Abstract
BACKGROUND
Calls for primary care providers (PCPs) to offer obese patients behavioral weight-loss counseling have not been accompanied by adequate guidance on how such care could be delivered. This randomized trial compared weight loss during a 2-year period in response to three lifestyle interventions, all delivered by PCPs in collaboration with auxiliary health professionals (lifestyle coaches) in their practices.
METHODS
We randomly assigned 390 obese adults in six primary care practices to one of three types of intervention: usual care, consisting of quarterly PCP visits that included education about weight management; brief lifestyle counseling, consisting of quarterly PCP visits combined with brief monthly sessions with lifestyle coaches who instructed participants about behavioral weight control; or enhanced brief lifestyle counseling, which provided the same care as described for the previous intervention but included meal replacements or weight-loss medication (orlistat or sibutramine), chosen by the participants in consultation with the PCPs, to potentially increase weight loss.
RESULTS
Of the 390 participants, 86% completed the 2-year trial, at which time, the mean (±SE) weight loss with usual care, brief lifestyle counseling, and enhanced brief lifestyle counseling was 1.7±0.7, 2.9±0.7, and 4.6±0.7 kg, respectively. Initial weight decreased at least 5% in 21.5%, 26.0%, and 34.9% of the participants in the three groups, respectively. Enhanced lifestyle counseling was superior to usual care on both these measures of success (P = 0.003 and P = 0.02, respectively), with no other significant differences among the groups. The benefits of enhanced lifestyle counseling remained even after participants given sibutramine were excluded from the analyses. There were no significant differences between the intervention groups in the occurrence of serious adverse events.
CONCLUSIONS
Enhanced weight-loss counseling helps about one third of obese patients achieve long-term, clinically meaningful weight loss. (Funded by the National Heart, Lung, and Blood Institute; POWER-UP ClinicalTrials.gov number, NCT00826774.)
Investigators are searching for new approaches to the treatment of obesity during routine medical visits.1–4 Trials in which primary care providers (PCPs) offer counseling about diet and activity (i.e., lifestyle counseling) have led to weight loss of 2.5 kg or less over study periods ranging from 6 to 18 months.5–7 Limited treatment contact is probably responsible for this modest degree of weight loss.1 Given the demands on providers’ time, an increase in the frequency of PCP counseling does not appear to be feasible.8 As an alternative, Tsai et al.9 trained medical assistants to deliver individual lifestyle counseling with the use of an abbreviated version of the Diabetes Prevention Program.10 Overweight patients who received eight brief counseling sessions (and met quarterly with their PCP to manage coexisting illnesses) lost 4.4 kg in 6 months, as compared with 0.9 kg for the patients in the control group, who were limited to quarterly PCP visits.
The present study expanded on this pilot investigation by assessing brief lifestyle counseling delivered monthly (primarily by medical assistants) in a 2-year, randomized trial conducted in the primary care setting. In addition to comparing the effectiveness of lifestyle counseling versus usual care, the study included a third intervention that was designed to increase weight loss by enhancing lifestyle modification with liquid meal replacements or with weight-loss medication, as suggested by a treatment algorithm.11 Both orlistat (GlaxoSmithKline) and sibutramine (Abbott) increase weight loss by approximately 3 to 5 kg, as compared with placebo,12–14 and the same is true for the addition of meal replacements to lifestyle modification.15,16 The primary aim of the study was to show that both brief and enhanced brief lifestyle counseling would result in significantly greater weight loss at 24 months than would usual care. Secondary aims included comparing the effectiveness of the two lifestyle interventions. (Our study began in September 2006; in October 2010, sibutramine was removed from the market17 because of reports of an increase in cardiovascular events among patients with preexisting cardiovascular disease.18,19)
METHODS
STUDY DESIGN
The Practice-based Opportunities for Weight Reduction trial at the University of Pennsylvania (POWER-UP) was one of three trials funded by the National Heart, Lung, and Blood Institute to assess behavioral interventions for weight loss in primary care practice.20 The trial was approved by the university’s institutional review board, and all participants provided written informed consent. Randomization began on January 9, 2008, and final outcome assessments were completed on February 11, 2011.
Participants were recruited and treated at six primary care practices owned by Penn Medicine. Six sites were selected from a total of 27 on the basis of providing care to 2000 or more adults and having at least two physicians and two auxiliary health providers on staff. The sites (three urban and three suburban) served a racially and economically diverse population.
The study design was proposed by six of the authors and was finalized in collaboration with members of the study steering committee.20 Data were gathered by one of the authors in collaboration with research coordinators and were analyzed by two of the authors. The lead author wrote the first draft of the manuscript, with subsequent input from all the authors, and vouches for the completeness and accuracy of the data and analyses.
STUDY PARTICIPANTS
Eligibility criteria included an age of 21 years or older, a body-mass index (BMI, the weight in kilograms divided by the square of the height in meters) of 30 to 50, and at least two of five components of the metabolic syndrome to increase the likelihood that the participants would have cardiovascular risk factors.21 (These criteria are described in detail in the study protocol, available with the full text of this article at NEJM.org.) Exclusion criteria were recent cardiovascular disease, other medical conditions contraindicating weight loss, blood pressure of 160/100 mm Hg or higher, medications that substantially affect body weight (e.g., glucocorticoids), substance abuse, severe psychiatric illness that could have affected adherence to the study, bariatric surgery, loss of 5% or more of initial body weight in the previous 6 months, and pregnancy or lactation.20 Antidepressant medications were permitted except for those associated with marked weight gain (e.g., lithium). The study was conducted according to the protocol.
SCREENING AND RANDOMIZATION
Participants were recruited with the use of multiple methods, including PCP referral and self-referral in response to in-clinic advertisements. Applicants completed two on-site screening visits to provide informed consent and to be assessed by means of standardized measures of height, weight, and cardiovascular risk factors. Participants were randomly assigned to interventions (in equal numbers) with the use of a computer-generated algorithm that was operated by the Investigational Drug Service at the University of Pennsylvania. Assignments were stratified by clinic, with randomly varied block sizes (3, 6, or 9).
INTERVENTIONS
All participants were prescribed the same goals with respect to diet and physical activity but were provided with different levels of support to achieve them (as described in the Supplementary Appendix, available at NEJM.org, and the study protocol). Participants whose weight was less than 113.4 kg were prescribed a balanced diet of 1200 to 1500 kcal per day (1500 to 1800 kcal per day for participants who weighed 113.4 kg or more), which consisted of approximately 15 to 20% kcal from protein, 20 to 35% kcal from fat, and the remainder from carbohydrate. All participants were instructed to gradually increase their physical activity to 180 minutes per week and were given a pedometer, a calorie-counting book,22 and handouts from Aim for a Healthy Weight.23
Usual Care
Participants assigned to usual care were scheduled for quarterly PCP visits during the 24 months of the study to address coexisting illnesses. At each visit, the PCP spent approximately 5 to 7 minutes reviewing the participant’s weight change and discussing the information provided in the handouts.23 The PCPs followed written protocols and were instructed not to provide specific behavioral strategies for changing eating and activity habits.
Brief Lifestyle Counseling
Participants assigned to brief lifestyle counseling were scheduled for the same quarterly PCP visits as the usual-care group but also spent 10 to 15 minutes each month with an auxiliary health care provider (medical assistant), referred to as a lifestyle coach, who delivered treatment by following abbreviated lessons from the Diabetes Prevention Program.9,10,24 Visits began with a weigh-in and then a review of participants’ recording of food intake, physical activity, and other goals prescribed in sequential monthly handouts (as listed in the Supplementary Appendix). For month 1 only, participants had two counseling visits to instruct them about how to record food and calorie intake in diaries provided. In year 2, they were permitted, every other month, to complete counseling visits by telephone (although <5% of visits were made by telephone).
Enhanced Brief Lifestyle Counseling
Participants assigned to enhanced lifestyle counseling had the same PCP and counseling visits as those assigned to brief lifestyle counseling. However, in consultation with their PCP, they also chose to take sibutramine, orlistat, or meal replacements to increase weight loss, beginning 1 month after treatment began. (Participants were allowed to choose among these options in order to make the additional treatment acceptable to them and also to reflect the way such interventions are most likely be selected in the primary care setting.) Sibutramine was provided at a dose of 10 mg per day, with the option of increasing the dose to 15 mg per day after 6 months if blood pressure and pulse measurements were within acceptable ranges.25 Orlistat was provided at a dose of 60 mg per meal,26 with the option of increasing the dose to 120 mg after 6 months. Participants who chose meal replacements were instructed to replace two meals and one snack each day with shakes or meal bars (Slim-Fast, Unilever) for the first 4 months and to replace one meal and one snack each day for the remainder of the study.27 (Both orlistat and the meal-replacement products were donated by the respective manufacturers, which had no role in the design of the study, data collection and analysis, or preparation of the manuscript.) Participants were allowed only one enhancement at a time (i.e., medication or meal replacements, all of which were provided without charge) but could switch between them with the approval of their PCP. After sibutramine was removed from the market, participants who took this medication switched to either meal replacements or orlistat. (This option was also offered in November 2009 after the Food and Drug Administration [FDA] issued an alert concerning the safety of sibutramine.)
PCPs AND LIFESTYLE COACHES
Thirty of 31 PCPs across the six sites participated as study providers; only 1 had considerable weight-management experience. Two or three lifestyle coaches were identified at each site on the basis of their good rapport with patients; none had experience with weight management, and none had to meet a body-mass index requirement to serve as a coach. Delivery of the interventions was standardized across sites with the use of detailed protocols. The year before the intervention began, study staff provided 6 to 8 hours of training to PCPs and lifestyle coaches. All providers were certified in intervention delivery at baseline and were recertified at 6-month intervals, according to previously described methods.27 Throughout the trial, the study staff met with PCPs and coaches (for 30 to 60 minutes) approximately quarterly and monthly, respectively, to review protocol implementation.
OUTCOMES AND ASSESSMENTS
The primary outcome was the change in body weight at month 24 in each of the lifestyle-counseling groups as compared with the usual-care group. Secondary outcomes included weight change in the enhanced-lifestyle-counseling group as compared with weight change in the lifestyle-counseling group and the percentages of participants in each of the three groups whose initial weight was decreased by 5% or more at 12 and 24 months and by 10% or more at 12 and 24 months. Weight was measured by a certified staff member at baseline and at 6, 12, 18, and 24 months with the use of a digital scale (Tanita BWB-800). Waist circumference, blood pressure, and fasting levels of blood glucose, triglycerides, and cholesterol (total, high-density lipoprotein [HDL], and low-density lipoprotein [LDL]) were assessed at baseline and at months 6, 12, and 24 with the use of standardized methods described previously25 (and in the protocol).
STATISTICAL ANALYSIS
Changes in weight in the intention-to-treat population were compared with the use of repeated-measures linear mixed-effects models (for continuous outcomes) and generalized-estimating-equation models (for categorical outcomes), which controlled for initial weight, age, sex, race or ethnic group, and study site. The study had 80% power to detect a 2.75-kg difference in weight change at month 24 between the usual-care and brief-lifestyle-counseling groups and between the usual-care and enhanced-lifestyle-counseling groups. Holm’s procedure28 was used to adjust for multiple comparisons and to identify significant differences in at least one of the two between-group comparisons (P = 0.025). For analyses of secondary outcomes, a P value of 0.05 or less was considered to indicate statistical significance.
RESULTS
BASELINE CHARACTERISTICS OF THE STUDY PARTICIPANTS
The participants included 311 women (79.7%) and 79 men with a mean (±SD) age of 51.5±11.5 years, a mean body weight of 107.7±18.3 kg, and a mean BMI of 38.5±4.7 (Table 1). Nearly 95% had completed high school or above; 59.0% identified themselves as white, 38.5% as black, and 4.6% as Hispanic. Participants who received enhanced lifestyle counseling weighed significantly less than those who received usual care (P = 0.02), a difference addressed in the analyses by the a priori decision to control for initial weight.
Table 1.
Baseline Characteristics of the Participants.*
| Characteristic | Usual Care (N = 130) |
Brief Lifestyle Counseling (N = 131) |
Enhanced Brief Lifestyle Counseling (N = 129) |
Total (N = 390) |
|---|---|---|---|---|
| Sex — no. of patients (%) | ||||
| Female | 98 (75.4) | 110 (84.0) | 103 (79.8) | 311 (79.7) |
| Male | 32 (24.6) | 21 (16.0) | 26 (20.2) | 79 (20.3) |
| Race or ethnic group — no. of patients (%)† | ||||
| White | 81 (62.3) | 75 (57.3) | 74 (57.4) | 230 (59.0) |
| Black | 46 (35.4) | 52 (39.7) | 52 (40.3) | 150 (38.5) |
| Asian | 2 (1.5) | 0 | 2 (1.6) | 4 (1.0) |
| More than one race | 1 (0.8) | 4 (3.1) | 1 (0.8) | 6 (1.5) |
| Self-reported Hispanic | ||||
| Yes | 6 (4.6) | 6 (4.6) | 6 (4.7) | 18 (4.6) |
| No | 124 (95.4) | 125 (95.4) | 123 (95.3) | 372 (95.4) |
| Education — no. of patients (%) | ||||
| Less than high school | 10 (7.7) | 5 (3.8) | 6 (4.7) | 21 (5.4) |
| High school | 25 (19.2) | 27 (20.6) | 26 (20.2) | 78 (20.0) |
| Some college or associate’s degree | 43 (33.1) | 50 (38.2) | 48 (37.2) | 141 (36.2) |
| Bachelor’s degree | 29 (22.3) | 26 (19.8) | 27 (20.9) | 82 (21.0) |
| Graduate or professional degree | 23 (17.7) | 23 (17.6) | 22 (17.1) | 68 (17.4) |
| Age — yr | 51.7±12.1 | 52.0±12.2 | 51.0±10.1 | 51.5±11.5 |
| Weight — kg‡ | 111.2±20.0 | 106.3±17.3 | 105.4±17.2 | 107.7±18.3 |
| Height — cm | 168.5±8.7 | 165.9±8.6 | 166.9±8.6 | 167.1±8.7 |
| Body-mass index§ | 39.0±4.8 | 38.5±4.6 | 37.8±4.7 | 38.5±4.7 |
| Waist circumference — cm‡ | 119.8±13.9 | 117.1±11.9 | 115.9±11.7 | 117.6±12.6 |
| Triglycerides — mg/dl | 120.5±58.9 | 120.7±69.5 | 111.5±59.4 | 117.5±62.7 |
| Cholesterol — mg/dl | ||||
| Low-density lipoprotein | 112.1±38.7 | 116.0±31.0 | 118.1±31.3 | 115.4±33.8 |
| High-density lipoprotein | 44.0±12.7 | 45.4±12.9 | 48.6±14.9 | 46.0±13.6 |
| Total | 181.7±46.8 | 185.5±35.9 | 189.1±35.5 | 185.5±39.7 |
| Fasting glucose — mg/dl‡ | 112.3±40.1 | 106.2±32.2 | 96.3±22.5 | 104.9±32.9 |
| Blood pressure — mm Hg | ||||
| Systolic | 120.9±18.4 | 122.8±15.6 | 120.5±14.7 | 121.4±16.3 |
| Diastolic | 76.0±10.4 | 75.9±11.3 | 76.5±9.7 | 76.2±10.4 |
| Medical conditions — no. of patients (%) | ||||
| Hypertension | 92 (70.8) | 93 (71.0) | 92 (71.3) | 277 (71.0) |
| Diabetes mellitus | 29 (22.3) | 26 (19.8) | 16 (12.4) | 71 (18.2) |
| Hypercholesterolemia | 78 (60.0) | 93 (71.0) | 82 (63.6) | 253 (64.9) |
Plus–minus values are means ±SD. P>0.05 for all comparisons except as otherwise noted. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129. To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for glucose to millimoles per liter, multiply by 0.05551.
Race and Hispanic ethnic group were self-reported.
P<0.05 for the comparison between the usual-care group and the enhanced-lifestyle-counseling group.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
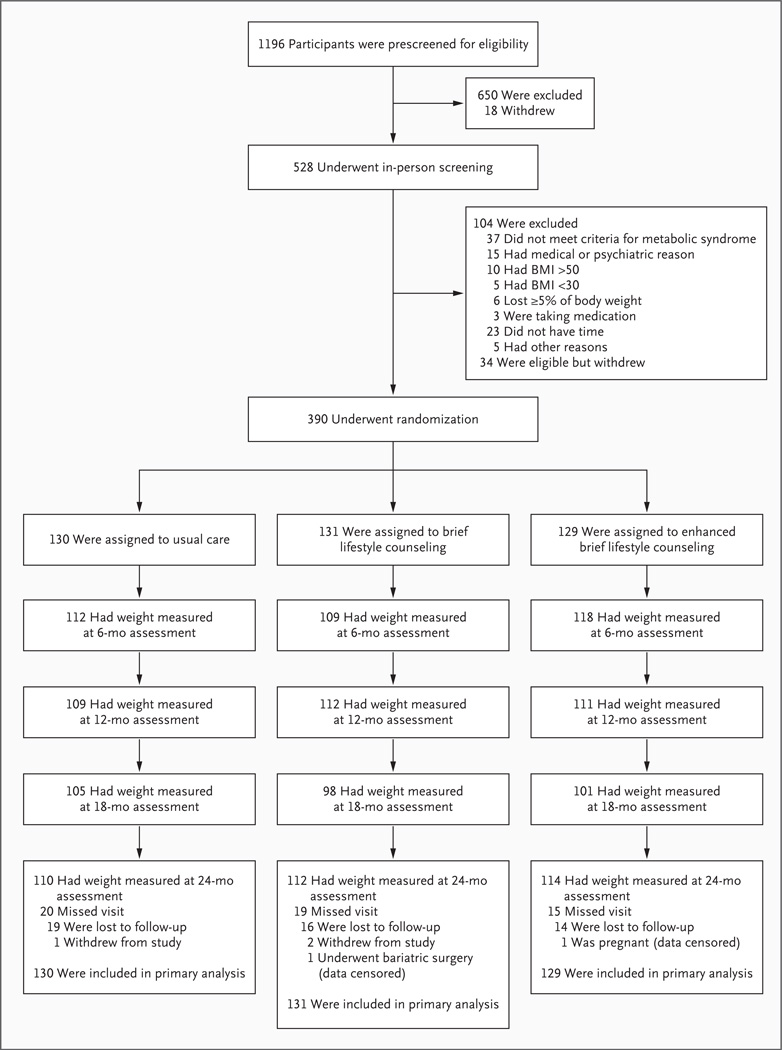
Approximately 86% of the participants in each group had their weight measured at 24 months (Fig. 1). Reasons for missed visits at the 24-month assessment included withdrawal from the study (3 participants), censoring of data because of pregnancy (1 participant) and bariatric surgery (1 participant), and loss to follow-up (49 participants).
Figure 1. Screening, Randomization, and Assessments of Study Participants.
Of the 129 participants randomly assigned to enhanced brief lifestyle counseling, 67, 38, and 24 initially chose to use meal replacements, sibutramine, and orlistat, respectively. BMI denotes body-mass index (the weight in kilograms divided by the square of the height in meters).
WEIGHT LOSS
Intention-to-Treat Population
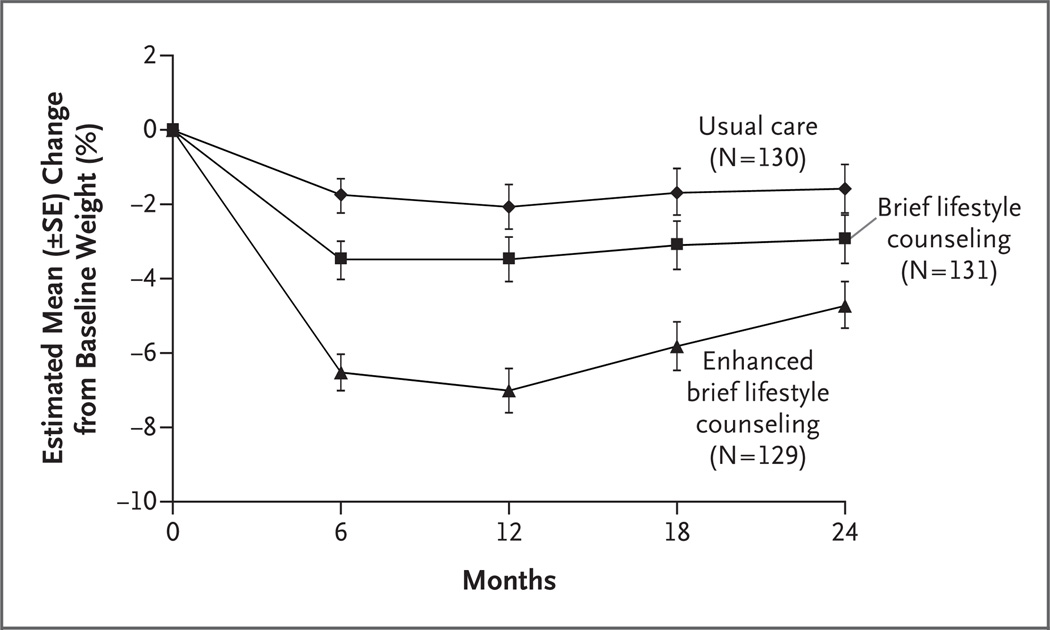
At month 24, the mean (±SE) weight loss among participants assigned to usual care, those assigned to brief lifestyle counseling, and those assigned to enhanced lifestyle counseling was 1.7±0.7, 2.9±0.7, and 4.6±0.7 kg, respectively. Enhanced lifestyle counseling resulted in significantly greater weight loss than did usual care (Table 2), whereas other between-group differences were not significant. (Sensitivity analyses, in which multiple imputation was used to assess the effects of missing data, yielded similar results.) Weight losses in all three groups differed significantly from one another at month 6, and the maximum weight loss was generally reached at month 12 (Table 2). The percent reduction in initial weight is shown in Figure 2 and Table 2; changes in the BMI are also shown in Table 2.
Table 2.
Estimated Mean Weight Loss, Percent Reduction in Body Weight, and Change in Body-Mass Index over a 24-Month Period in the Intention-to-Treat Population.*
|
Variable |
Usual Care (N = 130) |
Brief LC (N = 131) |
Enhanced Brief LC (N = 129) |
Brief LC vs. Usual Care |
P Value Enhanced Brief LC vs. Usual Care |
Enhanced Brief LC vs. Brief LC |
|---|---|---|---|---|---|---|
| Change in body weight (kg) | ||||||
| At month 6 | −2.0±0.5 | −3.5±0.5 | −6.6±0.5 | 0.03 | <0.001 | <0.001 |
| At month 12 | −2.3±0.6 | −3.4±0.6 | −7.1±0.6 | 0.23 | <0.001 | <0.001 |
| At month 18 | −1.9±0.7 | −3.0±0.7 | −5.8±0.7 | 0.22 | <0.001 | 0.004 |
| At month 24 | −1.7±0.7 | −2.9±0.7 | −4.6±0.7 | 0.22 | 0.003 | 0.08 |
| Change in weight (%) | ||||||
| At month 6 | −1.8±0.5 | −3.5±0.5 | −6.5±0.5 | 0.005 | <0.001 | <0.001 |
| At month 12 | −2.1±0.6 | −3.5±0.6 | −7.0±0.6 | 0.08 | <0.001 | <0.001 |
| At month 18 | −1.7±0.7 | −3.1±0.7 | −5.8±0.6 | 0.10 | <0.001 | 0.002 |
| At month 24 | −1.6±0.6 | −2.9±0.7 | −4.7±0.6 | 0.12 | <0.001 | 0.04 |
| Change in body-mass index† | ||||||
| At month 6 | −0.7±0.2 | −1.3±0.2 | −2.4±0.2 | 0.02 | <0.001 | <0.001 |
| At month 12 | −0.8±0.2 | −1.3±0.2 | −2.5±0.2 | 0.18 | <0.001 | <0.001 |
| At month 18 | −0.7±0.2 | −1.1±0.2 | −2.1±0.2 | 0.17 | <0.001 | 0.005 |
| At month 24 | −0.6±0.2 | −0.9±0.2 | −1.6±0.2 | 0.27 | 0.003 | 0.05 |
Plus–minus values are means ±SE. The data for the three intervention groups are model-based estimates for the intention-to-treat population. The numbers of participants for whom weight measurements were available at 6, 12, 18, and 24 months were as follows: for the group that received usual care, 112, 109, 105, and 110 participants, respectively; for the group that received brief lifestyle counseling (LC), 109, 112, 98, and 112 participants, respectively; and for the group that received enhanced brief LC, 118, 111, 101, and 114 participants, respectively.
Figure 2. Estimated Percent Reduction in Baseline Weight over a 24-Month Period in the Intention-to-Treat Population.
At baseline, participants who received enhanced lifestyle counseling chose one of three additional treatment enhancements: meal replacements (67 participants), sibutramine (38), or orlistat (24). An intention-to-treat analysis, based on participants’initial choice of enhancement, revealed a mean weight loss of 3.9±1.0, 5.5±1.3, and 4.6±1.7 kg in these three subgroups, respectively, at month 24, with no significant differences among them. Eleven participants (16%) who initially chose meal replacements switched to a different enhancement, as did 15 participants (39%) who chose sibutramine (including 9 in whom the drug was withdrawn in response to the FDA’s actions) and 8 participants (33%) who chose orlistat. For all the sibutramine-treated participants, the assessment at month 6 preceded the withdrawal of the medication from the market. Weight losses at this time point and the results of other assessments can be found in Figure 1 in the Supplementary Appendix.
Modified Intention-to-Treat Population
Weight loss at month 24 among the 129 participants who received enhanced lifestyle counseling was reanalyzed, excluding the 44 participants who received sibutramine at any time. The remaining 85 participants lost a mean of 4.3±0.8 kg at month 24, which was significantly greater than the loss for those who received usual care (1.7±0.7 kg) but did not differ significantly from the weight loss for those who received brief lifestyle counseling (2.9±0.7 kg). Table 1 in the Supplementary Appendix shows weight loss in this modified intention-to-treat population. An analysis of weight loss in the 66 participants in the enhanced-lifestyle-counseling group who used meal replacements (without exposure to sibutramine) for most of the trial revealed a loss of 4.1±0.9 kg at month 24, which was significantly greater than that in the usual-care group (P = 0.04) but did not differ significantly from the weight loss in the brief-lifestyle- counseling group (P = 0.30).
CATEGORICAL WEIGHT LOSS
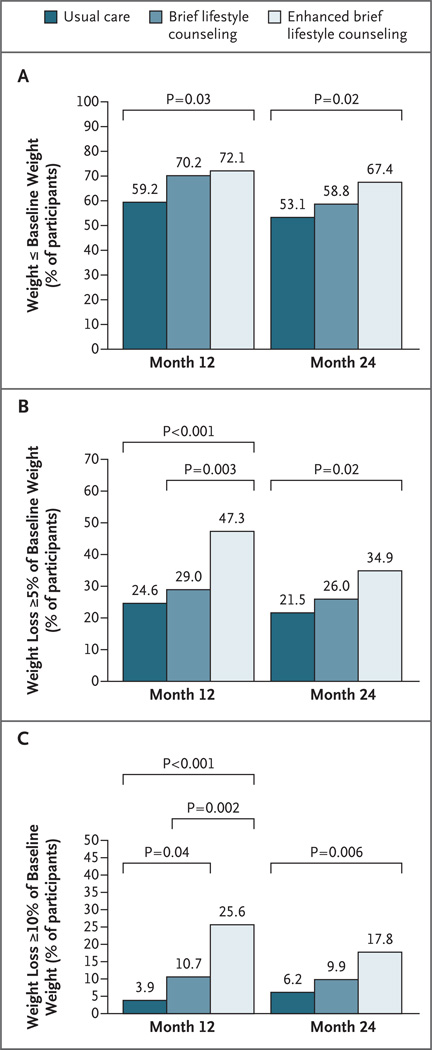
Figure 3 shows categorical weight loss in the intention-to-treat population: the percentages of participants in the three groups whose weight was at or below their baseline weight at months 12 and 24, the percentages of participants who had lost 5% or more of their initial weight at months 12 and 24, and the percentages of participants who had lost 10% or more of their initial weight at months 12 and 24. All categorical weight losses at 12 and 24 months were significantly greater in the group that received enhanced lifestyle counseling than in the group that received usual care. (Fig. 2 in the Supplementary Appendix shows the results for the modified intention-to-treat population.)
Figure 3. Categorical Weight Loss at 12 and 24 Months.
Panel A shows the percentage of participants in each group in the intention-to-treat population who were at or below their baseline weight at months 12 and 24. (Participants for whom data on weight were missing were assumed to have a weight above the baseline weight.) Panel B shows the percentage of participants who lost 5% or more of their baseline weight, and Panel C shows the percentage of participants who lost 10% or more of their baseline weight. (The percentage of participants who lost 5% or more of their baseline weight includes the percentage who lost 10% or more.)
ATTENDANCE AT SCHEDULED VISITS
Participants in the usual-care, lifestyle-counseling, and enhanced-lifestyle-counseling groups attended 71.8±28.6%, 69.0±29.1%, and 76.7±27.4% of the 8 scheduled PCP visits, respectively. The frequency of attendance (across groups) declined from year 1 (81.7±24.9%) to year 2 (61.0±39.2%) (P<0.001). Participants in the lifestyle-counseling group and those in the enhanced-lifestyle-counseling group attended 56.1±28.8% and 64.7±25.8% of the 25 scheduled coaching visits, respectively. Attendance was higher in the enhanced-lifestyle-counseling group than in the lifestyle-counseling group (P = 0.01) and declined across both groups from year 1 (72.1±25.4%) to year 2 (45.6±35.2%) (P<0.001). Figure 3 in the Supplementary Appendix shows that for each intervention group, higher attendance generally was associated with greater weight loss.
CHANGES IN CARDIOVASCULAR RISK FACTORS
Participants who received enhanced lifestyle counseling had significantly greater improvements in waist circumference and in HDL cholesterol and triglyceride levels at one or more assessments, as compared with the other two groups (in the intention-to-treat population) (Table 2A in the Supplementary Appendix). However, this group had significantly smaller reductions in LDL cholesterol levels at month 24. Blood pressure was essentially unchanged from normal baseline values.
ADVERSE EVENTS
A total of 73 hospitalizations for serious adverse events were reported by participants during the 2-year trial. This included 21 events in 16 participants in the usual-care group, 26 events in 20 participants in the brief-lifestyle-counseling group, and 26 events in 22 participants in the enhanced-lifestyle-counseling group, with no significant differences between groups (P = 0.556). There were no deaths. (See Table 3 in the Supplementary Appendix for a list of all events according to intervention group.) Only three events — two cholecystectomies and one case of syncope — were judged by the study physicians to be related to the intervention. Sibutramine was discontinued in five participants because of increases in blood pressure (≥10 mm Hg), in a sixth participant because of tachycardia, and in a seventh because of anxiety. Orlistat was discontinued in five participants because of gastrointestinal symptoms.
DISCUSSION
The principal finding of this study was that PCPs, collaborating with medical assistants, helped one group of their obese patients lose an average of 4.7% of their initial weight at 24 months. This loss, which was accompanied by improvements in cardiovascular risk factors, was achieved with enhanced brief lifestyle counseling, which combined quarterly PCP visits, brief lifestyle coaching delivered monthly, and the use of meal replacements or weight-loss medication. Thirty-five percent of the participants assigned to this intervention lost 5% or more of their initial weight, which is a common criterion for clinically meaningful weight loss.11,29 Long-term weight loss in the group that received enhanced lifestyle counseling (as well as in the group that received brief lifestyle counseling without enhancement) was greater than weight loss observed in other primary care trials,5–7,30,31 with the exception of a study involving extremely obese patients who were treated with intensive group lifestyle modification and weight-loss medications.32 Enhanced lifestyle counseling offers a model for treating obesity in primary care practices with the help of regular staff members (PCPs and medical assistants).
As compared with usual care, only the enhanced counseling led to a significant increase in weight loss at month 24; brief counseling without enhancement did not result in the 4-kg weight loss expected in light of the results of the pilot study.9 Participants who received the brief lifestyle counseling attended significantly fewer coaching sessions than did those who received the enhanced counseling, despite the fact that the same personnel delivered both interventions. In contrast, participants who received the usual care lost more than the expected 1 kg, probably because the weight-management support they were given (quarterly PCP visits, a calorie book, and a pedometer) to encourage them to remain in the study was greater than the support that would typically be provided in the primary care setting. Our findings suggest that PCPs may be able to assist one fifth of their obese patients in losing 5% or more of body weight by providing educational materials and briefly discussing weight management at quarterly visits.
Our study shows that combining quarterly PCP visits with brief monthly lifestyle coaching provided by medical assistants does not significantly increase weight loss, as compared with PCP visits alone. The use of specialized personnel (e.g., registered dietitians),15 as well as more intensive coaching (i.e., more than one session per month for the first 3 months), as recommended by the U.S. Preventive Services Task Force,1 could increase weight loss.33 However, both options would have considerable financial and logistic consequences for primary care practices, and as suggested by our attendance data, participants might not be willing to make additional office visits.
The strengths of this study include the randomized design, the provision of interventions by primary care personnel who treated obese patients in their local practices (rather than the provision of interventions by specialized personnel to highly selected volunteer subjects), and the high rate of study completion by participants (86%). Limitations included the provision of free treatment enhancements (which may limit the generalizability of the results); the need for longer follow-up; and the withdrawal of sibutramine from the market, which clouded interpretation of the findings for the group of participants who received enhanced brief lifestyle counseling. Nonetheless, the beneficial effects of this latter approach remained even after analyses were limited to persons who received only meal replacements or orlistat, each of which continues to be available. The study also confirmed the problem of weight regain despite ongoing counseling for weight-loss maintenance.34
Our data support the screening by PCPs of all adults for obesity, as well as efforts to help patients understand the health consequences of excess weight and the benefits of modest weight loss; these practices are consistent with prior recommendations.1,11 By providing enhanced lifestyle counseling, as described here, PCPs could help a considerable minority of obese persons achieve clinically meaningful weight loss,11,29 which they might not achieve if they were simply told to reduce their weight on their own. The treatment model used in this study awaits comparison with community-based approaches,24,35 as well as with electronically delivered interventions (including the Internet,36 mobile telephones,37 and telephone counseling33,38), which could result in equivalent or greater weight loss. Although our study has shown that primary care personnel can provide effective weight-management support, it has not addressed the more challenging question of who will pay for these or related weight-loss interventions.39
Supplementary Material
Acknowledgments
Supported by a grant (U01-HL087072) from the National Heart, Lung, and Blood Institute.
We thank the health care providers and their patients who participated in the study at the six primary care sites (individual practitioners and study research coordinators at the sites are listed in the Supplementary Appendix); Drs. Barbara Wells (project officer), Catherine Stoney (executive secretary), Jungnam Joo, Peter Kaufmann, and Caye Loria of the National Heart, Lung, and Blood Institute for contributing to the design and administration of the three POWER trials; Drs. David Goff (chair), Robert Kushner, Gbenga Ogedegbe, Amelie Ramirez, Nathan Stimson, and Barbara Tilley of the data and safety monitoring board for overseeing the conduct of the study; Drs. Hsin-Chieh Yeh (chair) and Gerald Jerome of the Resource Coordinating Unit at Johns Hopkins University for logistic support; Dr. Delia West for sharing the weight-loss maintenance protocol developed by the PRIDE (Program to Reduce Incontinence by Diet and Exercise) Research Group; and Dr. Meghan Butryn for assistance in developing the treatment materials used by the participants and the primary care personnel.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Preventive Services Task Force. Screening for obesity in adults: recommendations and rationale. Ann Intern Med. 2003;139:930–932. doi: 10.7326/0003-4819-139-11-200312020-00012. [DOI] [PubMed] [Google Scholar]
- 2.Simkin-Silverman LR, Conroy MB, King WC. Treatment of overweight and obesity in primary care practice: current evidence and future directions. Am J Life-style Med. 2008;2:296–304. [Google Scholar]
- 3.Kushner RF. Tackling obesity: is primary care up to the challenge? Arch Intern Med. 2010;170:121–123. doi: 10.1001/archinternmed.2009.479. [DOI] [PubMed] [Google Scholar]
- 4.Tsai AG, Wadden TA. Treatment of obesity in primary care practice in the United States: a systematic review. J Gen Intern Med. 2009;24:1073–1079. doi: 10.1007/s11606-009-1042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin PD, Dutton GR, Rhode PC, Horswell RL, Ryan DH, Brantley PJ. Weight loss maintenance following a primary care intervention for low-income minority women. Obesity (Silver Spring) 2008;16:2462–2467. doi: 10.1038/oby.2008.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christian JG, Bessesen DH, Byers TE, Christian KK, Goldstein MG, Bock BC. Clinic-based support to help overweight patients with type 2 diabetes increase physical activity and lose weight. Arch Intern Med. 2008;168:141–146. doi: 10.1001/archinternmed.2007.13. [DOI] [PubMed] [Google Scholar]
- 7.Ockene IS, Hebert JR, Ockene JK, et al. Effect of physician-delivered nutrition counseling training and an office-support program on saturated fat intake, weight, and serum lipid measurements in a hyperlipidemic population: Worcester Area Trial for Counseling in Hyperlipidemia (WATCH) Arch Intern Med. 1999;159:725–731. doi: 10.1001/archinte.159.7.725. [DOI] [PubMed] [Google Scholar]
- 8.Kushner RF. Barriers to providing nutrition counseling by physicians: a survey of primary care practitioners. Prev Med. 1995;24:546–552. doi: 10.1006/pmed.1995.1087. [DOI] [PubMed] [Google Scholar]
- 9.Tsai AG, Wadden TA, Rogers MA, Day SC, Moore RH, Islam BJ. A primary care intervention for weight loss: results of a randomized controlled pilot study. Obesity (Silver Spring) 2010;18:1614–1618. doi: 10.1038/oby.2009.457. [DOI] [PubMed] [Google Scholar]
- 10.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Heart Lung and Blood Institute (NHLBI) Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report: National Institutes of Health. Obes Res. 1998;6 Suppl 2:51S–209S. [Erratum, Obes Res 1998;6:464.] [PubMed] [Google Scholar]
- 12.Davidson MH, Hauptman J, DiGirolamo M, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA. 1999;281:235–242. doi: 10.1001/jama.281.3.235. [Erratum, JAMA 1999;281:1174.] [DOI] [PubMed] [Google Scholar]
- 13.James WP, Astrup A, Finer N, et al. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. Lancet. 2000;356:2119–2125. doi: 10.1016/s0140-6736(00)03491-7. [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Maglione M, Tu W, et al. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med. 2005;142:532–546. doi: 10.7326/0003-4819-142-7-200504050-00012. [DOI] [PubMed] [Google Scholar]
- 15.Ashley JM, St Jeor ST, Schrage JP, et al. Weight control in the physician’s office. Arch Intern Med. 2001;161:1599–1604. doi: 10.1001/archinte.161.13.1599. [DOI] [PubMed] [Google Scholar]
- 16.Heymsfield SB, van Mierlo CA, van der Knaap HC, Heo M, Frier HI. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes Relat Metab Disord. 2003;27:537–549. doi: 10.1038/sj.ijo.0802258. [DOI] [PubMed] [Google Scholar]
- 17.Food and Drug Administration. FDA drug safety communication: FDA recommends against the continued use of Meridia (sibutramine) ( http://www.fda.gov/Drugs/DrugSafety/ucm228746.htm).
- 18.James WPT, Caterson ID, Coutinho W, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363:905–917. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- 19.Curfman GD, Morrissey S, Drazen JM. Sibutramine — another flawed diet pill. N Engl J Med. 2010;363:972–1044. doi: 10.1056/NEJMe1007993. [DOI] [PubMed] [Google Scholar]
- 20.Yeh HC, Clark JM, Emmons KE, et al. Independent but coordinated trials: insights from the Practice-based Opportunities for Weight Reduction Trials Collaborative Research Group. Clin Trials. 2010;7:322–332. doi: 10.1177/1740774510374213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on the detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 22.The CalorieKing calorie, fat & carbohydrate counter. 8th ed. Conta Mesa, CA: Family Health; 2008. [Google Scholar]
- 23.National Heart, Lung, and Blood Institute. Aim for a healthy weight. Bethesda, MD: National Institutes of Health; 2005. Aug, (NIH publication no. 05-5213.) [Google Scholar]
- 24.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community: the DEPLOY Pilot Study. Am J Prev Med. 2008;35:357–363. doi: 10.1016/j.amepre.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353:2111–2120. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 26.Hauptman J, Lucas C, Boldrin MN, Collins H, Segal KR. Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med. 2000;9:160–167. doi: 10.1001/archfami.9.2.160. [DOI] [PubMed] [Google Scholar]
- 27.Wadden TA, West DS, Delahanty L, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 29.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen MD, D’Amico FJ, Merenstein JH. Weight reduction in obese hypertensive patients. Fam Med. 1991;23:25–28. [PubMed] [Google Scholar]
- 31.Kumanyika S, Fassbender J, Sarwer D, et al. One-year results of the Think Health! study of weight management in primary care practice. Obesity (Silver Spring) doi: 10.1038/oby.2011.329. (in press) [DOI] [PubMed] [Google Scholar]
- 32.Ryan DH, Johnson WD, Myers VH, et al. Nonsurgical weight loss for extreme obesity in primary care settings: results of the Louisiana Obese Subjects Study. Arch Intern Med. 2010;170:146–154. doi: 10.1001/archinternmed.2009.508. [DOI] [PubMed] [Google Scholar]
- 33.Digenio AG, Mancuso JP, Gerber RA, Dvorak RV. Comparison of methods for delivering a lifestyle modification program for obese patients: a randomized trial. Ann Intern Med. 2009;150:255–262. doi: 10.7326/0003-4819-150-4-200902170-00006. [DOI] [PubMed] [Google Scholar]
- 34.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heshka S, Anderson JW, Atkinson RL, et al. Weight loss with self-help compared with a structured commercial program: a randomized trial. JAMA. 2003;289:1792–1798. doi: 10.1001/jama.289.14.1792. [DOI] [PubMed] [Google Scholar]
- 36.Tate DF, Jackvony EH, Wing RR. A randomized trial comparing human email counseling, computer-automated tailored counseling, and no counseling in an Internet weight loss program. Arch Intern Med. 2006;166:1620–1625. doi: 10.1001/archinte.166.15.1620. [DOI] [PubMed] [Google Scholar]
- 37.Haapala I, Barengo NC, Biggs S, Surakka L, Manninen P. Weight loss by mobile phone: a 1-year effectiveness study. Public Health Nutr. 2009;12:2382–2391. doi: 10.1017/S1368980009005230. [DOI] [PubMed] [Google Scholar]
- 38.Donnelly JE, Smith BK, Dunn L, et al. Comparison of a phone vs clinic approach to achieve 10% weight loss. Int J Obes (Lond) 2007;31:1270–1276. doi: 10.1038/sj.ijo.0803568. [DOI] [PubMed] [Google Scholar]
- 39.Arterburn D, Westbrook EO, Wiese CJ, et al. Insurance coverage and incentives for weight loss among adults with metabolic syndrome. Obesity (Silver Spring) 2008;16:70–76. doi: 10.1038/oby.2007.18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.