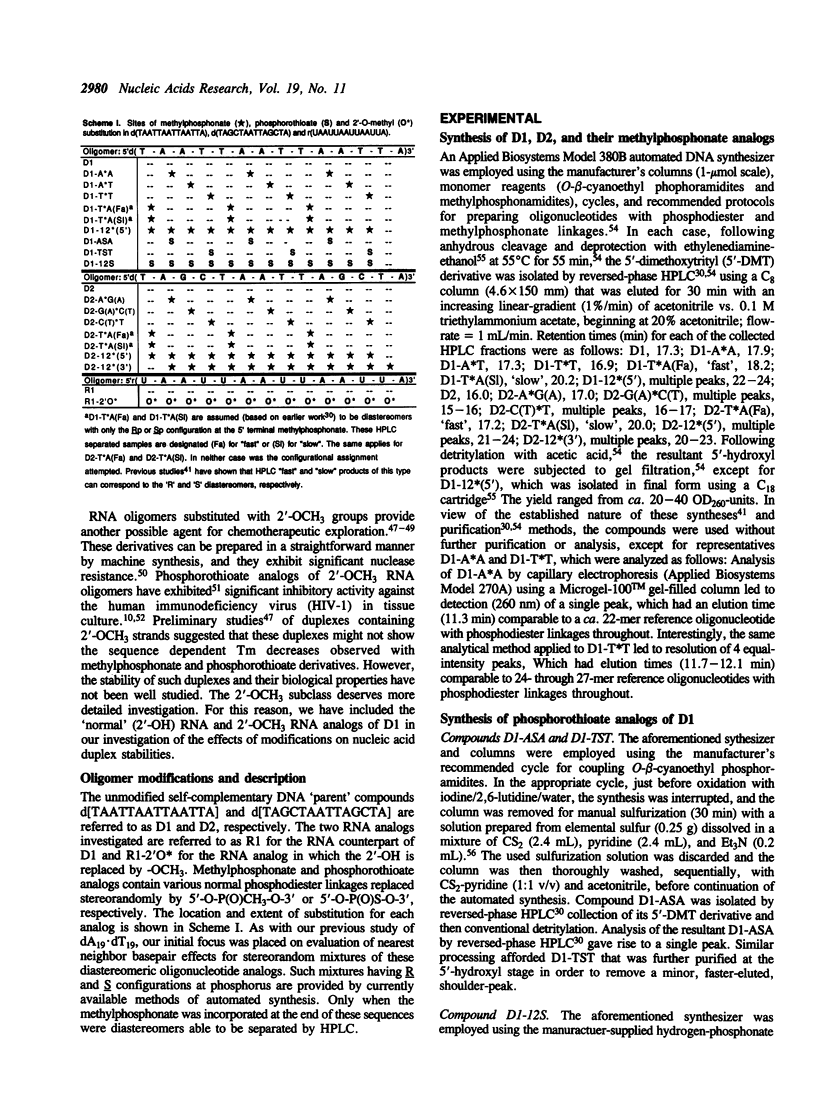
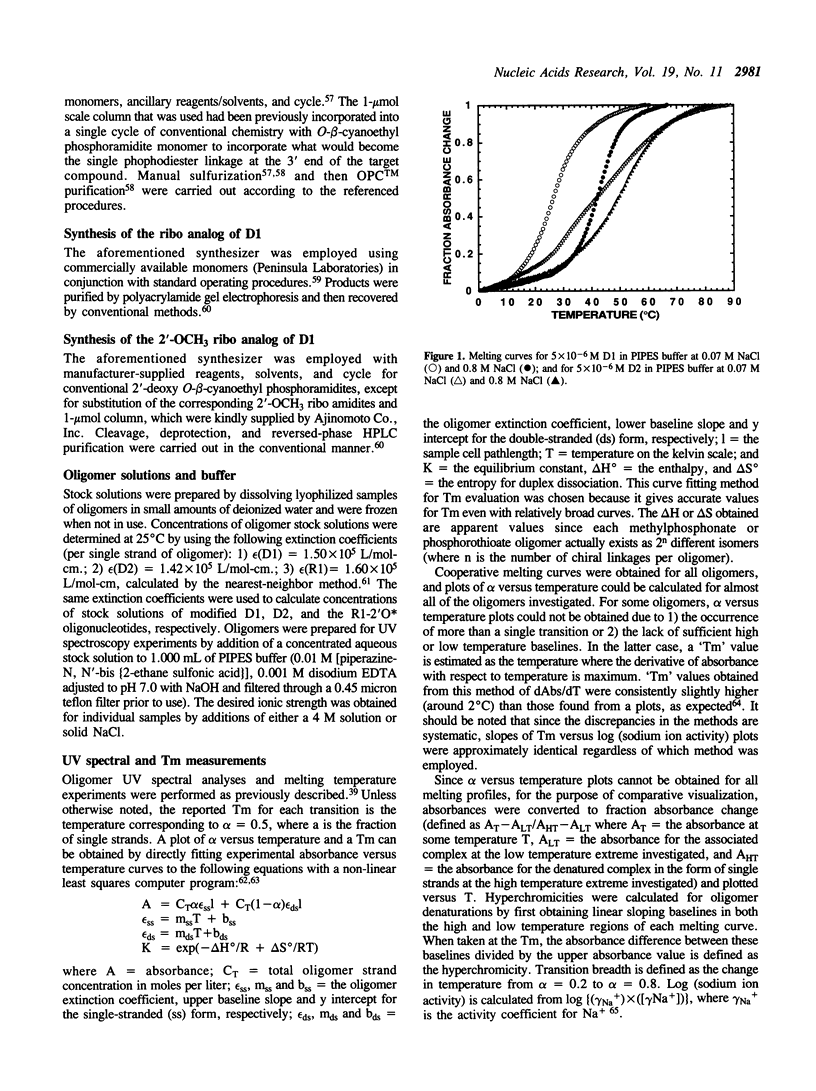
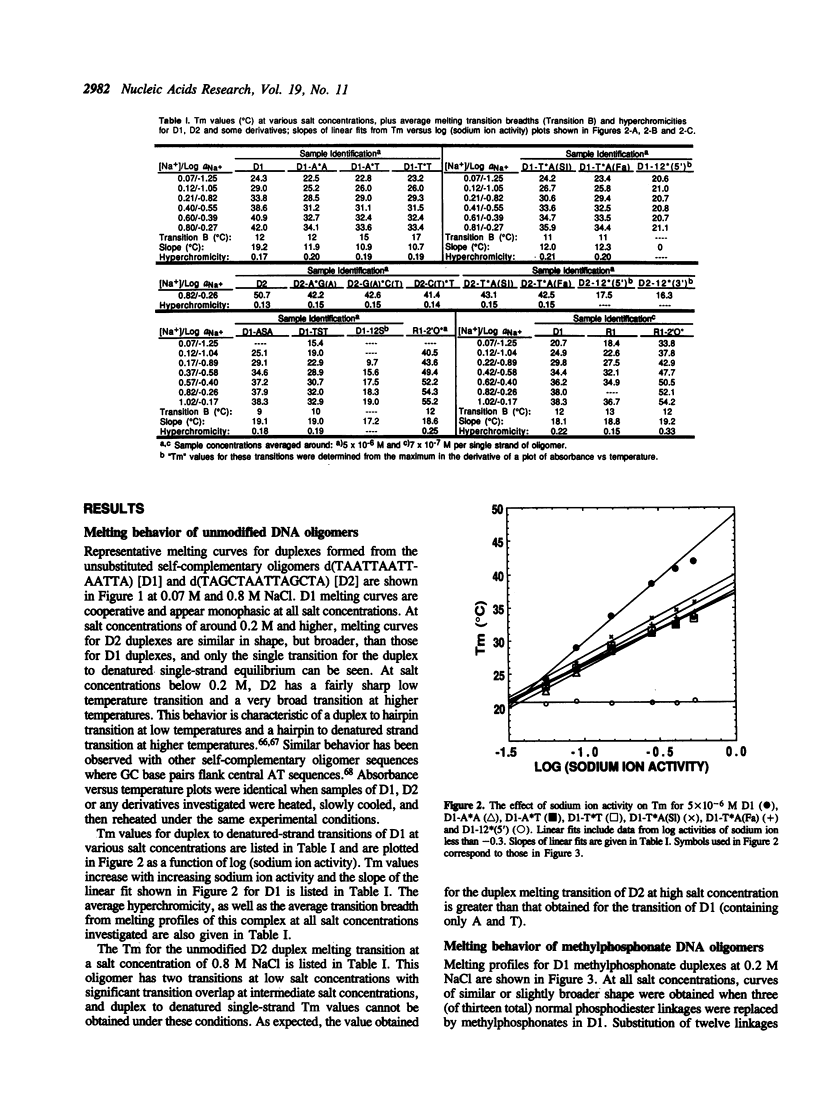
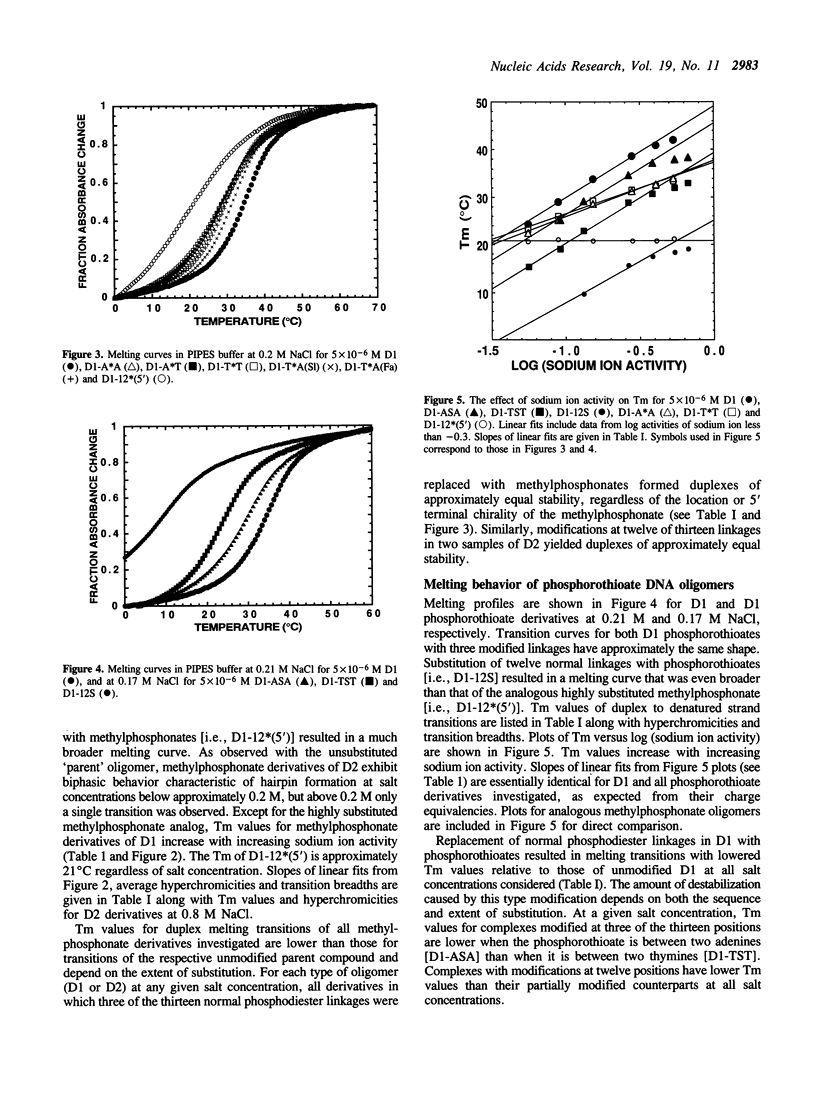
Abstract
The duplex stabilities of various phosphorothioate, methylphosphonate, RNA and 2'-OCH3 RNA analogs of two self-complementary DNA 14-mers are compared. Phosphorothioate and/or methylphosphonate analogs of the two sequences d(TAATTAATTAATTA) [D1] and d(TAGCTAATTAGCTA) [D2] differ in the number, position, or chirality (at the 5' terminal linkage) of the modified phosphates. Phosphorothioate derivatives of D1 are found to be less destabilized when the linkage modified is between adenines rather than between thymines. Surprisingly, no base sequence effect on duplex stabilization is observed for any methylphosphonate derivatives of D1 or D2. Highly modified phosphorothioates or methylphosphonates are less stable than their partially modified counterparts which are less stable than the unmodified parent compounds. The 'normal' (2'-OH) RNA analog of duplex D1 is slightly destabilized, whereas the 2'-OCH3 RNA derivative is significantly stabilized relative to the unmodified DNA. For the D1 sequence, at approximately physiological salt concentration, the order of duplex stability is 2'-OCH3 RNA greater than unmodified DNA greater than 'normal' RNA greater than methylphosphonate DNA greater than phosphorothioate DNA. D2 and the various D2 methylphosphonate analogs investigated all formed hairpin conformations at low salt concentrations.
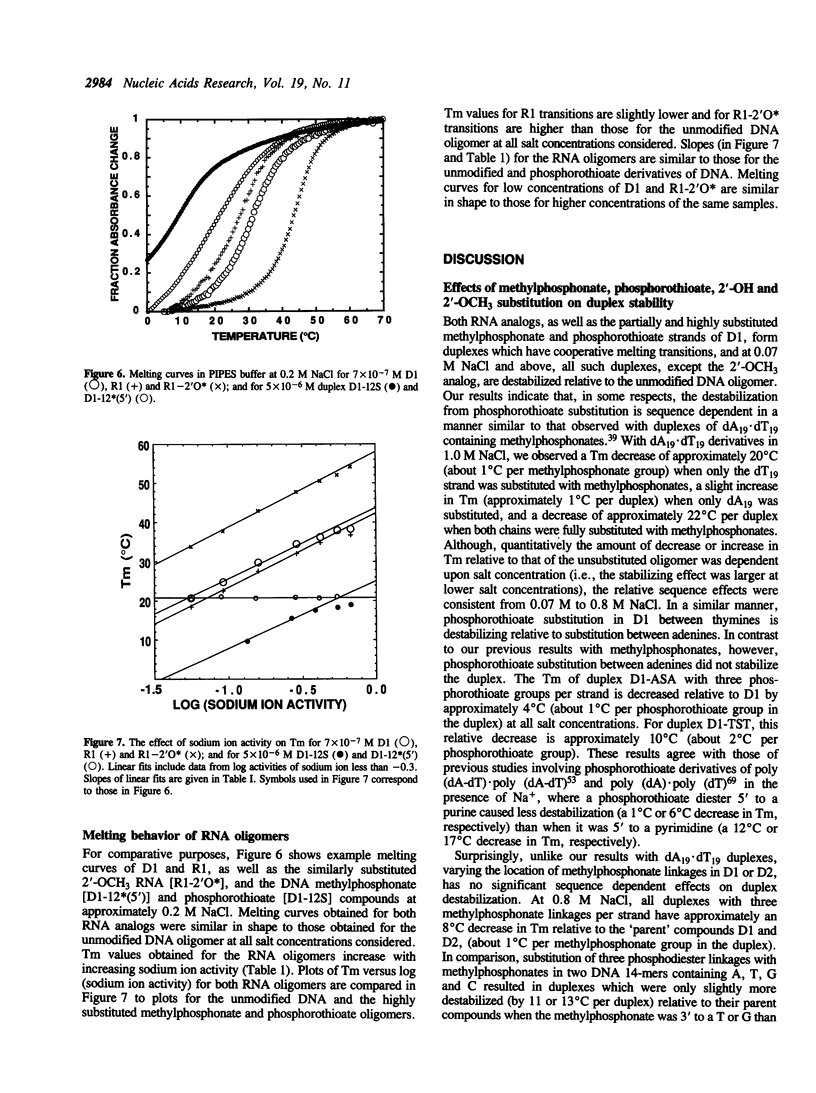
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S., Goodchild J., Civeira M. P., Thornton A. H., Sarin P. S., Zamecnik P. C. Oligodeoxynucleoside phosphoramidates and phosphorothioates as inhibitors of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7079–7083. doi: 10.1073/pnas.85.19.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal S., Ikeuchi T., Sun D., Sarin P. S., Konopka A., Maizel J., Zamecnik P. C. Inhibition of human immunodeficiency virus in early infected and chronically infected cells by antisense oligodeoxynucleotides and their phosphorothioate analogues. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7790–7794. doi: 10.1073/pnas.86.20.7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal S., Mayrand S. H., Zamecnik P. C., Pederson T. Site-specific excision from RNA by RNase H and mixed-phosphate-backbone oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1401–1405. doi: 10.1073/pnas.87.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott S., Hukins D. W., Dover S. D. Optimised parameters for RNA double-helices. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1392–1399. doi: 10.1016/0006-291x(72)90867-4. [DOI] [PubMed] [Google Scholar]
- Baker C., Holland D., Edge M., Colman A. Effects of oligo sequence and chemistry on the efficiency of oligodeoxyribonucleotide-mediated mRNA cleavage. Nucleic Acids Res. 1990 Jun 25;18(12):3537–3543. doi: 10.1093/nar/18.12.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borer P. N., Dengler B., Tinoco I., Jr, Uhlenbeck O. C. Stability of ribonucleic acid double-stranded helices. J Mol Biol. 1974 Jul 15;86(4):843–853. doi: 10.1016/0022-2836(74)90357-x. [DOI] [PubMed] [Google Scholar]
- Bower M., Summers M. F., Powell C., Shinozuka K., Regan J. B., Zon G., Wilson W. D. Oligodeoxyribonucleoside methylphosphonates. NMR and UV spectroscopic studies of Rp-Rp and Sp-Sp methylphosphonate (Me) modified duplexes of (d[GGAATTCC])2. Nucleic Acids Res. 1987 Jun 25;15(12):4915–4930. doi: 10.1093/nar/15.12.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslauer K. J., Frank R., Blöcker H., Marky L. A. Predicting DNA duplex stability from the base sequence. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3746–3750. doi: 10.1073/pnas.83.11.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D., Yu Z. P., Miller P., Blake K., Wei C., Kung H. F., Black R. J., Ts'o P. O., Chang E. H. Modulation of ras expression by anti-sense, nonionic deoxyoligonucleotide analogs. Oncogene Res. 1989;4(4):243–252. [PubMed] [Google Scholar]
- CDe Clercq E., Eckstein F., Sternbach H., Merigan T. C. The antiviral activity of thiophosphate-substituted polyribonucleotides in vitro and in vivo. Virology. 1970 Oct;42(2):421–428. doi: 10.1016/0042-6822(70)90285-0. [DOI] [PubMed] [Google Scholar]
- Cazenave C., Stein C. A., Loreau N., Thuong N. T., Neckers L. M., Subasinghe C., Hélène C., Cohen J. S., Toulmé J. J. Comparative inhibition of rabbit globin mRNA translation by modified antisense oligodeoxynucleotides. Nucleic Acids Res. 1989 Jun 12;17(11):4255–4273. doi: 10.1093/nar/17.11.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. H., Yu Z., Shinozuka K., Zon G., Wilson W. D., Strekowska A. Comparative inhibition of ras p21 protein synthesis with phosphorus-modified antisense oligonucleotides. Anticancer Drug Des. 1989 Oct;4(3):221–232. [PubMed] [Google Scholar]
- Chen T. L., Miller P. S., Ts'o P. O., Colvin O. M., Chem T. L. Disposition and metabolism of oligodeoxynucleoside methylphosphonate following a single i.v. injection in mice. Drug Metab Dispos. 1990 Sep-Oct;18(5):815–818. [PubMed] [Google Scholar]
- Chin D. J., Green G. A., Zon G., Szoka F. C., Jr, Straubinger R. M. Rapid nuclear accumulation of injected oligodeoxyribonucleotides. New Biol. 1990 Dec;2(12):1091–1100. [PubMed] [Google Scholar]
- Cosstick R., Eckstein F. Synthesis of d(GC) and d(CG) octamers containing alternating phosphorothioate linkages: effect of the phosphorothioate group on the B-Z transition. Biochemistry. 1985 Jul 2;24(14):3630–3638. doi: 10.1021/bi00335a035. [DOI] [PubMed] [Google Scholar]
- Dash P., Lotan I., Knapp M., Kandel E. R., Goelet P. Selective elimination of mRNAs in vivo: complementary oligodeoxynucleotides promote RNA degradation by an RNase H-like activity. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7896–7900. doi: 10.1073/pnas.84.22.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap B. E., Friderici K. H., Rottman F. 2'-O-Methyl polynucleotides as templates for cell-free amino acid incorporation. Biochemistry. 1971 Jun 22;10(13):2581–2587. doi: 10.1021/bi00789a026. [DOI] [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- Furdon P. J., Dominski Z., Kole R. RNase H cleavage of RNA hybridized to oligonucleotides containing methylphosphonate, phosphorothioate and phosphodiester bonds. Nucleic Acids Res. 1989 Nov 25;17(22):9193–9204. doi: 10.1093/nar/17.22.9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi H., Endo T., Kaji A. Enzymic synthesis of oligonucleotides containing methylphosphonate internucleotide linkages. Biochemistry. 1990 Sep 18;29(37):8747–8753. doi: 10.1021/bi00489a035. [DOI] [PubMed] [Google Scholar]
- Inoue H., Hayase Y., Imura A., Iwai S., Miura K., Ohtsuka E. Synthesis and hybridization studies on two complementary nona(2'-O-methyl)ribonucleotides. Nucleic Acids Res. 1987 Aug 11;15(15):6131–6148. doi: 10.1093/nar/15.15.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Hayase Y., Iwai S., Ohtsuka E. Sequence-dependent hydrolysis of RNA using modified oligonucleotide splints and RNase H. FEBS Lett. 1987 May 11;215(2):327–330. doi: 10.1016/0014-5793(87)80171-0. [DOI] [PubMed] [Google Scholar]
- Kibler-Herzog L., Kell B., Zon G., Shinozuka K., Mizan S., Wilson W. D. Sequence dependent effects in methylphosphonate deoxyribonucleotide double and triple helical complexes. Nucleic Acids Res. 1990 Jun 25;18(12):3545–3555. doi: 10.1093/nar/18.12.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer H., Sturtevant J. M. Heats of the helix-coil transitions of the poly A-poly U complexes. Biopolymers. 1968 Apr;6(4):491–512. doi: 10.1002/bip.1968.360060406. [DOI] [PubMed] [Google Scholar]
- Kulka M., Smith C. C., Aurelian L., Fishelevich R., Meade K., Miller P., Ts'o P. O. Site specificity of the inhibitory effects of oligo(nucleoside methylphosphonate)s complementary to the acceptor splice junction of herpes simplex virus type 1 immediate early mRNA 4. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6868–6872. doi: 10.1073/pnas.86.18.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlanche L. A., James T. L., Powell C., Wilson W. D., Uznanski B., Stec W. J., Summers M. F., Zon G. Phosphorothioate-modified oligodeoxyribonucleotides. III. NMR and UV spectroscopic studies of the Rp-Rp, Sp-Sp, and Rp-Sp duplexes, [d(GGSAATTCC)]2, derived from diastereomeric O-ethyl phosphorothioates. Nucleic Acids Res. 1986 Nov 25;14(22):9081–9093. doi: 10.1093/nar/14.22.9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer L. J., Hampel K., Lee J. S. Synthetic repeating sequence DNAs containing phosphorothioates: nuclease sensitivity and triplex formation. Nucleic Acids Res. 1989 Feb 25;17(4):1549–1561. doi: 10.1093/nar/17.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer L. J., Hampel K., Lee J. S. Synthetic repeating sequence DNAs containing phosphorothioates: nuclease sensitivity and triplex formation. Nucleic Acids Res. 1989 Feb 25;17(4):1549–1561. doi: 10.1093/nar/17.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter J. M., Agrawal S., Palese P., Zamecnik P. C. Inhibition of influenza virus replication by phosphorothioate oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1990 May;87(9):3430–3434. doi: 10.1073/pnas.87.9.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnikowski Z. J., Jaworska M., Stec W. J. Octa(thymidine methanephosphonates) of partially defined stereochemistry: synthesis and effect of chirality at phosphorus on binding to pentadecadeoxyriboadenylic acid. Nucleic Acids Res. 1990 Apr 25;18(8):2109–2115. doi: 10.1093/nar/18.8.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson J., Brown T., Duff G. Modulation of interleukin 1 beta gene expression using antisense phosphorothioate oligonucleotides. Lymphokine Res. 1990 Spring;9(1):35–42. [PubMed] [Google Scholar]
- Marcus-Sekura C. J. Techniques for using antisense oligodeoxyribonucleotides to study gene expression. Anal Biochem. 1988 Aug 1;172(2):289–295. doi: 10.1016/0003-2697(88)90447-2. [DOI] [PubMed] [Google Scholar]
- Marcus-Sekura C. J., Woerner A. M., Shinozuka K., Zon G., Quinnan G. V., Jr Comparative inhibition of chloramphenicol acetyltransferase gene expression by antisense oligonucleotide analogues having alkyl phosphotriester, methylphosphonate and phosphorothioate linkages. Nucleic Acids Res. 1987 Jul 24;15(14):5749–5763. doi: 10.1093/nar/15.14.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F. H., Tinoco I., Jr DNA-RNA hybrid duplexes containing oligo(dA:rU) sequences are exceptionally unstable and may facilitate termination of transcription. Nucleic Acids Res. 1980 May 24;8(10):2295–2299. doi: 10.1093/nar/8.10.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura M., Shinozuka K., Zon G., Mitsuya H., Reitz M., Cohen J. S., Broder S. Phosphorothioate analogs of oligodeoxynucleotides: inhibitors of replication and cytopathic effects of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7706–7710. doi: 10.1073/pnas.84.21.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura M., Zon G., Shinozuka K., Robert-Guroff M., Shimada T., Stein C. A., Mitsuya H., Wong-Staal F., Cohen J. S., Broder S. Regulation of viral expression of human immunodeficiency virus in vitro by an antisense phosphorothioate oligodeoxynucleotide against rev (art/trs) in chronically infected cells. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4244–4248. doi: 10.1073/pnas.86.11.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura M., Zon G., Shinozuka K., Stein C. A., Mitsuya H., Cohen J. S., Broder S. Synthesis of phosphorothioate analogues of oligodeoxyribonucleotides and their antiviral activity against human immunodeficiency virus (HIV). Gene. 1988 Dec 10;72(1-2):343–347. doi: 10.1016/0378-1119(88)90161-8. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Annan N. D., McParland K. B., Pulford S. M. Oligothymidylate analogues having stereoregular, alternating methylphosphonate/phosphodiester backbones as primers for DNA polymerase. Biochemistry. 1982 May 11;21(10):2507–2512. doi: 10.1021/bi00539a033. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Reddy M. P., Murakami A., Blake K. R., Lin S. B., Agris C. H. Solid-phase syntheses of oligodeoxyribonucleoside methylphosphonates. Biochemistry. 1986 Sep 9;25(18):5092–5097. doi: 10.1021/bi00366a017. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Yano J., Yano E., Carroll C., Jayaraman K., Ts'o P. O. Nonionic nucleic acid analogues. Synthesis and characterization of dideoxyribonucleoside methylphosphonates. Biochemistry. 1979 Nov 13;18(23):5134–5143. doi: 10.1021/bi00590a017. [DOI] [PubMed] [Google Scholar]
- Noble S. A., Fisher E. F., Caruthers M. H. Methylphosphonates as probes of protein-nucleic acid interactions. Nucleic Acids Res. 1984 Apr 11;12(7):3387–3404. doi: 10.1093/nar/12.7.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum G. E., Bloomfield V. A. Structural and electrostatic effects on binding of trivalent cations to double-stranded and single-stranded poly[d (AT)]. Biopolymers. 1990 Jan;29(1):13–27. doi: 10.1002/bip.360290105. [DOI] [PubMed] [Google Scholar]
- Puglisi J. D., Tinoco I., Jr Absorbance melting curves of RNA. Methods Enzymol. 1989;180:304–325. doi: 10.1016/0076-6879(89)80108-9. [DOI] [PubMed] [Google Scholar]
- Quartin R. S., Brakel C. L., Wetmur J. G. Number and distribution of methylphosphonate linkages in oligodeoxynucleotides affect exo- and endonuclease sensitivity and ability to form RNase H substrates. Nucleic Acids Res. 1989 Sep 25;17(18):7253–7262. doi: 10.1093/nar/17.18.7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartin R. S., Wetmur J. G. Effect of ionic strength on the hybridization of oligodeoxynucleotides with reduced charge due to methylphosphonate linkages to unmodified oligodeoxynucleotides containing the complementary sequence. Biochemistry. 1989 Feb 7;28(3):1040–1047. doi: 10.1021/bi00429a018. [DOI] [PubMed] [Google Scholar]
- Reed J. C., Stein C., Subasinghe C., Haldar S., Croce C. M., Yum S., Cohen J. Antisense-mediated inhibition of BCL2 protooncogene expression and leukemic cell growth and survival: comparisons of phosphodiester and phosphorothioate oligodeoxynucleotides. Cancer Res. 1990 Oct 15;50(20):6565–6570. [PubMed] [Google Scholar]
- Riley M., Maling B. Physical and chemical characterization of two- and three-stranded adenine-thymine and adenine-uracil homopolymer complexes. J Mol Biol. 1966 Sep;20(2):359–389. doi: 10.1016/0022-2836(66)90069-6. [DOI] [PubMed] [Google Scholar]
- Scheffler I. E., Elson E. L., Baldwin R. L. Helix formation by dAT oligomers. I. Hairpin and straight-chain helices. J Mol Biol. 1968 Sep 28;36(3):291–304. doi: 10.1016/0022-2836(68)90156-3. [DOI] [PubMed] [Google Scholar]
- Shibahara S., Mukai S., Morisawa H., Nakashima H., Kobayashi S., Yamamoto N. Inhibition of human immunodeficiency virus (HIV-1) replication by synthetic oligo-RNA derivatives. Nucleic Acids Res. 1989 Jan 11;17(1):239–252. doi: 10.1093/nar/17.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara S., Mukai S., Nishihara T., Inoue H., Ohtsuka E., Morisawa H. Site-directed cleavage of RNA. Nucleic Acids Res. 1987 Jun 11;15(11):4403–4415. doi: 10.1093/nar/15.11.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth J., Matthews G., Dale L., Baker C., Colman A. Antisense oligodeoxyribonucleotide-directed cleavage of maternal mRNA in Xenopus oocytes and embryos. Gene. 1988 Dec 10;72(1-2):267–275. doi: 10.1016/0378-1119(88)90152-7. [DOI] [PubMed] [Google Scholar]
- Stec W. J., Zon G., Uznanski B. Reversed-phase high-performance liquid chromatographic separation of diastereomeric phosphorothioate analogues of oligodeoxyribonucleotides and other backbone-modified congeners of DNA. J Chromatogr. 1985 Jun 19;326:263–280. doi: 10.1016/s0021-9673(01)87452-5. [DOI] [PubMed] [Google Scholar]
- Stein A., Iversen P. L., Subasinghe C., Cohen J. S., Stec W. J., Zon G. Preparation of 35S-labeled polyphosphorothioate oligodeoxyribonucleotides by use of hydrogen phosphonate chemistry. Anal Biochem. 1990 Jul;188(1):11–16. doi: 10.1016/0003-2697(90)90521-a. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Cohen J. S. Oligodeoxynucleotides as inhibitors of gene expression: a review. Cancer Res. 1988 May 15;48(10):2659–2668. [PubMed] [Google Scholar]
- Suggs J. W., Taylor D. A. Evidence for sequence-specific conformational changes in DNA from the melting temperatures of DNA phosphorothioate derivatives. Nucleic Acids Res. 1985 Aug 12;13(15):5707–5716. doi: 10.1093/nar/13.15.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidd D. M., Hawley P., Warenius H. M., Gibson I. Evaluation of N-ras oncogene anti-sense, sense and nonsense sequence methylphosphonate oligonucleotide analogues. Anticancer Drug Des. 1988 Aug;3(2):117–127. [PubMed] [Google Scholar]
- Ts'o P. O., Miller P. S., Aurelian L., Murakami A., Agris C., Blake K. R., Lin S. B., Lee B. L., Smith C. C. An approach to chemotherapy based on base sequence information and nucleic acid chemistry. Matagen (masking tape for gene expression). Ann N Y Acad Sci. 1987;507:220–241. doi: 10.1111/j.1749-6632.1987.tb45804.x. [DOI] [PubMed] [Google Scholar]
- Turner D. H., Sugimoto N., Freier S. M. RNA structure prediction. Annu Rev Biophys Biophys Chem. 1988;17:167–192. doi: 10.1146/annurev.bb.17.060188.001123. [DOI] [PubMed] [Google Scholar]
- Woolf T. M., Jennings C. G., Rebagliati M., Melton D. A. The stability, toxicity and effectiveness of unmodified and phosphorothioate antisense oligodeoxynucleotides in Xenopus oocytes and embryos. Nucleic Acids Res. 1990 Apr 11;18(7):1763–1769. doi: 10.1093/nar/18.7.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xodo L. E., Manzini G., Quadrifoglio F., van der Marel G. A., van Boom J. H. Oligodeoxynucleotide folding in solution: loop size and stability of B-hairpins. Biochemistry. 1988 Aug 23;27(17):6321–6326. doi: 10.1021/bi00417a018. [DOI] [PubMed] [Google Scholar]
- Zon G. Oligonucleotide analogues as potential chemotherapeutic agents. Pharm Res. 1988 Sep;5(9):539–549. doi: 10.1023/a:1015985728434. [DOI] [PubMed] [Google Scholar]
- Zuo E. T., Tanious F. A., Wilson W. D., Zon G., Tan G. S., Wartell R. M. Effect of base-pair sequence on the conformations and thermally induced transitions in oligodeoxyribonucleotides containing only AT base pairs. Biochemistry. 1990 May 8;29(18):4446–4456. doi: 10.1021/bi00470a027. [DOI] [PubMed] [Google Scholar]



