Abstract
Purpose
More than 50% of youth living with HIV (YLH) have unprotected sex. In previous studies, we reported effects of a motivational interviewing (MI)-based multi-risk reduction intervention, “Healthy Choices,” in improving motivation, depression and viral load in YLH. In this study we report the effect of the intervention on increasing condom use.
Methods
Six waves of longitudinal data (n = 142) across a period from baseline through 15 months post intervention were analyzed. The developmental trajectory modeling method was used for program effect evaluation.
Results
Three groups detected with distinct sexual risks were: Persistent low sexual risk (PLSR), delayed high sexual risk (DHSR), and high and growing sexual risk (HGSR) with regard to levels and time trajectories of condom use throughout the trial. Receiving Healthy Choices increased the likelihood to be in the PLSR group (63% vs. 32%, p < 0.01) and reduced the likelihood to be in the DHSR group (16% vs. 50%, p < 0.05). Receiving the intervention was also associated with progressive reductions in no-condom sex for PLSR youth (adjusted β = −0.325, p < 0.01) and HGSR youth (adjusted β = −0.364, p < 0.01).
Conclusion
The MI-based program Healthy Choices, when delivered in clinic settings, can prevent unprotected sex in subgroups of YLH, although more intensive interventions may be needed to change risk trajectories among those at highest risk of transmitting the AIDS virus. Developmental trajectory analysis provides an alternative approach to evaluate program effects for study samples that contain distinct subgroups.
Keywords: Healthy Choice, HIV positive youth, Sexual risk, Condom use, Randomized controlled trial, Trajectory analysis
Introduction
It is estimated that there are over 10 million adolescents and young adults who are living with HIV worldwide [1]. Among all the new infections in the United States, approximately half are adolescents and young adults [2]. Findings from diverse sources indicate that more than 50% of youth continue to engage in unprotected sex after being notified of their HIV infection [3–7]. Condom use remains the best method to prevent the spread of HIV through sexual contact given the challenges confronted in the efforts of vaccine development [8–10]. HIV prevention interventions delivered through various venues, including the internet, schools, and communities have been shown to be effective in increasing intention to use condom and condom use among youth who are at risk for HIV infection and in enhancing their knowledge, perceptions and self-efficacy regarding safer sex [11–19].
Youth who live with HIV (YLH) are a strategic population for HIV prevention [20], and HIV/AIDS clinics represent a natural and ideal venue for behavioral interventions targeting youth to curb the spread of HIV. Available data from a number of sources indicate the potential to deliver HIV prevention interventions to YLH at clinic settings for sexual risk reduction, including training of health professionals for program delivery and effective retention of the study participants [21–24]. Studies employing the pre- and post-test design have shown significant protective effects of theory-based intervention programs delivered in clinic settings [25]. Randomized controlled trials have shown a significant effect of clinic-based educational programs in reducing HIV risk and in improving HAART treatment effect among adults who live with HIV [26, 27]. However, there is a lack of data from randomized controlled trials for clinic-based prevention programs targeting YLH [28]. The only published randomized trial tested an 18-session intervention [29] that may be difficult to replicate in a clinic setting.
A methodological challenge to program evaluation is the assumption of a homogenous population with normally distributed outcome variables. Research data indicate that this assumption may not always be valid for various health risk behaviors, including sexual risk [11, 30]. For example, frequency of condom use among adolescents is typically not distributed normally, with a majority reporting no use [31]. In addition, responses to an intervention may also differ for participants with different baseline levels and development trajectories of the outcome variables [11].
In previous studies, we reported the initial effects of a behavioral intervention program “Healthy Choices”, in improving motivation, depression, and viral load reduction among YLH through a randomized controlled trial conducted via the Adolescent Medicine Trials Network for HIV/AIDS Interventions [32, 33]. In this study, we further assessed the effect of the same program on potential reductions in sexual risk behavior (reduction in number of unprotected intercourse acts).
Materials and Methods
Participants and Procedures
Participants of the original trial were recruited from five adolescent HIV clinics located in Baltimore, MD; Detroit, MI; Fort Lauderdale, FL; Los Angeles, CA; and Philadelphia, PA. All five study sites provided HIV primary care with an adolescent medicine specialist and provided the following onsite services: adherence, mental health, and risk reduction counseling, case management, HIV support groups, home visits, peer advocacy and outreach, and transportation.
Healthy Choices is a behavioral prevention intervention based on Motivational Interviewing (MI) technology [34, 35]. It consists of four sessions delivered to individual clients in the clinic settings by mental health clinicians with a master’s level of education. In session 1, a participant chooses one risk behavior to discuss first, and the interventionist elicits the views of a client using standard MI techniques. For effective risk reduction, the intervention focuses on structured personalized feedback on risk behaviors according to the baseline assessment, building motivation to initiate and maintain changes, decisional balance exercises to assess pros and cons of behavior changes, and actual plan for change. In session 2 (week 2), the intervention shifts to the second risk behavior using the same format. In the subsequent 2 sessions (weeks 6 and 10), the interventionist reviews the personalized behavior change plan; continues to monitor and encourage progresses, problem-solved barriers; and elicits strategies to maintain health behaviors and to prevent relapse.
Healthy Choices was adapted for YLH from a previous intervention, Positive Choices, tested with HIV-positive adult men who have sex with men [36]. Youth in the intervention group could work on two of three possible health risk behaviors based on their entry screening: substance use, sexual risk, or medication adherence. If they only had a substance use or adherence problem, they could still receive intervention for sexual risk as a prevention measure if they were sexually active, regardless of engagement in any unprotected sex act. Participants who were randomized to the intervention group received Healthy Choices plus standard multidisciplinary care and participants who were randomized into the control group received only the standard care.
Data for this analysis contained a subset of the participants who met criteria to target sexual risk (N = 142), with 71 being randomized into the intervention group and 71 into the control group. The detailed procedures for subject recruitment, behavioral intervention, and post-intervention assessment have been described elsewhere [32, 33]. Briefly, eligible participants were youth who were HIV-positive, 16 to 24 years of age, engaged in at least two of the three HIV risk behaviors (substance use, sexual risk behavior and adherence to antiretroviral treatment), and were able to complete questionnaires in English. Informed consent was obtained, and a waiver of parental permission was obtained for youth ages 16 and 17. Participants received $30 for the baseline visit with $5 increments for each subsequent follow-up visit at 3 months, 6 months, 9 months, 12 months and 15 months respectively.
Baseline assessment was conducted prior to intervention and within 30 days of the screening test. Assessment for program effect evaluation started immediately after the completion of the intervention and then followed at three-month intervals up to 15 months post intervention. Six waves of survey data were collected employing computer-assisted personal interviewing (CAPI) technology. All the surveys were conducted by trained researchers in clinic settings where privacy of the study participants could be ensured. Data collected through the CAPI were automatically saved on computer for use. No personal identifying information was recorded during the data collection and the interview sessions and a computer generated unique identifier was used to follow the individual participants and to index the data for longitudinal analysis.
Variables and Their Measurement
Sexual Risk Behavior
In this analysis, sexual risk for HIV infection was assessed using the prevalence rate of no condom use during sexual intercourse and the number of times of intercourse without a condom. The assessment was based on a detailed CAPI interview of sexual behavior in the previous three months, and the maximum times of no condom use in the past week was analyzed. In addition to the summarized number of no condom use as the main outcome measure, a dichotomized indicator variable was created to classify the participants as either at-risk (reported having unprotected intercourse at least once in the past 3 months) or not at-risk (reported no unprotected intercourse acts in the past 3 months).
Other Variables
Demographic variables were age (in years), race (two categories of African American vs. others), biological sex, and sexual orientation (dichotomous of heterosexual and others). In addition to summarizing sample characteristics, these variables were used as predictor variables in the multiple developmental trajectory analysis for program effect evaluation.
Statistical Analysis for Program Evaluation
The intention to treat (ITT) approach was used as guidance for program effect evaluation. Missing values on condom use at follow-up assessments were imputed using the Markov Chain Monte Carlo (MCMC) technique. Since data of the outcome variable (times of no condom use in the past 3 months) were skewed with large proportions of zeros (greater than 50%), a traditional comparison analysis was not relevant. We instead employed the method of discrete mixture model for developmental trajectory analysis [37, 38]. One advantage of developmental trajectory analysis is its capability to analyze data collected from non-normal samples by detecting distinct subgroups with improved homogeneity and to characterize the outcome variables for individual subgroups. This analytical approach has been successfully used in evaluation studies in cases where program effect could not be detected using conventional linear methodology with an assumption of normal distribution of data collected among homogeneous samples [30, 11].
By application of the developmental trajectory analysis, three pieces of information can be derived for program effect evaluation: 1) distinct risk groups with time trajectories reflecting changes in the level of no condom use over time, 2) likelihood for participants falling into different risk groups according to the time trajectory of individual participants, and 3) progressive change in sexual risk over time. Since participants in the intervention arm and the control arm were comparable at baseline through randomization, program effect was evident if an increase in the likelihood for a subject to be classified into the lower risk groups, or slower progression of the sexual risk in the time trajectory of a risk group, or both were associated with receiving the Healthy Choices intervention.
Statistical analysis was conducted using the software SAS (SAS Institute, Inc., Cary, NC). The PROC TRAJ [39] was used to assess the program effect by modeling the time trajectory of no condom use with intervention conditions entered as a major influential factor. In the trajectory analysis, model selection with regard to the number of distinct trajectory groups was based on the Bayesian Information Criterion (BIC) – Adding a new group must result in declines of 5 units of BIC. Furthermore, since more than half of the participants scored a zero on the outcome variable (e.g., times of no condom use), Zero-Inflated Poisson (ZIP) distribution was used for developmental trajectory modeling. To better assess intervention effect, age, gender and study sites (four dummy variables for five sites) were included as covariates, one at a time and then jointly, in modeling the developmental trajectories.
Results
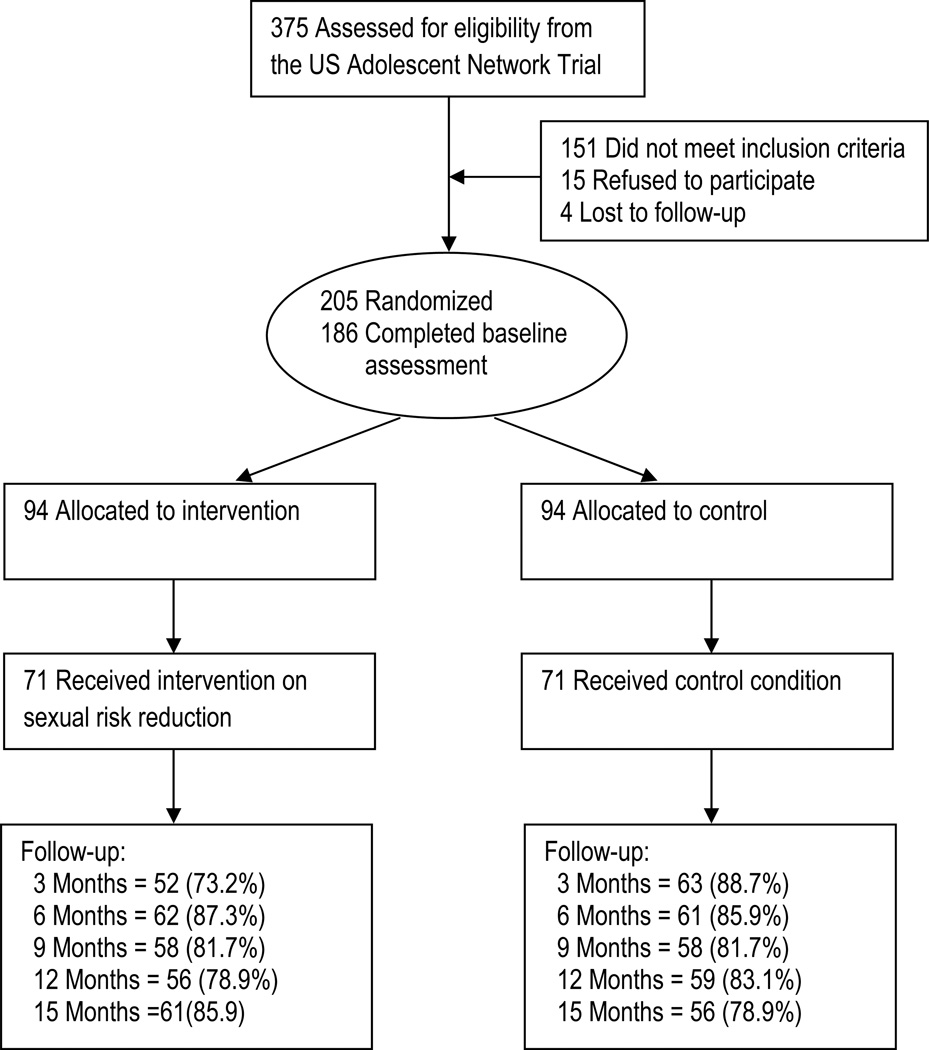
Among the 142 participants included at baseline, those who were retained at 3 months, 6 months, 9 months, 12 months, and 15 months post-intervention were 115 (81.0%), 123 (86.6%), 116 (81.7%), 115 (81.0%), and 117 (82.4%), respectively (Figure 1). There were no significant differences in the attrition rates between the intervention and the control group across the five waves of follow-up assessments except the assessment at 3 months post intervention in which the attrition rates were 73.2% and 88.7% for the intervention and the control group respectively (χ2 = 5.53, p < 0.05). Data in Table 1 indicate that the intervention group and the control group were also comparable at baseline with regard to demographic and condom use variables.
Figure 1.
Flow Chart of the Trial
Table 1.
Baseline Sample Characteristics
| Variables | Sexual risk subsample | Full sample | ||
|---|---|---|---|---|
| Intervention (n=71) |
Control (n=71) |
Intervention (n=94) |
Control (n=92) |
|
| Age in years | ||||
| Mean (SD) | 20.3 (2.5) | 20.5 (2.4) | 20.5 (2.4) | 20.5 (2.3) |
| Race/ethnicity | ||||
| African American | 58 (81.7%) | 58 (81.7%) | 80 (85.1%) | 75 (81.5%) |
| Other | 13 (18.3%) | 13 (18.3%) | 14 (14.9%) | 17 (14.9%) |
| Biological sex (transgender as male) | ||||
| Male | 32 (45.1%) | 41 (57.7%)a | 42 (44.7%) | 56 (60.9%) |
| Female | 39 (54.9%) | 30 (42.3%) | 52 (45.3%) | 36 (39.1%) |
| Sexual orientation | ||||
| Heterosexual | 41 (57.7%) | 36 (50.7%) | 56 (59.6%) | 47 (51.1%) |
| Sexual initiation | ||||
| Yes | 71 (100%) | 71 (100%) | 92 (97.9%) | 89 (96.7%) |
|
Log (times no condom) mean (SD) |
||||
| Anal, receptive | 0.3 (1.0) | 0.8 (2.6) | 0.2 (0.9) | 0.6 (2.2) |
| Anal, insertive | 0.3 (1.2) | 0.1 (0.4) | 0.3 (1.1) | 0.1 (0.4) |
| Vaginal | 3.9 (12.8) | 4.5 (14.4) | 4.9 (16.5) | 3.6 (12.8) |
| Oral (receive) | 4.1 (7.6) | 5.3 (11.2) | 4.3 (10.1) | 4.8 (10.0) |
| Oral (give) | 3.1 (6.0) | 5.2 (10.6) | 3.4 (9.9) | 5.4 (11.0) |
| All acts under influence | 3.1 (8.0) | 5.0 (10.6) | 2.4 (7.1) | 4.1 (9.5) |
| Risky acts under influence | 1.1 (3.2) | 2.2 (7.0) | 0.9 (2.8) | 1.8 (6.2) |
| All risky acts | 4.6 (12.5 | 5.7 (14.1) | 5.3 (16.1) | 4.6 (12.6) |
| All partners | 12.6 (19.9) | 18.1 (29.2) | 14.0 (33.5) | 16.2 (26.9) |
Note: Student t-test for the continuous variables (age, log times of no condom use) and Chi-square test for the categorical variables (the rest) indicated no significant differences in these variables between intervention and the control conditions for the 142 participants included in this analysis.
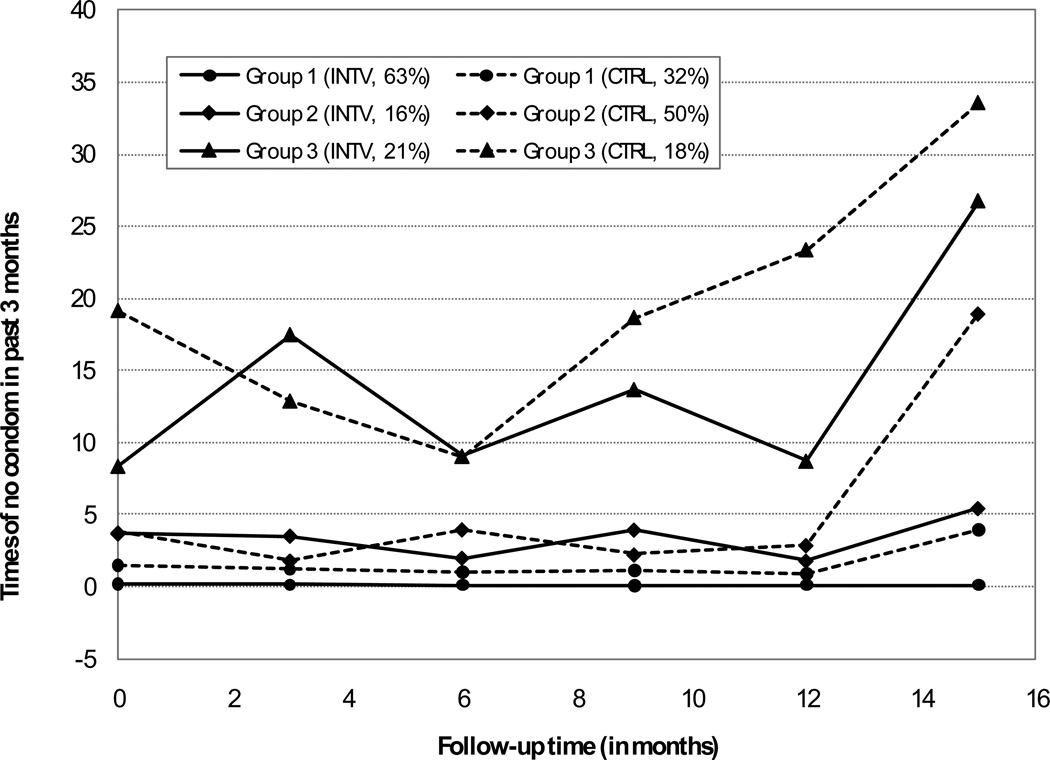
Using BIC criterion, the 142 participants were classified into three distinct groups through time trajectory analysis, based on the time patterns of condom use throughout the trial period from the baseline through 15 months post-intervention. The three groups were: 1) Persistent low sexual risk group or “PLSR”, 2) Delayed high sexual risk group or “DHSR”, and 3) High and growing sexual risk group or “HGSR” (Figure 2).
PLSR group was characterized by very low levels (zero to 2 times) of no condom use throughout the trial from baseline to 15 months post-intervention.
DHSR group began with low risk (1–3 times) but showed an upward trend in unprotected intercourse after 12 months post-intervention.
HGSR group was characterized by high levels (approximately 10 or more times) of no condom use during sex with an overall increasing trend over time.
Figure 2.
Comparison of Trajectories of No condom Use Between Intervention and Control Youths
Note: Group 1: Persistent Low Sexual Risk (PLSR), Group 2: Delayed High Sexual Risk (DHSR) and Group 3: High and Growing Sexual Risk (HGSR)
Data in the left two columns of Table 2 indicate that receiving Healthy Choices was associated with increased likelihood to be classified into the PLSR group (63% vs. 32%, χ2 = 7.69, odds ratio OR = 2.71, 95% CI = 1.33–5.52, p < 0.01) and with reduced likelihood to be classified into the DHSR group (16% vs. 50%, χ2 = 4.09, OR = 0.41, 95% CI = 0.17–0.99, p < 0.05). There were no differences between intervention and controls in the HGSR group.
Table 2.
Estimated Probabilities of Identified Sexual Risk Group Membership of No Condom Use and the Effect of Behavioral Intervention on the Trajectories of No Condom Use for Individual Risk Groups: Results from Developmental Trajectory Analysis
| Identified Group |
Probability of being in a sexual risk group |
Intervention effect on trajectory (beta coefficient) |
||
|---|---|---|---|---|
| Intervention | Control | Unadjusted | Adjusted | |
| Group 1 (PLSR) | 63% | 32%** | −0.356** | −0.325** |
| Group 2 (DHSR) | 16% | 50%* | −0.103* | −0.037 |
| Group 3 (HGSR) | 21% | 18% | −0.360** | −0.364** |
Note: PLSR : Persistent Low Sexual Risk, DHSR: Delayed High Sexual Risk, HDSR: High and Growing Sexual Risk. Covariates included in the adjusted model were age, gender and study sites;
p<0.05 and
p<0.01, indicating significant differences between the intervention and control youth.
Supplementing the results as indicated in Figure 2, the coefficients estimated from the trajectory analysis presented in the two columns on the right of Table 2 indicate that receiving the intervention was associated with reductions in number of times of unprotected sexual intercourse over the trial period for participants in all three groups (β = −0.356, p < 0.01 for PLSR group, β = −0.103, p < 0.05 for DHSR group, and β = −0.360, p < 0.01). After controlling for covariates, the intervention effect remained highly significant for participants in the HGSR group (adjusted β = −0.364, p < 0.01) as well as for participants in the PLSR group (adjusted β coefficient = −0.325, p < 0.01), but not for participants in the DHSR group.
It is worth mentioning that the mean times of no condom use for the PLSR were small even for the control group when the data were plotted together with other two groups in the same chart (Figure 2). Among subjects in this group, the level of unprotected sex was maintained at very low levels throughout the trial period compared to the control subjects who reported higher levels of unprotected with an increasing trend toward the last follow-up assessment. The significant effect from intervention was better manifested by the β coefficients as presented in Table 2.
Discussion and Conclusions
In this study, we reported results from a randomized controlled trial with 6 waves of longitudinal data extended to 15 months post-intervention to assess the effectiveness of “Healthy Choices” in reducing sexual risk in a multi-site sample of YLH. We have previously reported that this clinic-based 4-session MI intervention improved motivation, depression, and viral load [32, 33]. Findings of this study extended and further strengthened our previous findings of the utility of Healthy Choices to target multiple health behavior changes in YLH. Results in this analysis indicate that Healthy Choices had significant effects to reduce sexual risk among YLH with regard to both developmental trajectory and risk group membership. Receiving Healthy Choices significantly increased the likelihood of youth maintaining the persistent low risk group membership, and decreased membership in the delayed high risk group. Furthremore, receiving the intervention was associated with slow progression of the sexual risk trajectories among subjects in the persistent low risk group and the persistently high and growing sex risk group, reducing unprotected sex. Despite small sample size within individual trajectories, the substantial increases in within-group homogeneity enhanced statistical power for the study to effectively assess the program effect. Findings of studies suggest that Healthy Choices should be adopted for use in clinical settings for youth who are living with HIV.
It is worth noting that findings of this study indicate that YLH with different risk trajectories responded differently to Healthy Choices intervention. For the YLH with persistent low sexual risk over time, receiving Healthy Choices helped these youth to maintain low risk status and also to increase condom use during intercourse over time. For those YLH with high and growing risk, receiving Healthy Choices resulted in declines in sexual risk by increasing the frequency of condom use although group status did not change. Finally, while Healthy Choices participants were less likely to be in the delayed but growing risk group, those that were did not improve condom use over time when the major covariates (e.g., age, gender, and study sites) were controlled. Despite that small in groups size (16%), future studies should pay attention to this subgroup regarding changes with regard to changes in unprotected sex over time.
Compared to the conventional ITT-based evaluation approach, the method of discrete mixture model for trajectory analysis [37, 38, 39] showed its strengths in analyzing randomized trial data from a sample that contains distinct subgroups and outcome measures contain a large number of zeros. Data from such samples are not statistically normal and homogenous, therefore could not be efficiently analyzed if sample size is relatively small and the traditional linear comparative methods are used for program effect evaluation. The trajectory analysis method has been previously used in detecting significant program effects through randomized controlled trial for alcohol reduction in clinic settings [30] and for HIV risk reduction in school settings [11], which would not be detected otherwise. We are the first to successfully use this analytical approach to assess increases in condom use among youth living with HIV.
One limitation to the trajectory analysis is that the available computing methodology does not allow researchers to consider the effect from randomized trials that involve multiple sites. To minimize potential bias, we included study sites as covariates to account for between center differences, a major part of the intraclass correlation associated with multicenter design. Another limitation is that the study relied on self-report of condom use during sex, and reporting errors could not be ruled out without the use of biological markers such as sexually transmitted infection screening. Finally, the results of these secondary analyses must be confirmed in future trials. Third, although our recruitment yielded a retention rate of 80% at 15 months post intervention, the impact on program effect from the subjects who lost follow-up (up to 20%) could not be directly assessed. Lastly, despite the use of trajectory analysis, the sample size of 71 for experimental and control group are borderline with regard to statistical power for program effect evaluation given the three detected risk groups (PSLR, DHSR, and HGSR).
Results from previously reported studies suggest that the awareness of HIV infection through HIV diagnosis is not sufficient for many youth to change sexual risk behaviors as many YLH continue to have unprotected sex [3, 4, 7, 40]. There is an urgent need for effective prevention interventions to reduce sexual risk among these YLH. The one rigorously tested, successful intervention in the literature (Rotheram-Borus ref) may be difficult to implement in medical clinics because of its length. The current findings combined with previous findings of Healthy Choices effects on viral load, depression, and readiness to change, as well as a plethora of literature on the efficacy of MOtivaitonal Interviewing [35] suggest that MI-based interventions are worthy of implementation in HIV primary care and possibly adolescent medicine clinics at large.
Given the availability of MI trainers nationally and internationally (URL: www.Motivational interview.org), the detailed procedures for achieving fidelity, as well as recent studies suggesting that MI may be effectively delivered by paraprofessional staff such as those providing HIV counseling and testing [24] , a clinic-based MI intervention like Healthy Choices may be a useful and feasible approach to address multiple HIV risk behaviors in adolescents and young adults.
ACKNOWLEGEMENT
Funding/Support: The Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) supported this work through the grant U01-HD040533 from the National Institutes of Health, the National Institute of Child Health and Human Development (B. Kapogiannis and S. Lee), and supplemental funding from the National Institutes on Drug Abuse (N. Borek) and the National Institute of Mental Health (P. Brouwers and S. Allison).
We also acknowledge the contribution of the investigators and staff at the following ATN 004 sites that participated in this study: Children’s Diagnostic and Treatment Center (Ana Puga, MD, Esmine Leonard, BSN, and Zulma Eysallenne, RN); Children’s Hospital of Los Angeles (Marvin Belzer, MD, Cathy Salata, RN, and Diane Tucker, RN, MSN); University of Maryland (Ligia Peralta, MD, Leonel Flores, MD, and Esther Collinetti, BA); University of Pennsylvania and the Children’s Hospital of Philadelphia (Bret Rudy, MD, Mary Tanney, MPH, MSN, CPNP, Naini Seth, BSN, and Kelly Lannutti, BA); University of Southern California (Andrea Kovacs, MD); and Wayne State University orizons
Project (K. Wright, DO, P. Lam, MA, V. Conners, BA). We sincerely thank the youth who participated in this project. In loving memory of Dr. Kathryn Wright who initiated this work in Detroit.
Footnotes
Additional Contributions: The study was scientifically reviewed by the ATN’s Behavioral Leadership Group. Network, scientific, and logistical support was provided by the ATN coordinating center (C. Wilson and C. Partlow) at the University of Alabama at Birmingham. Network operations and data management support was provided by the ATN Data and Operations Center at Westat Inc (J. Korelitz, J. Davidson, and D.R. Harris).
REFERENCES
- 1.WHO. Progress Report 2006–2007. Geneva: World Health Organization; 2008. Highlights: Child and Adolescent Health and Development. [Google Scholar]
- 2.CDC. [cited 2009 October 15];HIV Prevention Strategic PLAN through 2005 [on line] 2005 Available from: http://www.cdc.gov/nchstp/od/hiv_plan/default.htm.
- 3.Rotheram-Borus MJ, Murphy DA, Kennedy M, et al. Health and risk behaviors over time among youth living with HIV. J Adolesc. 2001;24(6):791–802. doi: 10.1006/jado.2001.0432. [DOI] [PubMed] [Google Scholar]
- 4.Lightfoot M, Swendeman D, Rotheram-Borus MJ, et al. Risk behaviors of youth living with HIV: pre- and post-HAART. Am J Health Behav. 2005;29(2):162–171. doi: 10.5993/ajhb.29.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy DA, Durako SJ, Moscicki AB, et al. No change in health risk behaviors over time among HIV infected adolescents in care: role of psychological distress. J Adolesc Health. 2001;29(3 Suppl):57–63. doi: 10.1016/s1054-139x(01)00287-7. [DOI] [PubMed] [Google Scholar]
- 6.Sturdevant MS, Belzer M, Weissman G, et al. The relationship of unsafe sexual behavior and the characteristics of sexual partners of HIV infected and HIV uninfected adolescent females. J Adolesc Health. 2001;29(3 Suppl):64–71. doi: 10.1016/s1054-139x(01)00286-5. [DOI] [PubMed] [Google Scholar]
- 7.Kadivar H, Garvie PA, Sinnock C, et al. Psychosocial profile of HIV-infected adolescents in a Southern US urban cohort. AIDS Care. 2006;18(6):544–549. doi: 10.1080/13548500500228763. [DOI] [PubMed] [Google Scholar]
- 8.Letvin NL. Progress and obstacles in the development of an AIDS vaccine. Nat Rev Immunol. 2006;6(12):930–939. doi: 10.1038/nri1959. [DOI] [PubMed] [Google Scholar]
- 9.Lyles CM, Kay LS, Crepza N, et al. Best-evidence interventions: findings from a systematic review of HIV behavioral interventions for US populations at high risk, 2000–2004. Am J Public Health. 2007;97(1):133–143. doi: 10.2105/AJPH.2005.076182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemckert AA, Goudsmit J, Barouch DH. Challenges in the search for an HIV vaccine. Eur J Epidemiol. 2004;19(6):513–516. doi: 10.1023/b:ejep.0000032423.87658.68. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Lunn S, Deveaux L, et al. A cluster randomized controlled trial of an adolescent HIV prevention program among Bahamian youth: effect at 12 months post-intervention. AIDS Behav. 2009;13(3):499–508. doi: 10.1007/s10461-008-9511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmiege SJ, Broaddus MR, Levin M, et al. Randomized trial of group interventions to reduce HIV/STD risk and change theoretical mediators among detained adolescents. J Consult Clin Psychol. 2009;77(1):38–50. doi: 10.1037/a0014513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiClemente RJ, Wingood GM, Harrington KF, et al. Efficacy of an HIV prevention intervention for African American adolescent girls: a randomized controlled trial. JAMA. 2004;292(2):171–179. doi: 10.1001/jama.292.2.171. [DOI] [PubMed] [Google Scholar]
- 14.Sikkema KJ, Anderson ES, Kelly JA, et al. Outcomes of a randomized, controlled community-level HIV prevention intervention for adolescents in low-income housing developments. AIDS. 2005;19(14):1509–1516. doi: 10.1097/01.aids.0000183128.39701.34. [DOI] [PubMed] [Google Scholar]
- 15.Stanton BF, Li X, Kahihuata J, et al. Increased protected sex and abstinence among Namibian youth following a HIV risk-reduction intervention: a randomized, longitudinal study. AIDS. 1998;12(18):2473–2480. doi: 10.1097/00002030-199818000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Stanton BF, Li X, Ricardo I, et al. A randomized, controlled effectiveness trial of an AIDS prevention program for low-income African-American youths. Arch Pediatr Adolesc Med. 1996;150(4):363–372. doi: 10.1001/archpedi.1996.02170290029004. [DOI] [PubMed] [Google Scholar]
- 17.Villarruel AM, Jemmott JB, Jemmott LS. A randomized controlled trial testing an HIV prevention intervention for Latino youth. Arch Pediatr Adolesc Med. 2006;160(8):772–777. doi: 10.1001/archpedi.160.8.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markham CM, Shegog R, Leonard AD, et al. +CLICK: harnessing web-based training to reduce secondary transmission among HIV-positive youth. AIDS Care. 2009;21(5):622–631. doi: 10.1080/09540120802385637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fogarty LA, Heilig CM, Armstrong K, et al. Long-term effectiveness of a peer-based intervention to promote condom and contraceptive use among HIV-positive and at-risk women. Public Health Rep. 2001;116 Suppl 1:103–119. doi: 10.1093/phr/116.S1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox J, Fidler S. Sexual transmission of HIV-1. Antiviral Res. 2010;85(1):276–285. doi: 10.1016/j.antiviral.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Hirky AE, Kirshenbaum SB, Melendez RM, et al. The female condom: attitudes and experiences among HIV-positive heterosexual women and men. Women Health. 2003;37(1):71–89. doi: 10.1300/J013v37n01_05. [DOI] [PubMed] [Google Scholar]
- 22.Saleh-Onoya D, Reddy PS, Ruiter RA, et al. Condom use promotion among isiXhosa speaking women living with HIV in the Western Cape Province, South Africa: a pilot study. AIDS Care. 2009;21(7):817–825. doi: 10.1080/09540120802537823. [DOI] [PubMed] [Google Scholar]
- 23.da Silveira MF, dos Santos IS. Impact of an educational intervention to promote condom use among the male partners of HIV positive women. J Eval Clin Pract. 2006;12(1):102–111. doi: 10.1111/j.1365-2753.2005.00626.x. [DOI] [PubMed] [Google Scholar]
- 24.Naar-King S, Outlaw A, Green-Jones M, et al. Motivational interviewing by peer outreach workers: a pilot randomized clinical trial to retain adolescents and young adults in HIV care. AIDS Care. 2009;21(7):868–873. doi: 10.1080/09540120802612824. [DOI] [PubMed] [Google Scholar]
- 25.Butler RB, Schultz JR, Forsberg AD, et al. Promoting safer sex among HIV-positive youth with haemophilia: theory, intervention, and outcome. Haemophilia. 2003;9(2):214–222. doi: 10.1046/j.1365-2516.2003.00722.x. [DOI] [PubMed] [Google Scholar]
- 26.Antoni MH, Carrico AW, Duran RE, et al. Randomized clinical trial of cognitive behavioral stress management on human immunodeficiency virus viral load in gay men treated with highly active antiretroviral therapy. Psychosomatic Medicine. 2006;68(1):143–151. doi: 10.1097/01.psy.0000195749.60049.63. [DOI] [PubMed] [Google Scholar]
- 27.Chesney MA, Chambers DB, Taylor JM, et al. Coping effectiveness training for men living with HIV: Results from a randomized clinical trial testing a group-based intervention. Psychosomatic Medicine. 2003;65(6):1038–1046. doi: 10.1097/01.psy.0000097344.78697.ed. [DOI] [PubMed] [Google Scholar]
- 28.Silveira MF, dos Santos I. Impact of interventions promoting condom use among HIV-infected individuals. Rev Saude Publica. 2005;39(2):296–304. doi: 10.1590/s0034-89102005000200023. [DOI] [PubMed] [Google Scholar]
- 29.Rotheram-Borus MJ, Lee MB, Murphy DA, et al. Efficacy of a preventive intervention for youths living with HIV. Am J Public Health. 2001;91(3):400–405. doi: 10.2105/ajph.91.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gueorguieva R, Wu R, Pittman B, et al. New insights into the efficacy of naltrexone based on trajectory-based reanalyses of two negative clinical trials. Biol Psychiatry. 2007;61(11):1290–1295. doi: 10.1016/j.biopsych.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shew ML, Remafedi GJ, Bearinger LH, et al. The validity of self-reported condom use among adolescents. Sex Transm Dis. 1997;24(9):503–510. doi: 10.1097/00007435-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Naar-King S, Parsons JT, Murphy DA, et al. Improving health outcomes for youth living with the human immunodeficiency virus: a multisite randomized trial of a motivational intervention targeting multiple risk behaviors. Arch Pediatr Adolesc Med. 2009;163(12):1092–1098. doi: 10.1001/archpediatrics.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naar-King S, Parsons JT, Murphy D, Kolmodin K, Harris DR. A multisite randomized trial of a motivational intervention targeting multiple risks in youth living with HIV: initial effects on motivation, self-efficacy, and depression. J Adolesc Health. 2010;46(5):422–428. doi: 10.1016/j.jadohealth.2009.11.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller WR, Rollnick S. Ten Things that Motivational Interviewing Is Not. Behavioural and Cognitive Psychotherapy. 2009;37(2):129–140. doi: 10.1017/S1352465809005128. [DOI] [PubMed] [Google Scholar]
- 35.Suarez M, Mullins S. Motivational interviewing and pediatric health behavior interventions. Journal of Developmental and Behavioral Pediatrics. 29(5):417–428. doi: 10.1097/DBP.0b013e31818888b4. [DOI] [PubMed] [Google Scholar]
- 36.Velasquez MM, von Sterberg K, Johnson DH, et al. Reducing sexual risk behaviors and alcohol use among HIV-positive men who have sex with men: A Randomized clinical trial. Journal of Consulting and Clinical Psychology. 2009;77(4):657–667. doi: 10.1037/a0015519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagin DS. Analyzing developmental trajectories: A semiparametric, group-based approach. Psychological Methods. 1999;4(2):139–157. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- 38.Nagin DS, Tremblay RE. Analyzing developmental trajectories of distinct but related behaviors: A group-based method. Psychological Methods. 2001;6(1):18–34. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- 39.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research. 2001;29(3):374–393. [Google Scholar]
- 40.Outlaw A, Naar-King S, Janisse H, et al. Predictors of condom use in a multi-site study of high-risk youth living with HIV. AIDS Education and Prevention. 2010;22(1):1–14. doi: 10.1521/aeap.2010.22.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]