Abstract
Aims
Neuregulins (NRG) are growth factors that are synthesized by endothelial cells (ECs) and bind to erbB receptors. We have shown previously that NRG is proangiogenic in vitro, and that NRG/erbB signalling is important for autocrine endothelial angiogenic signalling in vitro. However, the role of NRG in the angiogenic response to ischaemia is unknown. We hypothesized that endothelial NRG is required for ischaemia-induced angiogenesis in vivo and that exogenous administration of NRG will enhance angiogenic responses after ischaemic insult.
Methods and results
An endothelial-selective inducible NRG knockout mouse was created and subjected to femoral artery ligation. Endothelial NRG deletion significantly decreased blood flow recovery (by 40%, P < 0.05), capillary density, αvβ3 integrin activation, and arteriogenesis after ischaemic injury. Isolated ECs from knockout mice demonstrated significantly impaired cord formation in vitro, suggesting that NRG signalling performs an important cell autonomous function. Recombinant human NRG (rNRG) has not only reversed the angiogenic defect in knockout mice but also accelerated blood flow recovery in wild-type mice.
Conclusion
Endothelial production of NRG is required for angiogenesis and arteriogenesis induced by ischaemic injury. Furthermore, exogenous administration of rNRG can enhance this process, suggesting a potential role for NRG in vascular disease.
Keywords: Endothelium, Angiogenesis, Ischaemia, Genetically altered mice, Growth factors
1. Introduction
Neuregulins (NRG) are a family of epidermal growth factor ligands that are expressed by vascular endothelial cells (ECs) and mediate signal transduction via binding to erbB receptors.1 There are four genes known to encode NRG, of which nrg-1 is the most extensively studied. The complexity of NRG signalling is further increased by the number and function of its family of receptors. The erbB receptor family consists of four members that function as homo- or heterodimers.2 Amplification and hyperactivation of erbB2 have been shown to be important for initiation and progression of aggressive forms of breast cancer,3 and treatment with the humanized monoclonal antibody, trastuzumab, which targets the erbB2 receptor, has dramatically improved the prognosis of patients with such tumours.4 ErbB2 overexpression is also associated with an increase in tumour-derived vascular endothelial growth factor (VEGF) expression and an increase in tumour vascularity,5 a property that is believed to contribute to metastatic potential.
While hyperactivation of the NRG/erbB pathway confers a greater metastatic potential for tumours, this signalling pathway may also be crucial for vascular responses to stress. We have previously shown that NRG is proangiogenic in vitro, using a collagen gel cord formation assay, and in vivo in a rat corneal angiogenesis assay.6 The observation that EC express NRG and erbB receptors suggests that this system may participate in autocrine angiogenic responses. In support of this idea, we recently demonstrated that EC release NRG in response to inflammatory cytokines, and this process is critical for ERK activation.7 Recent studies from our laboratory have demonstrated that EC also release NRG in response to hypoxia.8 Based on these findings, we hypothesized that EC NRG plays an important role in angiogenic responses to ischaemia.
To test this hypothesis, we generated an endothelial-selective inducible NRG knockout model to demonstrate that EC NRG is essential for angiogenesis and arteriogenesis in response to ischaemic injury induced by femoral artery ligation. Endothelial-specific NRG deletion results in a significant decrease in ischaemia-induced vascular growth and αvβ3 integrin activation. Interestingly, addition of exogenous human recombinant NRG-1 (rNRG) also accelerates blood flow recovery after femoral artery ligation in wild-type (WT) animals. These findings indicate that endothelial-derived NRG plays an important role in post-ischaemic angiogenic responses and may present a promising future target for treatment of ischaemic vascular disease.
2. Methods
2.1. Preparation of tamoxifen, rNRG, and mouse treatment
Tamoxifen was purchased from Sigma-Aldrich (MO, USA), diluted in ethanol to 100 mg/mL, and sonicated for 30 min and further diluted to 10 mg/mL in autoclaved sunflower oil (Sigma-Aldrich).9 Mice (except WT mice) were treated with vehicle (100 μL of 10% ethanol in autoclaved sunflower oil) or tamoxifen (1 mg) via intraperitoneal (i.p.) injection 3 days prior to surgery (to delete NRG), and daily thereafter.9 rNRG was purchased from R&D (MN, USA) and diluted in 0.1% BSA in PBS. rNRG-treated mice received daily i.p. injections of 2.5 μg of rNRG.10
2.2. Generation of mouse models
Inducible VE-cadherin promoter-driven Cre-ERT2 (VECad-Cre-ERT2) transgenic mice (generously provided by Dr Luisa Iruela-Arispe)11 were bred with Z/EG reporter mice.12 Cre recombinase is only expressed in EC and can only enter the nucleus after tamoxifen treatment; and, the Z/EG reporter gene will express green fluorescent protein (GFP) only when nuclear cre activity is present. These VECad-Cre-ERT2 GFP mice were treated with tamoxifen for 3 days.11 The second mouse model was generated by crossing the VECad-Cre-ERT2 mouse with a mouse carrying homozygous-floxed alleles (exons 7–9) of nrg-1 (a generous gift of Dr Carmen Birchmeier).13 All mice were 40–50 days of age to ensure maximum cre expression.11 Mice were euthanized with a single ip injection of pentobarbital (150 mg/kg).
2.3. Hindlimb surgery and flow measurements
All animal care protocols were approved by the Yale Institutional Animal Care and Use Committee and conform to guidelines of US National Institute of Health. Mice were anaesthetized with 0.2–5% inhaled isoflurane vaporized in O2 at a rate of 1.5 L/min via nosecone. Heart rate and body temperature were monitored continuously, and adequacy of anaesthesia was monitored throughout the procedure as adequate ventilatory rate and absent withdrawal to noxious stimuli. A small incision was made on the right leg to expose the femoral vasculature, and dual ligation of the femoral artery was performed distal to the profundus branch.
Flow measurements were performed using two different methods. Body temperature of the mice was kept constant at 37 ± 0.5°C. In method 1, a Moor laser Doppler imager (LDI; Moor Instruments, UK) was positioned to acquire blood flow distal to the area of ligation. Average hindlimb perfusion is expressed as the ratio of flow in the ischaemic to non-ischaemic hindlimb in equivalent regions of interest and normalized to surface area. In method 2, the PeriFlux System equipped with a laser Doppler perfusion module (LDPU; Perimed, OH, USA) was used. The probe was placed directly on the gastrocnemius muscle. Ischaemic and non-ischaemic limb perfusion was measured before, and immediately after surgery.14 Post-operative analgesia was maintained with buprenorphine for 24–48 h.
2.4. MicroSPECT-CT measurements of αvβ3 integrin activation
All animals were injected intravenously with ∼1.0 mCi of 99mTc-labelled Arg-Gly-Asp (RGD) peptide (99mTc-NC100692, GE Healthcare, NJ, USA), which binds to activated αvβ3 integrins. All images were acquired at post-operative day 7 with a hybrid microSPECT-CT scanner (X-SPECT, Gamma Medica, CA, USA) as described previously.15,16
2.5. Micro-CT-angiographic assessment of arterial size and density in the ischaemic hindlimb
The thoracic aorta of euthanized mice was cannulated and perfused with saline, then 4% paraformaldehyde for 5 min, and finally 20% Bismuth in 5% gelatine,17 as described elsewhere.18,19 Briefly, the arterial vasculature in the ligated hindlimb was imaged by a high-resolution micro-CT imaging system (GE eXplore Locus SP, GE Healthcare).17 Data are expressed as arterial segmental number, representing the total number of vessels, of a specified diameter, counted in equivalent volumes from 200 sections of the calf distal to the ligation.18,19
2.6. Capillary density measurements
Sections from gastrocnemius muscles were stained with an anti-CD31 (PECAM-1, BD Biosciences, San Jose, CA, USA) antibody and a secondary antibody labelled with Alexa 488 (Invitrogen, CA, USA). Six images were acquired from each slide. Quantification of the CD31 positive cells and fibres from ischaemic and non-ischaemic specimens was performed manually in a blinded fashion. The resultant data are presented as ratio of capillaries/muscle fibre in the ischaemic to non-ischaemic leg.
2.7. EC isolation, adenoviral transfection, cord formation, and western blotting
Skeletal muscle ECs were isolated from hindlimb muscles of homozygously NRG-floxed (FF) mice after digestion in type I collagenase (Sigma-Aldrich) as described elsewhere.7,19,20 The cell suspension was incubated with anti-PECAM-1-coated beads (BD Pharmingen, CA, USA). A second purification was performed using anti-ICAM-2 antibody-coated beads (BD Pharmingen).19 Early passage (3–5) NRG (FF) skeletal muscle EC were transduced for 48 h with cre recombinase (Adeno-cre) or control red fluorescent protein (Adeno-RFP; a generous gift from Dr Frank Giordano).21 Cells were stimulated with 25 ng/mL of recombinant human VEGF 165 (R&D, MN, USA) for 5 or 10 min or with 10% foetal bovine serum (FBS) for 10 min. Antibodies used for immunoblotting were as follows: anti-NRG (sc-348, Santa Cruz Biotechnology, CA, USA), anti-phospho p44/42 MAPK, and MAPK (Cell Signaling, MA, USA), anti-cre (Novagen, Darmstadt, Germany), and anti-actin (Santa Cruz Biotechnology).
2.8. Matrigel cord formation
Growth factor reduced Matrigel (175 μL; BD Biosciences) was placed in 48-well plates and allowed to polymerize at 37°C. Early (<3) passage skeletal muscle EC suspensions were plated at a density of 6 × 104 cells/well in DMEM containing 1% FBS or 50 ng/mL VEGF, purged in a 95% N2–5% CO2 chamber for 15 min in a sealed chamber, and then incubated overnight. Cords were counted manually (using phase contrast microscopy and Image J Software, National Institute of Health) in three fields from four replicate wells per experimental treatment.
2.9. Statistics
Student's t-test was used to compare two groups, and one- or two-way ANOVAs were performed where applicable, with the Bonferroni post hoc analysis for comparisons among paired groups. GraphPad Prism 3 software (GraphPad, CA, USA) was used for statistical comparisons.
3. Results
3.1. Generation of an inducible endothelial NRG deletion mouse model
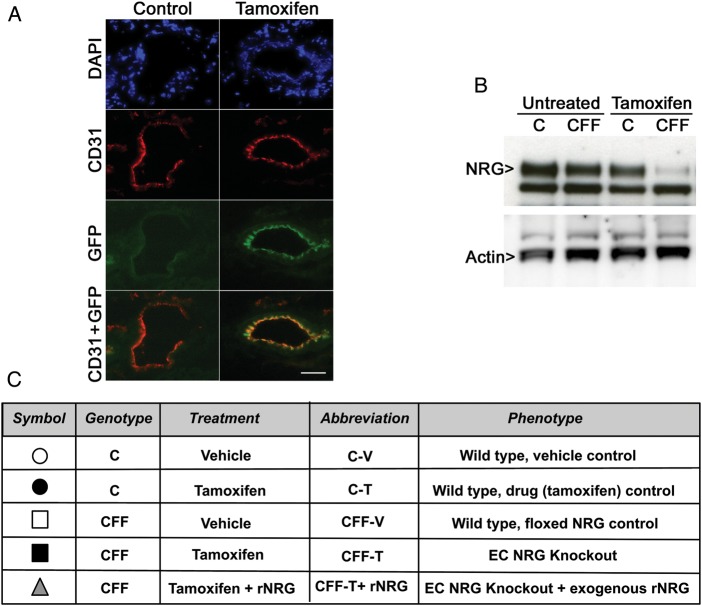
To determine the efficiency of cre induction and activity as well as the appropriate time course of induction with tamoxifen, we treated the VECad-Cre-ERT2 Z/EG reporter mice12 for 3 days11 with tamoxifen or vehicle. Skeletal muscle tissue was collected and immunostained for CD31 to identify ECs. As shown in Figure 1A, 3 days of tamoxifen treatment were sufficient to allow cre nuclear localization and gene recombination, as detected by the presence of GFP signal, specifically in vascular endothelium. However, mice injected with vehicle control for 3 days did not show endothelial GFP signal, as previously reported.11
Figure 1.
Tamoxifen treatment for 3–14 days is sufficient to allow cre-induced gene recombination. (A) Skeletal muscle sections from 3-day control or tamoxifen-treated VECad-Cre-ERT2 GFP mice were stained with CD31 (red) to delineate endothelial cells (ECs). GFP expression (green) is seen only in vascular endothelium of tamoxifen-treated animals. Scale bar: 25 µm. (B) Western blot for NRG expression in pooled purified skeletal muscle ECs before and after 14 days of tamoxifen treatment. (Note: arrowed top band is specific for NRG, bottom band is non-specific; see text.) (C) Endothelial NRG-deleted mice (CFF-T) were compared with three separate control groups: C mice treated with either vehicle or tamoxifen as cre transgene and drug controls, and CFF treated with vehicle as floxed NRG transgene controls. In rescue experiments, CFF-T mice were treated with recombinant NRG (CFF-T + rNRG).
Next, we crossed the VECad-Cre-ERT2 (C) mice with mice carrying homozygous-floxed alleles of the nrg-1 gene (FF).13 Because of the complex nature of this model, our endothelial NRG-deleted mice were compared with multiple controls, as summarized in Figure 1C. To demonstrate that tamoxifen induction effectively decreases NRG expression, purified skeletal muscle EC from C or CFF mice treated for 14 days with tamoxifen or vehicle were subjected to western blotting for NRG. NRG protein expression is decreased only after tamoxifen treatment, and only in CFF animals (Figure 1B). (The specificity of the indicated higher molecular weight band was verified using a NRG peptide competition assay. The lower band shown in Figure 1B was not competed away by preincubation of antibody with purified NRG peptide, demonstrating this band does not represent NRG (data not shown).) Based on these findings, we used a 3-day pretreatment of mice with tamoxifen (or vehicle) prior to femoral artery ligation. Tamoxifen (or vehicle) was continued post-operative for the duration of each experiment (for a maximum of 14 days).
3.2. Endothelial NRG deletion decreases blood flow recovery in response to ischaemia-induced injury
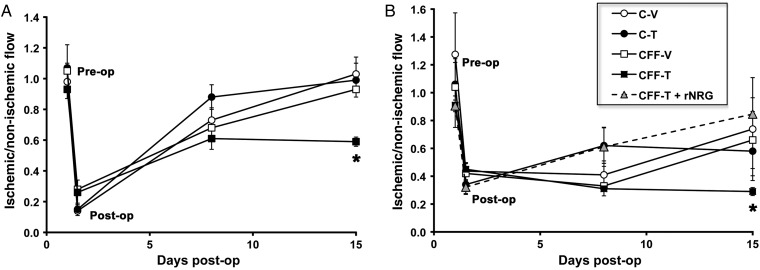
To test whether loss of EC NRG impairs angiogenic responses to ischaemic injury, we subjected mice with EC NRG deletion to femoral artery ligation and followed global flow recovery in the distal hindlimb by serial laser Doppler scanning. No significant differences in blood flow were seen between the groups immediately before or after hindlimb surgery (Figure 2A), suggesting that short-term NRG deletion does not impair basal flow. Flow recovery was detectable at day 7 and had returned to baseline by day 14 in all control groups. However, global distal hindlimb blood flow was 40% less in the CFF-T mice compared with all control groups at day 14 (Figure 2A).
Figure 2.
Endothelial NRG deletion decreases flow recovery after ischaemic injury and exogenous administration of rNRG rescues this impairment. (A) Mice were pretreated with vehicle or tamoxifen for 3 days prior to surgery and thereafter for 14 days. Flow recovery was measured using laser Doppler in the whole lower limb area pre- and post-surgery, and at days 7 and 14. Data are reported as the ratio of ischaemic to non-ischaemic flow per unit surface area. NRG deletion (CFF-T) resulted in a significant reduction in flow recovery. (n = 5–8/group, *P < 0.05 vs. all control groups). (B) Deep tissue flow was measured using a laser Doppler probe placed directly on the gastrocnemius muscle pre- and post-surgery and at days 3, 7, and 14. Compared with controls, NRG deletion significantly decreased flow recovery that was rescued by simultaneous treatment with ip rNRG (n = 4–6 per group, *P < 0.05 vs. all control groups).
The scanning Doppler method provides an assessment of global flow in both superficial and deeper tissues. We next aimed to determine if the effects of NRG deletion seen by this method were due to impaired flow in the hindlimb muscles directly affected by loss of femoral blood supply. To specifically measure this deep muscle flow distal to ligation, we used a small calibre Doppler measurement probe that was placed directly on the gastrocnemius muscle. As shown in Figure 2B, no differences in pre- or immediately post-surgery blood flow measurements were seen between any of the groups. All control groups began to recover flow by day 7 and, again, baseline flow was restored by day 14. By contrast, CFF-T animals showed impaired recovery at day 7, which persisted at day 14, resulting in flow that was 40–50% lower than the control groups. To confirm that NRG deletion was specifically responsible for impaired flow recovery, human recombinant NRG-1β(rNRG) was given to CFF-T mice. These mice were pretreated with tamoxifen for 3 days, and rNRG treatment was begun at the time of surgery and continued daily (in addition to continued tamoxifen). Administration of exogenous human rNRG rescued the decrease in flow in the CFF tamoxifen group (Figure 2B, triangles). This confirms that the impaired flow recovery obtained in the CFF-T mice is due to deletion of EC NRG.
3.3. Endothelial NRG deletion decreases arteriogenesis in response to ischaemia-induced injury
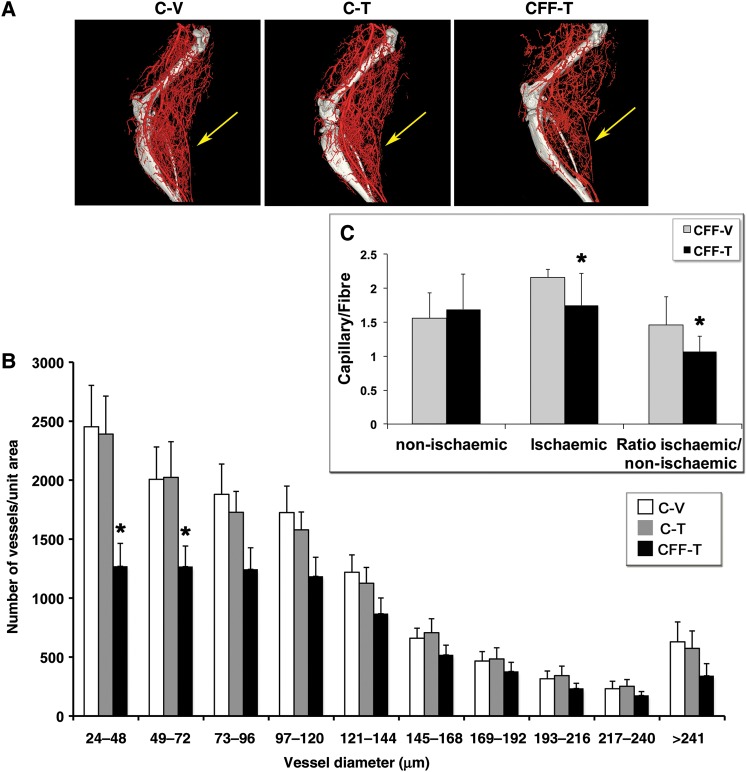
Effects of NRG on global and regional flow may be due to altered angiogenesis and/or arteriogenesis. To determine if endothelial NRG deletion affects arteriogenesis, C-V-, C-T-, and CFF-T-treated mice were subjected to femoral artery ligation. At day 14, mice were euthanized and the arterial vasculature in the ischaemic hindlimb was imaged by high-resolution micro-CT. In this procedure, only the arterial vasculature is quantified and all veins are excluded from the results reported.17 There was a significant decrease in arterial density in the EC NRG-deleted animals (CFF-T) compared with vehicle (C-V) and drug-treated (C-T) controls (Figure 3A and B, P < 0.001 for effect of NRG deletion on vessel density by two-way ANOVA). In particular, the most significant impact was seen in arteries size <72 μm in diameter, in which there was a 30–50% reduction in the number of vessels in the CFF-T animals, indicating a specific defect in the formation of smaller arteries in response to an ischaemic insult in the absence of endothelial NRG expression.
Figure 3.
Endothelial NRG deletion decreases ischaemia-induced arteriogenesis and capillary density. (A) Representative micro-CT arteriograms from hindlimbs of C-V-, C-T-, and CFF-T-treated animals at post-operative day 14 showing decreased arterial vasculature in the NRG-deleted group (CFF-T); yellow arrows show the region of gastrocnemius muscle with decreased vasculature. (B) Quantitative analysis of micro-CT angiograms from indicated (yellow arrow, A) calf region (n = 5–6/group, *P < 0.05 compared with either C-V or C-T groups). (C) Capillary density in the ischaemic and non-ischaemic hindlimb of CFF-V and CFF-T animals. There was a significant decrease in capillary density only in the CFF-T ischaemic region compared with CFF-V (n = 5–6/group, *P < 0.05 compared with CFF-V).
To further assess the effect of endothelial NRG deletion on ischaemic-induced microvascular angiogenic responses, gastrocnemius muscles were isolated from ischaemic and non-ischaemic legs as well as CD31 staining was used to delineate capillaries on sections of isolated muscle of mice at 1-week post-surgery. There was no significant difference in the capillary/muscle fibre density in the non-ischaemic legs of the control (CFF-V) animals vs. the knockouts (CFF-T; Figure 3C), suggesting that loss of NRG expression alone was not sufficient to cause loss of vasculature. In the ischaemic limbs of the control mice, capillary density was significantly higher than that in the ischaemic limbs of the knockout mice, further supporting the idea that endothelial NRG expression is required for microvascular angiogenesis (Figure 3C).
3.4. Endothelial NRG deletion decreases αvβ3 integrin activation
During angiogenesis, ECs proliferate, migrate, and differentiate to communicate with other supporting cells and form new vessels. These processes involve the activation of the adhesion protein receptor integrins, specifically αvβ3, on ECs.22 Activation of these integrins has been used as a marker of early angiogenesis prior to detectable changes in blood flow. Expression of αvβ3 integrins has also been shown to correlate with NRG expression in tumours.23 To test whether NRG deletion affects activated αvβ3 early in angiogenesis, a 99mTc-labelled peptide that contains an RGD (Arg-Gly-Asp) (99mTc-NC100692) motif was used to image integrin activation after ischaemic insult.24 Data were collected at day 7 post-surgery and treatment. We found that the ratio of radiotracer uptake in the ischaemic–non-ischaemic limb was comparable between C-V and C-T mice, but was significantly reduced in the CFF-T group, suggesting decreased αvβ3 activation in the ischaemic region of the NRG-deleted mice (Figure 4). Exogenous administration of rNRG also rescued the decrease in αvβ3 integrin activation seen in the NRG-deleted group (CFF-T). These findings suggest that NRG is important in early stages of angiogenesis (e.g. integrin activation) in response to ischaemia.
Figure 4.
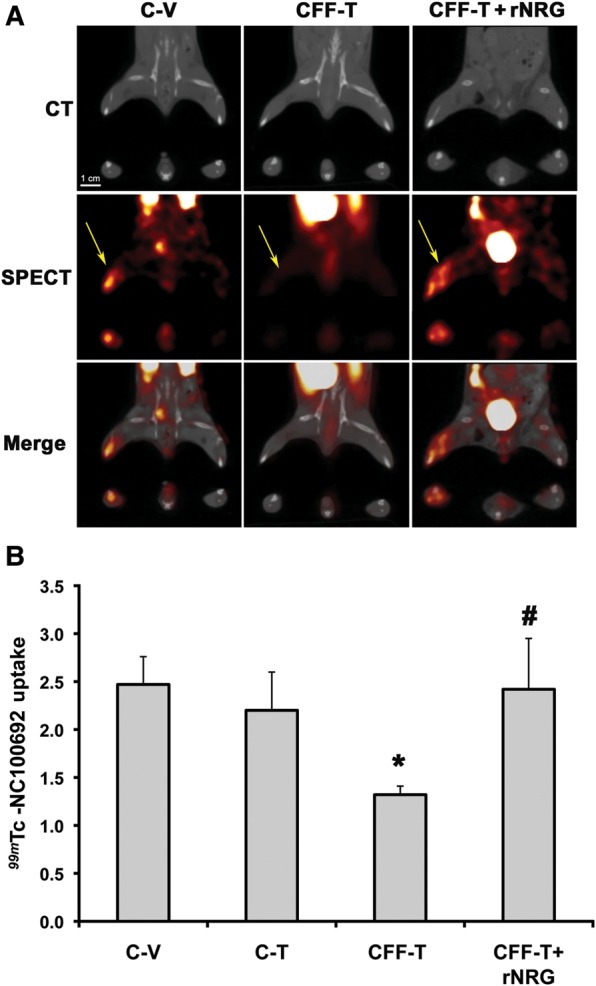
Endothelial NRG deletion decreases αvβ3 activation. (A) Representative micro-CT/SPECT images of C-V, C-T, CFF-T, and CFF-T + rNRG mice after 99mTc-NC10069 injection. Top panels show representative CT scans define coronal (top image) and axial (lower images, at level of ischaemic zone) anatomy. Middle panels show SPECT imaging of 99mTc. Bottom images are merged CT and SPECT data. Yellow arrows point to the uptake of the radiotracer in the ischaemic leg. (B) Quantification of αvβ3 integrin activation using the radiotracer 99mTc-NC10069 at day 7 post-surgery. NRG deletion (CFF-T) significantly decreased 99mTc-NC10069 uptake, which was rescued by exogenous administration of rNRG (CFF-T + rNRG) (n = 4–8/group, *P < 0.05 vs. C-V or C-T controls; #P < 0.05 vs. CFF-T).
3.5. Loss of endothelial NRG expression impairs Matrigel cord formation
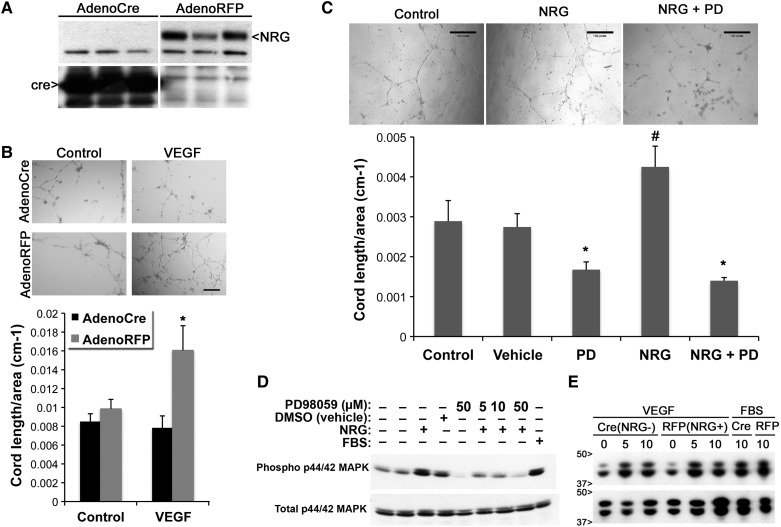
To further assess mechanisms by which loss of NRG impairs angiogenesis, we tested whether loss of NRG expression affects the ability of isolated, purified ECs to form cord-like structures in Matrigel. Skeletal muscle ECs from NRG FF mice were infected with adenovirus expressing cre recombinase (Adeno-Cre to delete NRG) or control protein (Adeno-RFP). As expected, cre expression efficiently decreased NRG expression in vitro (Figure 5A). These EC were then plated on Matrigel and stimulated with hypoxia + additional VEGF. We found that deletion of NRG expression significantly decreased cord formation (Figure 5B). These in vitro data support the hypothesis that NRG is important for endogenous autocrine angiogenic responses.
Figure 5.
Endothelial NRG deletion decreases cord formation. (A) Western blot for NRG in endothelial cells (ECs) purified from skeletal muscle of FF mice treated with AdenoCre or AdenoRFP (control). Cre expression efficiently decreased NRG protein expression in vitro (representative blot of n = 3–4 plates/treatment). (B) Isolated ECs were infected with AdenoCre or RFP, plated on growth factor reduced/1% FBS or VEGF-repleted Matrigel and placed in a hypoxia chamber overnight. Cords were manually quantified the following day (n = 4, *P < 0.05 vs. all other groups). Scale bar: 50 µm. (C) ECs were plated on Matrigel with NRGβ3 and/or PD98059 and incubated overnight (in room air) (*P < 0.05 vs. control and vehicle-treated cells, #P < 0.05 vs. all groups, n = 4). Scale bar: 50 µm. (D) Representative (n = 3) western blot for phospho- and total ERK protein expression in WT ECs stimulated with NRGβ3 and/or PD98059. (E) Representative (n = 5) western blot for phospho- and total ERK protein levels in FF ECs treated with Adeno-cre or Adeno-RFP and stimulated with VEGF or 10% FBS.
Activation of the ERK pathway is well known to promote angiogenesis in response to VEGF stimulation.25 To test whether NRG-stimulated cord formation also requires the activation of ERK, isolated ECs were treated with vehicle, NRGβ3, PD98059 (a MEK/ERK inhibitor), or NRGβ3 + PD98059. We found that addition of NRG alone increased cord formation; however, this was abolished in the presence of the ERK inhibitor (Figure 5C), indicating that ERK activation is necessary for NRG-mediated cord formation. NRG-dependent ERK activation was further investigated in isolated ECs treated with NRG and increasing doses of ERK inhibitor. We found that NRG can directly activate ERK (Figure 5D); however, NRG deletion does not affect VEGF-induced ERK activation (Figure 5E) suggesting alternate pathways may affect VEGF-induced cord formation in the absence of NRG.
3.6. Exogenous administration of human recombinant NRG-1β enhances flow recovery after femoral artery ligation
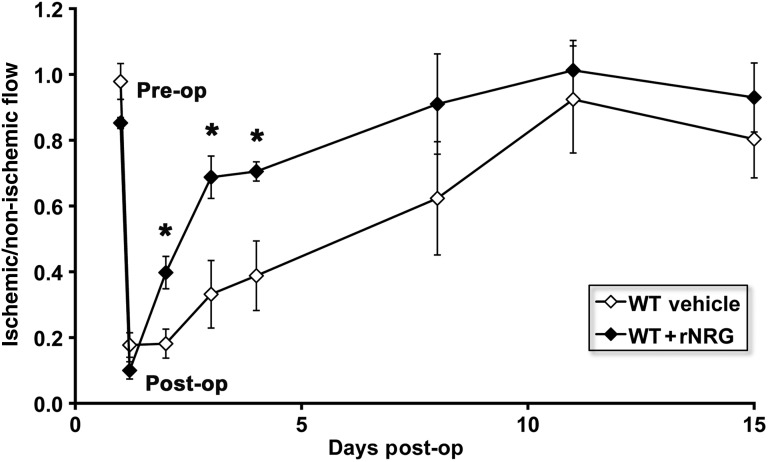
Our data show that administration of exogenous NRG can reverse impaired angiogenesis in the setting of decreased NRG expression in a transgenic model. To test the possibility of employing human recombinant NRG-1β(rNRG) as a therapeutic tool to enhance blood flow in the setting of ‘normal’ EC expression of NRG, we treated WT mice at the time of femoral artery ligation and thereafter for 14 days with i.p. injections of rNRG and measured flow recovery using the deep tissue Doppler probe (as described for Figure 2B). As shown in Figure 6, WT rNRG-treated mice showed significantly improved flow recovery at day 1, 2, and 3 post-surgery compared with controls, suggesting that increasing NRG can augment the endogenous angiogenic responses to ischaemic insult.
Figure 6.
Exogenous administration of human recombinant NRG enhances flow recovery following ischaemic injury. WT C57BL6 mice underwent femoral artery ligation and were treated with vehicle or rNRG (2.5 μg/g per day) for 14 days. Serial measurements of deep tissue flow are shown as the ratio of flow in the ischaemic–non-ischaemic limb. rNRG treatment significantly enhanced flow recovery at days 1, 2, and 3. (n = 4–7/group, *P < 0.05 vs. control).
4. Discussion
We show that endothelial-specific NRG deletion significantly impairs ischaemia-induced angiogenesis using two different methods to measure blood flow in the hindlimb. There is a significant decrease in flow recovery after femoral artery ligation in the absence of endothelial NRG. This impairment in flow recovery after NRG deletion is accompanied by reduced small artery and capillary numbers. These findings demonstrate for the first time that NRG plays an important role in the growth of smaller arteries and microvasculature in the setting of ischaemia, and impaired regeneration of these resistance vessels is responsible, in part, for the prolonged impairment in blood flow seen in these animals. Changes in vascular tone as a result of NRG deletion may also play a role, particularly, in early flow recovery in this model.
Our data also show that NRG deletion results in decreased levels of activated αvβ3 integrins during early stages (day 7) of ischaemia-induced angiogenesis. αvβ3 integrins are part of a family of heterodimeric cell surface glycoprotein molecules that are involved in cell adhesion, proliferation, migration, and differentiation, all steps crucial for formation of new vessels in response to ischaemia.22,26 Interruption of αvβ3 signalling (using RGD-targeted blocking peptides or antibodies) has been shown to disrupt angiogenesis.27 The complex link between NRG and αvβ3 has been studied in breast cancer cells, where NRG has been shown to increase expression of αvβ3 by a mechanism involving increased expression of the proangiogenic protein Cyr61.28 NRG has also been proposed to bind directly to αvβ3, and mutations in NRG that disrupt this binding result in significantly impaired erbB signalling in response to NRG.29 In addition, cross talk and even direct association between receptor tyrosine kinases (including erbB receptors) and αvβ3 has been shown.30 Although these data suggest that integrin activation may be an important target in the angiogenic response to NRG, further studies will be required to determine whether decreased αvβ3 activation, seen in NRG-deleted animals, is due to either a direct effect of loss of NRG induced αvβ3 expression or impaired erbB activation vs. other non-integrin pathways. Furthermore, our use of the RGD peptide to detect integrin activation does not allow full assessment of alternate forms of αvβ3 activation by NRG.
In this in vivo angiogenesis model, it can be difficult to discern whether the effects of endothelial NRG deletion on angiogenesis are purely autocrine/cell-type autonomous (involving a direct effect of NRG on ECs) or involve in a paracrine mechanism, whereby NRG activates a secondary angiogenic factor or response in other cell types.31 For example, in the setting of hindlimb ischaemia, mononuclear inflammatory cells can participate in the angiogenic response, an effect that appears to involve elaboration of inflammatory cytokines and chemokines by these cells.32,33 Interestingly, we have recently reported that certain inflammatory cytokines (e.g. IFN-γ and IL-6) can cause rapid cleavage and release of NRG by metalloproteinases. Blocking NRG cleavage or activity significantly reduces cytokine-induced endothelial signalling and in vitro angiogenesis.7 Thus, impaired endothelial angiogenic responses to the inflammation induced by ischaemia may also play a role in the overall effects of loss of NRG in vivo. Additionally, some endothelial precursor cells (EPCs) have recently been shown to express erbB receptors.34 NRG induced enhanced EPC survival, but did not alter mobilization or proliferation of EPC in these studies. This observation raises the possibility that NRG may alter EPC survival in the ischaemic limb, increasing their participation in angiogenic responses. Finally, some subsets of vascular smooth muscle cells (VSMCs) express erbB receptors,35,36 making these cells another potential target in the angiogenic response to NRG. Future studies may help elucidate the role of EPC and VSMC in NRG-mediated angiogenesis.
The role of NRG autocrine effects on endothelial responses was further studied in an isolated cell culture system of skeletal muscle endothelium. Deletion of NRG significantly impaired endothelial cord formation in vitro indicating that NRG plays an important cell autonomous role in this response (Figure 5). NRG-induced cord formation is ERK-dependent. Although NRG has been reported to induce the expression of VEGF in epithelial cancer cells,37 our previous work has shown that in vitro EC cord formation in response to NRG is VEGF-independent.6 In addition, our data do not support the idea that VEGF-induced ERK activation is impaired in the setting of loss of EC NRG, suggesting additional pathways, including αvβ3 activation, and other pathways (e.g. endothelial nitric oxide) may be involved in the angiogenic effects of NRG.
In conclusion, endothelial-derived NRG is important in the angiogenic and arteriogenic response to ischaemic limb injury. We hypothesize that the effects of endothelial NRG during vessel growth may be mediated by activating both αvβ3 integrins and ERK signalling. The exciting and novel finding that exogenous administration of rNRG rescues this phenotype and, more importantly, can enhance blood flow recovery in WT mice holds promise for manipulation of this signalling pathway in diseases such as diabetes and coronary artery disease where angiogenic responses (not only in skeletal muscle but also heart muscle) are impaired.
Funding
This work was supported by the National Heart and Blood Institute at the National Institutes of Health (R01HL80176 and K08HL04429 to K.S.R., R01HL077310 to R.R.R., R01HL65662 to A.J.S., and T32HL007950 to N.H.) and the American Heart Association (grant-in-aid 0355623T to K.S.R.).
Conflicts of interest: None declared.
References
- 1.Iivanainen E, Paatero I, Heikkinen SM, Junttila TT, Cao R, Klint P, et al. Intra- and extracellular signaling by endothelial neuregulin-1. Exp Cell Res. 2007;313:2896–2909. doi: 10.1016/j.yexcr.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 2.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dankort DL, Muller WJ. Signal transduction in mammary tumorigenesis: a transgenic perspective. Oncogene. 2000;19:1038–1044. doi: 10.1038/sj.onc.1203272. [DOI] [PubMed] [Google Scholar]
- 4.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 5.Loureiro RM, Maharaj AS, Dankort D, Muller WJ, D'Amore PA. ErbB2 overexpression in mammary cells upregulates VEGF through the core promoter. Biochem Biophys Res Commun. 2005;326:455–465. doi: 10.1016/j.bbrc.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 6.Russell KS, Stern DF, Polverini PJ, Bender JR. Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. Am J Physiol. 1999;277:H2205–2211. doi: 10.1152/ajpheart.1999.277.6.H2205. [DOI] [PubMed] [Google Scholar]
- 7.Kalinowski A, Plowes NJ, Huang Q, Berdejo-Izquierdo C, Russell RR, Russell KS. Metalloproteinase-dependent cleavage of neuregulin and autocrine stimulation of vascular endothelial cells. FASEB J. 2010;24:2567–2575. doi: 10.1096/fj.08-129072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedhli N, Huang Q, Kalinowski A, Palmeri M, Hu X, Russell RR, et al. Endothelium-derived neuregulin protects the heart against ischemic injury. Circulation. 2011;123:2254–2262. doi: 10.1161/CIRCULATIONAHA.110.991125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metzger D, Chambon P. Site- and time-specific gene targeting in the mouse. Methods. 2001;24:71–80. doi: 10.1006/meth.2001.1159. [DOI] [PubMed] [Google Scholar]
- 10.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 11.Monvoisin A, Alva JA, Hofmann JJ, Zovein AC, Lane TF, Iruela-Arispe ML. VE-cadherin-CreERT2 transgenic mouse: a model for inducible recombination in the endothelium. Dev Dyn. 2006;235:3413–3422. doi: 10.1002/dvdy.20982. [DOI] [PubMed] [Google Scholar]
- 12.Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- 13.Yang X, Arber S, William C, Li L, Tanabe Y, Jessell TM, et al. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron. 2001;30:399–410. doi: 10.1016/s0896-6273(01)00287-2. [DOI] [PubMed] [Google Scholar]
- 14.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, et al. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meoli DF, Sadeghi MM, Krassilnikova S, Bourke BN, Giordano FJ, Dione DP, et al. Noninvasive imaging of myocardial angiogenesis following experimental myocardial infarction. J Clin Invest. 2004;113:1684–1691. doi: 10.1172/JCI20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobrucki LW, Dione DP, Kalinowski L, Dione D, Mendizabal M, Yu J, et al. Serial noninvasive targeted imaging of peripheral angiogenesis: validation and application of a semiautomated quantitative approach. J Nucl Med. 2009;50:1356–1363. doi: 10.2967/jnumed.108.060822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zagorchev L, Oses P, Zhuang ZW, Moodie K, Mulligan-Kehoe MJ, Simons M, et al. Micro computed tomography for vascular exploration. J Angiogenes Res. 2010;2:7. doi: 10.1186/2040-2384-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhuang ZW, Gao L, Murakami M, Pearlman JD, Sackett TJ, Simons M, et al. Arteriogenesis: noninvasive quantification with multi-detector row CT angiography and three-dimensional volume rendering in rodents. Radiology. 2006;240:698–707. doi: 10.1148/radiol.2403050976. [DOI] [PubMed] [Google Scholar]
- 19.Lanahan AA, Chittenden TW, Mulvihill E, Smith K, Schwartz S, Simons M. Synectin-dependent gene expression in endothelial cells. Physiol Genomics. 2006;27:380–390. doi: 10.1152/physiolgenomics.00145.2006. [DOI] [PubMed] [Google Scholar]
- 20.Yu J, Lei L, Liang Y, Hinh L, Hickey RP, Huang Y, et al. An engineered VEGF-activating zinc finger protein transcription factor improves blood flow and limb salvage in advanced-age mice. FASEB J. 2006;20:479–481. doi: 10.1096/fj.04-3670fje. [DOI] [PubMed] [Google Scholar]
- 21.Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G, et al. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell. 2006;126:489–502. doi: 10.1016/j.cell.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 22.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 23.Menendez JA, Mehmi I, Griggs DW, Lupu R. The angiogenic factor CYR61 in breast cancer: molecular pathology and therapeutic perspectives. Endocr Relat Cancer. 2003;10:141–152. doi: 10.1677/erc.0.0100141. [DOI] [PubMed] [Google Scholar]
- 24.Hua J, Dobrucki LW, Sadeghi MM, Zhang J, Bourke BN, Cavaliere P, et al. Noninvasive imaging of angiogenesis with a 99mTc-labeled peptide targeted at alphavbeta3 integrin after murine hindlimb ischemia. Circulation. 2005;111:3255–3260. doi: 10.1161/CIRCULATIONAHA.104.485029. [DOI] [PubMed] [Google Scholar]
- 25.Munoz-Chapuli R, Quesada AR, Angel Medina M. Angiogenesis and signal transduction in endothelial cells. Cell Mol Life Sci. 2004;61:2224–2243. doi: 10.1007/s00018-004-4070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F. Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 1999;18:882–892. doi: 10.1093/emboj/18.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheppard D. Endothelial integrins and angiogenesis: not so simple anymore. J Clin Invest. 2002;110:913–914. doi: 10.1172/JCI16713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Kelly J, Chung A, Lemp N, Chumakova K, Yin D, Wang HJ, et al. Functional domains of CCN1 (Cyr61) regulate breast cancer progression. Int J Oncol. 2008;33:59–67. [PubMed] [Google Scholar]
- 29.Ieguchi K, Fujita M, Ma Z, Davari P, Taniguchi Y, Sekiguchi K, et al. Direct binding of the EGF-like domain of neuregulin-1 to integrins ({alpha}v{beta}3 and {alpha}6{beta}4) is involved in neuregulin-1/ErbB signaling. J Biol Chem. 2010;285:31388–31398. doi: 10.1074/jbc.M110.113878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soung YH, Clifford JL, Chung J. Crosstalk between integrin and receptor tyrosine kinase signaling in breast carcinoma progression. BMB Rep. 2010;43:311–318. doi: 10.5483/bmbrep.2010.43.5.311. [DOI] [PubMed] [Google Scholar]
- 31.Kuramochi Y, Cote GM, Guo X, Lebrasseur NK, Cui L, Liao R, et al. Cardiac endothelial cells regulate reactive oxygen species-induced cardiomyocyte apoptosis through neuregulin-1beta/erbB4 signaling. J Biol Chem. 2004;279:51141–51147. doi: 10.1074/jbc.M408662200. [DOI] [PubMed] [Google Scholar]
- 32.Wragg A, Mellad JA, Beltran LE, Konoplyannikov M, San H, Boozer S, et al. VEGFR1/CXCR4-positive progenitor cells modulate local inflammation and augment tissue perfusion by a SDF-1-dependent mechanism. J Mol Med. 2008;86:1221–1232. doi: 10.1007/s00109-008-0390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luft FC. Mononuclear inflammatory cells and angiogenesis. J Mol Med. 2008;86:1193–1195. doi: 10.1007/s00109-008-0401-8. [DOI] [PubMed] [Google Scholar]
- 34.Safa RN, Peng XY, Pentassuglia L, Lim CC, Lamparter M, Silverstein C, et al. Neuregulin-1beta regulation of embryonic endothelial progenitor cell survival. Am J Physiol Heart Circ Physiol. 2011;300:H1311–1319. doi: 10.1152/ajpheart.01104.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalinowski A, Hedhli N, Russell KS. Vascular smooth muscle cells express ErbB receptors and migrate in response to neuregulin. Circulation. 2009;120 S1168b. [Google Scholar]
- 36.Iivanainen E, Nelimarkka L, Elenius V, Heikkinen SM, Junttila TT, Sihombing L, et al. Angiopoietin-regulated recruitment of vascular smooth muscle cells by endothelial-derived heparin binding EGF-like growth factor. FASEB J. 2003;17:1609–1621. doi: 10.1096/fj.02-0939com. [DOI] [PubMed] [Google Scholar]
- 37.Bagheri-Yarmand R, Vadlamudi RK, Wang RA, Mendelsohn J, Kumar R. Vascular endothelial growth factor up-regulation via p21-activated kinase-1 signaling regulates heregulin-beta1-mediated angiogenesis. J Biol Chem. 2000;275:39 451–39 457. doi: 10.1074/jbc.M006150200. [DOI] [PubMed] [Google Scholar]