Abstract
Previous studies have shown the feasibility of using diffusion tensor imaging (DTI) as a noninvasive imaging modality to evaluate neurodegeneration in humans and animals. The axial and radial diffusivities derived from DTI were demonstrated to be sensitive markers for axonal and myelin damage, respectively. This study used DTI to evaluate optic nerve degeneration in wild-type and slow Wallerian degeneration (WldS) mutant mice. Longitudinal DTI was performed on optic nerves following high intraocular pressure-induced transient retinal ischemia. The axial diffusivity of wild-type nerves decreased 30% (P<0.05) at 3 days and 40% (P<0.05) at 5–30 days after transient elevation of intraocular pressure. In contrast, the axial diffusivity of WldS nerves did not change at 3 days; decreased by 20% (P<0.05) at 5 days, and continued to decrease by 30% (P<0.05) at 15 days and 40% (P<0.05) at 30 days after transient intraocular pressure elevation, suggesting delayed axonal damage in WldS mice. Radial diffusivity increased 200% (P<0.05) at 15–30 days in the wild-type mice and 100% (P<0.05) at 30 days in the WldS mice after transient intraocular pressure elevation, suggesting delayed myelin damage in WldS mice. DTI detected damage was confirmed with immunohistochemistry using phosphorylated neurofilament and myelin basic protein for assessing axonal and myelin integrity, respectively. These findings support the use of DTI not only to evaluate the progression of neurodegeneration but also to noninvasively demonstrate WldS mutation to delay the Wallerian degeneration.
Keywords: diffusion tensor imaging, axial diffusivity, radial diffusivity, WldS mouse, axon degeneration, myelin damage
Wallerian degeneration is an active process of axon self-destruction commonly seen in many neural disorders (Bjartmar et al., 2003; Medana and Esiri, 2003; Coleman, 2005; Saxena and Caroni, 2007; Beirowski et al., 2008). The slow Wallerian degeneration (WldS) mutant mouse exhibits a significant delay in Wallerian degeneration (Coleman et al., 1998). Gaining a better understanding of WldS neuroprotective mechanisms could lead to applying these mechanisms to the treatment of human neurodegenerative diseases. However, tools to noninvasively assess delayed neurodegeneration in the WldS mouse have been limited. Histological assessments are not suitable for longitudinal evaluation (Gillingwater et al., 2004; Chitnis et al., 2007; Beirowski et al., 2009). Longitudinal neurological assessments are informative (Chitnis et al., 2007), but the changes are not directly associated with specific pathologies. A noninvasive tool is imperative for a temporal evaluation of the neuroprotective effects of WldS.
Diffusion tensor imaging (DTI) is a noninvasive imaging modality sensitive to structural changes in tissue associated with injuries. Derived from DTI, the decreased axial diffusivity (AD, diffusion along the nerve fibers) and increased radial diffusivity (RD, diffusion across the nerve fibers) were demonstrated to be closely correlated with axonal and myelin damage, respectively (Song et al., 2002; Song et al., 2003; Sun et al., 2008). Because myelin disruption following axonal degeneration is a common process in degenerated nerves, the use of AD and RD would be ideal to noninvasively evaluate the deterioration processes involved in Wallerian degeneration. However, to the best of our knowledge, the use of DTI to delineate neurodegeneration in the WldS mouse has not been reported. In this study, we performed longitudinal DTI to evaluate optic nerve degeneration resulting from high intraocular pressure-induced retinal ischemia in WldS and wild-type mice (Song et al., 2003; Sun et al., 2008). After DTI, immunohistochemistry targeting the axonal and myelin integrity was performed to confirm the DTI findings.
EXPERIMENTAL PROCEDURES
Animals with a transient rise of intraocular pressure
All study procedures were performed according to the protocol approved by the Animal Studies Committee at Washington University in St. Louis, MO, USA. Five male 8–12-week-old WldS mutant mice (Hope Center Animal Models Core at Washington University, St. Louis, MO, USA) and five gender- and age-matched C57BL/6 (wild-type) mice (Harlan, Indianapolis, IN, USA) were anesthetized with an i.p. injection of the mouse cocktail containing ketamine 65 mg/kg and xylazine 13 mg/kg. Body temperature was monitored and maintained by a heating pad. A transient rise of intraocular pressure was produced as previously described (Song et al., 2003). Briefly, the intraocular pressure of the left eye was raised to and maintained at 100–120 mmHg for 60 min (injured eye). The contralateral eye was not cannulated (control eye). Mice were housed in a 12-h light-dark cycle environment at 20–22 °C with food and water available ad libitum before imaging.
DTI of mouse optic nerve
Longitudinal DTI was performed at 3, 5, 15, and 30 days after a transient rise of intraocular pressure. Mice were anesthetized with a mixture of oxygen and isoflurane (Baxter Healthcare Corporation, Deerfield, IL, USA) using an isoflurane vaporizer (DRE, Inc, Louisville, KY, USA). Five percent isoflurane was used for induction and 1.5% for maintenance. The core body temperature was maintained using a warm water circulating pad. Each mouse was immobilized by a custom-made head holder and placed in the center of a 9-cm inner diameter Helmholtz coil served as the radio frequency (RF) transmitter. A circular surface coil, 1.5-cm outer diameter, was placed on top of the mouse head as the signal receiver. The entire device was put in an Oxford Instruments 200/330 (4.7 T, 33 cm clear bore) magnet equipped with a 16-cm inner diameter, actively shielded Oxford gradient coil (18 G/cm, 200 µs rise time) (Oxford Instruments, Oxfordshire, UK). The magnet and the gradient coil were controlled by a Varian Unity-Inova console (Agilent Technologies, Santa Clara, CA, USA).
A conventional spin-echo sequence inserted with a Stejskal-Tanner diffusion-sensitizing gradient pair was used for acquisition of the required series of diffusion-weighted images for DTI analysis. The images were acquired with repetition time (TR) 1.7 s, spin-echo time (TE) 50 ms, time between application of gradient pulses (Δ) 25 ms, diffusion gradient pulse duration (δ) 8 ms, diffusion sensitizing gradient strength 9.1 G/cm, 4 scans averaged per k space line, slice thickness 0.5 mm (11 slices total), field of view 3 cm, and data matrix 256×256 (zero filled to 512×512). Diffusion sensitizing gradients were applied in six orientations. Two diffusion-sensitizing factors, or b values, were used: 0 and 0.838 ms/µm2. DTI data were obtained with an acquisition time of 3 h.
The diffusion tensor was calculated from diffusion-weighted images. The resulting tensor element maps were used to derive eigenvalues (λ1, λ2, and λ3) of the diffusion tensor by matrix diagonalization. On a pixel-by-pixel basis, quantitative indexes, including AD, RD, relative anisotropy (RA), and trace of the diffusion tensor (Tr), were derived using software written in Matlab (MathWorks, Natick, MA, USA) defined by the following equations:
| (1) |
| (2) |
| (3) |
| (4) |
Data analysis
Image analysis was performed on Image Browser (Varian, Inc., Palo Alto, CA, USA). The region of interest (ROI) was selected on optic nerves at 1–2 mm anterior to the optic chiasm (Fig. 1). This section was chosen because the left and right nerves are relatively parallel compared with other sections of the nerve (Banerjee et al., 2007). The ROI of 3×3 pixels in the center of each nerve minimized the partial volume effects (Sun et al., 2008). Data were presented as mean±standard deviation. Repeated measures analysis of variance (ANOVA) was carried out followed by the Bonferroni t-test with P<0.05 considered to be statistically significant. Statistical analysis was conducted using SigmaPlot 11 (Systat Software Inc., San Jose, CA, USA).
Fig. 1.
DTI color-coded relative anisotropy (RA) maps show region of interest (ROI) of the optic nerves. The color of this map indicates the primary direction of the fibers: red for left-right, blue for inward-outward, and green for top-bottom. The transactional view of left and right optic nerves appeared as two blue dots. An ROI was defined as a 3×3 square pixel in the center of each optic nerve to avoid a partial volume effect.
Immunohistochemistry examination
Another three WldS and three wild-type mice were sacrificed at 15 days after the high intraocular pressure-induced retinal ischemia. Mice were intracardially perfused with 4% paraformaldehyde. The brains were preserved in 4% paraformaldehyde for 1 day at 4 °C and decalcified for 1 week before the histological analysis. A 4-mm-thick coronal section (−1 to +3 mm of bregma) from each brain was embedded in paraffin. Tissue sections (3 µm) matching the anatomic position of DTI were deparaffinized for immunocytochemistry. Sections were rinsed in PBS for 3×10 min and incubated with PBS containing a 2% blocking solution (Invitrogen, Carlsbad, CA, USA) for 1 h. Sections were then incubated with a primary antibody for either mouse anti-SMI-31 (1:2000, a phosphorylated neurofilament marker, Invitrogen) or mouse anti-myelin basic protein (MBP) (1:1000, Abcam, Cambridge, MA, USA) in the blocking buffer at 4 °C overnight. After rinsing with PBS three times, sections were incubated in goat anti-mouse IgG conjugated with Cy3 (1:800, Jackson ImmunoResearch, West Grove, PA, USA) in PBS at room temperature for 1 h. After washing with PBS, sections were coverslipped using Vectashield Mounting Medium (Vector Laboratories, Burlingame, CA, USA).
Histological sections were examined with a Nikon Eclipse 80i fluorescence microscope equipped with a 60× oil objective, and images were captured with a CCD camera using MetaMorph software (Universal Imaging Corporation, Downingtown, PA, USA). A customized Cell C image analysis software tool was used to count the SMI-31-positive axons (Selinummi et al., 2005). This program was further modified to count MBP-positive axons by taking into consideration the ring-like structure of staining as a measure of the myelin integrity. For each histological section, counting was conducted on the entire captured image (150×110 µm2). Data were presented as mean±standard deviation. One-way ANOVA statistical analysis was performed to compare axonal and myelin counts between injured and control nerves.
RESULTS
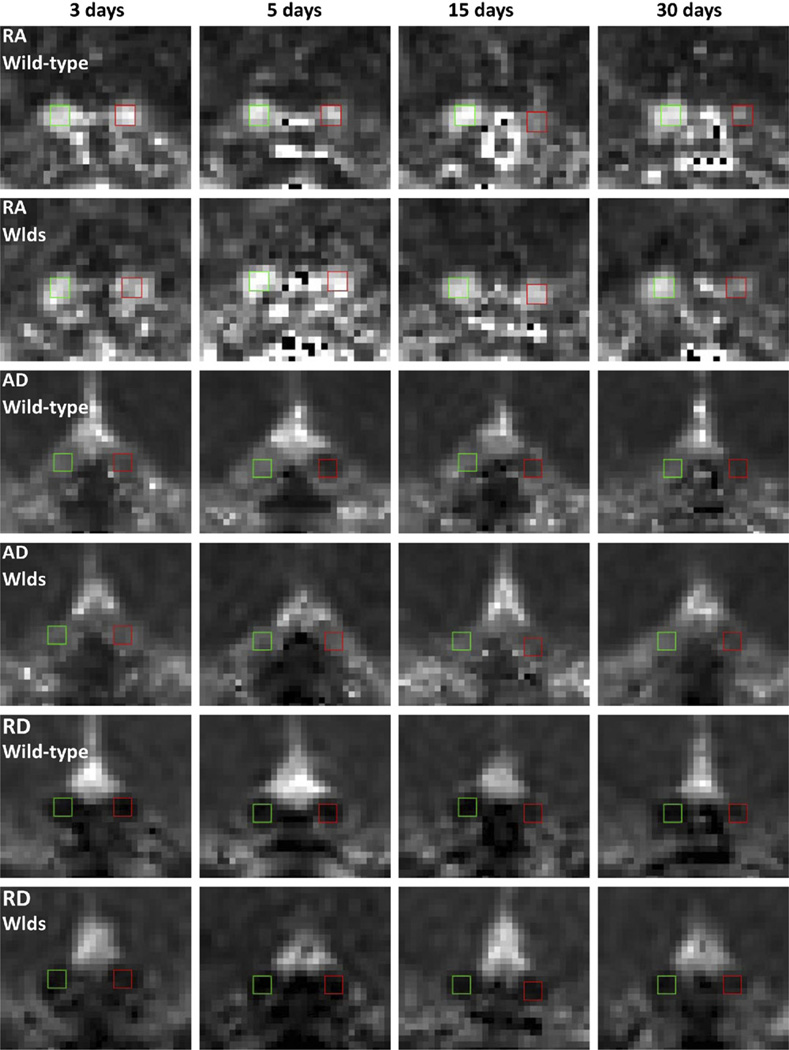
Derived from DTI, color-coded RA maps present the orientation of white matter tracts. The transverse views of left and right optic nerves are seen as two blue dots from the color-coded RA (Fig. 1). Expanded views of optic nerves at each time point are shown on RA, AD, and RD maps (Fig. 2). The left nerve (control) remained unchanged throughout the time course. The right nerve (injured) exhibited a gradual decrease in RA and AD and a gradual increase in RD in wild-type mice; these changes were delayed in WldS mice.
Fig. 2.
RA, AD, and RD maps of WldS and wild-type optic nerves after a transient rise of intraocular pressure. The normal and injured optic nerves were indicated by green and red boxes, respectively. RA appeared bright in the normal nerves. In the injured nerves, RA gradually decreased at 5–30 d in the wild-type mice and at 15–30 d in the WldS mice. AD appeared bright in the normal nerves, but significantly decreased in injured nerves at 3–30 d in the wild-type mice and at 15–30 d in WldS mice. RD appeared dark in the normal nerves, but gradually increased in injured nerves at 15–30 d in the wild-type mice and at 30 d in the WldS mice.
The DTI measurements of optic nerves were analyzed by repeated measures ANOVA (Fig. 3). The baseline RA and baseline RD showed significant differences (P<0.05) between the WldS and wild-type mice, while the baseline AD did not appear a significant difference between these two strains. The WldS optic nerves showed a 40% increase of baseline RD and a 10% decrease of baseline RA compared with wild-type healthy nerves. Repeated measures ANOVA also confirmed significant differences (P<0.05) between left (control) and right (injured) nerves for each DTI index (RA, AD, and RD) in each strain (WldS and wild-type mice). In the injured wild-type nerves, AD decreased by 30% (P<0.05) at day 3 and remained at 40–50% reduction (P<0.05) through days 5–30. In contrast, no significant change of AD was observed at day 3 in the WldS optic nerve. At day 5, AD decreased by 20% (P<0.05) and continued to decrease by 30% (P<0.05) at day 15 and 40% (P<0.05) at day 30. As for RD, in the wild-type optic nerves, significant 200% increases were seen at days 15 and 30 (P<0.05). In WldS optic nerves, RD did not change until 30 days by which time a 100% increase (P<0.05) had occurred. RA did not show significant changes at days 3 and 5 in the injured wild-type or WldS mice. In the injured wild-type mice, RA decreased by 40% (P<0.05) at day 15 and remained the same at 30 days (40% reduction, P<0.05). For the WldS nerves, RA decreased 20% (P<0.05) at day 15 and 40% (P<0.05) at day 30.
Fig. 3.
DTI time course of WldS and wild-type optic nerves after a transient rise of intraocular pressure. The black and white bars represent the mean measurements from the injured and normal nerves, respectively (n=5), and the error bar is the standard deviation. “*” indicates P<0.05. In the wild-type mice, AD decreased 30% at day 3 and remained a 40–50% reduction at days 5–30. In contrast, WldS AD showed no changes at day 3, while it gradually decreased from 20% (day 5) to 40% (day 30). As for RD, wild-type nerves showed a 200% increase at days 15–30. In contrast, WldS RD did not show significant changes until day 30 with an increase of 100%. RA showed a significant 40% decrease at day 15–30 in injured wild-type nerves. As for the WldS nerves, RA decreased 20% at day 15 and 40% at day 30.
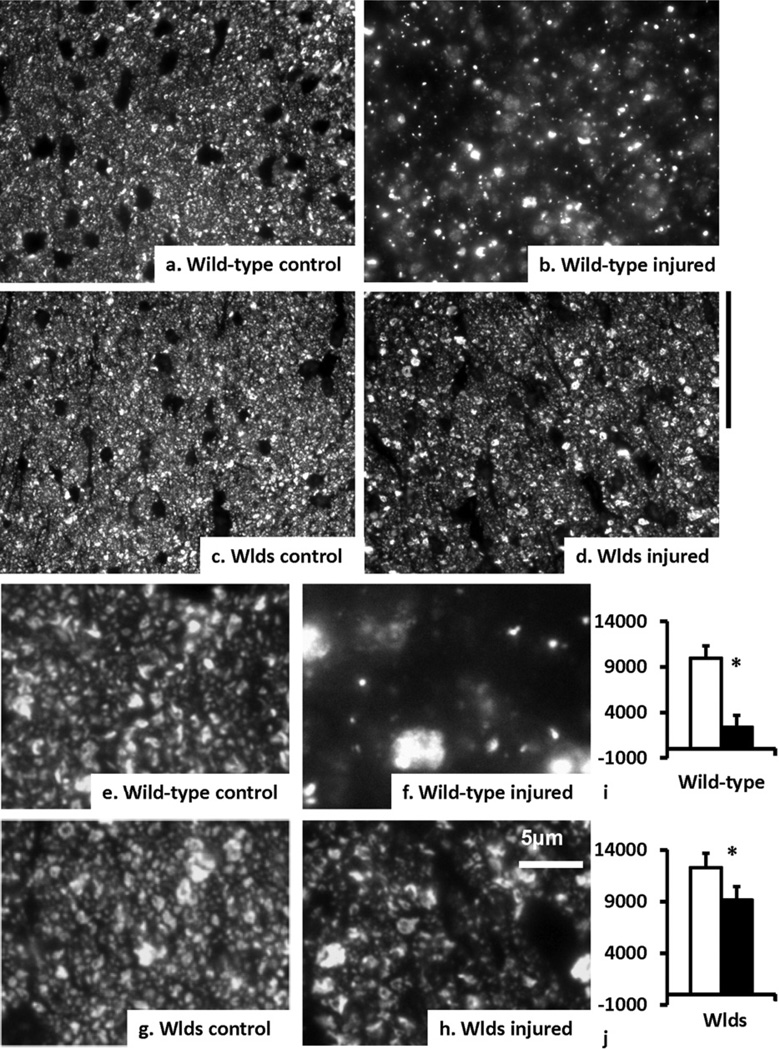
Upon histological examination, phosphorylated neurofilament staining (SMI-31) showed an 80% reduction (P<0.05) of SMI-31-positive axons in wild-type nerves but only a 30% reduction (P<0.05) in the WldS nerves at day 15 (Fig. 4). In contrast, MBP staining showed that the injured wild-type nerves lost 30% (P<0.05) of myelinated axons, while injured WldS nerves showed no detectable myelin loss at day 15 after retinal injury (Fig. 5).
Fig. 4.
SMI-31 immunoreactivity, residual axons in the optic nerves at day 15 after a transient rise of intraocular pressure. Microscopic photos showed SMI-31-labeling of phosphorylated neurofilaments in the wild-type control (a, e), wild-type injured (b, f), the WldS control (c, g), and WldS injured (d, h) optic nerves. The scale bar (50 µm) for (a–d) was put aside (d). The high-magnified histology was shown in (e–h) with a scale bar of 5 µm put by (h). The black and white bars represent the measurements from injured and normal nerves, respectively (i and j, n=3). The error bar is the standard deviation. The wild-type injured nerve lost 80% of axons, while the WldS injured nerve lost 30%. “*” indicates P<0.05.
Fig. 5.
MBP immunoreactivity, myelin integrity in the optic nerves at day 15 after a transient rise of intraocular pressure. Microscopic photos showed MBP in the wild-type control (a, e), wild-type injured (b, f), the WldS control (c, g), and WldS injured (d, h) optic nerves. The scale bar for (a–d) (50 µm) was put by (d). The high-magnified histology was shown in (e–h) with a scale bar of 5 µm put by (h). The black and white bars represent the measurements from injured and normal nerves, respectively (i and j, n=3). The error bar is the standard deviation. The wild-type injured nerve lost 30% myelinated axons, while the WldS nerve did not show detectable myelin lost. “*” indicates P<0.05.
DISCUSSION
AD and RD quantify axonal and myelin damage in Wallerian degeneration
Interest in using DTI to assess nerve degeneration in the human central nervous system (CNS) following stroke or trauma has increased significantly in recent years (Thomalla et al., 2004; Gupta et al., 2006; Yu et al., 2009; McDonald et al., 2010; Puig et al., 2010; Righini et al., 2010; Tasker et al., 2010; Liu et al., 2011). During this time, our laboratory has confirmed that DTI is a sensitive, noninvasive imaging modality for characterizing the temporal progression of Wallerian degeneration in mouse models of retinal ischemia and spinal cord injury (Song et al., 2003; Sun et al., 2008; Kim et al., 2010). The current study is one of the first to use DTI to investigate the impact of the slow Wallerian degeneration mutation on neurodegeneration. Axial and radial diffusivities were measured in vivo to quantify the integrity of axons and myelin, respectively. Delayed damage to axons and myelin in WldS mice compared with wild-type mice was confirmed by histology. By supporting DTI’s ability to distinguish differences in the progression of optic nerve degeneration following retinal ischemia in the WldS mouse compared with the wild-type mouse, this study suggests a future role for DTI in the evaluation of treatment efficacy.
The transient retinal ischemia model of mouse optic nerve degeneration chosen for this study has been widely used and validated by our laboratory and others (Büchi et al., 1991; Büchi, 1992; Adachi et al., 1996; Song et al., 2003; Sun et al., 2008; Sun et al., 2009). The damage to retinal ganglion cell (RGC) has been shown to be consistent between animals, although transient rise of intraocular pressure may also cause damage to other cells and tissues in the eye. This may include damage to different layers in the retina and, possibly, induction of glial activities in the retina (Adachi et al., 1996). Lastly, our findings in this experimental model (Song et al., 2003), such as the observation that the RGC layer is totally lost in 7 days, are consistent with the findings of Zheng and colleagues (2007) who reported 80% RGC loss in 7 days.
Myelin loss is known to occur later than axonal damage in nerves undergoing Wallerian degeneration. The late occurrence of myelin loss has been thought to be associated with the functional but impaired axons delaying the death of oligodendrocytes distant from the injury site (Dong et al., 2003). Because AD and RD are sensitive to axonal and myelin damage, respectively, combined information of AD and RD to delineate the neurodegeneration process may provide new insights into the myelin-axonal interaction and the mechanism of Wallerian degeneration. In the wild-type mice, consistent with our previous findings, AD quickly decreased 30% at day 3 followed by a 100% increase of RD at day 7 (Song et al., 2003; Sun et al., 2006a; Sun et al., 2008). The occurrences of the axonal and myelin disruption were also consistent with reported studies from other investigators. The axonal impairment was measured at day 3 followed by the loss of myelin at day 6 in mouse optic nerves after the anterior ischemic optic neuropathy (Goldenberg-Cohen et al., 2005). Thus, despite the diversity of the primary insults, these data support consistency of the Wallerian degeneration process in mouse optic nerves.
However, the use of AD and RD to compare the interaction of myelin disruption and colocalized axonal damage between the WldS and wild-type mice could be more complicated. In the degenerated optic nerves, for example, our data showed that the AD of injured WldS nerves slowly decreased from 30% to 40% from days 15–30, while the same amount of change occurred from days 3–5 in the injured, wild-type nerves. The occurrence of an ~100% increment of RD was observed at day 30 in the WldS mice and day 7 in the wild-type mice (Sun et al., 2006a). If we count the days between the occurrences of 30% decreased AD and 100% increased RD, it took 4 days (days 3–7) in a wild-type mice and ~15 days (days 15–30) in a WldS mice. However, if we count the days between the occurrences of 40% decreased AD and 100% increased RD, both strains showed a similar time period (days 5–7 for the wild-type mice and in around days 30 for the WldS mice). The time course with a higher sampling frequency will provide a better estimation of the occurrence of axonal and myelin damage in the degenerated nerves.
It should also be noted that although our study has achieved high spatial resolution compared to conventional MRI, the voxel size is still large compared with the dimension of axons. Consider two image voxels: one voxel contains 10% severely injured axons and the damage causes these axons to show a 90% decrease in AD; the other voxel contains 90% mildly injured axons and the mild damage only causes these axons to show a 10% decrease in AD. The overall AD of these two voxels may appear similar to each other, while the injury severity could be different. Higher spatial resolution of images is needed to increase the accuracy of DTI to better characterize the underlying damage. In summary, the sampling rate along the time course and the spatial resolution of images are two major limitations to precisely unveiling the underlying axonal-myelin interactions in degenerated nerves.
Water diffusion sensitive to tissue structural changes
Linkage of decreased AD to axonal damage is possibly related to the focal misalignment of neurofilaments, accumulation of organelles, and/or the beadings in injured axons to hinder water molecule movement along axons (Christman et al., 1994; Arfanakis et al., 2002; Smith and Jeffery, 2006; Budde and Frank, 2010; Xie et al., 2010). As for RD, the disruption of myelin sheaths would reduce the hindrance of water to move across fibers leading to increased RD. Our data demonstrated that both DTI indexes were sufficiently sensitive to detect the delayed axonal and myelin damage occurring in the degenerated nerves of WldS mice.
It should be noted that water diffusion is sensitive to tissue structural changes, and tissue structures can be altered by various pathologies. Thus, the use of AD and RD in complicated white matter damage may face challenges. We previously performed DTI on mice treated with a cuprizone diet, an animal model of demyelination and remyelination (Sun et al., 2006b). We found that RD failed to detect acute demyelination (4 weeks after cuprizone treatment) despite its capability to delineate chronic demyelination and remyelination (Sun et al., 2006b). Microglial or macrophage activation combined with axon swellings was speculated to complicate tissue structures, reducing the sensitivity of RD to detect acute demyelination in these mice (Xie et al., 2010; Tobin et al., 2011). In mice suffering from experimental autoimmune encephalomyelitis (Budde et al., 2009), RD was found uncorrelated with the histological staining intensity of MBP (Budde et al., 2009). Inflammation was suspected to alter tissue structure, reducing the sensitivity of RD to detect myelin changes. Collectively, these findings led to our speculation that severe inflammation, including profound cell infiltration and ramification, may reduce the sensitivity of RD to detect myelin damage.
One possible solution to untangle this complex challenge is to perform data simulation analysis to incorporate theoretical diffusion models into the morphological features of various pathological conditions (Alexander et al., 2010; Yablonskiy and Sukstanskii, 2010; Momot, 2011; Zhang et al., 2011). Recently, Budde and colleagues derived a biophysical model of neurite beading as a consequence of osmotic imbalance after ischemia to demonstrate that neurite beadings could cause up to 70% decreases of AD. Their simulation prediction was subsequently confirmed by animal experiments (Budde and Frank, 2010). Theoretically, one may incorporate inflammatory cell infiltration and its ramifications as additional factors in the morphology simulation combined with diffusion models. These approaches should improve understanding how DTI changes, in particular the AD and RD, in response to complicated white matter diseases.
Alternative assessments for WldS
To evaluate delayed axon and myelin degeneration in the WldS mouse, fluorescent imaging has been recently used as an alternative to traditional histological evaluation (Gillingwater et al., 2004; Chitnis et al., 2007; Beirowski et al., 2009). Like DTI, fluorescent imaging has the capability of detecting the injury process in live animals. Using a two-photon microscope, Kerschensteiner and colleagues showed the delayed axonal degeneration in individual fluorescent axons of injured spinal cords in WldS mice (Kerschensteiner et al., 2005). Wong and colleagues applied fiber-optic technology to confocal microendoscopy to improve imaging penetration for detection of the WldS protective effect on sciatic nerves (Wong et al., 2009). Although advanced fluorescent imaging techniques have enhanced resolution and signal penetration, their application to human studies is limited compared with DTI because they require genetic modification to produce fluorescent axons.
Challenges involved in this study included the consistency of imaging over the time course and minimizing the partial volume effects to depict small structural changes in mouse optic nerves. Imaging consistency was achieved by consistent imaging planning using the anatomical landmarks of mouse head as shown on the template scans as detailed previously (Sun et al., 2008). Our procedures have been previously peer-reviewed and published (Sun et al., 2008; Ou et al., 2009; Sun et al., 2009). The consistency of our longitudinal imaging can also be appreciated by the time-course data measured on the control nerves (white bars in Fig. 3). Imaging with high spatial resolution helped to minimize the partial volume effect. With imaging resolution of 117 µm × 117 µm (in-plane pixel size of 58 µm × 58 µm after zero-filling), the cross section of optic nerves consistently contained ~20 pixels (between 4 × 4–5 × 5 pixels; Fig. 1). The region of interest selected in the central 3-by-3 pixels of each nerve minimized the partial volume effects caused by the boundary of optic nerves. Along the optic nerves extending from eyes to optic chiasm, the left and right optic nerves are relatively parallel at about 1–2 mm anterior to the optic chiasm as compared with the other sections of the nerves (Banerjee et al., 2007). DTI measurements conducted on the cross-section views of this segment of nerves ensure measurement consistency and minimize partial volume effects.
Possible clinical implications
Wallerian degeneration has thought to be a common axonal deterioration process after various neural damages, including the cerebral ischemia (Hall and Yonkers, 1989; Brunelli and Brunelli, 1995; Thomalla et al., 2004; Sylaja et al., 2007; Puig et al., 2010), trauma (Hall and Yonkers, 1989; Fox and Faden, 1998; Kelley et al., 2006), multiple sclerosis (Perry and Anthony, 1999; Casanova et al., 2003; Dziedzic et al., 2010), Alzheimer’s disease (Serra et al., 2010), and Parkinson’s disease (Sajadi et al., 2004). Therapeutic interventions to delay Wallerian degeneration may have a wide application to these diseases (Hains et al., 2004; Stys, 2005). Given the effect of the WldS mutation to delay Wallerian degeneration, it is likely that WldS protein may be applied to the treatment of human neural diseases in the future (Perry and Anthony, 1999). To evaluate and monitor the repair process in patients under these therapies, a noninvasive neuroimaging examination is desired. Our findings supported the use of DTI as an adjunct to neurological tests performed on patients who receive therapeutic treatments and/or participate in clinical trials.
CONCLUSION
In conclusion, this study demonstrated the feasibility of DTI to noninvasively evaluate the delayed axon and myelin degeneration in WldS mice. Decreased AD and increased RD were sensitive to the axonal and myelin damage as confirmed by immunohistochemistry. The combined information of AD and RD offers a great opportunity to monitor myelin disruption in association with axonal damage in the degenerated nerves. Because DTI is available in clinical facilities, this study supported not only the use of DTI to evaluate disease progression but also to evaluate the efficacy of newly developed neuroprotective interventions.
Acknowledgments
This study was supported partly by grants provided by NMSS RG-3670, RG-3864, and CA-1012, NIH R01NS062830, and NIH R01NS054194. All experiments of this study were performed in the Biomedical MR Laboratory at Washington University. We also thank Dr. Sandra Hilliker for her review of the manuscript.
Abbreviations
- AD
axial diffusivity
- ANOVA
analysis of variance
- DTI
diffusion tensor imaging
- MBP
myelin basic protein
- RA
relative anisotropy
- RD
radial diffusivity
- RGC
retinal ganglion cell
- ROI
region of interest
- WldS
slow Wallerian degeneration
REFERENCES
- Adachi M, Takahashi K, Nishikawa M, Miki H, Uyama M. High intraocular pressure-induced ischemia and reperfusion injury in the optic nerve and retina in rats. Graefes Arch Clin Exp Ophthalmol. 1996;234:445–451. doi: 10.1007/BF02539411. [DOI] [PubMed] [Google Scholar]
- Alexander DC, Hubbard PL, Hall MG, Moore EA, Ptito M, Parker GJ, Dyrby TB. Orientationally invariant indices of axon diameter and density from diffusion MRI. Neuroimage. 2010;52:1374–1389. doi: 10.1016/j.neuroimage.2010.05.043. [DOI] [PubMed] [Google Scholar]
- Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002;23:794–802. [PMC free article] [PubMed] [Google Scholar]
- Banerjee D, Hegedus B, Gutmann DH, Garbow JR. Detection and measurement of neurofibromatosis-1 mouse optic glioma in vivo. Neuroimage. 2007;35:1434–1437. doi: 10.1016/j.neuroimage.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B, Babetto E, Coleman MP, Martin KR. The WldS gene delays axonal but not somatic degeneration in a rat glaucoma model. Eur J Neurosci. 2008;28:1166–1179. doi: 10.1111/j.1460-9568.2008.06426.x. [DOI] [PubMed] [Google Scholar]
- Beirowski B, Babetto E, Gilley J, Mazzola F, Conforti L, Janeckova L, Magni G, Ribchester RR, Coleman MP. Non-nuclear Wld(S) determines its neuroprotective efficacy for axons and synapses in vivo. J Neurosci. 2009;29:653–668. doi: 10.1523/JNEUROSCI.3814-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjartmar C, Wujek JR, Trapp BD. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci. 2003;206:165–171. doi: 10.1016/s0022-510x(02)00069-2. [DOI] [PubMed] [Google Scholar]
- Brunelli GA, Brunelli GR. Tissue changes at different periods of ischemia. Int Angiol. 1995;14:253–263. [PubMed] [Google Scholar]
- Büchi ER. Cell death in the rat retina after a pressure-induced ischaemia-reperfusion insult: an electron microscopic study. I. Ganglion cell layer and inner nuclear layer. Exp Eye Res. 1992;55:605–613. doi: 10.1016/s0014-4835(05)80173-3. [DOI] [PubMed] [Google Scholar]
- Büchi ER, Suivaizdis I, Fu J. Pressure-induced retinal ischemia in rats: an experimental model for quantitative study. Ophthalmologica. 1991;203:138–147. doi: 10.1159/000310240. [DOI] [PubMed] [Google Scholar]
- Budde MD, Frank JA. Neurite beading is sufficient to decrease the apparent diffusion coefficient after ischemic stroke. Proc Natl Acad Sci U S A. 2010;107:14472–14477. doi: 10.1073/pnas.1004841107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde MD, Xie M, Cross AH, Song SK. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J Neurosci. 2009;29:2805–2813. doi: 10.1523/JNEUROSCI.4605-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova B, Martínez-Bisbal MC, Valero C, Celda B, Martí-Bonmatí L, Pascual A, Landente L, Coret F. Evidence of Wallerian degeneration in normal appearing white matter in the early stages of relapsing-remitting multiple sclerosis: a HMRS study. J Neurol. 2003;250:22–28. doi: 10.1007/s00415-003-0928-0. [DOI] [PubMed] [Google Scholar]
- Chitnis T, Imitola J, Wang Y, Elyaman W, Chawla P, Sharuk M, Raddassi K, Bronson RT, Khoury SJ. Elevated neuronal expression of CD200 protects Wld(s) mice from inflammation-mediated neurodegeneration. Am J Pathol . 2007;170:1695–1712. doi: 10.2353/ajpath.2007.060677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman CW, Grady MS, Walker SA, Holloway KL, Povlishock JT. Ultrastructural studies of diffuse axonal injury in humans. J Neurotrauma. 1994;11:173–186. doi: 10.1089/neu.1994.11.173. [DOI] [PubMed] [Google Scholar]
- Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat Rev Neurosci. 2005;6:889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Conforti L, Buckmaster EA, Tarlton A, Ewing RM, Brown MC, Lyon MF, Perry VH. An 85-kb tandem triplication in the slow Wallerian degeneration (Wlds) mouse. Proc Natl Acad Sci U S A. 1998;95:9985–9990. doi: 10.1073/pnas.95.17.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Fazzaro A, Xiang C, Korsmeyer SJ, Jacquin MF, McDonald JW. Enhanced oligodendrocyte survival after spinal cord injury in Bax-deficient mice and mice with delayed Wallerian degeneration. J Neurosci. 2003;23:8682–8691. doi: 10.1523/JNEUROSCI.23-25-08682.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziedzic T, Metz I, Dallenga T, König FB, Müller S, Stadelmann C, Brück W. Wallerian degeneration: a major component of early axonal pathology in multiple sclerosis. Brain Pathol. 2010;20:976–985. doi: 10.1111/j.1750-3639.2010.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox GB, Faden AI. Traumatic brain injury causes delayed motor and cognitive impairment in a mutant mouse strain known to exhibit delayed Wallerian degeneration. J Neurosci Res. 1998;53:718–727. doi: 10.1002/(SICI)1097-4547(19980915)53:6<718::AID-JNR9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Gillingwater TH, Haley JE, Ribchester RR, Horsburgh K. Neuroprotection after transient global cerebral ischemia in Wld(s) mutant mice. J Cereb Blood Flow Metab. 2004;24:62–66. doi: 10.1097/01.WCB.0000095798.98378.34. [DOI] [PubMed] [Google Scholar]
- Goldenberg-Cohen N, Guo Y, Margolis F, Cohen Y, Miller NR, Bernstein SL. Oligodendrocyte dysfunction after induction of experimental anterior optic nerve ischemia. Invest Ophthalmol Vis Sci. 2005;46:2716–2725. doi: 10.1167/iovs.04-0547. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Saksena S, Hasan KM, Agarwal A, Haris M, Pandey CM, Narayana PA. Focal Wallerian degeneration of the corpus callosum in large middle cerebral artery stroke: serial diffusion tensor imaging. J Magn Reson Imaging. 2006;24:549–555. doi: 10.1002/jmri.20677. [DOI] [PubMed] [Google Scholar]
- Hains BC, Saab CY, Lo AC, Waxman SG. Sodium channel blockade with phenytoin protects spinal cord axons, enhances axonal conduction, and improves functional motor recovery after contusion SCI. Exp Neurol. 2004;188:365–377. doi: 10.1016/j.expneurol.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Hall ED, Yonkers PA. Mechanisms of neuronal degeneration secondary to central nervous system trauma or ischemia. J Neurotrauma. 1989;6:227–228. doi: 10.1089/neu.1989.6.227. [DOI] [PubMed] [Google Scholar]
- Kelley BJ, Farkas O, Lifshitz J, Povlishock JT. Traumatic axonal injury in the perisomatic domain triggers ultrarapid secondary axotomy and Wallerian degeneration. Exp Neurol. 2006;198:350–360. doi: 10.1016/j.expneurol.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat Med. 2005;11:572–577. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- Kim JH, Loy DN, Wang Q, Budde MD, Schmidt RE, Trinkaus K, Song SK. Diffusion tensor imaging at 3 hours after traumatic spinal cord injury predicts long-term locomotor recovery. J Neurotrauma. 2010;27:587–598. doi: 10.1089/neu.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Tian W, Li L, Kolar B, Qiu X, Chen F, Dogra VS. Hyperintensity on diffusion weighted image along ipsilateral cortical spinal tract after cerebral ischemic stroke: a diffusion tensor analysis. Eur J Radiol. doi: 10.1016/j.ejrad.2010.12.053. (In press) [DOI] [PubMed] [Google Scholar]
- McDonald CR, Hagler DJ, Jr, Girard HM, Pung C, Ahmadi ME, Holland D, Patel RH, Barba D, Tecoma ES, Iragui VJ, Halgren E, Dale AM. Changes in fiber tract integrity and visual fields after anterior temporal lobectomy. Neurology. 2010;75:1631–1638. doi: 10.1212/WNL.0b013e3181fb44db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medana IM, Esiri MM. Axonal damage: a key predictor of outcome in human CNS diseases. Brain. 2003;126:515–530. doi: 10.1093/brain/awg061. [DOI] [PubMed] [Google Scholar]
- Momot KI. Diffusion tensor of water in model articular cartilage. Eur Biophys J. 2011;40:81–91. doi: 10.1007/s00249-010-0629-4. [DOI] [PubMed] [Google Scholar]
- Ou X, Sun SW, Liang HF, Song SK, Gochberg DF. Quantitative magnetization transfer measured pool–size ratio reflects optic nerve myelin content in ex vivo mice. Magn Reson Med. 2009;61:364–371. doi: 10.1002/mrm.21850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Anthony DC. Axon damage and repair in multiple sclerosis. Philos Trans RSoc Lond B Biol Sci. 1999;354:1641–1647. doi: 10.1098/rstb.1999.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig J, Pedraza S, Blasco G, Daunis IEJ, Prats A, Prados F, Boada I, Castellanos M, Sánchez-González J, Remollo S, Laguillo G, Quiles AM, Gómez E, Serena J. Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor deficit 30 days after middle cerebral artery ischemic stroke. AJNR Am J Neuroradiol. 2010;31:1324–1330. doi: 10.3174/ajnr.A2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righini A, Doneda C, Parazzini C, Arrigoni F, Matta U, Triulzi F. Diffusion tensor imaging of early changes in corpus callosum after acute cerebral hemisphere lesions in newborns. Neuroradiology. 2010;52:1025–1035. doi: 10.1007/s00234-010-0745-y. [DOI] [PubMed] [Google Scholar]
- Sajadi A, Schneider BL, Aebischer P. Wlds-mediated protection of dopaminergic fibers in an animal model of Parkinson disease. Curr Biol. 2004;14:326–330. doi: 10.1016/j.cub.2004.01.053. [DOI] [PubMed] [Google Scholar]
- Saxena S, Caroni P. Mechanisms of axon degeneration: from development to disease. Prog Neurobiol. 2007;83:174–191. doi: 10.1016/j.pneurobio.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Selinummi J, Seppälä J, Yli-Harja O, Puhakka JA. Software for quantification of labeled bacteria from digital microscope images by automated image analysis. Biotechniques. 2005;39:859–863. doi: 10.2144/000112018. [DOI] [PubMed] [Google Scholar]
- Serra L, Cercignani M, Lenzi D, Perri R, Fadda L, Caltagirone C, Macaluso E, Bozzali M. Grey and white matter changes at different stages of Alzheimer’s disease. J Alzheimers Dis. 2010;19:147–159. doi: 10.3233/JAD-2010-1223. [DOI] [PubMed] [Google Scholar]
- Smith PM, Jeffery ND. Histological and ultrastructural analysis of white matter damage after naturally-occurring spinal cord injury. Brain Pathol. 2006;16:99–109. doi: 10.1111/j.1750-3639.2006.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Stys PK. General mechanisms of axonal damage and its prevention. J Neurol Sci. 2005;233:3–13. doi: 10.1016/j.jns.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Cross AH, Song SK. Evolving Wallerian degeneration after transient retinal ischemia in mice characterized by diffusion tensor imaging. Neuroimage. 2008;40:1–10. doi: 10.1016/j.neuroimage.2007.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Le TQ, Armstrong RC, Cross AH, Song SK. Differential sensitivity of in vivo and ex vivo diffusion tensor imaging to evolving optic nerve injury in mice with retinal ischemia. Neuroimage. 2006a;32:1195–1204. doi: 10.1016/j.neuroimage.2006.04.212. [DOI] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Trinkaus K, Cross AH, Armstrong RC, Song SK. Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn Reson Med. 2006b;55:302–308. doi: 10.1002/mrm.20774. [DOI] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Xie M, Oyoyo U, Lee A. Fixation, not death, reduces sensitivity of DTI in detecting optic nerve damage. Neuroimage. 2009;44:611–619. doi: 10.1016/j.neuroimage.2008.10.032. [DOI] [PubMed] [Google Scholar]
- Sylaja RN, Goyal M, Watson T, Hill MD. Wallerian-like degeneration after ischemic stroke revealed by diffusion-weighted imaging. Can J Neurol Sci. 2007;34:243–244. doi: 10.1017/s0317167100006120. [DOI] [PubMed] [Google Scholar]
- Tasker RC, Gunn Westland A, White DK, Williams GB. Corpus callosum and inferior forebrain white matter microstructure are related to functional outcome from raised intracranial pressure in child traumatic brain injury. Dev Neurosci. 2010;32:374–384. doi: 10.1159/000316806. [DOI] [PubMed] [Google Scholar]
- Thomalla G, Glauche V, Koch MA, Beaulieu C, Weiller C, Röther J. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage. 2004;22:1767–1774. doi: 10.1016/j.neuroimage.2004.03.041. [DOI] [PubMed] [Google Scholar]
- Tobin JE, Xie M, Le TQ, Song SK, Armstrong RC. Reduced axonopathy and enhanced remyelination after chronic demyelination in fibroblast growth factor 2 (Fgf2)-null mice: differential detection with diffusion tensor imaging. J Neuropathol Exp Neurol. 2011;70:157–165. doi: 10.1097/NEN.0b013e31820937e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F, Fan L, Wells S, Hartley R, Mackenzie FE, Oyebode O, Brown R, Thomson D, Coleman MP, Blanco G, Ribchester RR. Axonal and neuromuscular synaptic phenotypes in Wld(S), SOD1(G93A) and ostes mutant mice identified by fiber-optic confocal microendoscopy. Mol Cell Neurosci. 2009;42:296–307. doi: 10.1016/j.mcn.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Xie M, Tobin JE, Budde MD, Chen CI, Trinkaus K, Cross AH, McDaniel DP, Song SK, Armstrong RC. Rostrocaudal analysis of corpus callosum demyelination and axon damage across disease stages refines diffusion tensor imaging correlations with pathological features. J Neuropathol Exp Neurol. 2010;69:704–716. doi: 10.1097/NEN.0b013e3181e3de90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonskiy DA, Sukstanskii AL. Theoretical models of the diffusion weighted MRsignal. NMR Biomed. 2010;23:661–681. doi: 10.1002/nbm.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Zhu C, Zhang Y, Chen H, Qin W, Wang M, Li K. A longitudinal diffusion tensor imaging study on Wallerian degeneration of corticospinal tract after motor pathway stroke. Neuroimage. 2009;47:451–458. doi: 10.1016/j.neuroimage.2009.04.066. [DOI] [PubMed] [Google Scholar]
- Zhang H, Hubbard PL, Parker GJ, Alexander DC. Axon diameter mapping in the presence of orientation dispersion with diffusion MRI. Neuroimage. 2011;56:1301–1315. doi: 10.1016/j.neuroimage.2011.01.084. [DOI] [PubMed] [Google Scholar]
- Zheng L, Gong B, Hatala DA, Kern TS. Retinal ischemia and reperfusion causes capillary degeneration: similarities to diabetes. Invest Ophthalmol Vis Sci. 2007;48:361–367. doi: 10.1167/iovs.06-0510. [DOI] [PubMed] [Google Scholar]