MACS, HIV antibody was tested and, if negative, was tested semiannually in each participant. Specimens within the first 2 years of entry into the MACS were tested for HCV antibody and hepatitis B surface antigen (HBsAg). Participants who were anti-HCV positive had HCV RNA testing performed.
This cross-sectional study compared APRI values among 4 groups of MACS participants: HIV- and viral hepatitis uninfected, HIV monoinfected, viral hepatitis monoinfected, and HIV–viral hepatitis coinfected. In order to examine liver disease prior to HAART, the visit 1 year before HAART initiation was selected for HIV-infected subjects who received HAART. For the HIV-uninfected subjects and the HIV-infected non-HAART initiators, we selected the corresponding visit by frequency matching to the calendar year of the visit for the HAART initiators. Men who were taking antiretroviral therapy (ART) that was not HAART were included. All participants provided informed consent, and the institutional review boards at each site approved the study.
Laboratory Testing and Definitions
HIV status was determined using enzyme immunoassay (EIA) and confirmed with Western blotting, as previously described [19]. HBsAg was performed with EIA (Abbot Laboratories). HCV antibody testing was done with a third-generation EIA (ADVIA Centura HCV assay). If positive, HCV RNA testing was performed in the same sample using the COBAS AmpliPrep Taqman HCV assay or the COBAS TaqMan HCV test (lower limit of detection, 50 IU/mL and 43 IU/mL, respectively; Roche Diagnostic Systems). Starting in 2001, AST and alanine aminotransferase (ALT) testing was performed at each visit. For subjects without an AST/ALT result from the selected visit, testing was performed at the Johns Hopkins Hospital clinical laboratory using stored serum or plasma specimens frozen at −70°C until use. Quality control testing demonstrated that AST testing of the thawed specimens was reliable, but the ALT values were often lower in thawed samples compared with fresh samples. Thus, our analysis focused on APRI, which uses AST. Chronic hepatitis B infection was defined as a positive HBsAg at the most recent visit. Chronic hepatitis C infection was defined as HCV antibody and HCV RNA positive. Viral hepatitis was defined as chronic infection with either hepatitis B or hepatitis C.
HAART was defined according to guidelines from the US Department of Health and Human Services/Kaiser Panel [20]. The following regimens were considered to be HAART: (1) ≥2 nucleoside reverse transcriptase inhibitors (NRTIs) in combination with at least 1 protease inhibitor (PI) or 1 nonnucleoside reverse transcriptase inhibitor (NNRTI); (2) 1 NRTI in combination with at least 1 PI and at least 1 NNRTI; (3) ritonavir and saquinavir in combination with 1 NRTI and no NNRTIs; and (4) abacavir or tenofovir with 3 NRTIs in the absence of both PIs and NNRTIs.
Body mass index (BMI) was calculated as (body weight [kg]/height [m])2 and was categorized by a modification of the World Health Organization’s classification system: normal, <25 kg/m2; overweight, 25–30 kg/m2; or obese, ≥30 kg/m2. Age was categorized based upon the age distribution in the cohort: <40 years, 40–50 years, and ≥50 years. Because heavy alcohol use is uncommon in the MACS, we defined heavy alcohol use as a self-reported average of >2 drinks per day over the prior 6 months. CD4 T-cell count was measured in all participants and HIV RNA was measured in those with HIV infection. To account for a potential cohort effect, the calendar year of the MACS visit used for APRI testing was included in the analysis and was dichotomized into before 2001 and after 2001. Other characteristics included in the analysis were age, race, and self-reported current injection drug use (IDU).
APRI was defined as 100 × (AST/upper limit of normal)/platelet count (109/L) [14]. The upper limit of normal (ULN) was 30 units/L.
Statistical Analysis
Differences in characteristics across groups were compared using Wilcoxon rank-sum test (2-group comparisons) and Kruskal–Wallis rank test (≥3 group comparisons). Pearson χ2 test was used to compare categorical variables across groups.
The primary analysis was a comparison of differences in APRI using linear regression. The APRI scores were natural log-transformed for the regression analyses because the empirical APRI distribution was right-skewed. Multivariable regression models were developed using covariates that were statistically significant (P < .05) on univariate analysis or that were selected a priori. Those included in the final model were: HIV status, viral hepatitis status, the interaction between HIV and viral hepatitis infections, age, year of MACS visit, heavy alcohol use, BMI, and race. The analysis was performed among the entire cohort and then restricted to the HIV-infected men to determine factors associated with liver disease in the setting of HIV. To facilitate interpretation of the results, coefficients were reported as a fold change in APRI comparing those with and without the covariate of interest.
A secondary analysis was performed using a proportional odds logistic regression model to determine the relative odds of a higher liver fibrosis category with the following preestablished cutoffs: APRI ≤0.5 (mild/no fibrosis), APRI 0.5–1.5 (intermediate fibrosis), and APRI >1.5 (significant fibrosis) [14]. Stata 11.2 (StataCorp) was used for the analysis.
RESULTS
Study Population
A total of 1819 men were included in this analysis; 1170 were not infected with HIV or viral hepatitis, 509 were HIV monoinfected, 74 were hepatitis monoinfected (56 HCV only, 17 HBV only, 1 HCV-HBV coinfected), and 66 were HIV–viral hepatitis coinfected (36 HCV, 27 HBV, 3 HCV-HBV).
The median age was 45 years, with the hepatitis-monoinfected group being the oldest and the HIV-monoinfected group the youngest (median age, 48 and 42 years, respectively) (Table 1). The majority of participants were white. However, only 34% of the hepatitis-monoinfected men were white, most likely because more of these men were enrolled during the 2001–2003 recruitment period, which focused on nonwhite populations. The median BMI of the entire cohort was 25.4, and BMI was highest among the hepatitis-monoinfected men. Although the hepatitis-monoinfected and HIV-hepatitis–coinfected men were more likely to use injection drugs, IDU was low overall (1%). The median platelet count was normal in all groups but was lowest among the HIV–viral hepatitis–coinfected men (161 × 109/L), who also had the highest median AST (54 IU/mL).
Table 1.
Characteristics of the Cohort, by Disease Status
| Characteristic | No Infection (n = 1170) | HIV Monoinfection (n = 509) | Hepatitis Monoinfection (n = 74) | Coinfection (n = 66) | P Valuea |
| Age | |||||
| <40 | 302 (26) | 199 (39) | 5 (6.8) | 19 (29) | <.001 |
| 40–49 | 456 (39) | 217 (43) | 37 (50) | 33 (50) | |
| ≥50 | 412 (35) | 93 (18) | 32 (43) | 14 (21) | |
| Year of MACS visit | |||||
| <2001 | 286 (24) | 372 (73) | 14 (19) | 46 (70) | <.001 |
| ≥2001 | 884 (76) | 137 (27) | 60 (81) | 20 (30) | |
| White race | 864 (74) | 391 (77) | 25 (34) | 46 (70) | <.001 |
| Heavy alcohol use | 98 (8.5) | 34 (6.9) | 6 (8.3) | 6 (9.2) | .711 |
| BMI | |||||
| <25 | 472 (43) | 253 (55) | 24 (34) | 37 (58) | <.001 |
| 25–30 | 426 (38) | 162 (35) | 29 (41) | 20 (31) | |
| ≥30 | 212 (19) | 45 (10) | 18 (25) | 7 (11) | |
| Current IDU | 7 (0.6) | 6 (1.2) | 3 (4.2) | 2 (3.2) | .007 |
| Log10 HIV viral load, median (IQR) | 4.27 (3.58–4.83) | 4.16 (3.46–4.78) | .294b | ||
| CD4 count, median (IQR) | 394 (250–531) | 393 (160–524) | .695b | ||
| CD4 count ≤200 | 93 (18) | 19 (29) | .037b | ||
| Current lamivudine use | 75 (15) | 11 (17) | .7b | ||
| Current ART | |||||
| No therapy | 297 (60) | 40 (62) | .722b | ||
| Monotherapy | 80 (16) | 8 (12) | |||
| Combination therapy | 120 (24) | 17 (26) | |||
| Platelets, 109/L median (IQR) | 233 (201–269) | 208 (171–247) | 209 (176–247) | 161 (127–212) | .0001 |
| AST, IU/mL, median (IQR) | 22 (18–27) | 27 (22–35) | 31 (23–45) | 54 (38–78) | .0001 |
| Elevated ASTc | 70 (6.0) | 103 (20.2) | 27 (36.5) | 51 (77.3) | <.001 |
| ALT, IU/mL, median (IQR)d | 23 (18–30) | 25 (18–33) | 33 (26–51) | 70.5 (36–97) | .0001 |
| Elevated ALTc,d | 109 (12.8) | 24 (18.5) | 27 (45.8) | 19 (73.1) | <.001 |
All data are No. (%) unless otherwise indicated.
Abbreviations: ALT, alanine aminotransferase; ART, antiretroviral therapy; AST, aspartate aminotransferase; BMI, body mass index; HIV, human immunodeficiency virus type 1; IDU, intravenous drug use; IQR, interquartile range; MACS, Multicenter AIDS Cohort Study.
P values are for comparison across all groups (unless specified) using Kruskal-Wallis rank test for continuous variables and Pearson χ2 test for categorical variables.
Comparison limited to HIV infected using Wilcoxon rank-sum test for continuous variables and Pearson χ2 test for categorical variables.
Elevated AST and ALT defined as ≥1.25 × upper limit of normal (ULN), with ULN 30 IU/mL [21].
Limited to 1069 subjects with ALT results available (ALT not routine tested in the cohort prior to 2001): 854 no infection, 130 HIV monoinfection, 59 hepatitis monoinfection, 26 coinfection.
Among HIV-infected individuals, HIV RNA levels were similar between men with and without viral hepatitis. Although median CD4 T-cell counts did not differ significantly between groups, HIV–viral hepatitis–coinfected men were more likely than HIV-monoinfected men to have a CD4 count ≤200 cells/mL (29% vs 18%; P = .03). Because subject visits were chosen prior to HAART, most HIV-infected subjects (63%) were not taking any ART. Among those taking ART, the most common class was the NRTIs (38% of HIV-infected subjects); within this class, zidovudine was used most frequently (24%) (Table 2). A few individuals had either a PI or an NNRTI in their ART regimen (3.8%). Among the potentially hepatotoxic non-ART medications, trimethoprim/sulfamethoxazole was used most frequently (19.5%), followed by fluconazole (8.2%) and dapsone (3%) (Table 2).
Table 2.
Current Medication Use Among the 575 HIV-Infected Participants
| Medication | Current Use, No. |
| ART | |
| ≥1 NRTI | 220 (38%) |
| Zidovudine | 137 (24%) |
| Lamivudine | 86 (15%) |
| Stavudine | 58 (10%) |
| Didanosine | 44 (7.7%) |
| Abacavir | 1 (0.2%) |
| ≥1 PI | 16 (2.8%) |
| Ritonavir | 2 (0.3%) |
| ≥1 NNRTI | 6 (1%) |
| Nevirapine | 2 (0.3%) |
| Non-ART | |
| Trimethoprim/sulfamethoxazole | 112 (19%) |
| Fluconazole | 47 (8.2%) |
| Dapsone | 17 (3%) |
| Ketoconazole | 11 (1.9%) |
| Testosterone | 5 (0.9%) |
| Dehydroepiandrosterone | 4 (0.7%) |
| Nandrolone | 2 (0.3%) |
| Oxandrolone | 2 (0.3%) |
| Isoniazid | 1 (0.2%) |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus type 1; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Four men were on anti-HCV medication (2 HIV coinfected). Seven HBV-infected men were taking lamivudine (all HIV-HBV coinfected), and none were taking tenofovir or emtricitabine.
APRI Values by HIV and Hepatitis Status
The median APRI for the entire cohort was low at 0.31. The uninfected group had the lowest median APRI at 0.27 (interquartile range [IQR], 0.21–0.36). Notably, the median APRI values in the HIV-monoinfected group (0.42; IQR, 0.30–0.59) and hepatitis-monoinfected group (0.43; IQR, 0.28–0.66) were nearly identical and were significantly higher compared with the uninfected group (0.27; P < .001 respectively, compared with the HIV-monoinfected and hepatitis-monoinfected groups). The HIV-hepatitis–coinfected group had the highest median APRI at 1.0 (IQR, 0.56–1.6; P < .001 compared with all other groups).
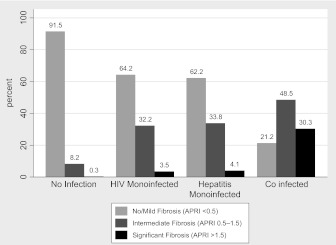
According to the APRI values, 80% of the men (n = 1457) had no or mild fibrosis, 17% (n = 317) had intermediate fibrosis, and 2.5% (n = 45) had significant fibrosis. The prevalence of significant fibrosis was highest in the HIV-hepatitis–coinfected men at 30%. However, interestingly, the prevalence was similar between the HIV-monoinfected and the hepatitis-monoinfected men (3.5% and 4.0%, respectively) (Figure 1). In pairwise comparison, each of these was significantly higher than in the uninfected men, among whom only 0.3% had significant fibrosis (P for each group comparison vs uninfected <.001; for HIV-hepatitis coinfected vs either HIV monoinfected or hepatitis monoinfected, P < .001).
Figure 1.
Categorization of hepatic fibrosis by human immunodeficiency virus (HIV) and viral hepatitis disease category.
Factors Associated With APRI
After establishing that APRI values differed significantly among the various HIV and viral hepatitis groups, we next aimed to determine factors associated with higher APRI. In univariate analysis of the entire cohort, HIV infection (1.66-FI, P < .001) and chronic viral hepatitis infection (2.04-FI, P < .001) were significantly associated with a higher APRI and remained so in multivariable analysis (Table 3). Age ≥50 years was also associated with a significantly higher APRI (1.11-FI, P = .004). The possibility of a synergistic effect of infection with both HIV and viral hepatitis was tested using an HIV-hepatitis interaction term. On multivariable analysis, HIV-hepatitis coinfection was associated with a 1.57-fold higher APRI (P < .001) than would be expected based on the individual contributions of each infection. The same factors remained statistically significant after adding type of chronic viral hepatitis (HBV or HCV) to the model (results not shown).
Table 3.
Factors Associated With Difference in ln(APRI), Entire Cohorta
| Unadjusted |
Adjusted |
|||
| Factor | Fold Change in APRI (95% CI) | P Value | Fold Change in APRI (95% CI) | P Value |
| HIV status | ||||
| Uninfected | 1 | 1 | ||
| Infected | 1.66 (1.57–1.76) | <.001 | 1.39 (1.31–1.49) | <.001 |
| Hepatitis status | ||||
| Uninfected | 1 | 1 | ||
| HBV or HCV infected | 2.04 (1.85–2.25) | <.001 | 1.52 (1.33–1.72) | <.001 |
| HIV-hepatitis interaction | ||||
| No | 1 | |||
| Yes | 1.57 (1.30–1.89) | <.001 | ||
| Age | ||||
| <40 | 1 | 1 | ||
| 40–50 | 1.03 (0.96–1.1) | .4 | 1.05 (0.98–1.11) | .161 |
| ≥50 | 0.96 (0.89–1.03) | .26 | 1.11 (1.03–1.19) | .004 |
Abbreviations: APRI, aspartate aminotransferase-to-platelet ratio index; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus type 1; ln, natural logarithm.
Model also adjusts for heavy alcohol use, body mass index, white race, and year of Multicenter AIDS Cohort Study visit.
After determining that HIV and viral hepatitis infections were independently and synergistically associated with higher APRI values, we next investigated whether this translated into a greater chance of being categorized as having hepatic fibrosis. We therefore performed multivariable proportional odds logistic regression and found that HIV infection (odds ratio [OR], 3.8; P < .001), chronic viral hepatitis infection (OR, 6.6; P < .001), and age ≥50 (OR, 1.6; P = .013) were each independently associated with increased odds of a higher fibrosis category.
To investigate factors associated with higher APRI in HIV-infected subjects, the next series of analyses was limited to the 575 HIV-infected men. In univariate analysis, viral hepatitis coinfection (2.32-FI compared with HIV monoinfected; P < .001), HIV RNA ≥100000 copies/mL (1.37-FI; P < .001), CD4 T-cell count ≤200 cells/mL (1.65-FI; P < .001), ART monotherapy (1.24-FI compared with no ART; P = .015), current trimethoprim/sulfamethoxazole use (1.26-FI; P = .002), and current fluconazole use (1.76-FI; P < .001) were all associated with a higher APRI (Table 4). After adjusting for age, BMI, alcohol use, race, and year of MACS visit, the covariates that remained significantly associated with APRI were viral hepatitis coinfection (2.34-FI; P < .001), CD4 T-cell count ≤200 cells/mL (1.23-FI; P = .022), and HIV RNA ≥100 000 copies/mL (1.26-FI; P = .007). Again, the same factors remained statistically significant after adding type of chronic viral hepatitis to the model (results not shown). When use of an NRTI was substituted for ART in the multivariate model, it was not significantly associated with APRI.
Table 4.
Factors Associated With Difference in ln(APRI), Among All HIV-Positive Participantsa
| Unadjusted |
Adjusted |
|||
| Factor | Fold Change in APRI (95% CI) | P Value | Fold Change in APRI (95% CI) | P Value |
| Hepatitis status | ||||
| Uninfected | 1 | 1 | ||
| HBV or HCV infected | 2.32 (1.95–2.76) | <.001 | 2.34 (1.97–2.79) | <.001 |
| White race | ||||
| No | 0.86 (.75–.99) | .036 | 0.96 (.84–1.11) | .61 |
| Yes | 1 | 1 | ||
| CD4 count ≤200 cells/mL | ||||
| No | 1 | 1 | ||
| Yes | 1.65 (1.43–1.9) | <.001 | 1.23 (1.03–1.47) | .022 |
| Viral load ≥100000 copies/mL | ||||
| No | 1 | 1 | ||
| Yes | 1.37 (1.16–1.61) | <.001 | 1.26 (1.07–1.49) | .007 |
| Current ART use | ||||
| No therapy | 1 | 1 | ||
| Monotherapy | 1.24 (1.04–1.47) | .015 | 0.93 (.77–1.11) | .396 |
| Combination therapy | 1.08 (.94–1.25) | .273 | 1.06 (.91–1.23) | .486 |
| Current trimethoprim/sulfamethoxazole use | ||||
| No | 1 | 1 | ||
| Yes | 1.26 (1.09–1.47) | .002 | 0.95 (.80–1.13) | .542 |
| Current fluconazole | ||||
| No | 1 | 1 | ||
| Yes | 1.76 (1.42–2.17) | <.001 | 1.21 (.95–1.54) | .118 |
Abbreviations: APRI, aspartate aminotransferase-to-platelet ratio index; ART, antiretroviral therapy; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus type 1; ln, natural logarithm.
Model also adjusts for age, heavy alcohol use, body mass index, and year of Multicenter AIDS Cohort Study visit.
Effect of Platelets on APRI Values
Because thrombocytopenia is common among patients with HIV infection [22] and platelet count is used in the APRI calculation, higher APRI values in HIV-infected individuals may be due to HIV infection rather than underlying liver disease. To further explore this, we repeated the analysis after stratifying subjects into low, middle, and high platelet categories based on the distribution of platelets in the cohort: <188 × 109/L (25th percentile), 188–263 × 109/L (middle 50%), or >263 × 109/L (75th percentile). HIV and hepatitis infections were each associated with significantly higher APRI values regardless of stratification by platelet count. The HIV-hepatitis interaction term was only statistically significant in the lowest platelet group. This is likely due to the small number of coinfected individuals in the models after stratification; there were 43, 19, and 4 coinfected men in the low, middle, and high platelet groups, respectively. We also repeated the analysis using natural logarithm (ln) AST as the outcome rather than ln(APRI) because AST alone is correlated with significant fibrosis on liver biopsy among HIV-infected individuals [23]. The inferences were similar in that both viral hepatitis and HIV were independently associated with higher values. Taken together, these analyses suggest that differences in platelets between the groups were not responsible for the associations with higher APRI values.
DISCUSSION
This study of 1819 men demonstrates that both HIV and viral hepatitis infections are independently associated with APRI, a noninvasive marker of hepatic fibrosis. In fact, HIV-monoinfected men were much more likely to have significant fibrosis, as measured by APRI, than HIV- and viral hepatitis–uninfected controls; surprisingly, their APRI scores were similar to those of the hepatitis-monoinfected men. Among the HIV-infected men, higher HIV RNA and lower CD4 T-cell count were associated with higher APRI, suggesting that HIV-associated immunosuppression or opportunistic infections may be associated with liver fibrosis.
Few studies have evaluated the prevalence of liver disease among HIV-monoinfected individuals, and all rely on noninvasive measurements of hepatic fibrosis [10–12, 24–28]. Our study is unique in that it directly compares HIV-monoinfected men with otherwise similar men who are HIV uninfected, hepatitis monoinfected, or HIV-hepatitis coinfected. We also evaluated APRI values prior to HAART initiation in an effort to exclude the potentially confounding effects of HAART on liver disease.
Four prior studies used APRI as a surrogate for liver disease in HIV monoinfection. The prevalence of significant hepatic fibrosis in these studies ranged from 2.6% to 8.3% [10, 11, 24, 25]. Our finding of a 3.5% prevalence of significant fibrosis among HIV-monoinfected men is therefore consistent with prior findings, although caution must be exercised in comparing APRI across studies because the ULN for AST used in calculations may vary. Unlike our study, the 2 cohorts with higher prevalence of significant fibrosis (7.4% and 8.3%) were predominantly African American and had a high proportion of IDU [10, 11]. In contrast, a third study of 540 HIV-monoinfected individuals, in which the majority were MSM without IDU, found 4% prevalence of significant hepatic fibrosis, a rate similar to ours [24]. None of these studies included hepatitis-monoinfected individuals as a comparison. It is notable that the prevalence of significant fibrosis, as determined by APRI, among the HIV-monoinfected men in our study was not only similar to other HIV-monoinfected cohorts but was also similar to the hepatitis-monoinfected men within our own cohort. In our cohort, high HIV RNA and low CD4 T-cell count were each independently associated with higher APRI values. This is consistent with prior studies of surrogate markers for hepatic fibrosis in HIV-positive cohorts [10–12]. However, the mechanism by which these factors affect liver disease, especially in the absence of viral hepatitis, deserves further investigation.
Although APRI has been validated as a surrogate for hepatic fibrosis in cohorts of HIV-HCV–coinfected individuals, it has not been validated in HIV monoinfection. Such a study is unlikely to be performed, however, because it would be neither feasible nor ethical to obtain liver biopsies in cohorts of HIV-monoinfected individuals without clinical indications for liver biopsy. Although high APRI in HIV may be due to thrombocytopenia, it is unlikely that our findings are primarily due to the effect of HIV on platelets. First, the results were consistent even after stratifying subjects by platelet count. Second, after substituting AST for APRI in the models, HIV infection was significantly associated with the outcome. This finding is consistent with other studies that have found AST elevations in individuals with HIV infection even in the absence of viral hepatitis coinfection, hepatotoxic medications, or antiviral treatment [7, 29].
The mechanism behind elevated AST in HIV infection is intriguing but poorly understood and is likely multifactorial. It is possible that HIV infects hepatocytes, though evidence of this has been inconclusive [9, 30, 31]. Even without directly infecting hepatocytes, HIV can induce hepatocyte apoptosis [31]. In addition, HIV has been demonstrated to infect and activate human hepatic stellate cells, thereby promoting liver injury and fibrosis. The HIV envelope protein gp120 may also cause fibrosis by increasing hepatic stellate cell chemotaxis and expression of proinflammatory chemokines [8, 32]. Other potential causes of AST elevations include opportunistic infections and medications [4]. Though few men in our cohort were on PIs or NNRTIs, a sizable minority were taking NRTIs, which can cause hepatotoxicity due to their ability to inhibit mitochondrial polymerase-γ. However, neither ART nor NRTI use was associated with APRI after adjusting for HIV RNA level and CD4 count. Of the potentially hepatotoxic non-ART medications, trimethoprim/sulfamethoxazole and fluconazole were used most frequently in our cohort but were not associated with higher APRI after adjusting for degree of immunosuppression.
As expected, infection with both HIV and viral hepatitis had a synergistic effect on APRI. This is consistent with prior studies demonstrating the deleterious effect of HIV infection on viral hepatitis–related liver disease [33–35]. In vitro experiments have shown that HIV and HCV cooperatively enhance hepatocyte apoptosis [36]. In the setting of viral hepatitis, HIV-associated immune dysregulation and increased gastrointestinal microbial translocation have also been implicated in promoting hepatic fibrosis [37, 38].
The primary limitation of this study is the reliance on an imperfect surrogate marker to assess liver disease. However, a study this size using liver biopsies is unlikely to be performed given its cost, invasiveness, and risk. A second limitation is that our primary analysis treated APRI as a continuous variable, which has not been validated. Our results were supported by our secondary analysis using APRI as a categorical variable. A third limitation is that chronic HCV- and HBV-infected subjects were categorized together in the analysis because of the relatively small number of HBV-monoinfected individuals. Even after adjusting for type of viral hepatitis, however, HIV infection, high HIV RNA, and low CD4 T-cell count remained significantly associated with higher APRI. A fourth limitation is that we did not analyze FIB-4, another surrogate marker of liver disease that was derived in an HIV-HCV–coinfected cohort [39]. This marker requires ALT, which we could not obtain reliably in all of our subjects. However, in an analysis of the men with available ALT, the FIB-4 and APRI results were comparable. Finally, because the MACS only includes men, this study cannot address whether HIV-monoinfected women would also have increased risk for liver disease.
A major strength of this study is the inclusion of otherwise similar HIV- and viral hepatitis–naive controls. To our knowledge, only 1 other study has investigated liver disease among a cohort of HIV- and HCV-positive and -negative individuals. However, HBV-infected subjects were included with the uninfected controls, and a head-to-head comparison across viral disease categories was not performed [12]. Other strengths include the large number of subjects, the assessment of other factors that are associated with liver disease, and the lack of confounding from HAART.
In summary, our study demonstrates that HIV and chronic viral hepatitis are each independent risk factors for elevated APRI, that there is a synergistic effect of coinfection with HIV and viral hepatitis, and that HIV-related advanced immunosuppression is associated with higher APRI. Additional studies are needed to understand the biological basis for liver disease in HIV-monoinfected individuals, to understand how immunosuppression affects liver disease, and to determine whether HAART mitigates liver disease.
Notes
Acknowledgments.
Data in this manuscript were collected by the with centers (principal investigators) located at the following MACS centers: Johns Hopkins Bloomberg School of Public Health (Joseph Margolick); Howard Brown Health Center and Northwestern University Medical School (John Phair, Steven Wolinsky); University of California, Los Angeles (Roger Detels, Oto Martinez-Maza); University of Pittsburgh (Charles Rinaldo); and Data Analysis Center (Lisa Jacobson).
Financial support.
This work was funded by the National Center for Research Resources (NCRR) (1KL2RR025006-01 to J. C. P.) and the National Institute on Drug Abuse (NIDA) (5R03DA026094-02 to C. L. T.). The MACS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute and the National Heart, Lung, and Blood Institute (UO1-AI-35042, UL1-RR025005 to G. C. R. C., UO1-AI-35043, UO1-AI-35039, UO1-AI-35040, UO1-AI-35041).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.TDCoAEoA-HIVdS Group. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D study. AIDS. 2010;24:1537–48. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- 2.Sulkowski MS. Viral hepatitis and HIV coinfection. J Hepatol. 2008;48:353–67. doi: 10.1016/j.jhep.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Staples CT, Jr, Rimland D, Dudas D. Hepatitis C in the HIV (human immunodeficiency virus) Atlanta V.A. (Veterans Affairs Medical Center) Cohort Study (HAVACS): the effect of coinfection on survival. Clin Infect Dis. 1999;29:150–4. doi: 10.1086/520144. [DOI] [PubMed] [Google Scholar]
- 4.Price JC, Thio CL. Liver disease in the HIV-infected individual. Clin Gastroenterol Hepatol. 2010;8:1002–12. doi: 10.1016/j.cgh.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meraviglia P, Schiavini M, Castagna A, et al. Lopinavir/ritonavir treatment in HIV antiretroviral-experienced patients: evaluation of risk factors for liver enzyme elevation. HIV Med. 2004;5:334–43. doi: 10.1111/j.1468-1293.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- 6.Rockstroh JK, Mocroft A, Soriano V, et al. Influence of hepatitis C virus infection on HIV-1 disease progression and response to highly active antiretroviral therapy. J Infect Dis. 2005;192:992–1002. doi: 10.1086/432762. [DOI] [PubMed] [Google Scholar]
- 7.Sterling RK, Chiu S, Snider K, Nixon D. The prevalence and risk factors for abnormal liver enzymes in HIV-positive patients without hepatitis B or C coinfections. Dig Dis Sci. 2008;53:1375–82. doi: 10.1007/s10620-007-9999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuyama AC, Hong F, Saiman Y, et al. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatology. 2010;52:612–22. doi: 10.1002/hep.23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackard JT, Sherman KE. HCV/HIV co-infection: time to re-evaluate the role of HIV in the liver? J Viral Hepat. 2008;15:323–30. doi: 10.1111/j.1365-2893.2008.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sulkowski MS, Mehta S, Montes De Oca R, Thomas D, Moore R. 13th Conference on Retroviruses and Opportunistic Infections. Denver, CO: 2006. Estimated prevalence of significant liver disease among 4,052 HIV-infected adults with and without chronic hepatitis B and C [abstract 842] [Google Scholar]
- 11.DallaPiazza M, Amorosa VK, Localio R, Kostman JR, Lo Re V., 3rd Prevalence and risk factors for significant liver fibrosis among HIV-monoinfected patients. BMC Infect Dis. 2010;10:116. doi: 10.1186/1471-2334-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackard JT, Welge JA, Taylor LE, et al. HIV mono-infection is associated with FIB-4-A noninvasive index of liver fibrosis—in women. Clin Infect Dis. 2011;52:674–80. doi: 10.1093/cid/ciq199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 14.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 15.Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726–36. doi: 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- 16.Nunes D, Fleming C, Offner G, et al. HIV infection does not affect the performance of noninvasive markers of fibrosis for the diagnosis of hepatitis C virus-related liver disease. J Acquir Immune Defic Syndr. 2005;40:538–44. doi: 10.1097/01.qai.0000184856.31695.bf. [DOI] [PubMed] [Google Scholar]
- 17.Nunes D, Fleming C, Offner G, et al. Noninvasive markers of liver fibrosis are highly predictive of liver-related death in a cohort of HCV-infected individuals with and without HIV infection. Am J Gastroenterol. 2010;105:1346–53. doi: 10.1038/ajg.2009.746. [DOI] [PubMed] [Google Scholar]
- 18.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–8. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 19.Dudley J, Jin S, Hoover D, Metz S, Thackeray R, Chmiel J. The Multicenter AIDS Cohort Study: retention after 9 1/2 years. Am J Epidemiol. 1995;142:323–30. doi: 10.1093/oxfordjournals.aje.a117638. [DOI] [PubMed] [Google Scholar]
- 20. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. October 14, 2011; 1–167. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 7 December 2011. [Google Scholar]
- 21.Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 22.Torre D, Pugliese A. Platelets and HIV-1 infection: old and new aspects. Curr HIV Res. 2008;6:411–8. doi: 10.2174/157016208785861140. [DOI] [PubMed] [Google Scholar]
- 23.Macias J, Mira J, Gilabert I, et al. Combined use of aspartate aminotransferase, platelet count and matrix metalloproteinase 2 measurements to predict liver fibrosis in HIV/hepatitis C virus-coinfected patients. HIV Med. 2011;12:14–21. doi: 10.1111/j.1468-1293.2010.00836.x. [DOI] [PubMed] [Google Scholar]
- 24.Al-Mohri H, Murphy T, Lu Y, Lalonde RG, Klein MB. Evaluating liver fibrosis progression and the impact of antiretroviral therapy in HIV and hepatitis C coinfection using a noninvasive marker. J Acquir Immune Defic Syndr. 2007;44:463–9. doi: 10.1097/QAI.0b013e318030ff8e. [DOI] [PubMed] [Google Scholar]
- 25.Vodkin I, Aouizerat B, Deeks S, Martin J, Peter M. 17th Conference on Retroviruses and Opportunistic Infections. San Francisco, CA: 2010. Factors associated with liver disease severity in a cohort of patients with HIV [abstract 649] [Google Scholar]
- 26.Merchante N, Perez-Camacho I, Mira JA, et al. Prevalence and risk factors for abnormal liver stiffness in HIV-infected patients without viral hepatitis coinfection: role of didanosine. Antivir Ther. 2010;15:753–63. doi: 10.3851/IMP1612. [DOI] [PubMed] [Google Scholar]
- 27.Castellares C, Barreiro P, Martin-Carbonero L, et al. Liver cirrhosis in HIV-infected patients: prevalence, aetiology and clinical outcome. J Viral Hepat. 2008;15:165–72. doi: 10.1111/j.1365-2893.2007.00903.x. [DOI] [PubMed] [Google Scholar]
- 28.Blanco F, Barreiro P, Ryan P, et al. Risk factors for advanced liver fibrosis in HIV-infected individuals: role of antiretroviral drugs and insulin resistance. J Viral Hepat. 2011;18:11–16. doi: 10.1111/j.1365-2893.2009.01261.x. [DOI] [PubMed] [Google Scholar]
- 29.Mata-Marin JA, Gaytan-Martinez J, Grados-Chavarria BH, Fuentes-Allen JL, Arroyo-Anduiza CI, Alfaro-Mejia A. Correlation between HIV viral load and aminotransferases as liver damage markers in HIV infected naive patients: a concordance cross-sectional study. Virol J. 2009;6:181. doi: 10.1186/1743-422X-6-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao P, Usami O, Suzuki Y, et al. Characterization of a CD4-independent clinical HIV-1 that can efficiently infect human hepatocytes through chemokine (C-X-C motif) receptor 4. AIDS. 2008;22:1749–57. doi: 10.1097/QAD.0b013e328308937c. [DOI] [PubMed] [Google Scholar]
- 31.Vlahakis SR, Villasis-Keever A, Gomez TS, Bren GD, Paya CV. Human immunodeficiency virus-induced apoptosis of human hepatocytes via CXCR4. J Infect Dis. 2003;188:1455–60. doi: 10.1086/379738. [DOI] [PubMed] [Google Scholar]
- 32.Bruno R, Galastri S, Sacchi P, et al. gp120 modulates the biology of human hepatic stellate cells: a link between HIV infection and liver fibrogenesis. Gut. 2010;59:513–20. doi: 10.1136/gut.2008.163287. [DOI] [PubMed] [Google Scholar]
- 33.Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30:1054–8. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 34.Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562–9. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 35.Colin JF, Cazals-Hatem D, Loriot MA, et al. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology. 1999;29:1306–10. doi: 10.1002/hep.510290447. [DOI] [PubMed] [Google Scholar]
- 36.Munshi N, Balasubramanian A, Koziel M, Ganju RK, Groopman JE. Hepatitis C and human immunodeficiency virus envelope proteins cooperatively induce hepatocytic apoptosis via an innocent bystander mechanism. J Infect Dis. 2003;188:1192–204. doi: 10.1086/378643. [DOI] [PubMed] [Google Scholar]
- 37.Kim AY, Chung RT. Coinfection with HIV-1 and HCV—a one-two punch. Gastroenterology. 2009;137:795–814. doi: 10.1053/j.gastro.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balagopal A, Philp FH, Astemborski J, et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135:226–33. doi: 10.1053/j.gastro.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]