Abstract
Interleukin-17 (IL-17) and IL-25 signaling induce the expression of genes that encode inflammatory factors and they are implicated in the pathology of various inflammatory diseases. Nuclear factor κB (NF-κB) activator 1 (Act1) is an adaptor protein and E3 ubiquitin ligase that is critical for IL-17 and IL-25 signaling, and it is recruited to their receptors through heterotypic interactions between their SEFIR [SEF (similar expression to fibroblast growth factor genes)/IL-17R] domains. Modeling of SEFIR domains has shown their structural similarity with the Toll-IL-1 receptor (TIR) domains of Toll-like receptors (TLRs) and the IL-1R. Whereas the BB′ loop of TIR is required for TIR-TIR interactions, we found that deletion of the BB′ loop from Act1 or IL-17RA (a common subunit of IL-17R and IL-25R) did not affect Act1–IL-17RA interactions. Instead, deletion of the CC′ loop from Act1 or IL-17RA abolished the interaction between Act1 and IL-17RA, suggesting that SEFIR and TIR domains interact in different manners. Surface plasmon resonance measurements showed that a peptide corresponding to the CC′ loop bound directly to IL-17RA. A cell-permeable decoy peptide based on the CC′ loop sequence inhibited IL-17- and IL-25-mediated signaling, and it inhibited IL-17- and IL-25-induced responses in vitro and pulmonary inflammation in vivo. Together, these findings provide the molecular basis for the specificity of SEFIR versus TIR domain interactions and consequent signaling. Moreover, we suggest that the CC′ loop motif of SEFIR domains is a promising target for therapeutic strategies against IL-17- and IL-25-asssociated inflammatory diseases.
INTRODUCTION
Homology-based cloning has revealed six members of the family of interleukin-17 (IL-17) cytokines, which are termed IL-17A to IL-17F. IL-17A (also known simply as IL-17), which is produced by the T helper 17 (TH17) subset of CD4+ T cells, is the prototypic IL-17 family member, and it exerts its actions either as a homodimer or as a heterodimer with IL-17F. IL-17 is required for host defense against extracellular microorganisms and is also involved in the pathogenesis of various human and animal autoimmune diseases, such as rheumatoid arthritis, multiple sclerosis (MS), experimental autoimmune encephalomyelitis (EAE, a mouse model of MS), and allergen-induced pulmonary inflammation (1–7). The main function of IL-17 is to coordinate local tissue inflammation through the increased production of proinflammatory and neutrophil-mobilizing cytokines and chemokines. IL-17 promotes the accumulation of neutrophils in the bronchoalveolar lavage (BAL) fluid of rats and mice (8, 9). Increased concentrations of IL-17 are found in the lungs and blood of allergic asthma patients and are correlated to the severity of asthma (10–12).
Another well-characterized IL-17 family member is IL-17E (also referred as IL-25), which is the most divergent member of the IL-17 family. IL-25 is produced by airway epithelial cells in response to allergens and it is produced by mouse CD4+ T cells that have a TH2 profile as well as by the human eosinophils and basophils, which are innate effector cells (13, 14). IL-25 plays a critical role in the initiation and propagation of the TH2-type immune response (14–17). Transgenic expression of IL-25 in mice as well as recombinant IL-25 induce TH2-type immunity and lead to increases in the concentrations of the TH2-type cytokines IL-4, IL-5, and IL-13, as well as increased eosinophilia and serum concentrations of immunoglobulin E (IgE) (13, 18, 19). In IL-25-deficient mice, expulsion of helminth parasites is delayed, indicating an impairment of the TH2-type immune response. Furthermore, endogenous IL-25 is critical in allergen-induced pulmonary airway hyperreactivity (AHR) and inflammation in a mouse model of asthma (16). Increased concentrations of IL-25 are detected in asthmatic lung tissues compared to those found in normal tissue, highlighting a role for IL-25 in allergic pulmonary inflammation (15).
Given studies that have defined critical roles for IL-17 (IL-17A) and IL-25 (IL-17E) in autoimmune inflammatory responses, a mechanistic understanding of IL-17- and IL-25-mediated signaling is required to develop new and improved therapeutics for the treatment of inflammatory diseases. IL-17R (IL-17RA and IL-17RC) and IL-25R (IL-17RB and IL-17RA) belong to the recently defined family of SEFIR proteins, which are characterized by the presence of a conserved SEFIR domain in their cytoplasmic regions (20). SEFIR domains share limited homology with Toll-IL-1 receptor (TIR) domains of Toll-like receptors (TLRs) and IL-1Rs, and SEFIR and TIR domains together constitute the STIR (SEFIR/TIR)-domain superfamily. Because TIR domains mediate TIR-TIR homotypic interactions, it was thought that the SEFIR domain might also mediate protein-protein interactions. We and others reported that the adaptor protein nuclear factor κb (NF-κB) activator 1 (Act1, encoded by the gene TRAF3IP2), which is also known as CIKS [connection to inhibitor of κB kinase (IKK) and stress-activated protein kinase (SAPK)/c-Jun N-terminal kinase (JNK)], is a key component in IL-17 and IL-25 signaling (9, 21–23). Act1 also contains a SEFIR domain and is a member of the SEFIR protein family. Upon stimulation with IL-17, Act1 is recruited to the IL-17R and the IL-25R through SEFIR-SEFIR domain interactions, which is followed by the recruitment of transforming growth factor b–activated kinase 1 (TAK1) and tumor necrosis factor (TNF) receptor–associated factor (TRAF) 6, which mediate downstream activation of NF-κB (9, 21, 24, 25). In addition, Act1 contains a helix-loop-helix domain at its N-terminus, two TRAF-binding sites, and a U-box-like region N-terminal to the SEFIR domain. We found that Act1 functions as an E3 ubiquitin ligase through its U-box-like region, and that this activity is essential for IL-17-mediated signaling pathways (26). Consistent with its role in signaling, Act1 is required for IL-17-dependent expression of genes encoding proinflammatory factors and for pulmonary neutrophilia, whereas Act1 deficiency abolishes IL-25-induced production of TH2-type cytokines and IL-25-dependent pulmonary eosinophilia (9).
Although heterodimeric interactions between the SEFIR domain of Act1 and that of the IL-17R are known, the molecular details of SEFIR-SEFIR interactions still remain unclear. Here, we investigated the molecular basis for the specificity of SEFIR versus TIR domains in protein-protein interactions and signaling. We applied our findings to a potential therapy for airway inflammation by developing cell-permeable inhibitory peptides that suppressed IL-17 and IL-25 signaling. Structural models of SEFIR domains showed their similarity to TIR domains, containing a central five-stranded β-sheet surrounded by five roughly parallel helices. Similar to TIR domains, the loop between the second strand and helix (the BB′ loop) of the SEFIR domain is quite prominent. Whereas previous studies have shown that the BB′ loop is required for TIR-TIR domain interactions, we found that deletion of the region containing the BB′ loop did not affect the interaction between Act1 and IL-17RA. Indeed, an additional helix (Cins) is inserted between the third strand (C) and third helix (C′) in the SEFIR domain. We defined a distinct interaction interface (the CC′ loop) within the Act1 SEFIR domain by mutagenesis and decoy peptide approaches. The CC′ loop in the Act1 SEFIR domain was required for the interaction of Act1 with IL-17RA (the common subunit of IL-17R and IL-25R), as well as for IL-17- and IL-25-dependent signaling. A cell-permeable decoy peptide designed from the CC′ loop of the Act1 SEFIR domain inhibited IL-17- and IL-25-mediated signaling and inflammatory gene expression in cultured cells. The decoy peptide also inhibited IL-17- and IL-25-induced pulmonary inflammation in mice, suggesting that the CC′ loop might be a promising therapeutic target region within Act1 for the treatment of IL-17- and IL-25-asssociated inflammatory diseases.
RESULTS
The SEFIR3 region is critical for interactions between the Act1 SEFIR domain and IL-17RA
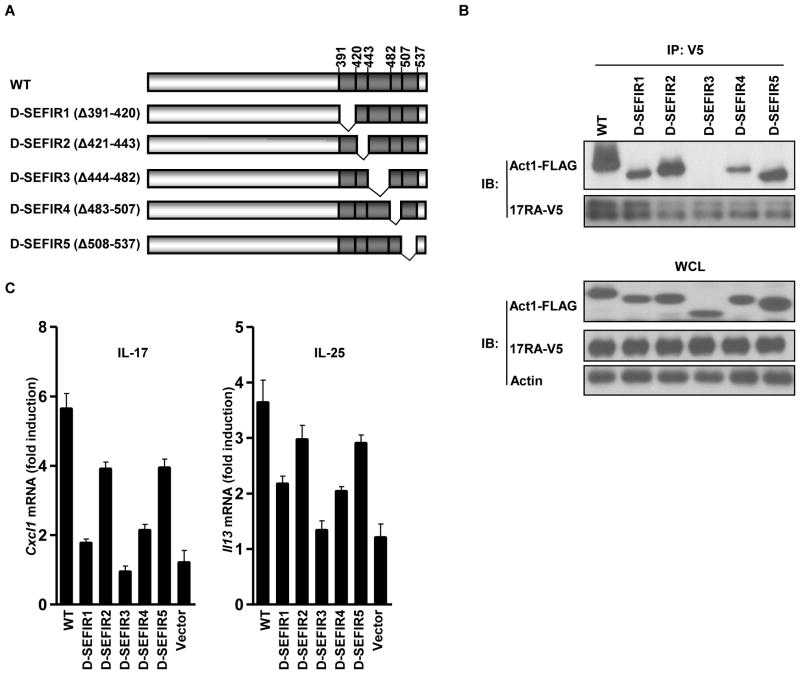
Act1 acts as an intermediate signaling component that is recruited to IL-17RA through heterodimeric interactions between their SEFIR domains (24, 25). We wanted to determine whether disruption of this SEFIR-SEFIR domain interaction was a feasible strategy to block IL-17 and IL-25 signaling. First, we mapped the SEFIR regions that were critical for this interaction by constructing deletion mutants of the Act1 SEFIR domain (Fig. 1A). These mutants, designated SEFIR1 to SEFIR5, were generated as a result of deletions of five exons that encode regions of the SEFIR domain, corresponding to amino acid residues 391 to 420, 421 to 443, 444 to 482, 483 to 507, and 508 to 537, respectively. We cotransfected HeLa cells with plasmids encoding FLAG-tagged wild-type Act1 or its SEFIR deletion mutants (D-SEFIR1 to D-SEFIR5) together with plasmid encoding the V5-tagged IL-17RA. Whereas D-SEFIR1, D-SEFIR2, D-SEFIR4, and D-SEFIR5 retained similar interactions with IL-17RA as occurred with wild-type Act1, the D-SEFIR3 deletion mutant of Act1 did not interact with the receptor (Fig. 1B). Furthermore, the D-SEFIR3 mutant Act1 was unable to restore IL-17-induced expression of Cxcl1 and IL-25-induced expression of Il13 in transfected Act1−/− mouse embryonic fibroblasts (MEFs) (Fig. 1C). These data demonstrate that SEFIR3 is a critical region for the interaction between Act1 and IL-17RA as well as for IL-17- and IL-25-dependent signaling.
Fig. 1.
The SEFIR3 region is critical for the interaction between the Act1 SEFIR domain and IL-17RA. (A) Schematic of wild-type (WT) mAct1 and mAct1 SEFIR deletion mutants. (B) HeLa cells were transiently cotransfected with plasmids encoding FLAG-tagged mouse Act1 or its deletion mutants together with plasmid encoding V5-tagged IL-17RA. Lysates of transfected cells were subjected to immunoprecipation (IP) with antibody against the V5 tag, after which they were analyzed by Western blotting (IB) with antibodies against FLAG and V5. WCL, whole-cell lysate. (C) Act1−/− MEFs expressing IL-17RB were reconstituted with either empty vector, FLAG-tagged mAct1, or FLAG-tagged deletion mutants of mAct1 by retroviral infection, after which they were treated with IL-17 (50 ng/ml) or IL-25 (100 ng/ml) for 3 hours. The abundances of Cxcl1 and Il13 messenger RNAs (mRNAs) were measured by real-time RT-PCR and the results are expressed as fold-induction calculated as a ratio of the abundance of a given mRNA in the treated sample to that in the untreated sample. The experiment was repeated five times and the data are shown as the mean ± the standard error of the mean (SEM). All other experiments were performed three times, with representative blots shown.
A CC′ loop peptide derived from SEFIR3 binds directly to IL-17RA
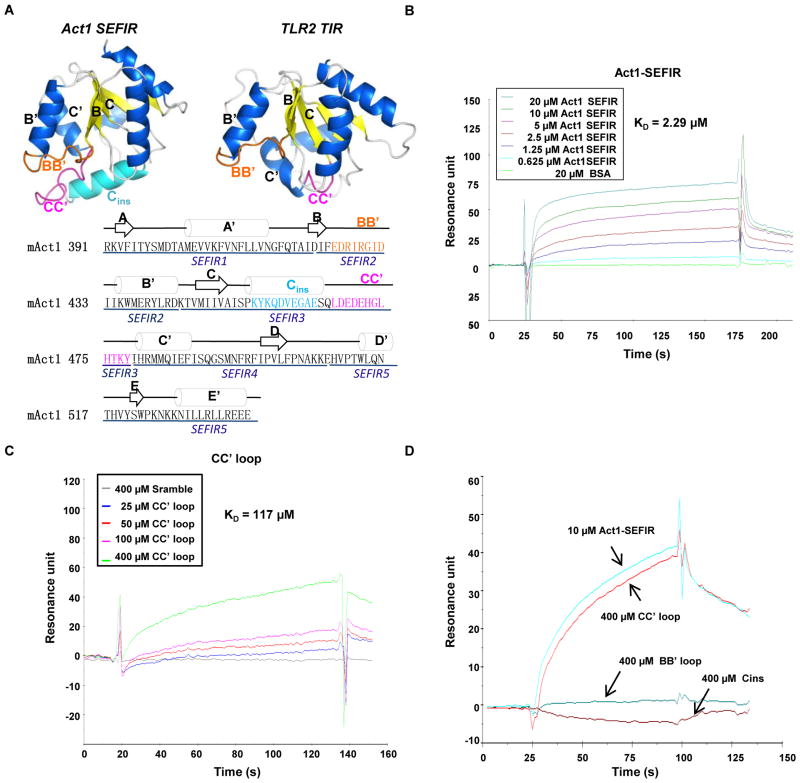
Based on sequence homology, SEFIR domains are thought to resemble TIR domains in their secondary and tertiary structures (20). We therefore carried out computational modeling of the SEFIR domain of Act1 with I-Tasser software (27). Although we did not explicitly specify any initial homology templates, most of the templates selected by I-Tasser were TIR domains from TLR2, TLR4, TLR10, and the TLR adaptor protein MyD88 (myeloid differentiation primary response gene 88). A homology model of the Act1 SEFIR domain showed a central five-stranded β-sheet structure surrounded by five roughly parallel helices (Fig. 2A), which closely resembled the canonical TIR domain fold; however, an additional helix (Cins) was inserted between the third strand (C) and third helix (C′) of the Act1 SEFIR domain. Similar to TIR domains, the loop between the second strand and the second helix (the BB′ loop) of the Act1 SEFIR domain was prominent. Whereas previous studies have shown that the BB′ loop is required for the TIR-TIR domain interaction, we found that deletion of a segment containing the BB′ loop (as occurred in the SEFIR2 mutant) (Fig. 1A and Fig. 2A) did not affect the interaction betweenAct1 and IL-17RA (Fig. 1B). The D-SEFIR3 Act1 mutant, from which the Cins helix and the CC′ loop (which connects Cins to the C′ helix) were deleted, did not interact with IL-17RA (Fig. 1B), suggesting that SEFIR domains interact with each other in a manner different from that of TIR domains.
Fig. 2.
A CC′ loop peptide corresponding to a region from SEFIR3 binds directly to IL-17RA. (A) Computational modeling of the SEFIR domain of mAct1 with I-Tasser in comparison to the TIR domain of TLR2. The SEFIR domain of Act1 consists of five-stranded β-sheets (A to D) and of five roughly parallel α-helices (A′, B′, C′, Cins and C′, D′, and E′) interconnected by loops. The critical structures are colored to highlight secondary structural elements: the BB′ loop is orange; the Cins helix is cyan; and the CC′ loop is pink. The amino acid sequence of the SEFIR domain of mAct1 is shown below the structures together with the modeled secondary structure. (B) SPR analysis of the binding of the mAct1 SEFIR domain to the IL-17RA SEFIR domain. Purified mAct1 SEFIR domain (0.625 to 20 μM) and bovine serum albumin (BSA, 20μM) were injected over the surface to which IL-17RA was immobilized. Binding signal is recorded as resonance units (RU). (C) SPR analysis of the binding of the CC′ loop peptide to the IL-17RA SEFIR domain. The CC′ loop peptide (25 to 400 μM) or a scrambled peptide (Scramble, 400 μM) were injected over the surface to which IL-17RA was immobilized. (D) Comparison of the binding of BB′ loop and the CC′ loop peptides to the IL-17RA SEFIR domain. The BB′ loop, Cins, and CC′ loop peptides (400 μM each) and the Act1 SEFIR domain (10 μM) were injected over the surface to which IL-17RAwas immobilized. Graphs are representative of three experiments.
The Cins helix and the CC′ loop are distinct putative surface-exposed sequences in the SEFIR3 segment of Act1. To determine whether the Cins helix and the CC′ loop interacted with IL-17RA, we used surface plasmon resonance (SPR) to test peptides derived from Cins (SPKYKQDVEGAESQ) and the CC′ loop (LDEDEHGLHTKY) for their ability to bind to the IL-17RA SEFIR domain. As expected, the recombinant Act1 SEFIR domain bound to the sensor-immobilized SEFIR of IL-17RA (Fig. 2B). We also found that the CC′ loop peptide, but not the Cins peptide, bound to the IL-17RA SEFIR domain (Fig. 2. C and D). Furthermore, a peptide derived from the BB′ loop of the Act1 SEFIR domain (EDRIRGID) did not bind to the IL-17RA SEFIR domain (Fig. 2D). This result was consistent with our earlier data that showed that deletion of the BB′ loop did not affect the interaction between Act1 and IL-17RA (Fig. 1B, D-SEFIR2 mutant). The SPR data suggested that the CC′ loop might mediate the interaction between the Act1 SEFIR domain and IL-17RA.
Mutations in the CC′ loop region of the Act1 SEFIR domain disrupt its interaction with IL-17RA and suppress IL-17- and IL-25-depedent signaling
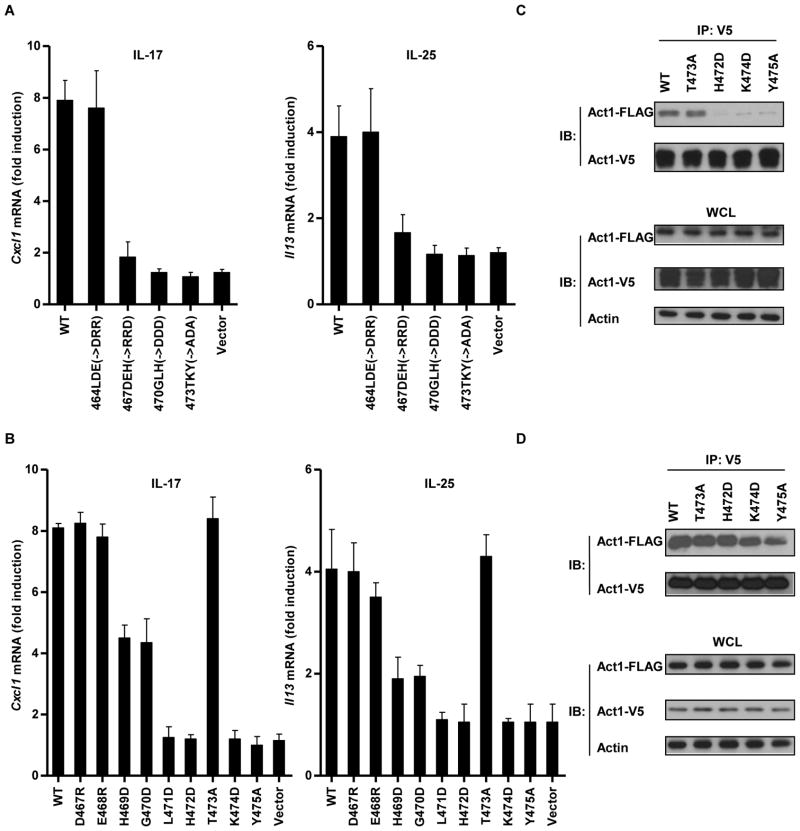
To identify the residues within the CC′ loop region that affected the interaction between Act1 and the IL-17RA, we introduced point mutations within the CC′ loop. First, we generated four mutants of Act1 that contained three amino acid (3-AA) point mutants by replacing each residue in the group of three with a residue of a different charge (Fig. 3A). Three of these mutant proteins (with point mutations located between residues 467 and 475) could not restore IL-17- and IL-25-mediated gene expression in Act1−/− MEFs (Fig. 3A), which suggested that these CC′ loop residues were critical for the interaction between Act1 and the IL-17RA. We next used nine single-residue point mutants to map specific residues that were critical for IL-17RA signaling in more detail. Four point mutants of Act1 (G470D, L471D, K474D, and Y475A) were unable to restore IL-17- or IL-25-mediated gene expression in Act1−/− MEFs (Fig. 3B). Of these mutants, three (L471D, K474D, and Y475A) exhibited almost no interaction with IL-17 RA (Fig. 3C). Because Act1 can form homodimers or homomeric multimers through its SEFIR domain (28), we tested whether residues important for the interaction between Act1 and IL-17RA were also important for Act1-Act1 associations. We found that the L471D, K474D, and Y475A mutants retained the ability to form Act1-Act1 interactions (Fig. 3D), suggesting that Act1 may use distinct interfaces for IL-17 RA interaction and for self-association. These results indicated that the CC′ loop region of the Act1 SEFIR domain was critical for Act1–IL-17RA interactions and for IL-17- and IL-25-dependent signaling.
Fig. 3.
Mutations in the CC′ loop region of the Act1 SEFIR domain disrupt its interaction with IL-17RA and inhibit IL-17 and IL-25 signaling. (A and B) Act1−/− MEFs expressing IL-17RB were reconstituted with empty vector, FLAG-tagged mAct1, or (A) FLAG-tagged 3-AA CC′ loop mutants or (B) single-AA CC′ loop mutants, as indicated, by retroviral infection, after which cells were treated with IL-17 or IL-25. The abundances of Cxcl1 and Il13 mRNAs were measured by real-time RT-PCR, and the fold-induction in mRNA amounts was calculated as a ratio of the amount of mRNA in the treated sample compared to that in the untreated sample. Data are the means ± SEMs from three experiments. (C) HeLa cells were transiently cotransfected with plasmids encoding FLAG-tagged mAct1 or the indicated single-AA point mutants of Act1 together with plasmid encoding V5-tagged IL-17RA. Cell lysates were subjected to immunoprecipation with antibody against V5, after which they were analyzed by Western blotting with antibodies against the FLAG and V5 tags. (D) HeLa cells were transiently cotransfected with plasmids encoding FLAG-tagged mAct1 or the indicated single-AA point mutants of Act1 together with plasmid encoding V5-tagged mAct1. Cell lysates were subjected to immunoprecipitation with antibody against the V5 tag, after which they were analyzed by Western blotting with antibodies against the FLAG and V5 tags. Data in (C) and (D) are representative of three experiments.
A cell-permeable CC′ loop decoy peptide disrupts the Act1–IL-17RA interaction and inhibits IL-17-dependent and IL-25-dependent signaling
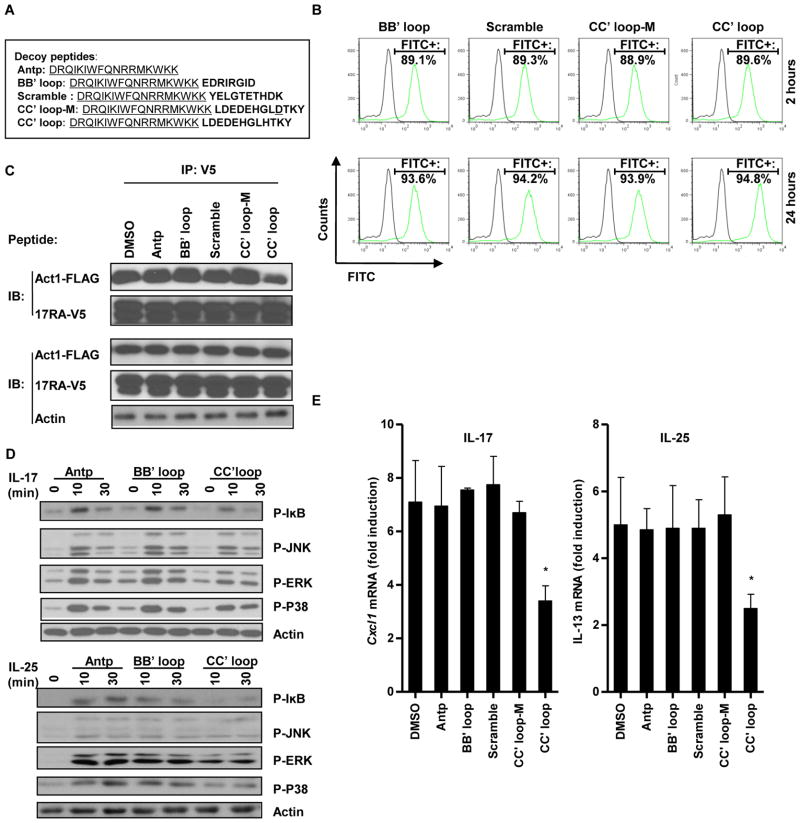
Decoy peptides are based on surface-exposed regions of the primary sequence of a protein to mimic a putative interaction interface (29). A well-folded decoy peptide often has the ability to bind to and occupy the docking site of the interacting partner of the original protein, and this binding then prevents the protein-protein interaction and inhibits downstream signaling. Cell-permeable TIR BB′ loop decoy peptides have been successfully used to inhibit TLR signaling (29). We designed cell-permeable decoy peptides that contained the BB′ loop or CC′ loop sequences of the Act1 SEFIR domain fused to the C-terminus of the translocating segment of the antennapedia homeodomain (Antp: DRQIKIWFQNRRMKWKK) (Fig. 4A). Antp sequence was identified in the homeodomain of the Drosophila transcription factor, antennapedia, and is well known for its ability to penetrate cell membrane and carry cargo peptides into cells (29). These decoy peptides were also tagged at their C-termini with fluorescein isothiocyanate (FITC) to enable their cell- penetrating efficiency to be evaluated by flow cytometric sanalysis. We found that all of the peptides had similar cell permeabilities, and that incubation of the cells with the peptides for 24 hours enabled maximal cell-penetrating efficiency without causing any noticeable adverse effects (Fig. 4B). The decoy peptides were then compared for their ability to inhibit the interaction between Act1 and the IL-17RA. The Act1 SEFIR domain CC′ loop peptide more efficiently inhibited the Act1–IL-17RA interaction than did the Act1 SEFIR domain BB′ loop peptide, the scrambled peptide, and the mutant (CC′ loop-M, H472→D) peptide (Fig. 4C). That the Act1 SEFIR domain CC′ loop decoy peptide inhibited the Act1–IL-17RA interaction suggested that the peptide might also block IL-17 and IL-25 signaling. Indeed, we found that the Act1 SEFIR domain CC′ loop peptide, but not the Act1 SEFIR domain BB′ loop peptide, partly attenuated IL-17- and IL-25-dependent signaling, as assessed by determining that amounts of phosphorylated IκB (pIκB), pJNK, phosphorylated extracellular signal–regulated kinase (pERK) and phosphorylated p38 mitogen-activated protein kinase (pp38 MAPK) by Western blotting (Fig. 4D). Moreover, the Act1 SEFIR domain CC′ loop peptide attenuated IL-17-mediated expression of Cxcl1 (~50% inhibition) and IL-25-mediated expression of Il13 (~60% inhibition), whereas the Act1 SEFIR domain BB′ loop peptide had no effect (Fig. 4E). In addition, the CC′ loop decoy peptide inhibited IL-17- and IL-25-dependent signaling and gene expression in a dose-and time-dependent manner (fig. S1, A and B).
Fig. 4.
A cell-permeable CC′ loop decoy peptide disrupts the interaction between Act1 and IL-17RA and inhibits IL-17R and IL-25R signaling. (A) Sequences of Antennapedia homeodomain (Antp) and decoy peptides with the Antp sequence underlined. (B) MEFs expressing IL-17RB were incubated for 2 or 24 hours with the indicated FITC-tagged peptides (200 μM) and were then analyzed by flow cytometry. Data show the analysis of FITC-positive cells from untreated (black lines) and FITC-tagged peptide-treated (green lines) samples. The percentages of FITC-positive cells in the treated samples are shown. (C) HeLa cells were cotransfected with plasmids encoding FLAG-tagged mAct1 and V5-tagged IL-17RA and were cultured for 24 hours, after which they were incubated for 24 hours with the indicated peptides (200 μM). IL-17RA was immunoprecipitated from the samples with antibody against the V5 tag, and samples were then subjected to Western blotting analysis with the indicated antibodies. (D) MEFs expressing IL-17RB were incubated with the indicated peptides (200 μM) for 24 hours after which they were treated with IL-17 or IL-25 and then analyzed by Western blotting with the indicated antibodies. (E) MEFs expressing IL-17RB were incubated with the indicated peptides (200 μM) for 24 hours, after which they were treated with IL-17 or IL-25 for 3 hours. The abundances of Cxcl1 and Il13 mRNAs were then measured by real-time RT-PCR. Data shown are means ± SEM from three individual experiments. *P < 0.05 (difference between samples treated with DMSO or the CC′ loop peptide). Representative blots are shown from three individual experiments.
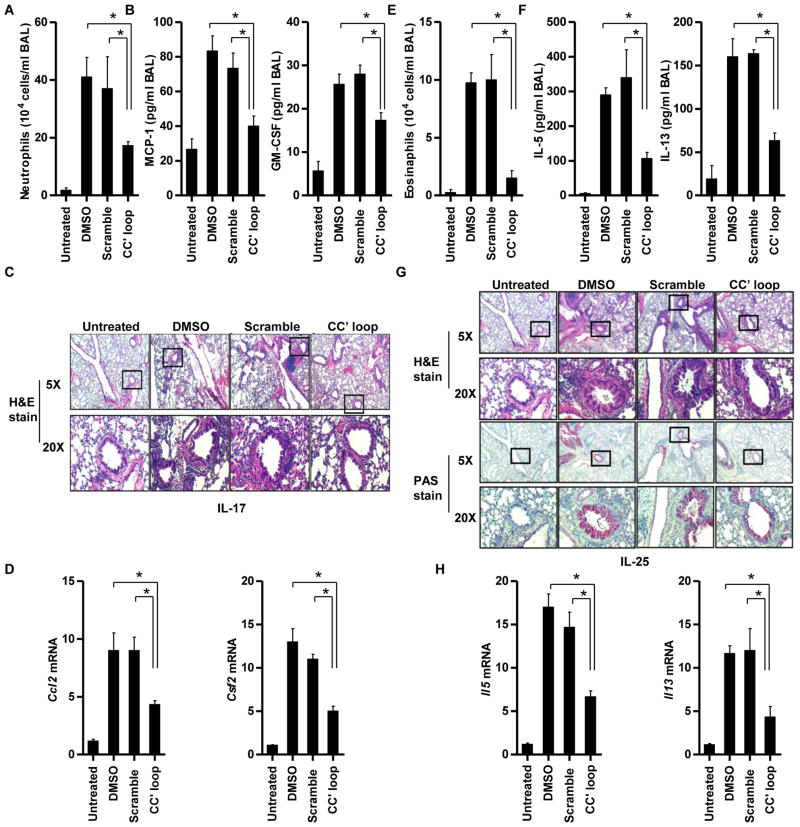
The cell-permeable CC′ loop decoy peptide inhibits IL-17- and IL-25-induced pulmonary inflammation
Because the Act1 SEFIR domain CC′ loop decoy peptide disrupted the Act1–IL-17RA interaction and inhibited IL-17- and IL-25-dependent signaling in cultured cells, we tested whether the peptide also blocked IL-17- and IL-25-dependent effector functions in vivo. We and others have shown that Act1 plays an essential role in IL-17- and IL-25-mediated pulmonary inflammation (9, 22). Thus, we examined whether the Act1 SEFIR domain CC′ loop decoy peptide ameliorated IL-17- and IL-25-induced airway inflammation in vivo. We pretreated BALB/c mice with scrambled peptide or with the Act1 SEFIR domain CC′ loop decoy peptide for 4 hours, after which we injected the mice intranasally with IL-17 or IL-25. We found that IL-17-induced neutrophilia and inflammation, as determined by measurement of neutrophil counts and the amounts of monocyte chemoattractant protein 1 (MCP-1, encoded by the gene Ccl2), a potent neutrophil-attracting chemokine, and granulocyte-macrophage colony-stimulating factor (GM-CSF, encoded by the gene Csf2) in the BAL fluid, were substantially reduced in mice treated with the CC′ loop decoy peptide compared to those in mice pretreated with the scrambled peptide (Fig. 5, A and B). Consistently, IL-17-induced airway recruitment of granulocytes was reduced in mice pretreated with the CC′ loop decoy peptide compared to that in mice pretreated with the scrambled peptide (Fig. 5C). The lower grade of pulmonary inflammation in the mice treated with the CC′ loop decoy peptide correlated with the decreased IL-17-dependent expression of genes encoding MCP-1 and GM-CSF in the lung tissues (Fig. 5D). Similarly, the Act1 SEFIR domain CC′ loop decoy peptide substantially attenuated IL-25-induced eosinophilia and TH2-type responses, as determined by measuring the numbers of eosinophils and the amounts of IL-5 and IL-13 in the BAL (Fig. 5. E and F). IL-25-induced airway recruitment of granulocytes, predominantly eosinophils, as well as mucin production, as determined by Periodic acid-Schiff (PAS) staining, were reduced in mice pretreated with the CC′ loop decoy peptide compared to that in mice pretreated with the scrambled peptide (Fig. 5G). The decreased pulmonary inflammation in Act1-deficient mice correlated with decreased expression of genes encoding the TH2-type cytokines IL-5 and IL-13 in the lungs (Fig. 5H). Furthermore, the CC′ loop decoy peptide inhibited IL-17- and IL-25-induced pulmonary inflammation in a dose-dependent manner (fig. S2, A to D). These results demonstrated that the Act1 SEFIR domain CC′ loop decoy peptide played a protective role during IL-17- and IL-25-mediated pulmonary inflammation.
Fig. 5.
A cell-permeable CC′ loop decoy peptide inhibits IL-17- and IL-25-induced pulmonary inflammation. Wild-type (WT) female BALB/c mice (n = 4 mice per group) were pretreated with DMSO, scrambled peptide, or the CC′ loop decoy peptide for 4 hours, after which they were administered saline, IL-17, or IL-25 intranasally. Mice were sacrificed and analyzed 16 hours after injection with IL-17 or 4 days after injection with IL-25. (A) Mice pretreated with the CC′ peptide showed reduced accumulation of neutrophils in BAL fluid after intranasal administration of IL-17 compared to that in BAL fluid from mice pretreated with the scrambled peptide. (B) The IL-17-associated cytokines MCP-1 and GM-CSF were measured by enzyme-linked immunosorbent assay (ELISA) analysis of the BAL from IL-17-treated mice. (C) H&E staining of lung tissue from IL-17-treated mice showed that IL-17-induced airway recruitment of granulocytes, was reduced in mice pretreated with the CC′ loop decoy peptide compared to that in mice pretreated with the scrambled peptide or DMSO. (D) Real-time RT-PCR analysis of gene expression in lung tissue from IL-17-treated mice. (E) Mice pretreated with the CC′ decoy peptide showed reduced accumulation of eosinophils in the BAL after intranasal administration of IL-25 compared to that in the BAL from mice pretreated with the scrambled peptide. (F) ELISA analysis of the IL-25-associated cytokines IL-5 and IL-13 in the BAL of IL-25-treated mice. (G) H&E and PAS staining of lung tissue from IL-25-treated mice showed that IL-25-induced airway recruitment of granulocytes, predominantly eosinophils, as well as mucin production, as determined by PAS staining, were reduced in mice pretreated with the CC′ loop decoy peptide compared to that in mice pretreated with the scrambled peptide or DMSO. (H) Real-time RT-PCR analysis of gene expression in lung tissue from IL-25-treated mice. All experiments were performed three times. *P < 0.05.
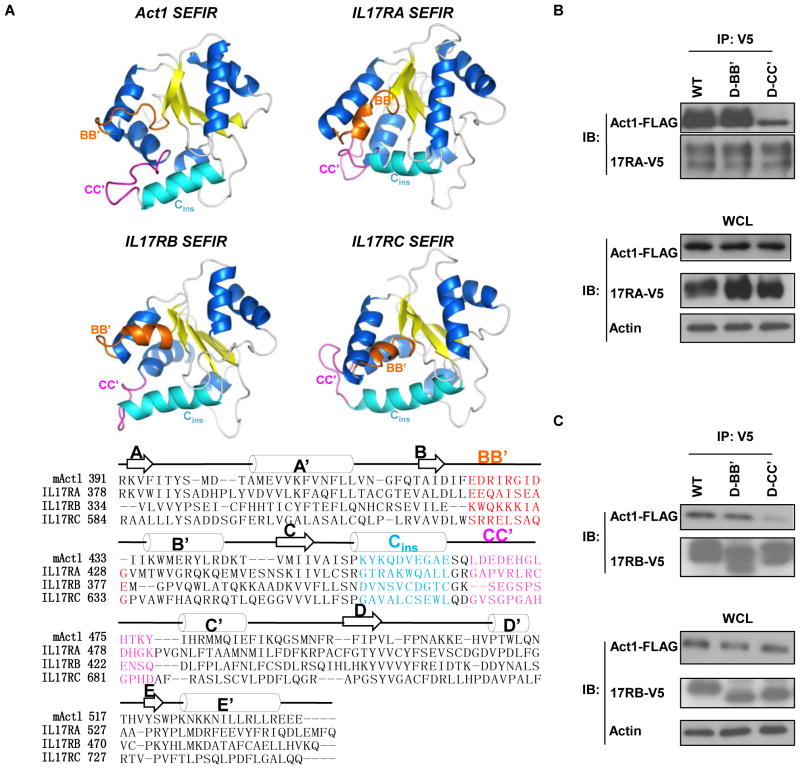
The CC′ loop motif is common among SEFIR family members
Because the CC′ loop of the Act1 SEFIR domain was critical for the interaction between Act1 and IL-17RA, we investigated whether the CC′ loop was present in the SEFIR domains of other SEFIR family members and whether it played similar roles. We generated homology models for the SEFIR domains of IL-17RA, IL-17RB, and IL-17RC, as described earlier for modeling the SEFIR domain of Act1. We found that the models of SEFIR domains from the IL-17Rs had similar structures, and each model had a BB′ loop, a Cins helix, and a CC′ loop as prominent features on the domain surface (Fig. 6A). We tested whether the CC′ loop of the IL-17RA SEFIR domain was required for the interaction between the IL17-RA SEFIR domain and Act1. We cotransfected HeLa cells with plasmids encoding V5-tagged IL-17RA or the corresponding BB′ loop (D-BB′) or CC′ loop (D-CC′) deletion mutants of the receptor together with plasmid encoding FLAG-tagged Act1. The CC′ loop deletion mutant of IL-17RA exhibited a weaker interaction with Act1 than did the wild-type receptor, whereas the BB′ loop mutant of IL-17RA retained the ability to interact with Act1 (Fig. 6B). We observed similar results for mutants of IL-17RB (Fig. 6C). These results suggested that the CC′ loop plays a conserved role, in multiple SEFIR family members, as a surface used for heterotypic SEFIR-SEFIR domain interactions.
Fig. 6.
The CC′ loop motif is a common motif among SEFIR family members and is critical for SEFIR-SEFIR interactions. (A) Computational modeling of the SEFIR domains of IL-17RA, IL-17RB, and IL-17RC in comparison with the SEFIR domain of Act1. The critical structures are colored to highlight secondary structure elements: the BB′ loop is orange; the Cins helix is cyan; and the CC′ loop is pink. (B) HeLa cells were transiently cotransfected with plasmids encoding V5-tagged IL-17RA or its D-BB′ or D-CC′ mutants together with plasmid encoding FLAG-tagged mAct1. Cell lysates were subjected to immunoprecipitation with antibody against the V5 tag, after which they were analyzed by Western blotting with antibodies against the FLAG and V5 tags. (C) HeLa cells were transiently cotransfected with plasmid encoding V5-tagged IL-17RB or its D-BB′ or D-CC′ mutants together with plasmid encoding FLAG-tagged mAct1. Cell lysates were subjected to immunopreciptation with antibody against the V5 tag, after which they were analyzed by Western blotting with antibodies against the FLAG and V5 tags. Blots shown are representative of three independent experiments.
DISCUSSION
Here, we identified an interaction interface (the CC′ loop) in the SEFIR domains of several interacting SEFIR family members, including IL-17RA, IL-17RB, IL-17RC, and Act1. Deletion of the CC′ loop from either Act1 or IL-17RA disrupted the interaction between these two proteins. The isolated CC′ loop of Act1 bound directly to IL-17RA, and point mutations in the CC′ loop region abolished IL-17- and IL-25-mediated signaling and target gene expression. Furthermore, a cell-permeable decoy peptide based on the CC′ loop interfered with IL-17- and IL-25-mediated signaling in cell culture and attenuated IL-17- and IL-25-induced pulmonary inflammation in mice. Together, our findings suggest that the CC′ loop motif of the SEFIR domain of Act1 is a promising therapeutic target to attenuate IL-17- and IL-25-asssociated inflammatory diseases.
The SEFIR domains share homology with the TIR domain, and together they constitute the STIR-domain superfamily (20). In TIR domains, the so-called BB′ loop is reported as a critical interface required for TIR-TIR domain interactions (29). In the case of SEFIR domains, we found that the BB′ loop was dispensable for the interaction between Act1 and IL-17RA, implying that SEFIR-SEFIR interactions use interaction interfaces that are distinct from those required for TIR-TIR domain interactions. In support of this, we and others have reported that Act1 is not recruited to TLRs, whereas the TLR adaptor protein MyD88 does not interact with IL-17Rs (21, 23). We found that the CC′ loop is a common surface-exposed structure in members of the SEFIR family, including IL-17RA, IL-17RB, IL-17RC, and Act1, suggesting that the CC′ loop might be a conserved motif for SEFIR-SEFIR interactions. These findings provide the molecular basis for the specificity of SEFIR versus TIR domains in protein-protein interactions and signaling.
Our studies open avenues for the development of blocking peptides or peptidomimetic compounds for treating IL-17- and IL-25-dependent inflammatory diseases. That IL-17 and IL-25 signaling participate in allergic airway inflammation makes the Act1 SEFIR domain CC′ loop decoy peptide a promising template for the development of future therapeutics for asthmatic patients. IL-17 has a more prominent role than does IL-25 in the pathogenic processes of human and animal autoimmune diseases, such as rheumatoid arthritis, inflammatory bowel disease, MS, and EAE. The Act1 SEFIR domain CC′ loop decoy peptide may also be useful against various IL-17-dependent autoimmune disorders; however, IL-25 plays a protective role in some aspects of autoimmune diseases, probably through its impact on TH2-type immune responses (30). Therefore, the development of receptor-specific SEFIR domain decoy peptides will be necessary to target specific inflammatory processes.
Although the CC′ loop is required for the interaction of Act1 with receptors (through heterotypic SEFIR-SEFIR domain interactions), other surface-exposed regions in the SEFIR domain may be important for receptor-receptor and Act1-Act1 homotypic SEFIR domain interactions. Previous studies have demonstrated the occurrence of IL-17RA–IL-17RB (IL-25R) and IL-17RA–IL-17RC (IL-17R) interactions (24, 25). We are applying both mutagenesis and decoy peptide approaches to study the homotypic interactions of SEFIR domains and their impact on IL-17 versus IL-25 signaling, and to identify additional, receptor-specific, SEFIR decoy peptides.
MATERIALS AND METHODS
Plasmids
Complementary DNA (cDNA) encoding FLAG-tagged mouse Act1 (mAct1) and its deletion and point mutants, including D-SEFIR1 to D-SEFIR5, 464LDE(→DRR), 467DEH(→RRD), 470GLH(→DDD), 473TKY(→ADA), D467R, E468R, H469D, G470D, L471D, H472D, T473A, K474D, and Y475A were subcloned into the plasmid pMSCV-IRES-GFP. cDNA encoding Myc-tagged mouse IL-17RB was also subcloned into pMSCV-IRES-GFP. cDNA encoding V5-tagged human IL-17RA and its mutants, D-BB′ (residues 416 to 427 deleted) and D-CC′ (residues 416 to 427 deleted), were subcloned into the vector pcDNA3.1. cDNA encoding V5-tagged mouse RB and its mutants D-BB′ (residues 367 to 377 deleted) and D-CC′ (residues 394 to 407 deleted) were subcloned into pcDNA3.1.
Cell culture and reagents
Primary MEFs were isolated from mouse embryos at day 14.5 as described previously (23). Human embryonic kidney (HEK) 293 cells and HeLa cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin G (100 μg/ml), and streptomycin (100 μg/ml). MEFs expressing IL-17RB were established by retroviral infection with pMSCV-IL-17RB-IRES-GFP. Recombinant IL-17 (IL-17A), IL-17E (IL-25) and tumor necrosis factor α (TNF-α) were purchased from R&D Systems. Protein A sepharose beads, FuGENE and protease inhibitor cocktail were obtained from Roche. An antibody against actin was purchased from Santa Cruz Biotechnology. Antibody against the FLAG tag (M2) was obtained from Sigma-Aldrich. Antibody against the hemagglutinin (HA) tag was obtained from Covance. Trizol reagent and antibody against V5 were purchased from Invitrogen. All peptides were synthesized by Biomatik. The cell-permeable peptides (with Antp sequence) were subject to N-terminal acetylation to increase their stability.
Expression and purification of IL-17RA and mAct1-SEFIR proteins
The cDNA encoding a SEFIR domain–containing fragment of human IL17RA (IL17RA-SEFIR, amino acid residues 351 to 616) was subcloned into a modified pET-28 vector, with a N-terminal 6× His tag and a tobacco etch virus protease (TEV) recognition site (ENLYFQG). The IL17RA SEFIR domain was expressed and purified by double-Nickel-Nitrilotriacetic Acid (Ni-NTA) affinity methods as previously described (31). The His-tag was subsequently removed from the eluted fusion protein by rTEV, and IL17RA-SEFIR was further purified as flow-through material from a second subtracting Ni-NTA column. Size-exclusion chromatography on a superdex s200 high resolution column was used as a final step for purification (32). The cDNA encoding a SEFIR domain containing a fragment of mouse Act1 (mAct1-SEFIR, residues 391 to 537) was subcloned into a modified pET28b vector that expresses maltose-binding protein (MBP) with an N-terminal 6× His tag and a C-terminal TEV recognition site. Recombinant mAct1-SEFIR was expressed and purified as previously described (33). The His-tagged MBP moiety was cleaved from the fusion protein by rTEV after the first Ni-NTA column purification. Recombinant mAct1-SEFIR was subsequently collected as flow-through from a second subtracting Ni-NTA column.
Modeling of the Act1 SEFIR domain
A homology model of the Act1 SEFIR domain was generated with I-Tasser (27), based on an input sequence of human Act1 amino acid residues 399 to 574 (which correspond to residues 391 to 555 of mAct1) with no additional input information. Structures of TIR domains from TLR2, TLR4, TLR10, and MyD88 constituted most of the top ten templates selected by I-Tasser for homology modeling. Whereas the I-Tasser output C-scores suggested that the models were of modest quality, the top four models were very consistent with average root-mean-square deviations (Rmsd < 1Å). The model with the best C-score was energy minimized in CNS software package (34) and manually rebuilt to correct stereochemical errors and eliminate clashes with the software program COOT (35). Homology models of the IL17RA, IL17RB, and IL17RC SEFIR domains were generated in a similar manner. All modeling data were summarized in Table S1.
Transfection, retroviral infection and coimmunoprecipation
All transfections were conducted with FuGENE according to the manufacturer’s instructions. For reconstitution assays in MEFs, cells were infected by retroviral supernatant as described previously (23). Briefly, viral supernatant was obtained by transfecting Phoenix cells with 5 μg of retroviral construct derived from pMSCV-IRES-GFP for 48 hours. MEFs were infected with viral supernatant for 24 hours and GFP-positive cells were sorted out to establish stable cell lines for downstream assay. For coimmunoprecipations, cell extracts were incubated with antibody (1 μg) and protein A beads (20 μl). After overnight incubation, beads were washed four times with lysis buffer, resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and analyzed by Western blotting according to standard procedures.
SPR analysis
Binding of purified Act1 SEFIR domain or peptides to IL-17RA was conducted on a Biacore 3000. Purified IL-17RASEFIR domain was immobilized on a CM5 sensor chip in 10 mM acetic acid (pH 4.5). Purified Act1 SEFIR domain or peptides were allowed to flow over the chip in a buffer of 10 mM Hepes (pH 7.4), 150 mM NaCl, and 0.005% surfactant P20. The chip was regenerated with 1 M NaCl. Binding KD values were determined by Biaevaluation software.
Real-time polymerase chain reaction assays
Total RNA was extracted with Trizol reagent according to the manufacturer’s instructions. The cDNAs were synthesized and real-time reverse transcription polymerase chain reaction (RT-PCR) assays were performed as described previously (23).
Intranasal injection of mice with IL-17 and IL-25
Wild-type BALB/c female mice were pretreated with dimethyl sulfoxide (DMSO, the solvent for peptides), scrambled peptide, or the CC′ loop decoy peptide for 4 hours, after which they underwent intranasal administration of saline, IL-17 (4 μg/mouse), or IL-25 (4 μg/mouse). For IL-25-treated mice, decoy peptides were administrated daily through intranasal injection after pretreatment. Mice were anesthetized with iso urane and sacri ced. BAL fluid was collected 16 hours after injection with IL-17 or 4 days after injection with IL-25. A total of 0.7 ml of HL-1 medium (BioWhittaker) was used to obtain BAL uid through trachea with a blunt needle and a 1-ml syringe. Cytospin slide preparations were obtained with a Shandon CytoSpin III Cytocentrifuge (Shandon/Thermo Scienti c). Differential leukocyte counts were obtained on cytospin slide preparations after incubation with Diff Quik Giemsa stain. Cytokines in the BAL were analyzed by MultipleX plate (Invitrogen). H&E and PAS staining were performed on lung tissues after fixation in 10% neutral-buffered formalin and paraffin embedding to detect pulmonary inflammation and for assessment of goblet cell metaplasia and mucin accumulation in airway epithelial cells.
Statistical analyses
Statistical analyses were performed by one-way analysis of variance (ANOVA), followed by multiple pair-wise comparisons of the treatments, or by the Student’s t test, where appropriate.
Supplementary Material
Fig. S1. A cell-permeable CC′ loop decoy peptide inhibits IL-17- and IL-25-dependent gene expression in a dose- and time-dependent manner.
Fig. S2. A cell-permeable CC′ loop decoy peptide inhibits IL-17- and IL-25-induced pulmonary inflammation in mice in a dose-dependent manner.
Table S1. SEFIR domain homology modeling data.
Acknowledgments
We thank S. Yadav (Molecular Biotechnology Core, Lerner Research Institute, Cleveland Clinic, OH, USA) for helping with SPR (Biacore) assay.
Funding: This work was supported by a Senior Investigator Award from the American Asthma Foundation and National Institutes of Health Grants (R01HL098935-01A1 and R01NS071996) (to X.L.), and by NIH AI081928, NS062287, and the Oklahoma Agricultural Experiment Station at Oklahoma State University under project OKL02618 (to J.D.).
Footnotes
Author contributions: C.L. performed all in vitro experiments with assistance from W.Q., Z.K., C.W., F.G., W.Y., and C.Z.; S.S. and C.L. performed all in vivo experiments; P.S., Y.H., and J.D. purified all recombinant proteins; S.M. conducted structural modeling; P.F., M.A., T.H., S.M., J.D., and X.L. interpreted results and provided scientific advice; C.L., S.S., S.M., and X.L. designed experiments and wrote manuscript; and X.L. oversaw the whole study.
Competing interests: The authors declare no competing interests.
REFERENCES AND NOTES
- 1.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 2.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 3.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Garcia I, Zhao Y, Ju S, Gu Q, Liu L, Kolls JK, Lu B. IL-17 signaling-independent central nervous system autoimmunity is negatively regulated by TGF-beta. J Immunol. 2009;182:2665–2671. doi: 10.4049/jimmunol.0802221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu Y, Shen F, Crellin NK, Ouyang W. The IL-17 pathway as a major therapeutic target in autoimmune diseases. Ann N Y Acad Sci. 2010 doi: 10.1111/j.1749-6632.2010.05825.x. [DOI] [PubMed] [Google Scholar]
- 6.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoshino H, Lotvall J, Skoogh BE, Linden A. Neutrophil recruitment by interleukin-17 into rat airways in vivo. Role of tachykinins. Am J Respir Crit Care Med. 1999;159:1423–1428. doi: 10.1164/ajrccm.159.5.9806008. [DOI] [PubMed] [Google Scholar]
- 9.Swaidani S, Bulek K, Kang Z, Liu C, Lu Y, Yin W, Aronica M, Li X. The critical role of epithelial-derived Act1 in IL-17- and IL-25-mediated pulmonary inflammation. J Immunol. 2009;182:1631–1640. doi: 10.4049/jimmunol.182.3.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hellings PW, Kasran A, Liu Z, Vandekerckhove P, Wuyts A, Overbergh L, Mathieu C, Ceuppens JL. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003;28:42–50. doi: 10.1165/rcmb.4832. [DOI] [PubMed] [Google Scholar]
- 11.Kolls JK, Kanaly ST, Ramsay AJ. Interleukin-17: an emerging role in lung inflammation. Am J Respir Cell Mol Biol. 2003;28:9–11. doi: 10.1165/rcmb.2002-0255PS. [DOI] [PubMed] [Google Scholar]
- 12.Linden A. Rationale for targeting interleukin-17 in the lungs. Curr Opin Investig Drugs. 2003;4:1304–1312. [PubMed] [Google Scholar]
- 13.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 14.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angkasekwinai P, Park H, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, Sturton G, Wong SH, McKenzie AN. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2007;120:1324–1331. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 17.Cheung PF, Wong CK, Ip WK, Lam CW. IL-25 regulates the expression of adhesion molecules on eosinophils: mechanism of eosinophilia in allergic inflammation. Allergy. 2006;61:878–885. doi: 10.1111/j.1398-9995.2006.01102.x. [DOI] [PubMed] [Google Scholar]
- 18.Sharkhuu T, Matthaei KI, Forbes E, Mahalingam S, Hogan SP, Hansbro PM, Foster PS. Mechanism of interleukin-25 (IL-17E)-induced pulmonary inflammation and airways hyper-reactivity. Clin Exp Allergy. 2006;36:1575–1583. doi: 10.1111/j.1365-2222.2006.02595.x. [DOI] [PubMed] [Google Scholar]
- 19.Tamachi T, Maezawa Y, Ikeda K, Kagami S, Hatano M, Seto Y, Suto A, Suzuki K, Watanabe N, Saito Y, et al. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J Allergy Clin Immunol. 2006;118:606–614. doi: 10.1016/j.jaci.2006.04.051. [DOI] [PubMed] [Google Scholar]
- 20.Novatchkova M, Leibbrandt A, Werzowa J, Neubuser A, Eisenhaber F. The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem Sci. 2003;28:226–229. doi: 10.1016/S0968-0004(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 21.Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem. 2006;281:35603–35607. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- 22.Claudio E, Sonder SU, Saret S, Carvalho G, Ramalingam TR, Wynn TA, Chariot A, Garcia-Perganeda A, Leonardi A, Paun A, et al. The adaptor protein CIKS/Act1 is essential for IL-25-mediated allergic airway inflammation. J Immunol. 2009;182:1617–1630. doi: 10.4049/jimmunol.182.3.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 24.Rickel EA, Siegel LA, Yoon BR, Rottman JB, Kugler DG, Swart DA, Anders PM, Tocker JE, Comeau MR, Budelsky AL. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol. 2008;181:4299–4310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
- 25.Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, Tocker J, Peschon J. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177:36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 26.Liu C, Qian W, Qian Y, Giltiay NV, Lu Y, Swaidani S, Misra S, Deng L, Chen ZJ, Li X. Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Sci Signal. 2009;2:ra63. doi: 10.1126/scisignal.2000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mauro C, Vito P, Mellone S, Pacifico F, Chariot A, Formisano S, Leonardi A. Role of the adaptor protein CIKS in the activation of the IKK complex. Biochem Biophys Res Commun. 2003;309:84–90. doi: 10.1016/s0006-291x(03)01532-8. [DOI] [PubMed] [Google Scholar]
- 29.Toshchakov VY, Vogel SN. Cell-penetrating TIR BB loop decoy peptides a novel class of TLR signaling inhibitors and a tool to study topology of TIR-TIR interactions. Expert Opin Biol Ther. 2007;7:1035–1050. doi: 10.1517/14712598.7.7.1035. [DOI] [PubMed] [Google Scholar]
- 30.Kleinschek MA, Owyang AM, Joyce-Shaikh B, Langrish CL, Chen Y, Gorman DM, Blumenschein WM, McClanahan T, Brombacher F, Hurst SD, et al. IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med. 2007;204:161–170. doi: 10.1084/jem.20061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng J, Davies DR, Wisedchaisri G, Wu M, Hol WG, Mehlin C. An improved protocol for rapid freezing of protein samples for long-term storage. Acta Crystallogr D Biol Crystallogr. 2004;60:203–204. doi: 10.1107/s0907444903024491. [DOI] [PubMed] [Google Scholar]
- 32.Krumm B, Meng X, Li Y, Xiang Y, Deng J. Structural basis for antagonism of human interleukin 18 by poxvirus interleukin 18-binding protein. Proc Natl Acad Sci U S A. 2008;105:20711–20715. doi: 10.1073/pnas.0809086106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng J, Lewis PA, Greggio E, Sluch E, Beilina A, Cookson MR. Structure of the ROC domain from the Parkinson’s disease-associated leucine-rich repeat kinase 2 reveals a dimeric GTPase. Proc Natl Acad Sci U S A. 2008;105:1499–1504. doi: 10.1073/pnas.0709098105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunger AT. Version 1.2 of the Crystallography and NMR system. Nat Protoc. 2007;2:2728–2733. doi: 10.1038/nprot.2007.406. [DOI] [PubMed] [Google Scholar]
- 35.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. A cell-permeable CC′ loop decoy peptide inhibits IL-17- and IL-25-dependent gene expression in a dose- and time-dependent manner.
Fig. S2. A cell-permeable CC′ loop decoy peptide inhibits IL-17- and IL-25-induced pulmonary inflammation in mice in a dose-dependent manner.
Table S1. SEFIR domain homology modeling data.