Introduction
The mammalian nervous system contains multipotent neural stem cells which have the capacity to generate new neurons and glia throughout the lifespan of an animal. Populations of neural stem and progenitor cells (NSC/NPCs) predominately reside in the subventricular zone (SVZ) lining the lateral ventricles and the dentate gyrus (DG) of the hippocampus (Altman & Das, 1965; Lois & Alvarez Buylla, 1993). These cells and their progeny are highly migratory and capable of functional integration into the existing neuronal circuitry of the olfactory bulb and hippocampus (van Praag et al., 2002). Beyond their capacity for migration, NSC/NPCs are capable of altering their mitogenic rate and maturational fate during the normal aging process and in response to a variety of brain insults and diseases (Ming & Song, 2005). These behavioral changes imply that these multipotent cells may play critical roles in brain homeostasis and in the innate reparative processes observed following trauma and in disease states. Such promising findings have underscored the need to develop novel tools to investigate these cell populations in vivo in order to elucidate the many functional roles of neural stem/progenitor cells.
One direct way to obtain information regarding the possible functional roles of these cells is to study developmental or reparative processes in animals where NSC/NPC numbers have been altered. In the absence of NSC/NPC-depleted transgenic animals, methodologies such as whole brain irradiation or anti-mitotic agent administration have exploited the mitotic activity of these cells to deplete them from the CNS (Shors et al., 2002; Tada et al., 1999, 2000). While such investigations have provided some insight into the contribution of NSC/NPCs to cognition, injury repair processes and brain homeostasis, both methodologies are limited in their ability to target region-specific stem/progenitor cell populations. This shortcoming, as well as those associated with the systemic effects of anti-mitotic drug administration, precludes a clearer understanding of the precise functional roles of region-specific NSC/NPCs populations. To this end, we describe a methodology to generate region-specific NSC/NPC depleted animals in an expeditious and reproducible manner.
The discrete localization of neural stem/progenitor cells in neurogenic regions, in combination with their mitotic activity, renders them susceptible targets for elimination by focal irradiation. Utilizing this approach, we are able to generate animals with a sustained depletion of NSC/NPCs in either the SVZ or dentate gyrus. By spatially restricting the irradiation further, animals can also be generated that are depleted of these cell populations in either neurogenic region in a unilateral manner. Collectively, this methodology can be used to generate a wide range of novel NSC/NPC-depleted animal models, which can significantly expand the array of experimental tools used to determine the functional importance of these cell populations.
Methods
X-irradiation procedure and BrdU labeling
Unilateral X-irradiation was used to eliminate dividing populations of NSC/NPCs from the left SVZ of newborn rat pups. Postnatal day 0 (P0) Sprague Dawley rats were anesthetized by hypothermia, and a lead shield was placed over their body, exposing only the surface of the head overlying the left SVZ (fig. 1a). This was achieved by cutting a right triangle (horizontal leg: 5mm; vertical leg: 9mm; hypotenuse:10mm) out of the lead shield and placing the horizontal leg along the midline of the cranium. Using a Faxitron X-ray machine, pups were administered a single dose of irradiation at varying intensities (5 Gray (Gy), 10Gy or 15Gy; n=4/dose) in order to determine the dose that most effectively depletes proliferating NSC/NPCs in the SVZ. Seven days later, animals received a single intraperitoneal injection of 300mg/kg bromodeoxyuridine (BrdU; Sigma) to label proliferating cells. Forty-eight hours later, animals were sacrificed and brains cut coronally (60µm) and processed for BrdU immunohistochemistry as previously described (Hagood et al., 2006). To determine the duration that mitotically active cells remained depleted in the irradiated SVZ, P0 pups were unilaterally exposed to 15Gy X-irradiation and BrdU was administered at varying time points following irradiation (7 days, 14 days, 1 month, 2 months or 3 months; n=3/time point). Animals were sacrificed forty-eight hours following BrdU administration and immunohistochemistry was performed on coronal sections throughout the SVZ.
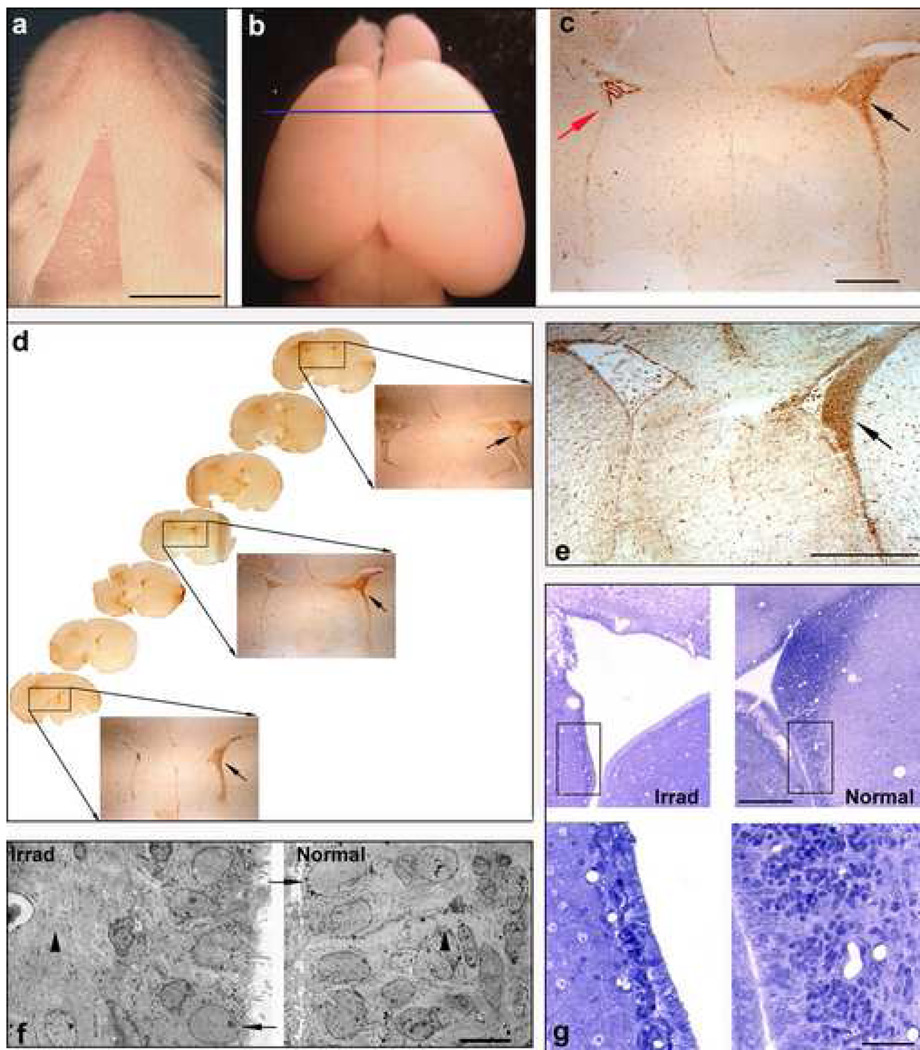
Figure 1. Unilateral X-irradiation eliminates proliferating cells from the ipsilateral SVZ.
(a) Dorsal view of a P9 rat that was unilaterally irradiated at P0. The area devoid of hair represents the irradiated field (bar = 5mm). (b) Brain of the rat shown in a. Blue line represents the position of the section shown in c. (c) Coronal section of the brain of a P9 rat that was unilaterally X-irradiated at P0 and injected with BrdU at P7. Section was immunstained for BrdU and shows the striking reduction of BrdU-positive cells within the SVZ on the irradiated side (red arrow) versus the untreated side (black arrow). Only the choroid plexus is stained brown (bar = 1mm). (d) Coronal sections throughout the SVZ of the rat shown in a immunostained for BrdU showing the depletion of proliferating cells (BrdU+) throughout the rostro-caudal extent of the left SVZ as compared to the untreated right SVZ. (e) Coronal section of the rat brain shown in b immunostained for nestin (bar = 1mm). (f) Electron micrographs of the normal and X-irradiated SVZ showing the elimination of dividing cell populations (arrowheads) and the persistence of post-mitotic ependymal cells (arrows) in the X-irradiated SVZ (bar = 5µm). (g) Semithins (upper panels) showing the depletion of cells residing in the X-irradiated SVZ versus the untreated side (bar = 500µm). Areas in the black boxes are shown at higher magnification in the lower panels (bar = 50µm).
In order to selectively target NSC/NPCs in the hippocampus, rats aged P0 to 1 month were anesthetized and a lead shield was placed over their body, exposing only the surface of the head overlying the rostro-caudal extent of the left hippocampus (see fig. 4, top panel). This was achieved by placing a rectangular opening (2mm×4mm) in the lead shield directly over the hippocampus.
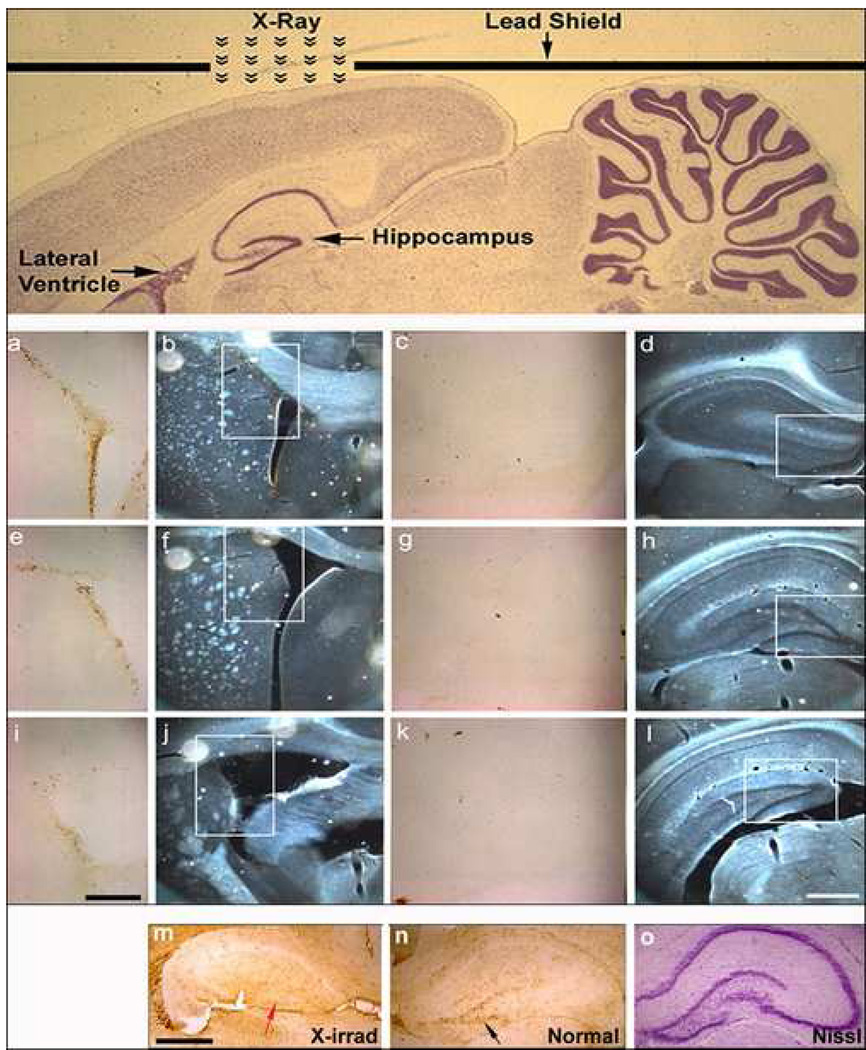
Figure 4. X-irradiation can be used to deplete neural stem/progenitor cell populations in a region specific manner.
By altering the placement of the lead shield, as shown in the Nissl-stained sagittal section in the upper panel, NSC/NPCs can be depleted from the rat hippocampus while being spared in the SVZ. The lower panels show BrdU-immunostained sections (a,c,e,g,i,k; bar = 250µm) from regions of the SVZ and hippocampus of a P30 rat irradiated at P28 (white boxes in the phase contrast images; b,d,f,h,j,l; bar = 500µm) that demonstrate the selective depletion of neural stem/progenitor cells from the hippocampus. (m–o) Targeted hippocampal irradiation at the time of birth (P0) eliminates NSC/NPCs from the hippocampus during the developmental formation of the dentate gyrus (bar = 300µm). Note the conspicuous absence of BrdU+ cells in the irradiated hippocampus (m, red arrow) as compared to the untreated side (n, black arrow).
To eliminate NSC/NPCs from either neurogenic region in a bilateral manner, a mirror-image opening was added to the original lead shield configuration.
BrdU quantification and statistical analysis
The total number of BrdU-positive cells in the X-irradiated and untreated SVZ was determined using the optical fractionator method of quantification and the Olympus CAST Stereology program (Gundersen et al., 1988). Specifically, BrdU-positive cells within a known fraction of the SVZ are quantified and the total number of cells is extrapolated by multiplying the number of cells counted by the reciprocal of the fraction of tissue that has been quantified. Values are presented as mean ± SEM (p< 0.05) and statistical significance was determined using single-factor ANOVA with a post hoc Tukey’s W-procedure.
Nestin immunohistochemistry
Due to their mitotic capacity, the populations residing in the SVZ that are vulnerable to X-irradiation include neural stem cells (Type B cells), neural progenitors (Type C cells) and some migrating neuroblasts (Type A cells). To confirm the NSC/NPC-identity of the cells depleted by X-irradiation, coronal sections throughout the SVZ of unilaterally irradiated P7 rats were immunostained for the protein nestin (an intermediate filament expressed by NSC/NPCs (Lendahl et al., 1990)) as previously described (Hagood et al., 2006).
Ultrastructural analysis
Newborn rat pups were unilaterally irradiated and at P7 were perfused with 4% paraformaldehyde/.1% glutaraldehyde in 0.1M PO4 and brains processed for electron microscopy. Briefly, brains were cut in 1mm coronal sections and those containing the SVZ were rinsed with phosphate buffer, osmicated, dehydrated and infiltrated with epon/araldite. Both semithin (1µm) and thin sections (80nm) of the irradiated and untreated SVZ were cut, stained and viewed with a Jeol electron microscope.
Proteomic analysis
P0 pups were unilaterally X-irradiated (15Gy), euthanized at P7and SVZ tissue was processed for 2D-PAGE (Kendrick Laboratories (Madison, WI) and stained with a mass spectrometry-compatible special silver stain as previously described (Colello et al., 2002). Three duplicate gel sets from the X-irradiated and untreated SVZ were generated (samples pooled from four P7 rat pups; n=12). A manual and computer automated subtractive comparative analysis between gel sets of the X-irradiated and untreated SVZ was performed to reveal proteins that were significantly down-regulated in the X-irradiated SVZ. Protein spots representing a decline in expression commensurate with the percent reduction (>85%) of the mitotically active cells present in the X-irradiated SVZ as compared to the untreated SVZ were considered proteins of interest. Liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS; performed by the Stanford Mass Spectrometry Laboratory, Stanford University) was used to determine the amino acid sequence of proteins, which was compared to theoretical MS spectra of known proteins using a MASCOT database search.
Results
Targeted X-irradiation depletes proliferating cells from the subventricular zone
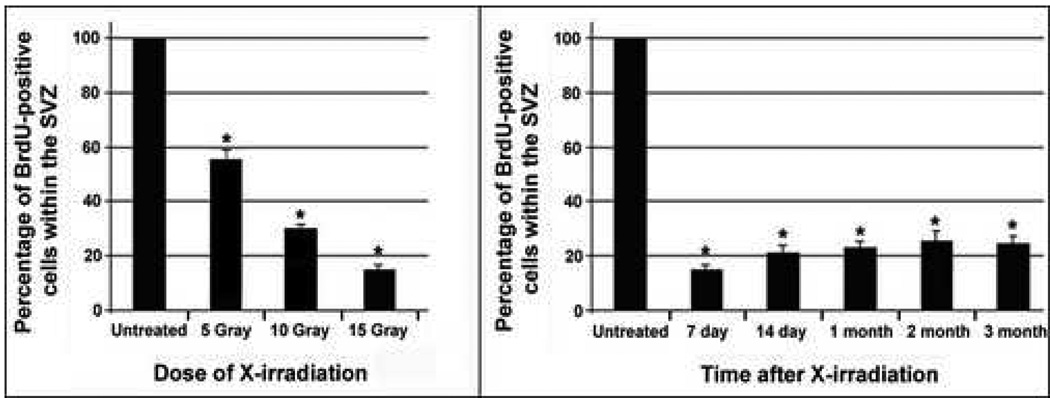
Targeted, unilateral X-irradiation was used to eliminate dividing populations of NSC/NPCs from the left SVZ of newborn rat pups. To determine the dose of X-irradiation that most effectively depletes the subventricular zone of proliferating NSC/NPC populations, newborn rat pups were unilaterally X-irradiated with varying doses of irradiation (5Gy, 10Gy, 15Gy) and administered BrdU seven days later to label mitotically active cells. A comparison of BrdU incorporation in the X-irradiated versus untreated SVZ revealed a dramatic decline in the number of BrdU-labeled cells throughout the rostro-caudal extent of the treated SVZ (fig. 1c,d). Using stereological methodologies to determine the number of BrdU-positive cells in both the normal and X-irradiated SVZ, we found that 15Gy was the most effective dose for maximally eliminating proliferating cells in the SVZ while inducing minimal tissue damage. Approximately 140,000 proliferating cells were observed within the normal non-irradiated SVZ. 47% of dividing cells were eliminated upon exposure to 5Gy irradiation, while 68% and 85% of BrdU-positive cells were eliminated following 10Gy and15Gy exposures, respectively (fig. 2a). Since the untreated SVZ was used as a control in this study, we wanted to ensure that cell numbers in the untreated SVZ were not altered by the unilateral X-irradiation treatment. The number of dividing cells observed in naïve animals was comparable to that of the untreated SVZ of unilaterally X-irradiated animals, indicating that unilateral administration of X-irradiation solely affected proliferation levels in the exposed SVZ (data not shown).
Figure 2. Unilateral X-irradiation eliminates proliferating cells from the ipsilateral SVZ in a dose-dependent manner and for up to three months.
(left) Graph showing the number of BrdU-positive cells found within the X-irradiated SVZ (as a percentage of the untreated SVZ) at various exposure doses. Newborn rats were unilaterally X-irradiated at P0, administered BrdU at P7 and left to survive for two days. X-irradiation at all doses significantly reduced the number of BrdU-labeled cells in the irradiated SVZ as compared to the untreated SVZ in a dose-dependent manner (*p<0.05; mean ± SEM; n=4/ dose). (right) Graph showing cell proliferation levels in the X-irradiated SVZ (as a percentage of the untreated SVZ) at various time points following a unilateral exposure of 15Gy X-irradiation at P0. The percent of BrdU-positive cells eliminated by a single 15Gy treatment is maintained throughout a three-month survival period (*p<0.05; mean ± SEM; n=3/ time point).
Immunohistochemistry, ultrastructural analysis and proteomic methodologies confirm the neural stem/progenitor cell-identity of cells targeted by X-irradiation
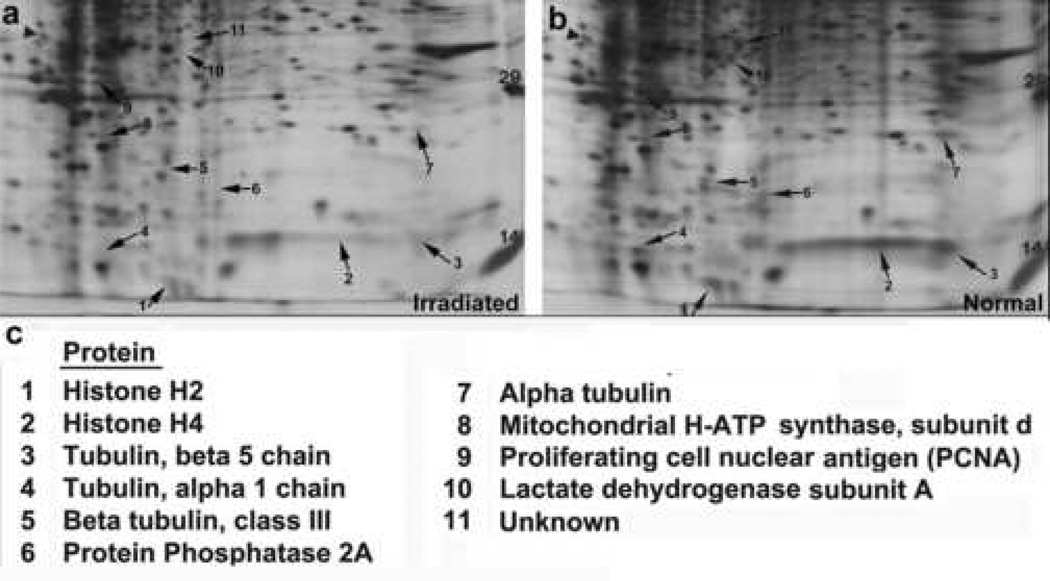
Once the optimal dose for eliminating proliferating cells from the SVZ was established, we next set out to determine the duration that mitotically active cells remained depleted in the irradiated SVZ. P0 pups were unilaterally exposed to 15Gy X-irradiation and BrdU was administered at 7 days, 14 days, 1 month, 2 months or 3 months following irradiation to label dividing cells. The proliferating population of NSC/NPCs remained largely depleted for up to 3 months following irradiation, the latest time point assessed (fig. 2b). This inability of the X-irradiated SVZ to repopulate itself demonstrates that slowly dividing stem cells (Type B cells) as well as their more rapidly dividing progeny (Type A and C cells) residing in this region were targeted by our irradiation paradigm. Immunostaining for nestin, a known NSC/NPC-expressed protein (Lendahl et al., 1990), revealed very few nestin-positive cells in the irradiated SVZ as compared to the normal SVZ, thereby further confirming the NSC/NPC-identity of cells targeted by the X-irradiation procedure (fig. 1e). Ultrastructural analysis of the X-irradiated SVZ further verified the absence of the NSC/NPC populations while also revealing that ependymal cells persist following irradiation (fig. 1f,g). This indicates that post-mitotic cell populations are not eliminated by exposure to the levels of X-irradiation administered in this study and therefore further validates the selective nature of the X-irradiation procedure. Lastly, a proteomic analysis of the protein profiles found within the normal SVZ versus the X-irradiated SVZ revealed an absence or significant down-regulation of several known NSC/NPC-associated proteins in the X-irradiated SVZ (fig. 3). Many of the proteins identified through this analysis are proteins known to be expressed in rapidly dividing progenitor cell populations (histones H2 and H4, the α- and β-tubulin family of proteins) (Stein, et al., 1996). Moreover, several of the identified proteins have previously been associated specifically with neural stem/progenitor and/or precursor cell populations in the brain (β-tubulin, class III, PCNA, protein phosphatase 2A, mitochondrial H+-ATP synthase, lactate dehydrogenase) (Doetsch et .al, 1997; Menezes et al., 1995; Memberg, et al., 1995; Maurer, et al., 2004; Ramalho-Santos et al., 2002). The absence of these proteins in the X-irradiated SVZ further supported the notion that these neural stem/progenitor cell populations were depleted in this X-irradiation experimental model.
Figure 3. The elimination of neural stem/progenitor cells by X-irradiation is confirmed by proteomic analysis of NSC/NPC-associated proteins.
Standard format, 10% SDS, silver-stained 2D gels showing proteins expressed in the normal SVZ (a) versus the X-irradiated SVZ (b) of P7 rats unilaterally irradiated at P0 (MW range of 10–40kDa proteins are shown). Arrows point to distinct proteins that are present in the normal SVZ and absent or significantly downregulated in the X-irradiated SVZ. (c) Selected proteins that display this expression pattern were identified using LC-ESI-MS/MS. Several of those proteins are expressed by NSC/NPC populations.
Focal X-irradiation depletes neural stem/progenitor cells from the dentate gyrus
We next set out to determine whether a similar approach could be used to selectively target NSC/NPCs in the hippocampal dentate gyrus and to ascertain the applicability of this model in rats of varying ages. Specifically, rats ranging in age from P0 to 1 month were exposed to a single dose of targeted X-irradiation (15Gy) to the hippocampus and administered BrdU to label proliferating cells (fig. 4). The presence of BrdU+ cells in the SVZ and their conspicuous absence in the DG demonstrated that focal irradiation can be used to exclusively target NSC/NPCs from this neurogenic region of the brain, while sparing those residing in the SVZ (fig. 4). Depending on the age of the animal, the effect of targeted X-irradiation to the DG differed. Delivery of X-irradiation to the neonatal P0 rat resulted in a complete absence of the dentate gyrus, due to the fact that this anatomical structure is not completely formed until three weeks after birth in rodents (fig. 4m,n,o). On the contrary, X-irradiation administration to the DG of 28 day old rats resulted solely in the elimination of BrdU+ proliferating cells in this neurogenic region.
Discussion
The critical functions that neural stem/progenitor cells are thought to perform in the brain combined with their potential therapeutic application has precipitated a sense of urgency in our desire to better understand these cell types and the manner in which they function. One approach to obtain information regarding NSC/NPC function is to study developmental or reparative processes in animals depleted of these cell populations. Through a series of immunohistochemical, ultrastructural and proteomic investigations, we demonstrate that focal X-irradiation can be utilized to selectively eliminate NSC/NPCs from either the SVZ or dentate gyrus, in a unilateral or bilateral manner. Employing this methodology, we are able to create several NSC/NPC-depleted animal models which should prove valuable tools to aid in our understanding of the functions of regionally distinct neural stem/progenitor cell populations.
Utilizing targeted X-irradiation and the cell proliferation marker BrdU, we demonstrated that both the SVZ and DG can be depleted of proliferating cells in a dose dependent manner, for a sustained period of time. Our findings are in agreement with previous investigations demonstrating the effectiveness of X-irradiation in eliminating proliferating populations of neural stem and progenitor cells in embryonic, juvenile and adult rats (Jensh et al., 1995; Tada et al., 1999, 2000) as well as mice (Raber et al., 2004; Mizumatsu et al., 2003). The current study represents the first of its kind in neonatal rats, a period when NSC/NPC numbers and proliferation levels are at the highest in the postnatal brain. It is also distinct from previous investigations in that focal X-irradiation, as opposed to whole brain irradiation or systemic anti-mitotic agents, is utilized to specifically eliminate proliferating NSC/NPC populations residing in either the SVZ or the dentate gyrus. Such targeted X-irradiation allows for the generation of several combinations of NSC/NPC-depleted animal models, whereby either neurogenic region can be targeted in either a unilateral or bilateral manner. The doses of X-irradiation administered in this study (5–15Gy) were chosen because they had previously been shown to eliminate NSC/NPCs from the neurogenic regions of adult animals without inducing the damage to post-mitotic CNS cells and/or white matter tracts observed at higher doses (≥20Gy). A single exposure to 15Gy X-irradiation was the most effective of the doses assessed and resulted in the ablation of over 85% of BrdU-positive proliferating cells in the SVZ while inducing little noticeable histological damage.
Upon establishing that focal X-irradiation depletes proliferating cell populations from the neurogenic regions, we next set out to verify that the eliminated cells were indeed neural stem/progenitor cells. This was achieved, in part, by determining the duration that mitotic activity was suppressed in the X-irradiated SVZ. By the latest time point assessed (3 months post-irradiation) proliferation levels in the X-irradiated SVZ remained depleted to 20% of the untreated side. This inability of the X-irradiated SVZ to repopulate itself suggests that slowly dividing stem cells (Type B cells) as well as their more rapidly dividing progeny (Type A and C cells) were targeted by our X-irradiation paradigm. This is based on previous studies demonstrating the capacity of resident neural stem cells to repopulate the SVZ within two weeks following the selective ablation of rapidly dividing progenitor cell populations using anti-mitotic agents (Doetsch et al., 1999). The absence of a similar regenerative response by NSCs in the current study indicates that focal administration of 15Gy X-irradiation to the SVZ diminishes stem cell numbers. Immunostaining for the NSC/NPC-associated marker, nestin, further substantiated the targeted nature of the irradiation experimental model to specifically eliminate SVZ stem/progenitor cells. Additionally, a proteomic analysis revealed the absence of several other NSC/NPC-associated proteins in the irradiated SVZ, further implying the depletion of these cell types (Doetsch et .al, 1997; Menezes et al., 1995; Memberg, et al., 1995; Maurer, et al., 2004; Ramalho-Santos et al., 2002). Data derived from the current and previous studies also provide compelling evidence that the remaining cells types residing in the SVZ, i.e. ependymal cells and postmitotic astrocytes, are not affected in a similar manner by irradiation. Specifically, our ultrastructural analysis revealed that ependymal cells persist in the X-irradiated SVZ. Also, previous studies utilizing a similar X-irradiation model to investigate the developing optic nerve demonstrate that post-mitotic astrocytes are not eliminated by doses of irradiation that are 3-fold those used in the current study (Colello et al., 1994, 2002). Collectively, these observations point to the selective and targeted nature of the irradiation experimental model to specifically eliminate stem and progenitor cells.
The establishment of these NSC/NPC-depleted animal models can provide powerful new tools to enhance our understanding of neural stem cell biology. Ongoing studies in our lab have begun to establish the feasibility of selectively eliminating populations of NSC/NPCs from either neurogenic region at varying time points throughout the lifespan of an animal. The targeted (unilateral or bilateral) elimination of NSC/NPCs from the major neurogenic regions provides a means to determine both the anatomical and functional contribution of these cell types at various stages during postnatal development and throughout the lifespan of an animal. Clarification of the role that these cells play in shaping the cellular make-up and anatomical organization of the brain can be achieved by performing a histological comparison between the brain hemisphere harboring the X-irradiated SVZ/dentate gyrus and the untreated hemisphere of a unilaterally irradiated animal. Utilizing such a unilateral exposure paradigm also provides an internal control within each experimental animal. Incidentally, we have observed that focal X-irradiation (15Gy) to the left SVZ of newborn rats results in a left cerebral hemisphere that is substantially diminished in size. Further histological and morphological examination will allow us to determine the precise contribution of SVZ stem/progenitor cells during this period of postnatal development. With regard to neural stem cell function, previous investigations have utilized both whole brain X-irradiation and anti-mitotic drugs in an effort to establish an association between cognitive function and neurogenesis. While these investigations have demonstrated a compelling link between the two processes, the global effects of whole brain irradiation and the systemic effects of anti-mitotic drugs have prevented these studies from establishing the precise role that each neurogenic region plays in cognition. Investigations into the functional consequences of targeted elimination of NSC/NPCs residing in either the SVZ or the dentate gyrus could potentially bridge this gap in our knowledge (Raber et al., 2004). Furthermore, previous (unpublished) studies in our lab have utilized whole brain X-irradiation to establish a link between the heightened levels of neurogenesis observed following traumatic brain injury and the recovery of cognitive function. Again, the methodological nature of these studies did not allow us to discern the specific contributions of neurogenesis in the DG or the SVZ to cognitive recovery. Such information could be obtained by utilizing targeted X-irradiation to specifically block injury-induced neurogenesis in either the SVZ or dentate gyrus and assessing the effects on cognitive recovery. An additional application of this methodology was described herein. By combining unilateral X-irradiation with proteomic methodologies, we were able to establish the absence of several NSC/NPC-associated proteins in the X-irradiated SVZ as compared to the untreated side. It should be noted that this combined paradigm represents a novel methodology for elucidating the repertoire of proteins expressed by NSC/NPCs in vivo. The conspicuous lack of known molecular/antigenic markers that are exclusive to neural stem cells has been a major impediment to our understanding of NSCs as well as our ability to study these cell populations. Thus novel methodologies for elucidating their protein expression profiles are needed. To this end, studies in our lab have begun to utilize this combined X-irradiation/proteomics paradigm to elucidate the proteins expressed by NSC/NPC populations residing in both the SVZ and the dentate gyrus. The results of these studies will allow for a comparison of NSC/NPC protein expression profiles from the two major neurogenic regions and will allow us to address the homogeneity/heterogeneity of region-specific neural stem/progenitor cell populations
In summary, we have exploited the mitogenic activity of neural stem/progenitor cells within the subventricular zone and dentate gyrus to selectively eliminate these cell populations using X-irradiation. The targeted nature of this approach makes it superior to previously utilized whole brain X-irradiation or pharmacological methods in that it can produce animal models that are depleted of NSC/NPCs in either neurogenic region, in a unilateral or bilateral manner. The reproducibility, versatility and ease of generation make these experimental animal models a valuable tool to aid in our understanding of the properties and functions of neural stem/progenitor cells, under both normal and pathological conditions in the brain.
Acknowledgements
This work was supported by NIH-NINDS NS048377. Microscopy was performed at the VCU-Dept. of Anatomy & Neurobiology Microscopy Facility, supported, in part, with funding from NIH-NINDS center core grant (5P30NS047463).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Colello RJ, Fuss B, Fox MA, Alberti J. A proteomic approach to rapidly elucidate oligodendrocyte-associated proteins expressed in the myelinating rat optic nerve. Electrophoresis. 2002;23:144–151. doi: 10.1002/1522-2683(200201)23:1<144::AID-ELPS144>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Colello RJ, Pott U, Schwab ME. The role of oligodendrocytes and myelin on axon maturation in the developing rat retinofugal pathway. J Neurosci. 1994;14:2594–2605. doi: 10.1523/JNEUROSCI.14-05-02594.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci USA. 1999;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A. Some new and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Hagood SK, McGinn MJ, Colello RJ. Characterizing the mitogenic effect of basic fibroblast growth factor in the adult rat striatum. J. Neurotrauma. 2006;23:205–215. doi: 10.1089/neu.2006.23.205. [DOI] [PubMed] [Google Scholar]
- Jensh RP, Eisenman LM, Brent RL. Postnatal neurophysiologic effects of prenatal X-irradiation. Int J Radiat Biol. 1995;67:217–227. doi: 10.1080/09553009514550271. [DOI] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci USA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer MH, Feldmann RE, Jr, Futterer CD, Butlin J, Kuschinsky W. Comprehensive proteome expression profiling of undifferentiated versus differentiated neural stem cells from adult rat hippocampus. Neurochem Res. 2004;29:1129–1144. doi: 10.1023/b:nere.0000023600.25994.11. [DOI] [PubMed] [Google Scholar]
- Memberg SP, Hall AK. Dividing neuron precursors express neuron-specific tubulin. J Neurobiol. 1995;27:26–43. doi: 10.1002/neu.480270104. [DOI] [PubMed] [Google Scholar]
- Menezes JRL, Smith CM, Nelson KC, Luskin MB. The division of neuronal progenitor cells during migration in the neonatal mammalian forebrain. Mol. Cell. Neurosci. 1995;6:496–508. doi: 10.1006/mcne.1995.0002. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Stein GS, Stein JL, Van Wijnen AJ, Lian JB. Transcriptional control of cell cycle progression: the histone gene is a paradigm for the G1/S phase and proliferation/differentiation transitions. Cell Biol. Int. 1996;20:41–49. doi: 10.1006/cbir.1996.0007. [DOI] [PubMed] [Google Scholar]
- Tada E, Parent JM, Lowenstein DH, Fike JR. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience. 2000;99:33–41. doi: 10.1016/s0306-4522(00)00151-2. [DOI] [PubMed] [Google Scholar]
- Tada E, Yang C, Gobbel GT, Lamborn KR, Fike JR. Long-term impairment of subependymal repopulation following damage by ionizing irradiation. Exp Neurol. 1999;160:66–77. doi: 10.1006/exnr.1999.7172. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]